Abstract
Prenatal cocaine exposure remains a major public health concern because of its adverse impact on cognitive function in children and adults. We report that prenatal cocaine exposure produces significant deficits in reversal learning, a key component of cognitive flexibility, in a mouse model. We used an olfactory reversal learning paradigm and found that the prenatally cocaine-exposed mice showed a marked failure to learn the reversed paradigm. Because brain-derived neurotrophic factor (BDNF) is a key regulator of cognitive functions, and because prenatal cocaine exposure increases the expression of BDNF and the phosphorylated form of its receptor, tyrosine kinase B (TrkB), we examined if BDNF-TrkB signaling is involved in mediating the reversal learning deficit in the prenatally cocaine exposed mice. Systemic administration of a selective TrkB receptor antagonist restored normal reversal learning in the prenatally cocaine-exposed mice, suggesting that increased BDNF-TrkB signaling may be an underlying mechanism of reversal learning deficit. Our findings provide novel mechanistic insights into the reversal learning phenomenon and may have significant translational implications, because impaired cognitive flexibility is a key symptom in psychiatric conditions of developmental onset.
Keywords: Cognitive flexibility, brain derived neurotrophic factor, cocaine
Introduction
Reversal learning is a measure of behavioral or cognitive flexibility in human subjects and animal models [1–3]. Cognitive flexibility is the ability to switch from one learned response choice to another under changing reward contingencies. It is a key component of a group of higher order cognitive functions known as executive functions, which are critically important for success in intellectual, social, interpersonal and professional activities [4]. Human cognitive flexibility is the mental ability to seamlessly switch between different concepts or simultaneously tackle multiple mental tasks, and it requires coordination of cognitive processes including previously learned responses, working memory and attention [4,5]. Cognitive flexibility is acquired in infancy and continues to be critical for cognitive functions throughout life. While the precise etiology of impaired cognitive flexibility, i.e. cognitive inflexibility, is not known, it can occur with aging and also as a result of drug abuse and addiction [6–8]. Additionally, cognitive inflexibility is a key symptom of neuro-psychiatric conditions of developmental origin including schizophrenia, autism spectrum disorder, attention deficit hyperactivity disorder and obsessive compulsive disorder [3,4,9–11]. Advances in neuroscience suggest that cognitive inflexibility is the result of impairment in neural networks of the frontal lobes of the brain [1,3,12]. However, the precise molecular mechanisms underlying cognitive inflexibility are not well understood.
Cocaine abuse by pregnant women continues to be a major public health concern throughout the world. A 2009 study estimated that approximately 7.5 million children were exposed to cocaine prenatally in the United States alone [13]. Prenatal cocaine exposure produces significant deficits in cognitive function, some of which can last a lifetime [14–19]. Although a link has been reported between cocaine exposure and cognitive inflexibility [20–22], the molecular mechanisms underlying such association have not been established.
We used a mouse model of prenatal cocaine exposure [23–28] to examine its impact on reversal learning, a key component of cognitive flexibility. We found that prenatal cocaine exposure produced significant deficits in reversal learning. Earlier work has demonstrated that prenatal cocaine exposure impairs brain-derived neurotrophic factor (BDNF) signaling in the embryonic and adult mouse brain [27] (Review in [29]). Specifically, we have shown that prenatally cocaine-exposed adult mice show increased expression of BDNF and the phosphorylated form of its receptor TrkB in the frontal cortex [30] Given the critical role of BDNF in a variety of cognitive functions [31–33], we examined whether excessive BDNF-TrkB signaling is associated with impaired reversal learning in a prenatal cocaine exposure mouse model. Our results demonstrate that systemic administration of a selective TrkB antagonist restored the reversal learning phenotype and decreased TrkB phosphorylation in the frontal cortex, highlighting BDNF-TrkB signaling as a potential mechanism underlying the reversal learning deficits produced by prenatal cocaine exposure.
Materials and Methods
Animals
All experimental procedures were in full compliance with institutional guidelines at Florida State University and the NIH Guide for the Care and Use of Laboratory Animals Timed-pregnant Swiss Webster mice were used. The day of vaginal plug detection was considered embryonic day 0 (EO) and the day of birth postnatal day 0 (P0). Pregnant dams of comparable body weight were assigned to prenatal saline or cocaine exposure groups on the 6th day of pregnancy. Singly-housed dams received twice daily (7 AM and 7 PM) subcutaneous injections of cocaine (20 mg/kg/injection: total daily dose = 40 mg/kg/day) or saline (same volume and time of administration as the cocaine injection) from the 8th day of pregnancy until the day of birth. The dose of cocaine used here (20 mg/kg; subcutaneous) results in a fetal plasma cocaine concentration of 2.3µM (30 min post-injection) and 0.6µM (120 min post-injection), which is comparable to the 1.1 µM concentration reported in humans following 0.6mg/kg intravenous cocaine administration [34]. All offspring were cross-fostered to drug-naive dams within 2 days of birth to eliminate potential effects of the experimental interventions on mother-infant interactions. Offspring were weaned on P21. For the behavioral tests, two mice were chosen from five litters for each prenatal treatment group. Male mice were used for olfactometry around P90, after which brain tissue samples were collected for protein biochemistry. Only male mice were used because our previous data showed that prenatal cocaine exposure produced changes in BDNF-TrkB signaling in male but not female mice [30].
Olfactometry
Mice were trained in a “go no-go” operant conditioning paradigm to recognize an odorant using a computerized, 8-channel liquid dilution olfactometer (Knosys LD-8, Tampa, FL.) as previously described [30,31]. Briefly, the automated olfactometer permitted precise odorant discrimination and odor-reversal paradigms by achieving reliable control over timing of onset and offset of stimulus presentation and precise control over monitoring of behavior. During olfactometry, mice were water deprived by restricting water intake to 1 – 1.5 ml per day and maintained at 85% of their pre-deprivation body weight. Positively entrained odors (referred as S+) were paired with a water reward whereas negatively entrained odors (S−) received no reward and a 10 second punishment period while the mice waited to initiate a new trial. Ethyl acetate (5%, EA) or acetophenone (1%, AP) were used as odors and water was used as the diluent.
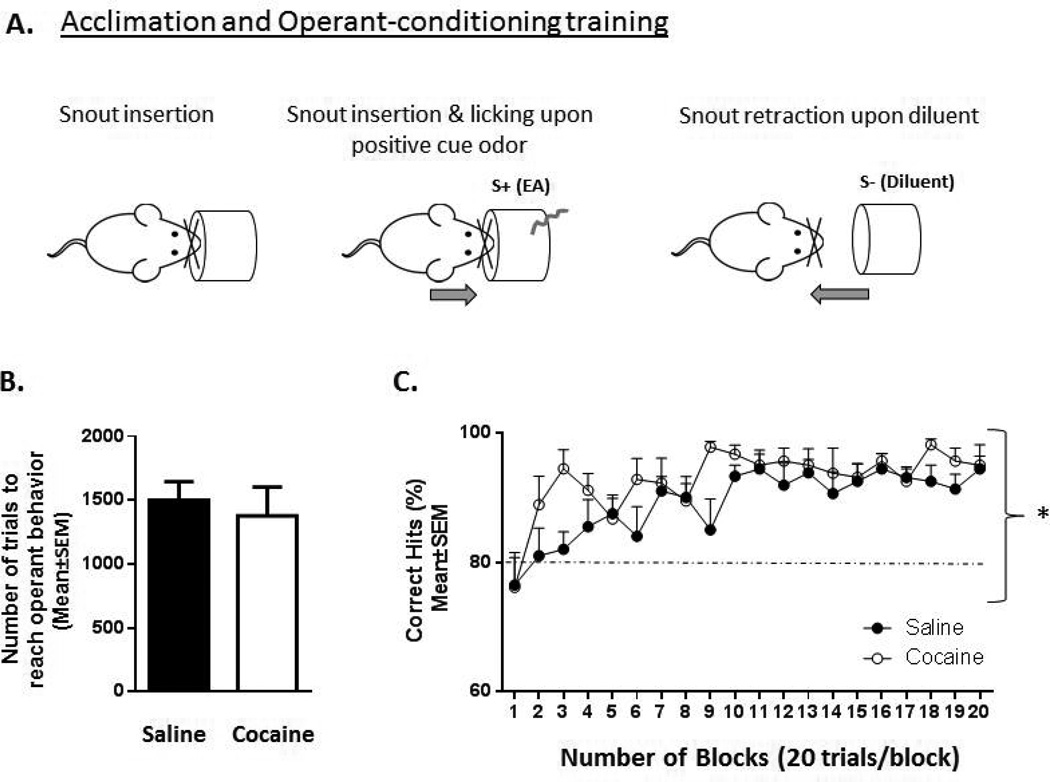
Acclimation and operant-conditioning training: (Fig. 1A)
Figure 1.
Schematic illustration of the acclimation and operant training phases (A), and the performance of prenatally saline- or cocaine-exposed adult mice during operant training (B, C). A. Mice insert their snouts into an odor-sampling port to detect a positively entrained odor cue (S+, ethyl acetate, EA) or a negatively entrained odor cue (S− diluent (water)). Right arrow = snout insertion and water reinforcement. Left arrow = snout withdrawal and punishment or timeout. B. The number of trials performed prior to reaching operant behavior training criteria. Student’s t-test, p > 0.05, saline (n = 10) mice, cocaine (n = 9) mice. C. Performance (mean±SEM % correct hits) of prenatally saline-versus cocaine-exposed mice over 20 blocks of trials. One block = 20 random trials of 10 S+ and 10 S− cues. Two-way analysis of variance (ANOVA), n= 10 saline-exposed mice and 9 cocaine-exposed mice. *p < 0.0001; EA = ethyl acetate. Dashed line = 80% criteria. Saline = prenatally saline-exposed mice, Cocaine = prenatally cocaine-exposed mice.
Following acclimation to the test equipment, operant training was performed using a step-wise battery of tasks that began with reward reinforcement for learning to lick the spout to receive 2 µl water on a random-interval schedule averaging 15 seconds (s) whereby mice could insert their snouts into the odor sampling port (Fig. 1A). All mice completed this stage and advanced to the next stage after 30 reinforcements. During the second stage, mice were reinforced for lick decisions paired with EA that was used as the initial positively-entrained odor (S+). Here trials were initiated by the mouse inserting its snout in the odor-sampling port and was followed by a 2 s delivery of training odor (Fig. 1A). The odor presentation was binned in ten 0.2 s periods whereby a criterion response for the mouse to receive water reinforcement was defined as a lick detected in at least six of the 0.2 s segments. A criterion response was scored as a “hit”, produced reinforcement, and initiated a 5 s interval. Mice were offered a total of 119 trials/session for a total of at least four sessions before being trained for operant conditioning. During the go no-go operant training mice inserted their snout into the odor-sampling port to initiate a trial and waited until the odor stimulus was presented. The mouse sampled the stimulus and then responded (i.e. licked) or did not respond, depending on the type of stimulus presented. S+ and S− individual stimuli were presented in random order with the restriction that there be an equal number of each in each session of 20 trials, which was defined as a block. Mice were given the opportunity to complete 10 blocks of 20 trials, or a maximum of 200 trials per daily session. The mice were water reinforced for correct decisions. The percentage of correct responses per trial was determined by the formula: % correct responses = [(HITs + Correct Rejections)/20] × 100, where a HIT is defined as a criterion response in the presence of S+, and a Correct Rejection (CR) is a failure to make a criterion response in the presence of S−. A miss was defined as a failure to make a criterion response in the presence of S+, and a false alarm (FA) was a criterion response in the presence of S−. [35, Thiebaud et al., 2014,36].
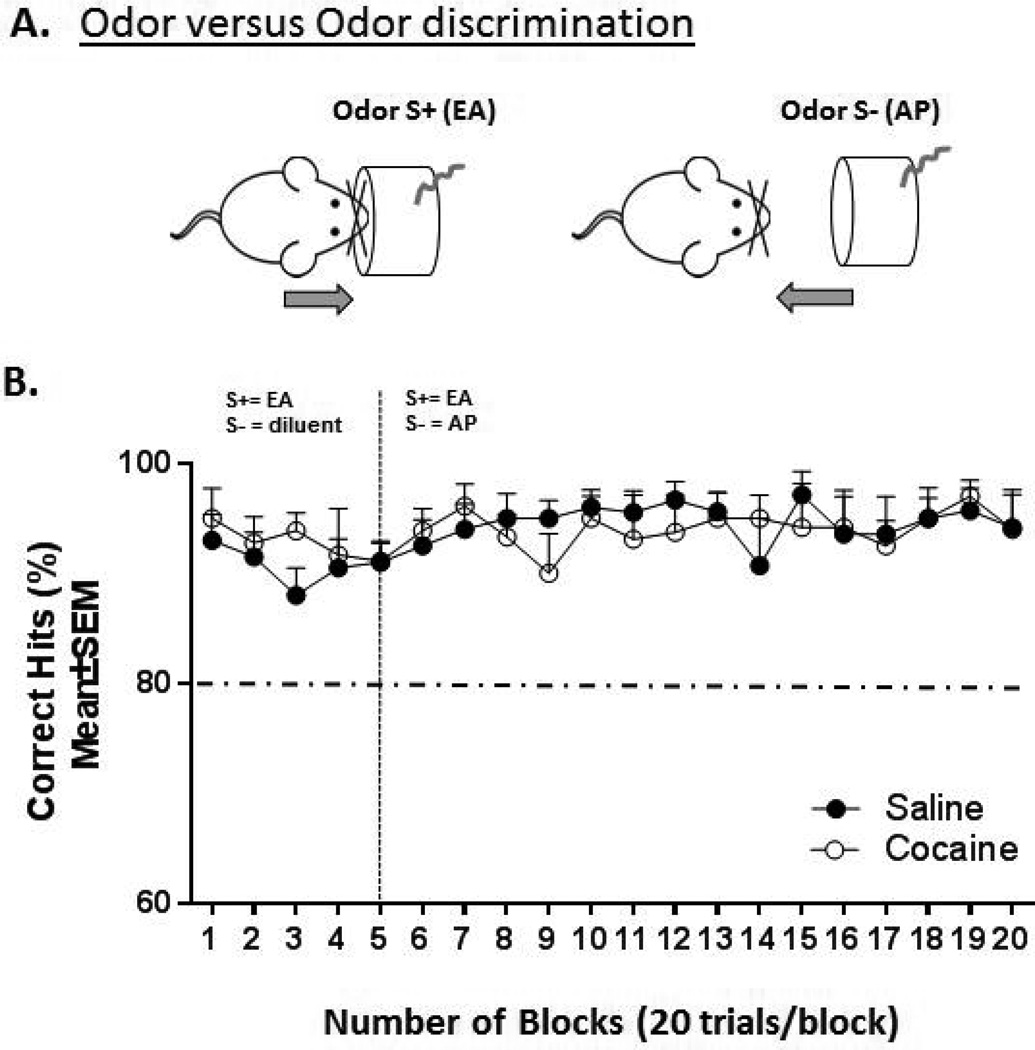
Odor discrimination training (Figure 2A)
Figure 2.
Schematic illustration of the odor versus odor discrimination task (A) and performance of prenatally saline- or cocaine-exposed mice in this task (B). A. Mice are initially trained using EA as the positive odor cue (S+ EA) and water as the negative odor cue (S− diluent). On the 5th block, the S+ stimulus remains as EA but a second odor AP (acetophenone) is substituted as the negative odor cue to achieve an odor-versus.-odor discrimination paradigm. B. Line graph of the performance (mean±SEM % correct hits) of prenatally saline versus prenatally cocaine-exposed mice over 20 blocks. Dashed horizontal line = criterion of 80% correct hits, dashed vertical line = introduction of new S−. Saline = prenatally saline-exposed mice (n = 10), cocaine = prenatally cocaine-exposed mice (n = 9).
After mice achieved 80% correct decisions (defined criteria), the S− was switched from diluent to a second odorant, AP, to examine ability to discriminate between two odors, EA (S+) versus AP (S−).
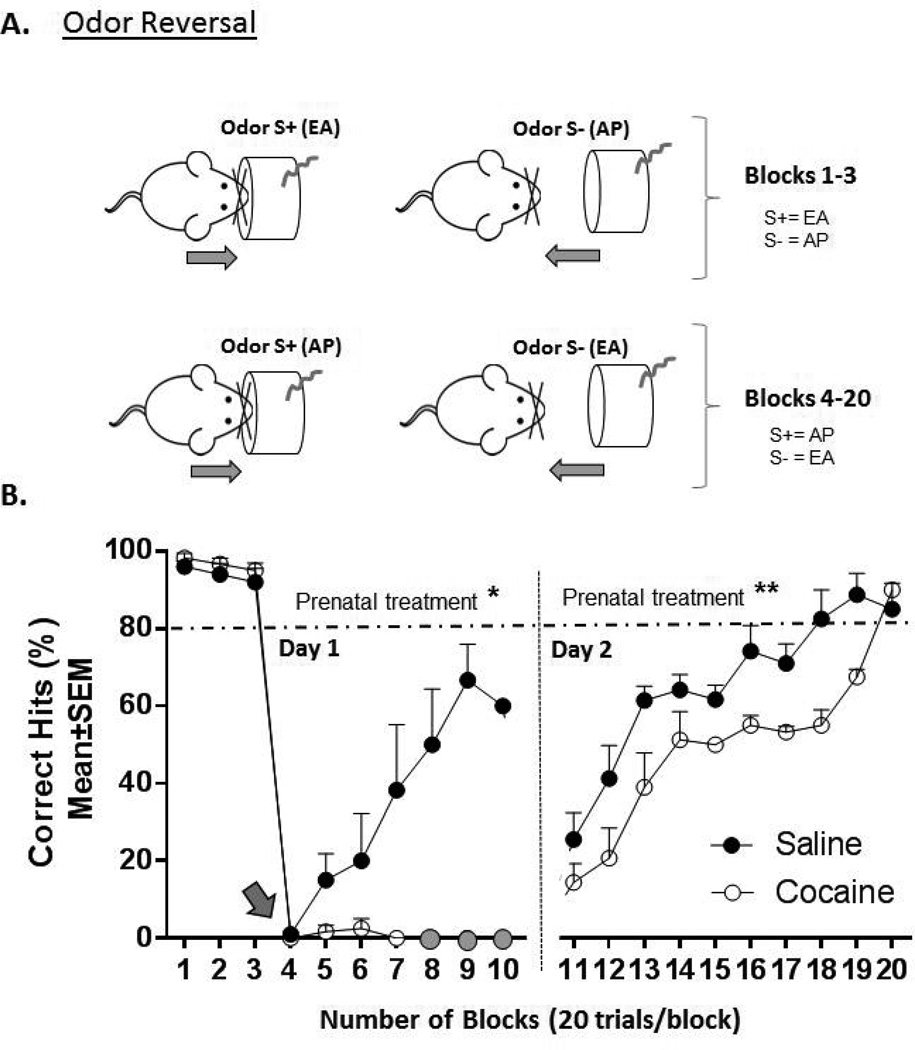
Odor-reversal Learning (Figure 3A)
Figure 3.
Schematic illustration of the olfactory reversal paradigm (A) and the performance of prenatally saline- or cocaine-exposed mice in this paradigm (B). A. Following odor versus odor discrimination training, mice are presented with EA as S+ and AP as S− (blocks 1–3) prior to reversing the S+ cue to the S− cue (i.e. previously unrewarded odor; blocks 4–20: gray arrow in B). Because mice can only be challenged with 10 blocks, or 200 trials per day, the reversal learning paradigm requires a 2 consecutive day training period. Day 1 = Blocks 1–10; Day 2 = Blocks 11–20. B. Performance (mean±SEM % correct hits) of prenatally saline– and cocaine-exposed mice over 20 blocks on days 1 and 2 of reversal. Two-way ANOVA revealed significant main effects of the prenatal treatment on day 1 (* = p < 0.001) and day 2 (**p < 0.0001). Filled gray circles = mice performing neither nose insertions nor withdrawals (not included in analysis). Saline= prenatally saline-exposed mice (n = 10), cocaine = prenatally cocaine-exposed mice (n = 9).
Once mice achieved performance criterion on the odor versus odor discrimination paradigm for 2 days, we tested the ability to relearn a reinforcement task if the S+ stimulus was switched to the S− stimulus[37].
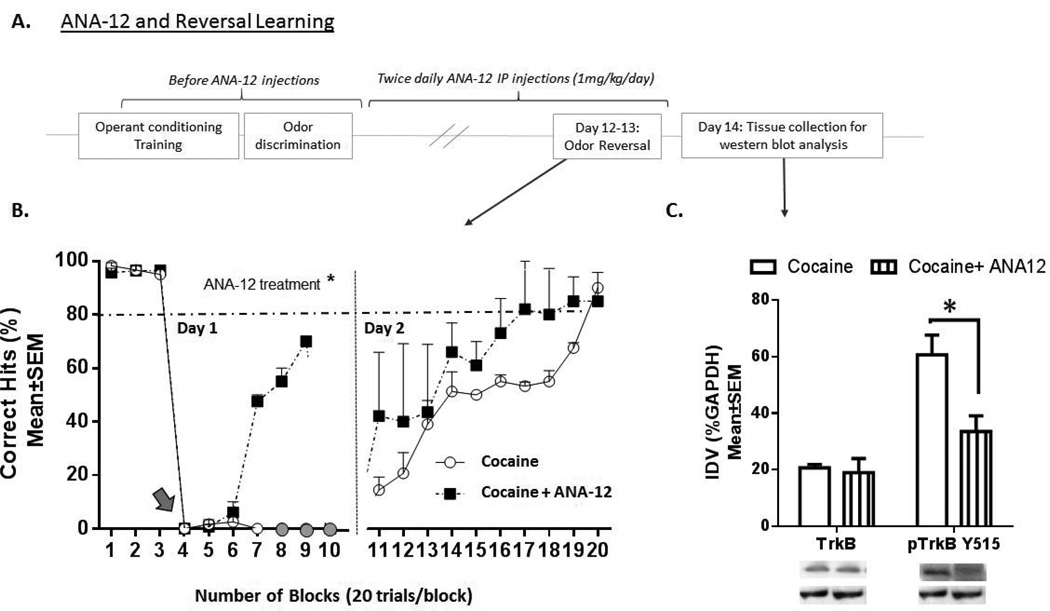
Administration of N-[2-[[(Hexahydro-2-oxo-1H-azepin-3-yl) amino] carbonyl] phenyl]-benzo[b]thiophene-2-carboxamide (ANA-12) (Figure 4A)
Figure 4.
Schematic illustration of ANA-12 administration during the olfactory reversal-learning paradigm (A). Following acclimation, operant training and odor versus odor discrimination phases, the mice from the prenatal cocaine exposure group were administered ANA-12 (1 mg/kg/day) for 13 consecutive days. On the 11th day, the mice were re-tested for their ability to discriminate between the S+ and S− odors (odor versus odor discrimination). On day 12 the reward contingency to which the mice were accustomed until then was reversed on block 4 (gray arrow in B). B. Performance (mean±SEM % correct hits) of prenatally cocaine-exposed mice with or without ANA-12 over 20 blocks in the reversal learning paradigm on day 1 (blocks 1–10) and day 2 (blocks 11–20). Two-way ANOVA revealed significant main effects of prenatal drug treatment on day 1 of reversal (* = p <0.0001). Cocaine = prenatally cocaine-exposed mice (n = 9), Cocaine + ANA-12 = prenatally cocaine-exposed mice receiving ANA-12 (n = 7). C. Bar graph of the integrated density values (IDV) for TrkB and pTrkB-Y515 expression (mean±SEM) for prenatally cocaine-exposed mice without ANA-12 administration (Cocaine, open bars) and with ANA-12 administration (cocaine + ANA-12, striped bars). Data were normalized to loading control GAPDH. Representative western blots are shown for TrkB/pTrkB-Y515 (MW = 91 kDa) and GAPDH (MW = 37 kDa). n = 4 for each of the two treatments, * = p<0.05 (2-way ANOVA followed by Bonferroni post-hoc test).
In a separate set of studies, upon successful completion of the training phase and odor discrimination phase, mice from the prenatal cocaine exposure group were administered ANA-12 (Sigma Aldrich, St. Louis, MO; SML0209) dissolved in 1% DMSO twice daily [0.5 mg/kg × 2; intraperitoneal (i.p.)] for 13 consecutive days [38,39]. On days 8–10 of the ANA-12 administration, the mice were water deprived (to 85% of pre-deprivation weight). On the 11th day, the mice were re-tested for their ability to discriminate between the S+ and S− odors. On days 12–13, the S+ stimulus was switched to the S− stimulus to monitor the animals’ ability to reversal learn over a two-day interval.
Western Blotting
Mice were administered on overdose of isoflurane to harvest brain tissues. Frontal cortex or striatum were micro-dissected from each hemisphere based on anatomical landmarks [40–42] and samples from the two hemispheres were pooled. Whole-tissue extracts were used for western blot [43,44]. The following antibodies were used: Anti- BDNF diluted 1:700 (Aviva Systems Biology, San Diego, CA; ARP41970_P050), anti-TrkB diluted 1:600 (Novus Biologicals, Lilleton, CO; NB100-92063) anti-pTrkBY515 1:1000 (Abcam, Cambridge, MA; Ab109684), and anti-GAPDH 1:1000 (Abcam, Cambridge, MA; MAB374). Integrated density values (% IDV) were calculated for each sample by dividing the optical density values of the bands for the protein of interest by the density values for the loading control (GAPDH). IDVs were measured only from those membranes with distinct bands at the appropriate molecular weight.
Statistical analyses
Main effects of prenatal treatment and trial block number were analyzed using an ordinary two-way analysis of variance (2-way ANOVA). Post-hoc tests for multiple comparisons were performed using a Bonferroni correction. A two-tailed Student’s t-test was used when differences between only two groups were analyzed.
Results
Operant training
Mice successfully learned operant behaviors regardless of prenatal treatment. The mean number of trials required to reach operant behaviors was not significantly different between prenatally saline- and cocaine-exposed mice (Fig. 1B; Student’s t-test: saline: 1500 ± 143 (n=10) cocaine: 1378 ± 225 (n = 9), t= 0.4679, df=17, p >0.05). The odor (EA) versus diluent (water) discrimination task (Fig. 1C) revealed significant main effect of prenatal treatment (2-way ANOVA; F(19, 309) = 4.382, p < 0.0001); significant main effect of time (i.e. block number; 2-way ANOVA; F(1,309) = 12.94, p <0.001) without any prenatal treatment × block number interaction (F(19, 309) = 0.8996, p >0.05).
Odor versus Odor discrimination
Once mice had achieved steady-state operant-conditioning (S+ EA, S− diluent) for 2 days, challenging them with a two-choice odorant discrimination (S+ EA, S− AP) revealed no significant main effect of prenatal treatment (2-way ANOVA; F(1,202) = 0.3129, p> 0.05) or number of blocks (2-way ANOVA; F(14, 202) = 0.3729, p > 0.05,Fig. 2B).
Reversal Learning
All mice routinely performed at or above criterion (≥80% correct hits) in the odor versus odor discrimination task following 2-days of training. We then tested their ability to reversal learn over a 2-day interval. On day 1, mice were presented with the familiar S+ EA, S− AP, odor versus odor paradigm for the first three blocks (Fig. 3B), but then on block 4 (Fig. 3B, grey arrow), the reward contingency was reversed such that the S+ stimulus (EA-rewarded) was switched to the S− stimulus (AP-unrewarded) and the mice were now required to switch their responses to the S+ and S− odors that they had displayed in earlier blocks (blocks 1, 2 and 3; Fig. 3B). On block 4, mice from both prenatal treatment groups fell to a poor level of discrimination (0% correct hits). They were allowed to continue working for a total of 7 blocks (blocks 4–10) on day 1 and 10 blocks (blocks 11–20) on day 2 (Fig. 3B). On day 1, prenatally saline-exposed mice gradually learned the reversed paradigm (i.e. exhibited the correct responses to the reversed paradigm) and their performance improved significantly starting on block 6 (block 4 versus block 6 and 7; p<0.05, p<0.001 respectively) (Fig. 3B). Unlike the prenatally saline-exposed group, the prenatally cocaine-exposed group continued the incorrect responses through blocks 5, 6 and 7 (block 4 versus block 5, 6, 7 all p>0.05; Fig. 3B) and failed to reversal learn. Furthermore, during blocks 8–10 the prenatally cocaine-exposed mice failed to show any response at all (stopped nose exploration, grey filled circles, Fig. 3B). Since the prenatally cocaine-exposed mice did not at all perform the task on blocks 8–10, assignment of a value of zero to such performance would not have been appropriate for statistical analysis (neither correct nor incorrect hits were performed by the mice). Therefore, blocks 8–10 were not included in the analysis (closed circles). A two-way ANOVA revealed main effect of prenatal treatment (F(1,29)= 11.13, p< 0.001) but no main effect of block number (2-way ANOVA; F(3,29)= 2.152, p> 0.05), or prenatal treatment X block number interaction (2-way ANOVA; F(3,29)= 2.181, p> 0.05). Bonferroni post-hoc pair-wise comparisons did not indicate significant effect of prenatal treatment within any given block (all p >0.05). On day 2, both cocaine- and saline-exposed mice improved performance over number of blocks (2-way ANOVA; F(9,80) = 12.10, p < 0.0001), however, the performance of the prenatally cocaine-exposed mice was significantly lower than that of saline-exposed mice (2-way ANOVA, F(1,80) = 18.69, p < 0.0001).
Effects of TrkB antagonism on reversal learning
Next we examined whether administration of ANA-12, a selective antagonist of the TrkB receptor, influenced reversal learning in the prenatally cocaine-exposed mice. Upon successful completion of the acclimation and odor discrimination phases, the mice were administered ANA-12 for 13 consecutive days (Fig. 4A). On day 12 of the ANA-12 administration paradigm, prenatally cocaine-exposed mice receiving ANA-12 injections performed at or above criterion (>80% correct hits) in the odor versus odor discrimination task (see Fig. 4B, Blocks 1–3). However, in striking contrast to the prenatally cocaine-exposed mice that were not injected with ANA-12 (circles), the ANA-12-administered prenatally cocaine-exposed mice demonstrated a significant improvement in performance on the first day of the odor-reversal paradigm (Fig.4B, Blocks 4–10). Here, the reversal learning performance of the ANA-12 administered, prenatally-cocaine-exposed mice (squares) was significantly different than that of prenatally cocaine exposed mice without ANA-12 administration (circles) (2-way ANOVA, F(1,22) = 191.0, p < 0.0001). Bonferroni post-hoc pair-wise comparisons showed a significant increase in percentage correct hits on block 7 in cocaine-exposed ANA-12 administered mice when compared to cocaine-exposed mice that did not receive ANA-12 (t-19.44, df=22, p<0.001). Similar to that of saline-exposed mice (Fig. 3B), mice administered ANA-12 exhibited improvement over that of mice without ANA-12 starting at blocks 6–7 (Bonferroni post-hoc test block 4 versus block 6 and 7; p<0.01, p<0.0001 respectively). By the second day of reversal learning, the performance of the two groups of prenatally cocaine-exposed mice was not significantly different [2-way ANOVA; ANA-12 treatment (F(1,74)= 3.004, p> 0.05), block number treatment (F(9,74)= 1.718, p> 0.05) and ANA-12 treatment × block number interaction (F(9,74)= 0.1271, p>0.05)].
Effect of ANA-12 administration on TrkB expression
Total TrkB and pTrkB-Y515 expression was examined in the frontal cortex of the prenatally cocaine-exposed mice and prenatally cocaine-exposed mice injected with ANA-12 (Fig. 4C). A two-way ANOVA showed significant effects of drug treatment (F(1, 10) = 6.448, p< 0.05), protein expression (F(1, 10) = 23.05, p< 0.001), and interaction between drug treatment × protein expression (F(1, 10) = 5.046, p< 0.05). Bonferroni post-hoc multiple comparison test indicated a significant decrease in pTrkBY515 expression in prenatally cocaine-exposed mice treated with ANA-12 compared to the prenatally cocaine-exposed mice (t=3.655, df=10, p<0.01), and no significant difference between the two groups in total TrkB expression (t=0.1938, df=10, p>0.05, Fig. 4C).
BDNF and TrkB expression in the striatum
The dorsal striatum is another brain region (apart from the frontal cortex) associated with regulation of reversal learning [6,45–49]. We analyzed BDNF, total and phosphorylated TrkB-Y515 expression in this region to determine if prenatal cocaine exposure produced an effect on BDNF-TrkB signaling. A two-way ANOVA showed no significant main effect of prenatal treatment on BDNF, total TrkB or phosphorylated TrkB-Y515 expression in the dorsal striatum (2-way ANOVA, p>0.05).
Discussion
Our findings demonstrate that prenatal cocaine exposure produces significant reversal learning deficits in an olfactory learning paradigm, and that the selective TrkB receptor antagonist ANA-12 restores the reversal learning to control levels. Because it is well known that reversal learning, an indicator of cognitive flexibility in rodents, is governed by the frontal cortex, the decrease in BDNF-TrkB signaling measured in this region in response to ANA-12 administration is likely the driver of this behavioral phenotype. Thus, reversal learning deficit mediated by increased BDNF-TrkB signaling emerges as a significant consequence of prenatal cocaine exposure.
We chose an olfactory reversal learning paradigm because mice perform better in an olfactory learning paradigm than in paradigms reliant on visual or spatial stimuli. In fact, visuo-spatial learning is not optimal for evaluating albino mice [50], such as the Swiss-Webster strain of mice used here. The fact that both the prenatally saline- and cocaine-exposed mice performed equivalently in the number of trials to reach operant behavior (Fig. 1B) suggests that in adult mice there were no behavioral changes in cognition to pair an odor with a water reinforcement or reward. It is interesting that the prenatally cocaine-exposed mice appeared to distinguish between an odor and water cue faster than the prenatally saline-exposed mice (Fig 1C). However, the two groups of mice did not show significant differences in their ability to discriminate between two odors. C57BL6/J mice are known to achieve performance criteria within 6 blocks [31] for initial odor versus water discrimination training, not unlike the Swiss-Webster strain in the current study. Because the odor versus water discrimination plateaus after these initial few blocks, the difference between the two prenatal treatment groups in this ability is unlikely to be biologically relevant. This is supported by the fact that the two prenatal treatment groups do not show significant differences in the more difficult odor versus odor discrimination task (Fig. 2B). We also note that the Swiss-Webster strain require 2 days to reach criteria during the odor reversal paradigm, unlike the observed C57BL6/J strain which require only 1 day [31].
The pause in snout insertion immediately upon reversal in the prenatally cocaine-exposed mice is a novel behavioral finding and has not been reported previously for the prenatally cocaine exposed mice. On the second post-reversal session (Day 2), although the prenatally cocaine-exposed mice performed the task, they achieved fewer correct decisions in comparison to controls. The pause in behavior observed following reversal and the subsequent recovery on the second post- reversal session is indicative of a delayed learning response. Although prenatally cocaine-exposed rats required more sessions to learn a reversed condition stimulus task and exhibited decreased accuracy in the first 10 responses following reversal, they did not display a complete halt in behavior reported here [51].
We analyzed BDNF-TrkB signaling because of the well-established involvement of this signaling mechanism in cognitive function [27–29] and its impairment by cocaine exposure [reviewed in [26]]. We focused on BDNF-TrkB signaling in the frontal cortex because reversal learning, even if assayed via olfactory (or other chemosensory) stimuli, as in the present study, is a frontal cortex-dependent cognitive function [30, 61–63]. Among the other brain regions involved in the regulation of reversal learning, we can rule out involvement of the striatum because neither BDNF nor TrkB (total or phosphorylated TrkB-Y505) is significantly altered by the prenatal cocaine exposure in the striatum. However, since we administered ANA-12 systemically, and since we did not measure BDNF and TrkB expression in the orbito-frontal cortex, nucleus accumbens or hippocampus, the other brain regions associated with reversal learning [39–44] we cannot rule out the role of BDNF-TrkB signaling in these other brain regions.
A single administration of ANA-12 at the same dose used in this study produces anxiolytic and antidepressant effects in mice [38]. However, whether repeated ANA-12 administration, such as that used here, impacts reversal learning or other cognitive behaviors is yet unknown. Isoflurane general anesthesia (1.5% isoflurane for 2 hr) is reported to reduce BDNF expression in the hippocampus of aged (15-month old) mice [52]. For euthanasia we used significantly shorter duration of isoflurane exposure (approximately 1 min) in the present study. In addition, we found a significant increase (rather than a decrease) in BDNF expression in the frontal cortex in the prenatally cocaine exposed mice. Therefore, it is unlikely that the acute isoflurane exposure in the present study contributed to the increase in BDNF levels. However, we cannot rule out the potential contribution of isoflurane exposure to BDNF expression in the experimental and control groups in the present study. Prenatal cocaine exposure is reported to affect locomotor activity in rodent models [53]. However, we did not find significant changes in open field activity in our prenatal cocaine exposure mouse model (data not shown). Therefore, the reversal learning deficits in the prenatally cocaine exposed mice reported here are unlikely to be influenced by alterations in locomotor activity. Finally, there are well-characterized TrkB signaling pathways in the olfactory bulb [54–56] that could have been affected by the systemic ANA-12. For example, phosphorylation of a voltage-dependent potassium channel (Kv1.3) by BDNF would be predicted to enhance olfactory ability. However, the prenatally cocaine exposed mice did not show enhanced or deficient odor versus odor discrimination ability. Moreover, odor reversal learning is not governed by the olfactory bulb.
Our data on BDNF expression and BDNF-TrkB signaling are from samples pooled from the medial and dorsal prefrontal cortices. An earlier report on the effects of prenatal cocaine exposure in mice [64] showed no change in BDNF expression in the medial prefrontal cortex at adulthood. It is possible that the increases in BDNF produced by prenatal cocaine exposure occur predominantly in the dorsal prefrontal cortex. However, additional studies would be required to resolve this issue.
Preclinical models of prenatal cocaine exposure have focused predominantly on behaviors such as drug reinforcement, behavioral sensitization or reward [57–63]. A consistent observation from these studies is that the prenatal cocaine-exposure decreases preference for cocaine, suggesting prima facie that prenatal cocaine exposure may not be “deleterious”. These observations are in contrast to the data from human studies, which suggest that prenatal cocaine exposure produces deleterious consequences [14–19]. Our present data demonstrate that reversal learning deficit is an “adverse” cognitive phenotype in the mouse model. Since reversal learning is a measure of cognitive flexibility, our findings could have significant implications for understanding the molecular mechanisms mediating human cognitive inflexibility. In other words, increased BDNF-TrkB signaling reported here may underlie cognitive inflexibility associated with neuropsychiatric conditions.
The involvement of frontal cortical BDNF-TrkB signaling in the reversal learning deficits in our mouse model is supported by at least three lines of evidence. First, the prenatally cocaine-exposed mice show significant up-regulation of BDNF expression and TrkB phosphorylation in the frontal cortex [30]. Second, ANA-12, a TrkB receptor antagonist restores reversal learning to control levels. Finally, ANA-12 administration downregulated phosphorylated TrkB expression in the frontal cortex of the prenatally cocaine-exposed mice. Collectively, these data demonstrate a link between increased frontal cortical BDNF-TrkB signaling and reversal learning deficit following prenatal cocaine exposure. Although previous reports had shown significant changes in behavioral phenotypes associated with BDNF signaling (e.g. fear extinction) as a result of prenatal cocaine exposure [64–66], a mechanistic link between the behavioral changes, prenatal cocaine exposure and BDNF signaling had not been established. The only study to our knowledge that examined frontal cortical BDNF signaling and reversal learning used a model of high fat diet exposure in rats and reported a link between decreased BDNF signaling and auditory discrimination reversal learning deficit [67]. Therefore, the present findings make a significant novel contribution to the field by demonstrating a link between prenatal cocaine exposure, BDNF and reversal learning.
BDNF induces dimerization and subsequent phosphorylation of TrkB receptors at two sites, each site associated with distinct intracellular signaling pathways: 1) TrkB-Y705, the autophosphorylation activation site and 2) TrkB-Y515, the shc adaptor protein docking site, which leads to MAPK/ERK activation [68–71]. Our previous study showed an increase in phosphorylation of TrkB-Y515 and not TrkB-Y705 in the frontal cortex of the prenatally cocaine-exposed mice [30]. Therefore, in the present study we focused on TrkB-Y515.
In summary, our data demonstrate that prenatal cocaine exposure produces reversal learning deficits that are associated with increased BDNF-TrkB signaling in the frontal cortex. These data may facilitate further investigations into the mechanistic bases of human cognitive inflexibility, which are presently not fully understood.
Acknowledgments
We gratefully acknowledge the technical assistance of Penny Coshatt and undergraduate scholars in the research, namely Sarah E. Lowe, Nicole Benjamin, Lewyn Boyette, Chris Calixte, Benjamin McMichael, Zachary Panzarella, Michael Porter, Esther Rosier, Alina Stimmell, Sean Sullivan and Margaret Yip. We gratefully acknowledge insightful comments on the earlier versions of the manuscript by Joseph Biederman and Thomas J. Spencer.
Funding: This work was supported by the Jim and Betty Anne Rodgers Chair Funds (PGB), Florida State University GAP Fund (PGB) and the National Institutes of Health [NIH; NIDCD T32 DC000044 (DAF), R01 DC013080 (DAF), NIDA R21 DA033641 (G.S.V.)].
Footnotes
- DMCC - Designed the study, generated the prenatally saline- and cocaine-exposed mice, performed ANA-12 injections, collected tissue samples, analyzed the data, created the figures, and wrote the manuscript
- GAB – Performed olfactometry experiments, analyzed data and wrote the manuscript
- ENC – Generated the prenatally saline- and cocaine-exposed mice and collected tissue for western blot
- KAM - Generated the western blot data
- MNH – Generated the western blot data
- GS-V - Analyzed the molecular data, and wrote the manuscript
- DAF - Supervised the olfactometry experiments, analyzed data and wrote the manuscript
- PGB - Designed the study, analyzed the data, and wrote the manuscript
Financial Disclosures: Authors report no financial interests or potential conflicts of interest.
References
- 1.Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex. 2010;20:1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glascher J, Hampton AN, O'Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb Cortex. 2009;19:483–495. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: An updated perspective. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dajani DR, Uddin LQ. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends in neurosciences. 2015;38:571–578. doi: 10.1016/j.tins.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Cruz AM, Ragozzino ME, Mosconi MW, Pavuluri MN, Sweeney JA. Human reversal learning under conditions of certain versus uncertain outcomes. NeuroImage. 2011;56:315–322. doi: 10.1016/j.neuroimage.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AD, Nippak PM, Murphey H, Ikeda-Douglas CJ, Muggenburg B, Head E, Cotman CW, Milgram NW. Visuospatial impairments in aged canines (Canis familiaris): the role of cognitive-behavioral flexibility. Behavioral neuroscience. 2002;116:443–454. [PubMed] [Google Scholar]
- 8.Matzel LD, Light KR, Wass C, Colas-Zelin D, Denman-Brice A, Waddel AC, Kolata S. Longitudinal attentional engagement rescues mice from age-related cognitive declines and cognitive inflexibility. Learn Mem. 2011;18:345–356. doi: 10.1101/lm.2034711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Frontiers in neuroscience. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried R, Chan J, Feinberg L, Pope A, Woodworth KY, Faraone SV, Biederman J. Clinical correlates of working memory deficits in youth with and without ADHD: A controlled study. Journal of clinical and experimental neuropsychology. 2016;38:487–496. doi: 10.1080/13803395.2015.1127896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remijnse PL, van den Heuvel OA, Nielen MM, Vriend C, Hendriks GJ, Hoogendijk WJ, Uylings HB, Veltman DJ. Cognitive inflexibility in obsessive-compulsive disorder and major depression is associated with distinct neural correlates. PloS one. 2013;8:e59600. doi: 10.1371/journal.pone.0059600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 13.Chae SM, Covington CY. Biobehavioral outcomes in adolescents and young adults prenatally exposed to cocaine: evidence from animal models. Biol Res Nurs. 2009;10:318–330. doi: 10.1177/1099800408330395. [DOI] [PubMed] [Google Scholar]
- 14.Akyuz N, Kekatpure MV, Liu J, Sheinkopf SJ, Quinn BT, Lala MD, Kennedy D, Makris N, Lester BM, Kosofsky BE. Structural brain imaging in children and adolescents following prenatal cocaine exposure: preliminary longitudinal findings. Developmental neuroscience. 2014;36:316–328. doi: 10.1159/000362685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandstra ES, Morrow CE, Accornero VH, Mansoor E, Xue L, Anthony JC. Estimated effects of in utero cocaine exposure on language development through early adolescence. Neurotoxicology and teratology. 2011;33:25–35. doi: 10.1016/j.ntt.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Lester BM, Neyzi N, Sheinkopf SJ, Gracia L, Kekatpure M, Kosofsky BE. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA pediatrics. 2013;167:348–354. doi: 10.1001/jamapediatrics.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Developmental neuroscience. 2009;31:159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen JW, Bennett DS, Carmody DP, Wang Y, Lewis M. Adolescent risk-taking as a function of prenatal cocaine exposure and biological sex. Neurotoxicology and teratology. 2014;41:65–70. doi: 10.1016/j.ntt.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmody DP, Bennett DS, Lewis M. The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicology and teratology. 2011;33:61–68. doi: 10.1016/j.ntt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chelonis JJ, Gillam MP, Paule MG. The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotoxicology and teratology. 2003;25:437–446. doi: 10.1016/s0892-0362(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 21.Verdejo-Garcia A, Verdejo-Roman J, Albein-Urios N, Martinez-Gonzalez JM, Soriano-Mas C. Brain substrates of social decision-making in dual diagnosis: cocaine dependence and personality disorders. Addiction biology. 2015 doi: 10.1111/adb.12318. [DOI] [PubMed] [Google Scholar]
- 22.Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: a mouse model demonstrating neuroanatomic and behavioral abnormalities. Journal of child neurology. 1994;9:234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- 24.Kosofsky BE, Wilkins AS. A mouse model of transplacental cocaine exposure. Clinical implications for exposed infants and children. Annals of the New York Academy of Sciences. 1998;846:248–261. [PubMed] [Google Scholar]
- 25.Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex. 2004;14:665–675. doi: 10.1093/cercor/bhh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubrusly RC, Bhide PG. Cocaine exposure modulates dopamine and adenosine signaling in the fetal brain. Neuropharmacology. 2010;58:436–443. doi: 10.1016/j.neuropharm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy DM, Zhang X, Darnell SB, Sangrey GR, Yanagawa Y, Sadri-Vakili G, Bhide PG. Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13400–13411. doi: 10.1523/JNEUROSCI.2944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy DM, Bhide PG. Prenatal cocaine exposure decreases parvalbumin-immunoreactive neurons and GABA-to-projection neuron ratio in the medial prefrontal cortex. Developmental neuroscience. 2012;34:174–183. doi: 10.1159/000337172. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy DM, Brown AN, Bhide PG. Regulation of BDNF expression by cocaine. Yale J Biol Med. 2012;85:437–446. [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy DMKA, Cannon EN, Huizenga MN, Darnell SB, Bhide PG, Sadri-Vakil G. Prenatal cocaine exposure alters BDNF-TrkB signaling in the embryonic and adult brain. Companion Paper- Developmental Neuroscience. 2016 doi: 10.1159/000453609. [DOI] [PubMed] [Google Scholar]
- 31.Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poo MM. Neurotrophins as synaptic modulators. Nature reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 33.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins AS, Genova LM, Posten W, Kosofsky BE. Transplacental cocaine exposure. 1: A rodent model. Neurotoxicology and teratology. 1998;20:215–226. doi: 10.1016/s0892-0362(97)00125-6. [DOI] [PubMed] [Google Scholar]
- 35.Slotnick B, Restrepo D. Olfactometry with mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] 2005;Chapter 8(Unit 8):20. doi: 10.1002/0471142301.ns0820s33. [DOI] [PubMed] [Google Scholar]
- 36.Thiebaud N, Johnson MC, Butler JL, Bell GA, Ferguson KL, Fadool AR, Fadool JC, Gale AM, Gale DS, Fadool DA. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:6970–6984. doi: 10.1523/JNEUROSCI.3366-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron. 2011;69:1176–1187. doi: 10.1016/j.neuron.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. The Journal of clinical investigation. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology. 2014;76(Pt B):269–275. doi: 10.1016/j.neuropharm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain research. 2007;1156:31–45. doi: 10.1016/j.brainres.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG. Transgenerational transmission of hyperactivity in a mouse model of ADHD. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:2768–2773. doi: 10.1523/JNEUROSCI.4402-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, McCarthy DM, Sharma N, Bhide PG. Dopamine receptor and Galpha(olf) expression in DYT1 dystonia mouse models during postnatal development. PloS one. 2015;10:e0123104. doi: 10.1371/journal.pone.0123104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nature neuroscience. 2013;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behavioural brain research. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- 46.Schwabe K, Enkel T, Klein S, Schutte M, Koch M. Effects of neonatal lesions of the medial prefrontal cortex on adult rat behaviour. Behavioural brain research. 2004;153:21–34. doi: 10.1016/j.bbr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 47.Watson DJ, Stanton ME. Spatial discrimination reversal learning in weanling rats is impaired by striatal administration of an NMDA-receptor antagonist. Learn Mem. 2009;16:564–572. doi: 10.1101/lm.1448009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural brain research. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behavioural brain research. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behavioural brain research. 2011;216:531–542. doi: 10.1016/j.bbr.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 51.Heyser CJ, Spear NE, Spear LP. Effects of prenatal exposure to cocaine on conditional discrimination learning in adult rats. Behavioral neuroscience. 1992;106:837–845. doi: 10.1037//0735-7044.106.5.837. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Zhang M, Li H, Sun X, Hao S, Ji M, Yang J, Li K. BDNF pathway is involved in the protective effects of SS-31 on isoflurane-induced cognitive deficits in aging mice. Behavioural brain research. 2016;305:115–121. doi: 10.1016/j.bbr.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 53.Martin MM, Graham DL, McCarthy DM, Bhide PG, Stanwood GD. Cocaine-induced neurodevelopmental deficits and underlying mechanisms. Birth defects research Part C. Embryo today : reviews. 2016;108:147–173. doi: 10.1002/bdrc.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. The Journal of physiology. 2002;542:413–429. doi: 10.1113/jphysiol.2002.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biju KC, Mast TG, Fadool DA. Olfactory sensory deprivation increases the number of proBDNF-immunoreactive mitral cells in the olfactory bulb of mice. Neuroscience letters. 2008;447:42–47. doi: 10.1016/j.neulet.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mast TG, Fadool DA. Mature and precursor brain-derived neurotrophic factor have individual roles in the mouse olfactory bulb. PloS one. 2012;7:e31978. doi: 10.1371/journal.pone.0031978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rocha BA, Mead AN, Kosofsky BE. Increased vulnerability to self-administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology. 2002;163:221–229. doi: 10.1007/s00213-002-1140-0. [DOI] [PubMed] [Google Scholar]
- 58.Crozatier C, Guerriero RM, Mathieu F, Giros B, Nosten-Bertrand M, Kosofsky BE. Altered cocaine-induced behavioral sensitization in adult mice exposed to cocaine in utero. Brain research Developmental brain research. 2003;147:97–105. doi: 10.1016/j.devbrainres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Malanga CJ, Riday TT, Carlezon WA, Jr, Kosofsky BE. Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biological psychiatry. 2008;63:214–221. doi: 10.1016/j.biopsych.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malanga CJ, Ren JQ, Guerriero RM, Kosofsky BE. Augmentation of cocaine-sensitized dopamine release in the nucleus accumbens of adult mice following prenatal cocaine exposure. Developmental neuroscience. 2009;31:76–89. doi: 10.1159/000207496. [DOI] [PubMed] [Google Scholar]
- 61.Malanga CJ, Pejchal M, Kosofsky BE. Prenatal exposure to cocaine alters the development of conditioned place-preference to cocaine in adult mice. Pharmacology, biochemistry, and behavior. 2007;87:462–471. doi: 10.1016/j.pbb.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malanga CJ, Kosofsky BE. Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Brain research Developmental brain research. 2003;147:47–57. doi: 10.1016/j.devbrainres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 63.Stanwood GD, Levitt P. Repeated i.v. cocaine exposure produces long-lasting behavioral sensitization in pregnant adults, but behavioral tolerance in their offspring. Neuroscience. 2003;122:579–583. doi: 10.1016/j.neuroscience.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 64.Kabir ZD, Katzman AC, Kosofsky BE. Molecular mechanisms mediating a deficit in recall of fear extinction in adult mice exposed to cocaine in utero. PloS one. 2013;8:e84165. doi: 10.1371/journal.pone.0084165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kabir ZD, Lourenco F, Byrne ME, Katzman A, Lee F, Rajadhyaksha AM, Kosofsky BE. Brain-derived neurotrophic factor genotype impacts the prenatal cocaine-induced mouse phenotype. Developmental neuroscience. 2012;34:184–197. doi: 10.1159/000337712. [DOI] [PubMed] [Google Scholar]
- 66.Snyder-Keller A, Sam C, Keller RW., Jr Enhanced susceptibility to cocaine- and pentylenetetrazol-induced seizures in prenatally cocaine-treated rats. Neurotoxicology and teratology. 2000;22:231–236. doi: 10.1016/s0892-0362(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 67.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behavioural brain research. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Current opinion in neurobiology. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 69.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual review of neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annual review of neuroscience. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 71.Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L. Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. Journal of neurodevelopmental disorders. 2009;1:185–196. doi: 10.1007/s11689-009-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]