Abstract
In vascular plants, the polysaccharide-based walls of water-conducting cells are strengthened by impregnation with the polyphenolic polymer lignin. The fine-scale patterning of lignin deposition in water-conducting cells is shown here to vary phylogenetically across vascular plants. The extent to which water transport in xylem cells can be modified in response to changes in the ionic content of xylem sap also is shown to vary in correlation with variation in lignification patterns, consistent with the proposed mechanism for hydraulic response through size change of middle-lamella pectins. This covariation suggests that the fine-scale distribution of hydrophilic polysaccharides and hydrophobic lignin can affect hydraulic as well as mechanical properties, and that the evolutionary diversification of vascular cells thus reflects biochemical as well as morphological innovations evolved to fulfill opposing cell functions of transport and structural support.
Keywords: cell wall, chemistry, hydraulics, lignin, plant
The cells of land plants have walls constructed primarily of polysaccharides, including cellulose, hemicelluloses, and pectins. In some cells, notably the water-conducting tracheids and vessel elements of xylem tissues in vascular plants, walls are strengthened by secondary walls with complex patterns of thickening and by impregnation with the polyphenolic polymer lignin. This evolutionary innovation allowed for both whole-plant structural support and resistance to cell collapse under the tension of water transport. It has long been understood that the size and shape of conducting cells reflect the conflicting architectural demands of mechanical support and water flow (1). The recent in vivo demonstration (2) that ionic concentration can alter flow rates in the xylem of some plants opens the possibility that the opposing functional requirements of conducting cells extend to the chemistry of their walls. Here, we demonstrate that lignin placement within conducting cell walls covaries with the capacity of xylem to modify flow rate. This covariation suggests that the fine-scale distribution of hydrophilic polysaccharides and hydrophobic lignin can affect hydraulic as well as biomechanical properties of conducting cells in vascular plants.
It has been thought that since the Silurian–Devonian evolution of tracheid lignification (3–8), tracheid wall structure has been stable (4, 9). Different disciplines, however, offer divergent descriptions of that structure. Industrial wood and paper chemists indicate that lignification is greatest in the thin primary wall and middle lamellae between cells (10). In contrast, botanists (5), paleobotanists (4, 11), and molecular biologists (12) emphasize lignification of the secondary wall. This discrepancy arises at least in part because available methods of investigating lignin distribution are limited in either spatial or chemical resolution and because of a research emphasis on important timber-producing trees. X-ray spectromicroscopy (13–15) allows investigation of fine-scale lignin distributions; here, we apply this emerging technique to a phylogenetically diverse set of vascular plants for comparison with phylogenetic variations in xylem flow-rate responses to changes in sap ionic concentrations.
Methods
X-Ray Spectromicroscopy. Samples consisted of 100- to 150-nm ultramicrotome sections of stem or petiole vascular tissue that had been excised from dried plant specimens and embedded in epoxy. All wood samples were from the sapwood of small, leafy, 2- to 3-year-old branches. X-ray spectromicroscopy is currently limited by access to a unique analytical facility (X-1A beamline, National Synchrotron Light Source, Brookhaven National Laboratory, Upton, NY). Nonetheless, in terms of chemical and spatial resolution, it affords an effective alternative to more commonly used techniques such as transmission electron microscopy, UV spectrometry, and traditional light microscopy staining techniques. The technique takes advantage of the fact that different types of molecular bonds differentially absorb x-rays of different frequencies. Xylem samples were examined at 285–286 eV (1 eV = 1.602 × 10–19 J) (Fig. 1). Because absorption in this frequency range is by aromatic carbon, images effectively map the distribution of lignin, the only major aromatic constituent of plant cell walls. Each sample was imaged either on the aromatic carbon absorption peak (285.2 eV) or just off (286 eV), depending on slight differences in thickness between samples. Epoxy produces a characteristic, extremely sharp, and easily detectable x-ray absorption spike; it does not infiltrate the walls, and the 100-nm spatial resolution of the analyses prevents the presence of epoxy in the cell lumen from altering assessment of native wall chemistry. Samples also were examined at the carboxylate absorption peak (288.5 eV). Sources of x-ray absorption at 288.5 eV will include nonaromatic wall polysaccharides, thereby allowing verification that a lack of x-ray absorption at 285.2 eV indicates unlignified wall material rather than a hole in the section.
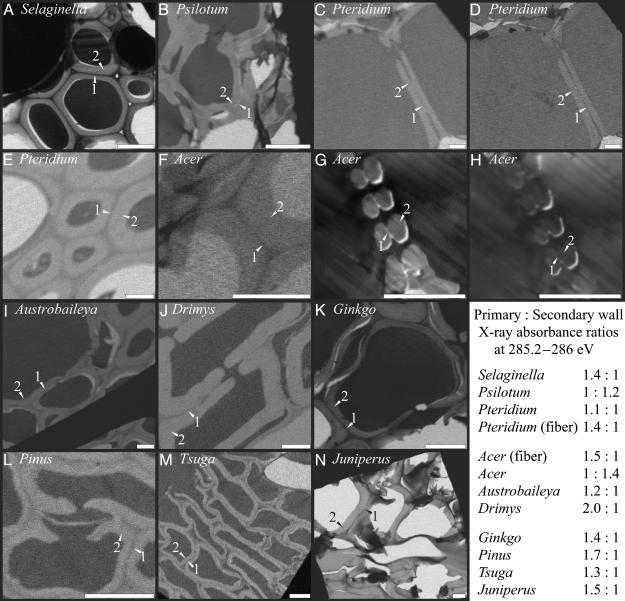
Fig. 1.
X-ray microscopy images of xylem cells with darker shades indicating greater x-ray absorbance and lignin abundance. Images were taken at the 285.2- to 286-eV absorption peak for aromatic carbon except for D and H, which were taken at the 288.5-eV absorption peak for carboxylate carbon most prevalent in pectins. All images are of water-conducting cells except for E and F (sclerenchyma cells). Primary walls (1) and secondary walls (2) are labeled in each image. Nearly black or white areas found in cell lumens are epoxy or holes in the section. (Scale bar, 6 μm.)
Measurement of Xylem Hydraulic Properties. Deionized water or KCl (20 mM solution) was pushed at 0.05 MPa pressure through 10-cm-long segments of fresh stem or frond petiole with outflow to a balance (model 210, Sartorius) with accuracy of 0.0001 g for determination of flow rate. Because temperature and driving force remained constant throughout the measurement, changes in the measured flow rate reflect changes in stem hydraulic conductance. KCl and deionized water measurements were performed on all samples, which were flushed with deionized water before and between measurements. Five replicate samples were used for each species. Further details are available elsewhere (2).
Results
In Ginkgo and conifer tracheids, the compound primary wall (primary wall and middle lamella together) is more heavily lignified than the secondary wall. The same is true of two basal angiosperms in which vessel elements are either relatively unmodified or absent, Austrobaileya and Drimys. However, a more derived angiosperm, the eudicot Acer, has unlignified compound primary walls between vessel elements. Spore-bearing vascular plants exhibit a range of lignification patterns: the lycopod Selaginella possesses highly lignified compound primary walls similar to those of conifers, and the leafless pteridophyte Psilotum develops unlignified compound primary walls similar to those of Acer, whereas Pteridium, a dennstaedtiacean fern, represents an intermediate state in which all layers exhibit equal, light lignification. Where data are available, the chemistry of the compound primary wall within pits, i.e., the pit membrane, is consistent with that of the primary wall adjacent to the pits (e.g., Fig. 1 B and J–L); however, observations of pit membranes lie near the current edge of x-ray spectroscopic resolution. Expected advances in microscope capabilities should allow more quantitative assessment of pit membrane chemistry in the future.
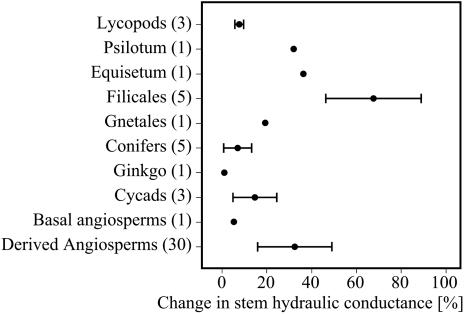
Observed differences in lignification (Fig. 1) correspond well with variations in ion-mediated changes in xylem hydraulic properties among different taxa (Fig. 2). Ferns and Psilotum showed a strong flow increase in response to low concentrations of potassium ions in the xylem sap relative to the flow of deionized water. More derived angiosperms also exhibited a large response, but the basal angiosperm Drimys showed only a weak flow increase, as did lycopods, conifers, and Ginkgo. This correlation between lignification patterns and the capacity for ion-mediated, reversible modification of xylem flow rate supports the hypothesis (2) that observed changes in xylem conductance are due to volume changes of middle-lamella pectins. Zwieniecki et al. (2) demonstrated that the addition of potassium ions to xylem water increased flow rates in the vessels of woody angiosperms and hypothesized that observed changes in conductance were mediated by middle-lamella pectins, which acted as a hydrogel, expanding or contracting with changes in ionic concentration. Our new measurements strengthen this hypothesis and suggest that the chemistry of the compound primary cell wall further contributes to the observed variations among taxa in the capacity to modify xylem conductance. Divergences in pit structure across the vascular plants may contribute to overall differences in xylem conductance, but these anatomical distinctions do not correlate with observed variations in the degree of ion-mediated changes in xylem hydraulic properties.
Fig. 2.
Percent increase of stem hydraulic conductance (mean ± standard deviation) measured with 20 mM KCl solution over conductance determined with deionized water. Numbers in parentheses reflect number of different species used in the analysis.
Discussion
Lignin contributes to the mechanical properties of the cell wall, influencing both rigidity and compressional strength (16). Low-lignin mutants of Arabidopsis thaliana develop irregularly shaped xylem vessels with reduced mechanical strength (17). Similar deformation has been observed under extreme xylem tensions (18), demonstrating the importance of the mechanical properties of conduit walls in water transport (19). Primary-wall lignification also influences the mechanical interactions between cells. For example, adjacent tracheids commonly pull apart in trees with decreased primary-wall lignification (20). This, then, is where the functional tradeoff of cell wall chemistry arises. Weak lignification of compound primary walls should enhance ion-mediated changes in xylem flow rate, but it also will diminish cell adhesion among tracheids and vessels, reducing the effective capacity of conducting cells to withstand mechanical stresses associated with water transport under tension. The functional conflict between optimal sites of lignin deposition for hydraulic regulation and the maximization of structural support is partially accommodated by variations within individual cells, so that cell corners away from pits and walls in contact with nonconducting cells tend to have more highly lignified compound primary walls (e.g., Fig. 1 I and K). However, in the spore-bearing and flowering plants for which hydraulic regulation may be a possibility, water-conducting cells play only a secondary role in structural support. In these taxa, support is provided by sclerenchyma cells with heavy primary-wall lignification (Fig. 1 E and F). Thus, the evolutionary derivation of both vessel elements and fibers from ancestral tracheids, long discussed in terms of the important structural and transport effects of diverging cell dimensions and wall anatomy, also must be evaluated in terms of divergent wall chemistry.
Evolutionary variations in lignification therefore represent fine-scale modification of conducting cell development related to alternative functional optima for sites of lignin deposition. This evolutionary diversification is reflected in the diversity of views on lignification found in the literature. Wood chemistry research is typically conducted on conifers, notably Pinus, for which lignification is greatest in the compound primary wall (10). Molecular work emphasizing secondary wall lignification has relied upon the Zinnia leaf model system (12, 21), a derived angiosperm for which our work would support weak primary-wall lignin content, perhaps compounded by the possibilities that lignification of the middle lamella may be compromised in the loose cells of tissue cultures and that leaf tracheids may differ in lignification pattern from wood.
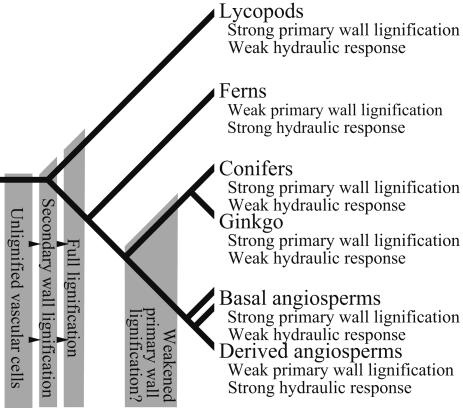
The diverse tracheid wall anatomies found among Early Devonian vascular plants (8) trend from unlignified conducting cells (3) through increasingly lignified tracheids, initially in the secondary wall (4, 5, 8, 11) (Fig. 3). Such small, simple plants could have relied exclusively on turgor pressure for structural support (16), but the evolution of larger, more complex plants beginning in the later Early and Middle Devonian likely required xylem-based structural support and, therefore, compound primary-wall lignification. Pending ecophysiological investigation of the role of hydrogel transport regulation, it is hypothesized that this mechanism plays an important role in balancing photosynthetic resource distribution in lineages with large laminate leaves. Thus, the Late Devonian and Carboniferous diversification of pteridophyte and seed plant lineages with fern-like foliage may have relied on a decrease in compound primary-wall lignification in tracheids and a concomitant increase in reliance on fibers for support. Lineages such as conifers and Ginkgo that continued to rely on xylem for structural support, and, therefore, maintained primary-wall lignification, have linear leaves or small leaves with simple laminae.
Fig. 3.
Phylogenetic distribution of lignification patterns and ionic mediation of xylem hydraulic properties. Descriptions of terminal taxa are based on current analyses of living plants. Gray boxes indicate inferred lignification states for extinct ancestral vascular plant and seed plant groups based on previous x-ray spectromicroscopic and taphonomic studies of fossils (3, 4, 6, 8, 11), except for the state of ancestral seed plants hypothesized here based on comparison of anatomy and reconstructed whole-plant morphology with that of extant plants analyzed in this study. Hypotheses concerning ancestral states are testable with further geochemical analysis of fossils.
Hypotheses concerning the physiological significance of specific lignification patterns can be tested with future investigation of the vasculature of different organ types as well as a more ecologically and phylogenetically diverse range of taxa; as much divergence may be expected among more derived angiosperms as documented here between derived and basal taxa. Furthermore, the demonstration that x-ray spectromicroscopy can resolve patterns of lignification in anatomically preserved fossils (3, 13) opens the way for direct paleontological tests of hypotheses of physiological and functional divergence of tracheids in Paleozoic plants.
Acknowledgments
We thank E. Wheeler, K. Niklas, and J. Sperry for insightful reviews of the manuscript. This research was supported by the Andrew W. Mellon Foundation, U.S. Department of Agriculture Grant NRICGP 2001-35100-10615 (to N.M.H.), National Science Foundation Grant ERA 0106816 (to A.H.K.), and a National Research Council/National Aeronautics and Space Administration Astrobiology Institute associateship (to C.K.B.). X-ray microscopy, performed on the X-1A beamline, was developed by the research group of J. Kirz and C.J. at Stony Brook University with support from the Office of Biological and Environmental Research at the U.S. Department of Energy (DE-FG02-89ER60858) and National Science Foundation (DBI-9605045) at the National Synchrotron Light Source, a Department of Energy-supported facility.
Author contributions: C.K.B. and M.A.Z. designed research; C.K.B., M.A.Z., G.D.C., and S.W. performed research; C.J. contributed new reagents/analytic tools; C.K.B., M.A.Z., A.H.K., and N.M.H. analyzed data; and C.K.B. wrote the paper.
References
- 1.Esau, K. (1953) Plant Anatomy (Wiley, New York).
- 2.Zwieniecki, M. A., Melcher, P. J. & Holbrook, N. M. (2001) Science 291, 1059–1062. [DOI] [PubMed] [Google Scholar]
- 3.Boyce, C. K., Cody, G. D., Fogel, M. L., Hazen, R. M., Alexander, C. M. O. D. & Knoll, A. H. (2003) Int. J. Plant Sci. 164, 691–702. [Google Scholar]
- 4.Wang, D. M., Hao, S. G. & Wang, Q. (2003) Int. J. Plant Sci. 164, 415–427. [Google Scholar]
- 5.Friedman, W. E. & Cook, M. E. (2000) Philos. Trans. R. Soc. London B 355, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenrick, P. & Crane, P. R. (1991) Bot. Gaz. 152, 355–356. [Google Scholar]
- 7.Kenrick, P. & Crane, P. R. (1997) Nature 389, 33–39. [Google Scholar]
- 8.Edwards, D. (1993) Plant Cell Environ. 26, 57–72. [Google Scholar]
- 9.Schmid, R. (1967) Am. J. Bot. 54, 720–729. [Google Scholar]
- 10.Sjöström, E. (1993) Wood Chemistry: Fundamentals and Applications (Academic, San Diego).
- 11.Kenrick, P., Edwards, D. & Dales, R. C. (1991) Palaeontology 34, 751–766. [Google Scholar]
- 12.McCann, M. C. (1997) Trends Plant Sci. 2, 333–338. [Google Scholar]
- 13.Boyce, C. K., Cody, G. D., Fesser, M., Jacobsen, C., Knoll, A. H. & Wirick, S. (2002) Geology 30, 1039–1042. [Google Scholar]
- 14.Jacobsen, C. & Kirz, J. (1998) Nat. Struct. Biol. 5, S650–S653. [DOI] [PubMed] [Google Scholar]
- 15.Cody, G. D., Botto, R. E., Ade, H. & Wirick, S. (1996) Int. J. Coal Geology 32, 69–86. [Google Scholar]
- 16.Niklas, K. (1992) Plant Biomechanics: An Engineering Approach to Plant Form and Function (Univ. of Chicago Press, Chicago).
- 17.Jones, L., Ennos, A. R. & Turner, S. R. (2001) Plant J. 26, 205–216. [DOI] [PubMed] [Google Scholar]
- 18.Cochard, H., Froux, F., Mayr, S. & Coutand, C. (2004) Plant Physiol. 134, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacke, U. G., Sperry, J. S., Pockman, W. T., Davis, S. D. & McCulloh, K. (2001) Oecologia 126, 457–461. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson, L. A. (2002) IAWA J. 23, 161–178. [Google Scholar]
- 21.Boudet, A. M., Lapierre, C. & Grima-Pettenati, J. (1995) New Phytol. 129, 203–236. [DOI] [PubMed] [Google Scholar]