Abstract
After total gastrectomy, anastomosis-related complications such as leak or stricture can be highly morbid. Between July 2005 and December 2015, a linear-stapled side-to-side esophagojejunostomy with hand-sewn closure of the common enterotomy (modified Orringer technique) was used for Roux-en-Y reconstruction after prophylactic total gastrectomy in 22 germline CDH1 mutation carriers and after therapeutic total gastrectomy in 18 patients diagnosed with gastric adenocarcinoma. All operations were performed by the same surgeon. No patient in either cohort developed a clinically evident anastomotic leak, one patient (2.5%) developed a contained radiographic leak that healed without intervention, and one patient (2.5%) developed an anastomotic stricture treated by endoscopic dilatation 7 months after operation. These rates were lower than radiographic leak and stricture rates in a comparison group of 32 patients who received a completely hand-sewn esophagojejunostomy (6.3% and 3.1%, respectively). Here we describe how to perform the linear-stapled esophagojejunostomy anastomosis.
Keywords: Gastric adenocarcinoma, hereditary diffuse gastric cancer, CDH1 mutation, total gastrectomy, esophagojejunostomy, anastomotic leak, anastomotic stricture
Introduction
Gastric cancer is the fifth leading cause of cancer worldwide and accounts for over 700,000 cancer-related deaths every year.[1] Early gastric cancer has an excellent prognosis when treated with endoscopic resection or gastrectomy with regional lymphadenectomy.[2-5] Patients with locally advanced gastric cancer often receive multimodality treatment with surgery, chemotherapy, and/or chemoradiation.[6, 7] A small percentage of gastric adenocarcinomas (Lauren diffuse type) arise in individuals with germline mutation of the CDH1 gene, which encodes the E-cadherin cell adhesion protein.[8-10] For germline CDH1 mutation carriers, the cumulative risk of developing diffuse gastric adenocarcinoma by 80 years of age is up to 80%, and the average age of diagnosis is 38 years old.[11, 12] Prophylactic total gastrectomy is recommended in healthy individuals with germline CDH1 mutation.[13]
The creation of the esophagojejunal anastomosis after total gastrectomy can be technically demanding, and reconstruction-related complications such as anastomotic leak and stricture account for a significant proportion of post-operative morbidity.[14-17] A recent retrospective review examined the adverse events within 90 days of operation for 238 patients who received total gastrectomy for gastric cancer.[18] Esophagojejunal anastomotic leak requiring invasive intervention was the most common major adverse event (11%). Even among the usually younger and healthier individuals that receive prophylactic total gastrectomy, esophageal anastomotic leak rates between 8-26% have been reported.[19-23] Anastomotic stricture rates of up to 21% have been reported following esophagojejunostomy with the highest rates occurring with circular staplers.[24-28]
In 2000, Orringer et al. described a technique for cervical esophagogastric anastomosis following transhiatal esophagectomy using a linear stapler to create a side-to-side anastomosis between the posterior wall of the esophagus and the gastric conduit, followed by closing the common enterotomy with two layers of running suture.[29] Prior to the use of this anastomosis, hand-sewn esophagogastric or esophagocolic anastomoses were performed in over 1000 patients with leak rates between 10-15%.[30] After employing the stapled anastomosis technique, clinically significant leaks were observed in only 3 of 114 patients (2.7%).
In this article, we report a single surgeon's experience with a modified version of the Orringer anastomosis in 22 CDH1 mutation carriers who underwent prophylactic total gastrectomy and 18 patients diagnosed with gastric adenocarinoma who underwent therapeutic total gastrectomy. The technique for the linear-stapled side-to-side esophagojejunal anastomosis is highlighted and described. Clinicopathologic factors and treatment outcomes are compared between patients who received the stapled esophagojejunostomy and a group of 32 patients who received a completely hand-sewn esophagojejunostomy.
Methods
A retrospective review of patients who underwent prophylactic or therapeutic total gastrectomy by a single surgeon (S.S.Y.) was performed for the time period between July 2005 and December 2015. Institutional Review Board approvals were obtained from both institutions at which the surgeon performed the operations (Massachusetts General Hospital, Boston, MA; Memorial Sloan Kettering Cancer Center, New York, NY). Patient demographics, clinical history, operative details, and postoperative outcomes were collected and recorded in prospectively maintained databases.
Operative Technique
The peritoneal cavity is entered via a vertical midline incision from xiphoid to umbilicus. A Bookwalter retractor is used to gain adequate exposure of the foregut structures. During gastric and nodal dissection, wide exposure of the distal esophagus is obtained to allow adequate visualization and performance of the esophagojejunal anastomosis (Fig. 1a). The left lateral segment of the liver is completely mobilized by dividing the left triangular ligament and is retracted to the right of the esophageal hiatus. The phrenoesophageal ligament is completely divided, and the abdominal esophagus and lower thoracic esophagus are mobilized to a length of approximately 6-8 cm above the gastroesophageal junction (GEJ). The anterior and posterior vagal trunks are divided. In obese patients or for tumors encroaching the esophagus, the esophageal hiatus can be opened anteriorly to improve exposure of the distal thoracic esophagus. After this dissection, the orogastric tube is removed.
Figure 1.
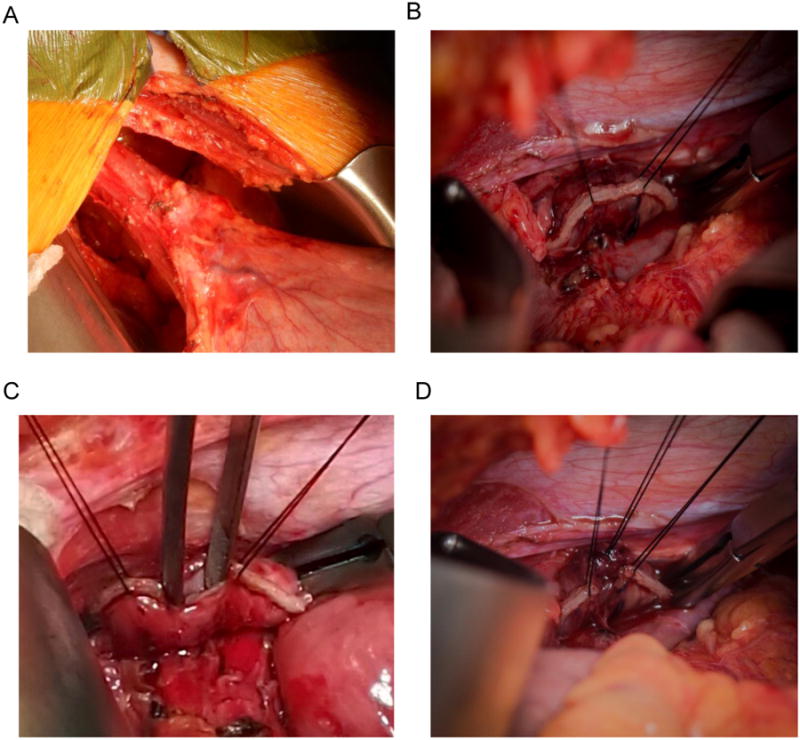
(a) The distal esophagus is mobilized to a length of 6 to 8 cm above the gastroesophageal junction.
(b)Two stay sutures of 3-0 silk are placed 15-20 mm apart in the midportion of the esophageal staple line. In preparation for the anastomosis, a 10 mm esophagotomy will be created in between the stay sutures by cutting out the three rows of staples.
(c)The lumen of the esophagus with whitish mucosa is demonstrated.
(d) To prevent false passage of the stapler anvil, the layers of the esophageal wall are sutured together. The two anastomotic stay sutures and the suture through the anterior wall layers are visible.
The esophagus is transected approximately 2 cm proximal to the GEJ for prophylactic cases and 4-5 cm proximal to gross tumor in therapeutic cases using the Endo GIA™ Ultra Universal Short stapler with a 60 mm articulating medium/thick (purple) reload with Tri-Staple™ technology (Covidien, North Haven, CT). The cartridge is maximally articulated to transect the esophagus in a transverse fashion. A Satinsky clamp can be placed across the esophagus 6-8 cm proximal to the GEJ to prevent the esophagus from retracting cephalad. In preparation for reconstruction, at least 5 cm of the remaining esophagus must be completely mobilized, paying particular attention to mobilization of the posterior wall.
Reconstruction is performed with a Roux-en-Y esophagojejunostomy in retrocolic fashion. The jejunum is divided approximately 20 cm distal to the ligament of Treitz with an Endo GIA™ stapler with a 60 mm medium/thick (purple) reload. The intervening mesentery is divided as much as necessary to allow a 60 cm Roux limb to reach the esophagus without tension through a window in the transverse mesocolon.
The esophagojejunal anastomosis is created using a modification of a technique described by Orringer et al., thus termed the modified Orringer technique.[29] Two anastomotic stay sutures of 3-0 silk are placed 15-20 mm apart just proximal to the midportion of the esophageal staple line (Fig. 1b). Placing these sutures just proximal to the staple line helps prevent tearing of the esophageal wall when tension is placed on these sutures. A 10 mm esophagotomy is created in the midportion of the staple line by cutting out the three rows of staples between the stay sutures. The lumen of the esophagus should be identified between the two smooth whitish layers of mucosa (Fig. 1c). All layers of the anterior wall, including mucosa and muscularis, are sewn together by a 3-0 silk interrupted suture, and all layers of the posterior wall are sewn together in similar fashion (Fig. 1d). This step is performed to prevent false passage of the stapler anvil. A large Kelly clamp can be used to investigate the esophageal lumen to determine the appropriate angle for insertion of the stapler anvil and to determine if tension-free passage up to 5 cm is possible.
An enterotomy is created on the antimesenteric border of the jejunal Roux limb approximately 6 cm distal to the transection staple line. The large jaw of the Endo GIA™ stapler with a 45 mm medium/thick (purple) reload is placed into the jejunum (Fig. 2a), and the antimesenteric portion of the Roux limb is brought posterior to the esophagus. The small jaw is placed into the esophageal lumen (Fig. 2b). Prior to firing the stapler, the esophagotomy and the Roux limb enterotomy must be exactly aligned at the proximal end of the stapler anvil. The esophagus tends to retract cephalad, and the Roux limb tends to slide caudad. If these two orifices are misaligned, a greater length of the jejunum is incorporated in the staple line, resulting in a common enterotomy that is larger than necessary. Once the orifices are aligned without any intervening tissue between the esophagus and the Roux limb, the stapler is slowly fired, creating three rows of staples on each side of the staple line.
Figure 2.
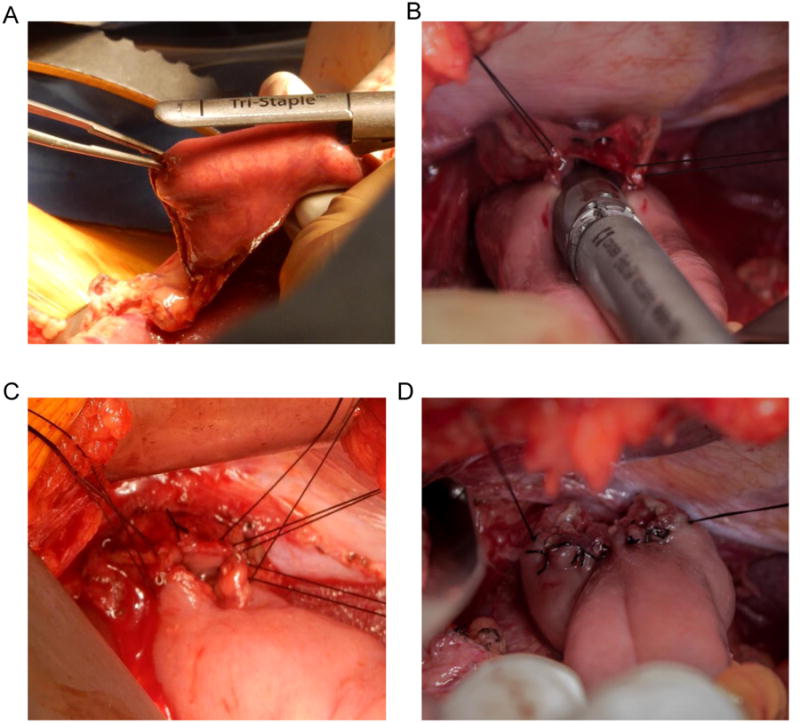
(a) The large jaw of the Endo GIA™ stapler with a 45 mm medium/thick (purple) reload is placed into the enterotomy created on the antimesenteric border of the jejunal Roux limb approximately 6 cm distal to the transection staple line.
(b) The small jaw of the stapler is placed into the esophageal lumen.
(c) The common enterotomy is closed with hand-sewn, interrupted 3-0 silk sutures in a single-layer fashion.
(d) The completed esophagojejunal anastomosis with hand-sewn closure of the common enterotomy.
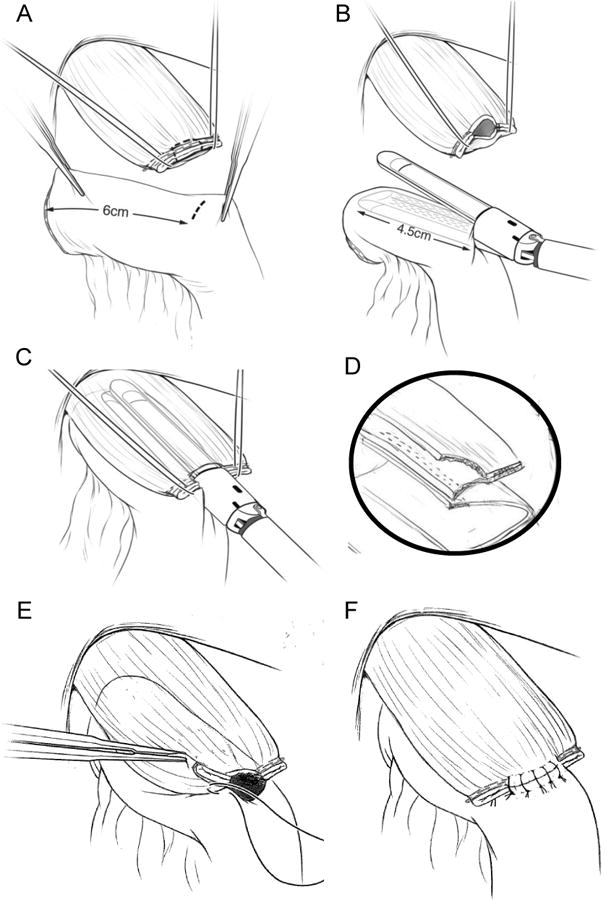
The common enterotomy is closed with hand-sewn, interrupted 3-0 silk sutures in a single-layer fashion. The corner sutures are placed first, and the untied ends are kept together with a Halsted clamp. The esophageal bites of the corner stitches occur just posterior to the staple line of the original esophageal transection on the posterior wall of the esophagus (Fig. 2c). The remaining sutures are then placed from anatomical left to right, and the untied ends for each suture are kept together with a Halsted clamp. The esophageal bites of these subsequent stitches are placed anterior to the staple line of the original esophageal transection on the anterior wall of the esophagus. A long, fine needle driver is typically used for suturing in this narrow space. It is important to avoid excessive torque on the needle, as the esophageal wall lacks serosa and can tear relatively easily. Thus, forceps are used to grasp the tip of the needle as it exits the esophageal wall and pull the remainder of the needle through the tissue to minimize torque. Once all sutures have been placed, they are tied from anatomical left to right, and the ends are cut (Fig. 2d). Figure 3 shows line drawings which demonstrate the key steps in the creation of this esophagojejunostomy anastomosis.
Figure 3. (a)-(f) Line drawings demonstrating performance of the esophagojejunostomy anastamosis.
In the postoperative period, patients are kept nil per os (NPO) until day 3-5, and an upper gastrointestinal series with contrast is obtained to rule out a subclinical anastomotic leak or stenosis. If no abnormality is identified, patients are advanced from sips to a mechanical soft diet in the ensuing three days. The patient is subsequently discharged on a mechanical soft diet for 2-3 weeks.
Results
Thirty-two patients who underwent prophylactic total gastrectomy for germline CDH1 mutation were identified from the examined time period. Table 1 summarizes the clinicopathologic and treatment characteristics of this group. Median age at time of operation was 42 years (range, 16-58 years), and 22 patients were female (69%). A small proportion of patients presented with accompanying gastrointestinal symptoms, and abdominal pain or discomfort was the most common symptom (13%). All patients had a documented family history of gastric cancer. Twelve patients had at least one family member with history of breast cancer (38%). A hand-sewn end-to-side esophagojejunal anastomosis was performed in the first ten consecutive patients, as previously described.[31] Thereafter, a linear-stapled side-to-side esophagojejunostomy with hand-sewn closure of the common enterotomy (i.e., modified Orringer anastomosis) was employed, as described above.[29]
Table 1. Clinicopathologic and treatment characteristics in patients with germline CDH1 mutation who underwent prophylactic total gastrectomy.
| Prophylactic Total Gastrectomy | All patients (n=32) n or median (% or range) | Hand-sewn anastomosis (n=10) n or median (% or range) | Modified Orringer anastomosis (n=22) n or median (% or range) |
|---|---|---|---|
|
| |||
| Age (years) | 42 (16-58) | 42 (26-50) | 42 (16-58) |
|
| |||
| Gender | |||
| Male | 10 (31.3) | 6 (60.0) | 4 (18.2) |
| Female | 22 (68.7) | 4 (40.0) | 18 (81.8) |
|
| |||
| Body mass index | 25.6 (18.0-41.8) | 25.6 (22.1-41.8) | 25.8 (18.0-36.3) |
|
| |||
| Gastrointestinal symptoms at presentationa | |||
| Abdominal pain or discomfort | 4 (12.5) | 3 (30.0) | 1 (4.5) |
| Nausea or vomiting | 2 (6.2) | 1 (10.0) | 1 (4.5) |
| Dyspepsia or heartburn | 2 (6.2) | 1 (10.0) | 1 (4.5) |
| Dysphagia | 1 (3.1) | 0 (0.0) | 1 (4.5) |
| Diarrhea | 2 (6.2) | 1 (10.0) | 1 (4.5) |
| None | 23 (71.9) | 5 (50.0) | 18 (81.8) |
|
| |||
| Family history | |||
| Gastric cancer | 32 (100.0) | 10 (100.0) | 22 (100.0) |
| Family members with CDH1 mutation | 29 (90.7) | 9 (90.0) | 20 (90.9) |
| Breast cancer | 12 (37.5) | 4 (40.0) | 8 (36.4) |
|
| |||
| Operative time (minutes) | 190 (124-308) | 229 (187-308) | 167 (124-267) |
|
| |||
| Estimated intraoperative blood loss (milliliters) | 100 (50-1000) | 300 (100-800) | 100 (50-1000) |
|
| |||
| Length of stay (days) | 8 (7-12) | 7 (7-8) | 8 (7-12) |
|
| |||
| Anastomotic leak | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Anastomotic stricture | 1 (3.1) | 1 (10.0) | 0 (0.0) |
|
| |||
| 30-day morbidity | 3 (9.4) | 1 (10.0) | 2 (9.1) |
|
| |||
| 30-day mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Weight loss (nadir % from baseline) | 15.8 (0.6-43.2) | 15.7 (8.9-43.2) | 16.0 (0.7-28.8) |
|
| |||
| Presence of microscopic foci of carcinoma | |||
| Noninvasive (intramucosal or in situ) | 9 (28.2) | 7 (70.0) | 2 (9.1) |
| Invasive (≥T1a) | 19 (59.4) | 1 (10.0) | 18 (81.8) |
| Noninvasive and Invasive | 2 (6.2) | 1 (10.0) | 1 (4.5) |
| None | 2 (6.2) | 1 (10.0) | 1 (4.5) |
|
| |||
| Lymph nodes resected (n) | 13 (1-40) | 13 (6-18) | 13 (1-40) |
Symptoms not mutually exclusive
For patients who received prophylactic total gastrectomy with an entirely hand-sewn anastomosis (n=10), no patient experienced an esophagojejunal anastomotic leak as documented by esophagram (performed in all 10 patients) or clinical symptoms. One patient (10%) developed anastomotic stricture requiring endoscopic dilatation at 6 months after the operation. Within 30 post-operative days, one patient developed pulmonary embolism which was treated with low molecular weight heparin bridge to warfarin.
In the patient cohort receiving prophylactic total gastrectomy with the modified Orringer anastomosis (n = 22), no patient experienced an anastomotic leak as documented by esophagram (performed in 21 of 22 patients) or clinical symptoms. No patients developed anastomotic stricture that required endoscopic dilatation. Within 30 postoperative days, one patient developed a superficial surgical site infection which was treated with a course of oral antibiotics, and one patient developed mild pancreatitis requiring re-admission on post-operative day 16 for supportive care.
Among all patients who received prophylactic total gastrectomy, the 30-day mortality rate was 0%, and the median nadir weight loss was 16% (range <1-43%) from baseline weight. At least one focus of intramucosal and/or early invasive carcinoma was present in 30 resected specimens (94%). Formal lymph node dissections were not performed, but perigastric lymph nodes were generally incorporated in the prophylactic total gastrectomy specimen. The median number of nodes resected was 13 (range, 1-40 nodes).
During the study period, 40 patients diagnosed with clinically apparent gastric adenocarcinoma underwent therapeutic total gastrectomy by the same surgeon. The first 22 consecutive patients had a hand-sewn esophagojejunostomy anastomosis while the subsequent 18 had a modified Orringer anastomosis. Table 2 summarizes the clinicopathologic and treatment characteristics of this group. Median age at time of operation was 67 years (range, 40-92 years), and 18 patients (45%) were female. Nineteen patients (48%) had diffuse-or mixed-type gastric cancer; Lauren histologic type was unspecified in nine patients. Formal D1+ or D2 lymphadenectomy was performed in all cases, and the median number of lymph nodes resected was 39 (range, 9-94 nodes). Twenty patients (50%) received neoadjuvant chemotherapy or chemoradiation.
Table 2. Clinicopathologic and treatment characteristics in patients who underwent therapeutic total gastrectomy for gastric cancer.
| Therapeutic Total Gastrectomy | All patients (n=40) n or median (% or range) | Hand-sewn anastomosis (n=22) n or median (% or range) | Modified Orringer anastomosis (n=18) n or median (% or range) |
|---|---|---|---|
|
| |||
| Age (years) | 67 (40-92) | 67 (40-82) | 68 (42-92) |
|
| |||
| Gender | |||
| Male | 22 (55.0) | 10 (45.5) | 12 (66.7) |
| Female | 18 (45.0) | 12 (54.5) | 6 (33.3) |
|
| |||
| Body mass index | 25.8 (17.3-42.1) | 25.5 (17.3-42.1) | 27.1 (20.8-34.0) |
|
| |||
| Gastrointestinal symptoms at presentationa | |||
| Abdominal pain or discomfort | 20 (50.0) | 11 (50.0) | 9 (50.0) |
| Nausea or vomiting | 18 (45.0) | 11 (50.0) | 7 (38.9) |
| Dyspepsia or heartburn | 5 (12.5) | 4 (18.2) | 1 (5.6) |
| Dysphagia | 4 (10.0) | 2 (9.1) | 2 (11.1) |
| Gastrointestinal bleed / Microcytic anemia | 9 (22.5) | 5(22.7) | 4 (22.2) |
| None | 1 (2.5) | 1 (4.5) | 0 (0.0) |
|
| |||
| Family history | |||
| Gastric cancer | 5 (12.5) | 4 (18.2) | 1 (5.6) |
| Breast cancer | 9 (22.5) | 5 (22.7) | 4 (22.2) |
|
| |||
| Neoadjuvant treatment | |||
| Chemotherapy | 17 (42.5) | 11 (50.0) | 6 (33.3) |
| Chemoradiation therapy | 3 (7.5) | 1 (4.5) | 2 (11.1) |
|
| |||
| Adjuvant treatment | |||
| Chemotherapy | 13 (32.5) | 8 (36.4) | 5 (27.8) |
| Chemoradiation therapy | 8 (20.0) | 6 (27.3) | 2 (11.1) |
|
| |||
| Operative time (minutes) | 266 (187-485) | 301 (224-485) | 242 (187-451) |
|
| |||
| Estimated intraoperative blood loss (milliliters) | 400 (100-1300) | 400 (250-1300) | 300 (100-1000) |
|
| |||
| Adjacent organ resectiona | |||
| Distal pancreatectomy | 3 (7.5) | 2 (9.1) | 1 (5.6) |
| Splenectomy | 12 (30.0) | 11 (50.0) | 3 (16.7) |
|
| |||
| Length of stay (days) | 9 (5-24) | 9.5 (5-24) | 8 (5-14) |
|
| |||
| Anastomotic leak | |||
| Radiographic | 3 (7.5) | 2 (9.1) | 1 (5.6) |
| Clinical | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Anastomotic stricture | 1 (2.5) | 0 (0.0) | 1 (5.6) |
|
| |||
| 30-day morbidity | 13 (32.5) | 9 (40.9) | 4 (22.2) |
|
| |||
| 30-day mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Weight loss (nadir % from baseline) | 17.3 (3.5-32.6) | 15.8 (6.3-29.6) | 18.6 (3.5-32.6) |
|
| |||
| Lauren histologic type | |||
| Intestinal | 12 (30.0) | 2 (9.1) | 10 (55.6) |
| Diffuse | 16 (40.0) | 13 (59.1) | 3 (16.7) |
| Mixed | 3 (7.5) | 0 (0.0) | 3 (16.7) |
| Unspecified | 9 (22.5) | 7 (31.8) | 2 (11.1) |
|
| |||
| Pathologic TNM stageb | |||
| Stage 0 | 3 (7.5) | 2 (9.1) | 1 (5.6) |
| Stage I | 10 (25.0) | 4 (18.2) | 6 (33.3) |
| Stage II | 10 (25.0) | 7 (31.8) | 3 (16.7) |
| Stage III | 17 (42.5) | 9 (40.9) | 8 (44.4) |
|
| |||
| Lymph nodes resected (n) | 39 (9-94) | 37 (9-83) | 39 (15-94) |
Categories not mutually exclusive
American Joint Committee on Cancer, 7th edition
Thirteen patients (33%) developed complications within 30 days of therapeutic total gastrectomy. Complications included superficial surgical site infection (n = 2), aspiration pneumonia (n = 1), infectious colitis (n = 2), adhesive bowel obstruction requiring laparotomy (n = 1), deep venous thrombus managed with oral anticoagulation (n = 2), and perihepatic abscess requiring drainage (n = 1). Among patients who received hand-sewn anastomoses after therapeutic total gastrectomy (n = 22), two patients developed subclinical radiographic anastomotic leak documented on esophagram. These radiographic leaks healed without intervention. There were no strictures requiring dilation. For patients who received the modified Orringer anastomosis after therapeutic total gastrectomy (n = 18), one patient developed a subclinical radiographic leak which healed without intervention, and one patient developed an esophagojejunal anastomotic stricture requiring endoscopic dilatation 7 months after the operation. No patient developed a clinically evident esophagojejunal anastomotic leak that required image-guided drainage. Among all patients, no post-operative deaths occurred within 30 days of operation.
When examining all 72 patients who underwent prophylactic or therapeutic total gastrectomy, patients who received a hand-sewn anastomosis (n = 32) had a subclinical radiological anastomotic leak rate of 6.3% and an anastomotic stricture rate of 3.1% (Table 3). Following adoption of the modified Orringer anastomosis (n = 40), subclinical radiological anastomotic leak and stricture rates were 2.5% and 2.5%, respectively.
Table 3. Anastomosis-related complications after total gastrectomy.
| Total gastrectomy | All patients (n=72) n (%) | Hand-sewn anastomosis (n=32) n (%) | Modified Orringer anastomosis (n=40) n (%) |
|---|---|---|---|
|
| |||
| Anastomotic leak | |||
| Radiographic | 3 (4.2) | 2 (6.3) | 1 (2.5) |
| Clinical | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Anastomotic stricture | 2 (2.8) | 1 (3.1) | 1 (2.5) |
Discussion
In this article, we describe a single surgeon's experience with a linear-stapled side-to-side esophagojejunostomy reconstruction in 22 consecutive patients who underwent prophylactic total gastrectomy for CDH1 mutation and 18 consecutive patients who underwent therapeutic total gastrectomy for gastric adenocarcinoma. Using this anastomosis, there were no clinically evident anastomotic leaks. Routine post-operative upper gastrointestinal contrast studies identified a subclinincal leak in one patient (2.5%), which healed without intervention. Only one patient (2.5%) developed a symptomatic stricture that required endoscopic dilatation. Thus, this modified Orringer technique may be a safe and reliable means of performing the esophagojejunal anastomosis following total gastrectomy.
Historically, reconstruction-related complications after total gastrectomy were associated with cause-specific mortality rates between 13-30%.[17, 32-34] The improved post-operative outcomes from more recent published series has been partially attributed to the decrease in esophagojejunal anastomotic leak rates, reported as low as <5% from large East Asian studies.[35-37] However, anastomotic leaks continue to prolong hospital stays and increase healthcare costs, and studies have suggested that anastomotic complications not only worsen post-operative outcomes but can also adversely affect long-term disease-specific survival.[17]
Large randomized control studies from East Asia and Europe have previously demonstrated no difference in anastomotic leak or stricture rates between hand-sewn and stapled anastomoses in total gastrectomy.[35, 38, 39] Nonetheless, the widespread use and availability of mechanical stapling devices and the growing expertise of minimally invasive approaches has largely shifted the method of esophagojejunal anastomosis construction to stapled anastomosis. Small case series utilizing novel techniques have been reported, but no single consensus method has been widely adopted or definitively shown to be superior in outcomes.[28, 40-42] Table 4 summarizes the esophagojejunal anastomosis-related complication rates after total gastrectomy from the most recent surgical case series of comparable size to this study cohort.
Table 4. Surgical case series of anastomotic complications after total gastrectomy (Publication date 1990-2016, study n ≥70).
| First Author | Study period | Approacha | Anastomosis Methodb | n | EJ Leak (%) | EJ Stenosis (%) |
|---|---|---|---|---|---|---|
| Fujimoto S[38] | 1983-1989 | O | Hand | 180 | 2.2 | 0.6 |
| O | Circular | 199 | 3.5 | 3.0 | ||
|
| ||||||
| Seufert RM[39] | 1987-1988 | O | Hand | 40 | 0 | 2.5 |
| O | Circular | 40 | 2.5 | 0 | ||
|
| ||||||
| Kim CB[44] | 1987-1992 | O | Circular | 100 | 2.0 | 2.0 |
|
| ||||||
| Yoo CH[45] | 1990-2000 | O | Circular | 185 | 4.9 | 8.1 |
|
| ||||||
| Kawamura Y[46] | 1997-2012 | LA | Linear | 259 | 5.0 | NR |
|
| ||||||
| Jeong GA[47] | 1998-2005 | LA | Circular | 131 | 2.3 | 0 |
|
| ||||||
| Sierzega M[17] | 1999-2004 | O | Circular | 690 | 5.9 | NR |
|
| ||||||
| Hyodo M[35] | 2001-NR | O | Circular | 390 | 0.5 | 1.0 |
|
| ||||||
| Son MW[48] | 2001-2008 | O | Circular | 106 | 1.9 | 2.8 |
|
| ||||||
| Ito H[49] | 2001-2012 | O | Circular | 166 | 4.2 | 3.0 |
| LA | Circular | 46 | 0 | 4.3 | ||
| TL | Circular | 117 | 1.7 | 1.7 | ||
|
| ||||||
| Wada N[50] | 2001-2012 | LA | Circular | 100 | 4.0 | 0 |
|
| ||||||
| Nagai E[51] | 2002-2010 | LA | Linear | 94 | 2.1 | 0 |
|
| ||||||
| Lee JH[52] | 2003-2010 | O + LA | Circular | 753 | 1.5 | 3 |
|
| ||||||
| Lee MS[53] | 2003-2010 | O | Circular | 228 | 2.2 | 1.8 |
|
| ||||||
| Jeong O[54] | 2003-2011 | O | Circular | 122 | 2.5 | 1.6 |
| LA | Circular | 122 | 7.4 | 1.6 | ||
| 2004- 2013 | LA | Circular | 203 | 4.4 | NR | |
|
| ||||||
| Kim DJ[55] | 2004-2014 | LA | Circular | 94 | 5.3 | 3.2 |
|
| ||||||
| Usui S[56] | 2004-2014 | LA | Circular | 78 | 0 | 0 |
|
| ||||||
| Morimoto M[41] | 2005-2013 | LA | Linear | 77 | 0 | 1.3 |
|
| ||||||
| Lee SR[57] | 2006-2009 | O | Circular | 50 | 6.0 | 0 |
| LA | Circular | 34 | 5.9 | 5.9 | ||
|
| ||||||
| Tsunoda S[58] | 2006-2012 | TL | Linear | 112 | 1.8 | 0 |
|
| ||||||
| Chen K[42, 59] | 2006-2014 | TL | Hand | 42 | 0 | 0 |
| Linear | 32 | 0 | 6.3 | |||
| Circular | 18 | 5.6 | 5.6 | |||
|
| ||||||
| Ichikawa D[60] | 2007-2013 | LA | Circular | 88 | 0 | 3.4 |
|
| ||||||
| Kanaji S[36] | 2008-2011 | O | Circular | 185 | 4.9 | NR |
|
| ||||||
| Kim HS[61] | 2009-2010 | TL | Linear | 124 | 1.6 | 1.6 |
|
| ||||||
| Yoon HM[26] | 2009-2011 | LA | Circular | 65 | 4.6 | 4.6 |
| RA | Circular | 36 | 0 | 2.8 | ||
|
| ||||||
| Shim JH[62] | 2009-2011 | O | Hand | 35 | 2.9 | 0 |
| LA | Linear + Circular | 35 | 8.6 | 22.9 | ||
|
| ||||||
| Song JH[63] | 2009-2013 | O | Circular | 134 | 0 | NR |
| LA | Circular | 74 | 0 | NR | ||
|
| ||||||
| Zhang G[64] | 2010-2011 | LA | Circular | 73 | 0 | 0 |
|
| ||||||
| Kim HS[27, 65] | 2011 | O | Linear | 207 | 1.4 | 0.5 |
| TL | Linear | 139 | 0.7 | 0 | ||
|
| ||||||
| Li P[66] | 2012 | LA | Circular | 108 | 0.9 | NR |
O = open; LA = laparoscopy-assisted; TL = totally laparoscopic; RA = robot-assisted
Hand = manually hand-sewn; Linear = linear stapler; Circular = circular stapler (including transoral delivery of anvil) n = number of patients; EJ = esophagojejunal anastomosis; NR = not recorded
Many centers, particularly in East Asian countries, perform circular stapled anastomoses. However, circular staplers carry their own set of challenges, including technical difficulty in the properly positioning the stapler anvil. Systems of transoral delivery of the anvil have been developed, but they carry the potential risks of injuring the esophageal mucosa and potential bacterial seeding of the abdominal cavity.[43] The modified Orringer technique described here may have a flatter learning curve based on the pervasive use and familiarity of the Endo GIA™ linear stapler among general surgery practices and academic centers.
While similar anastomosis-related outcomes were achieved in patients who received therapeutic total gastrectomy, it is important to note that CDH1 mutation carriers represent a unique population. For a number of reasons, the operative morbidity is likely significantly lower in the prophylactic total gastrectomy population compared to total gastrectomy performed for diagnosed invasive gastric cancer. Indeed, in our study cohort, patients who received therapeutic total gastrectomy were advanced in age, and many received neoadjuvant therapy or adjacent organ resection – all of which could contribute to post-operative morbidity. A formal radical lymphadenectomy, which can contribute to complications, is also not routinely performed in prophylactic total gastrectomy.
The post-operative quality of life for the patient is critically important, particularly for patients with germline CDH1 mutation as they often receive prophylactic total gastrectomy before any appreciable gastrointestinal symptoms arise. Long-term follow-up for patients who received prophylactic total gastrectomy was difficult, as they were typically discharged from the surgical clinic after 12 months. In that period, all patients were provided nutritional consultation and were prescribed a daily multivitamin with ferrous sulfate and monthly vitamin B12 injections. No statistically significant differences in post-gastrectomy dumping syndrome, persistent reflux symptoms, or weight loss were identified when comparing patients who received modified Orringer anastomosis to patients who received a completely hand-sewn anastomosis. However, a more robust, large-scale trial examining the functional status of varying reconstructive techniques will be necessary to overcome the limitations of the present study.
In summary, a linear-stapled side-to-side esophagojejunostomy with hand-sewn closure of the common enterotomy for Roux-en-Y reconstruction after total gastrectomy can be performed safely with anastomotic leak and stricture rates less than 5%. This manuscript describes one of the largest Western experiences in the operative management of CDH1 mutation carriers.
Acknowledgments
The authors thank Wenjing Wu in the Department of Communications at Memorial Sloan Kettering Cancer Center for creating the line illustrations.
Grant Support: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosures: The authors declare no conflict of interest.
Statement of Author Contribution: Study conception and design: K.K.C. and S.S.Y.
Acquisition of data: K.K.C., M.S.P., and S.S.Y.
Analysis and interpretation of data: K.K.C., M.S.P., and S.S.Y.
Drafting of manuscript: K.K.C., M.S.P., and S.S.Y.
Critical revision: K.K.C., M.S.P., and S.S.Y.
Final approval of version to be published: K.K.C., M.S.P., and S.S.Y.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends-An Update. Cancer epidemiology, biomarkers ' prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. The British journal of surgery. 2008;95(9):1131–5. doi: 10.1002/bjs.6295. [DOI] [PubMed] [Google Scholar]
- 3.Hiki N, Sano T, Fukunaga T, Ohyama S, Tokunaga M, Yamaguchi T. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. Journal of the American College of Surgeons. 2009;209(3):297–301. doi: 10.1016/j.jamcollsurg.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. The British journal of surgery. 2010;97(4):558–62. doi: 10.1002/bjs.6944. [DOI] [PubMed] [Google Scholar]
- 5.Min YW, Min BH, Lee JH, Kim JJ. Endoscopic treatment for early gastric cancer. World journal of gastroenterology. 2014;20(16):4566–73. doi: 10.3748/wjg.v20.i16.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. The New England journal of medicine. 2001;345(10):725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 8.Lynch HT, Silva E, Wirtzfeld D, Hebbard P, Lynch J, Huntsman DG. Hereditary diffuse gastric cancer: prophylactic surgical oncology implications. The Surgical clinics of North America. 2008;88(4):759–78. vi–vii. doi: 10.1016/j.suc.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. E-cadherin alterations in hereditary disorders with emphasis on hereditary diffuse gastric cancer. Progress in molecular biology and translational science. 2013;116:337–59. doi: 10.1016/B978-0-12-394311-8.00015-7. [DOI] [PubMed] [Google Scholar]
- 10.Tan RY, Ngeow J. Hereditary diffuse gastric cancer: What the clinician should know. World journal of gastrointestinal oncology. 2015;7(9):153–60. doi: 10.4251/wjgo.v7.i9.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA oncology. 2015;1(1):23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 12.Pharoah PD, Guilford P, Caldas C International Gastric Cancer Linkage C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121(6):1348–53. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 13.van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. Journal of medical genetics. 2015;52(6):361–74. doi: 10.1136/jmedgenet-2015-103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang H, Piso P, Stukenborg C, Raab R, Jahne J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2000;26(2):168–71. doi: 10.1053/ejso.1999.0764. [DOI] [PubMed] [Google Scholar]
- 15.Meyer L, Meyer F, Dralle H, Ernst M, Lippert H, Gastinger I, et al. Insufficiency risk of esophagojejunal anastomosis after total abdominal gastrectomy for gastric carcinoma. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2005;390(6):510–6. doi: 10.1007/s00423-005-0575-2. [DOI] [PubMed] [Google Scholar]
- 16.Sauvanet A, Mariette C, Thomas P, Lozac'h P, Segol P, Tiret E, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. Journal of the American College of Surgeons. 2005;201(2):253–62. doi: 10.1016/j.jamcollsurg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Sierzega M, Kolodziejczyk P, Kulig J Polish Gastric Cancer Study G. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. The British journal of surgery. 2010;97(7):1035–42. doi: 10.1002/bjs.7038. [DOI] [PubMed] [Google Scholar]
- 18.Selby LV, Vertosick EA, Sjoberg DD, Schattner MA, Janjigian YY, Brennan MF, et al. Morbidity after Total Gastrectomy: Analysis of 238 Patients. Journal of the American College of Surgeons. 2015;220(5):863–71 e2. doi: 10.1016/j.jamcollsurg.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis FR, Mellinger JD, Hayashi A, Lorelli D, Monaghan KG, Carneiro F, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery. 2001;130(4):612–7. doi: 10.1067/msy.2001.117099. discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 20.Hebbard PC, Macmillan A, Huntsman D, Kaurah P, Carneiro F, Wen X, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Annals of surgical oncology. 2009;16(7):1890–5. doi: 10.1245/s10434-009-0471-z. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Kingham K, Ford JM, Rosing J, Van Dam J, Jeffrey RB, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Annals of surgical oncology. 2011;18(9):2594–8. doi: 10.1245/s10434-011-1648-9. [DOI] [PubMed] [Google Scholar]
- 22.Seevaratnam R, Coburn N, Cardoso R, Dixon M, Bocicariu A, Helyer L. A systematic review of the indications for genetic testing and prophylactic gastrectomy among patients with hereditary diffuse gastric cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2012;15(Suppl 1):S153–63. doi: 10.1007/s10120-011-0116-3. [DOI] [PubMed] [Google Scholar]
- 23.Haverkamp L, van der Sluis PC, Ausems MG, van der Horst S, Siersema PD, Ruurda JP, et al. Prophylactic Laparoscopic Total Gastrectomy with Jejunal Pouch Reconstruction in Patients Carrying a CDH1 Germline Mutation. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2015;19(12):2120–5. doi: 10.1007/s11605-015-2963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. Journal of surgical oncology. 2009;100(5):392–5. doi: 10.1002/jso.21345. [DOI] [PubMed] [Google Scholar]
- 25.Nunobe S, Hiki N, Tanimura S, Kubota T, Kumagai K, Sano T, et al. Three-step esophagojejunal anastomosis with atraumatic anvil insertion technique after laparoscopic total gastrectomy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2011;15(9):1520–5. doi: 10.1007/s11605-011-1489-7. [DOI] [PubMed] [Google Scholar]
- 26.Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surgical endoscopy. 2012;26(5):1377–81. doi: 10.1007/s00464-011-2043-0. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. Journal of laparoendoscopic ' advanced surgical techniques Part A. 2013;23(4):323–31. doi: 10.1089/lap.2012.0389. [DOI] [PubMed] [Google Scholar]
- 28.Zuiki T, Hosoya Y, Kaneda Y, Kurashina K, Saito S, Ui T, et al. Stenosis after use of the double-stapling technique for reconstruction after laparoscopy-assisted total gastrectomy. Surgical endoscopy. 2013;27(10):3683–9. doi: 10.1007/s00464-013-2945-0. [DOI] [PubMed] [Google Scholar]
- 29.Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. The Journal of thoracic and cardiovascular surgery. 2000;119(2):277–88. doi: 10.1016/S0022-5223(00)70183-8. [DOI] [PubMed] [Google Scholar]
- 30.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Annals of surgery. 1999;230(3):392–400. doi: 10.1097/00000658-199909000-00012. discussion -3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandalai PK, Lauwers GY, Chung DC, Patel D, Yoon SS. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery. 2011;149(3):347–55. doi: 10.1016/j.surg.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Isozaki H, Okajima K, Ichinona T, Hara H, Fujii K, Nomura E. Risk factors of esophagojejunal anastomotic leakage after total gastrectomy for gastric cancer. Hepato-gastroenterology. 1997;44(17):1509–12. [PubMed] [Google Scholar]
- 33.Doglietto GB, Papa V, Tortorelli AP, Bossola M, Covino M, Pacelli F, et al. Nasojejunal tube placement after total gastrectomy: a multicenter prospective randomized trial. Archives of surgery. 2004;139(12):1309–13. doi: 10.1001/archsurg.139.12.1309. discussion 13. [DOI] [PubMed] [Google Scholar]
- 34.Pacelli F, Papa V, Rosa F, Tortorelli AP, Sanchez AM, Covino M, et al. Four hundred consecutive total gastrectomies for gastric cancer: a single-institution experience. Archives of surgery. 2008;143(8):769–75. doi: 10.1001/archsurg.143.8.769. discussion 75. [DOI] [PubMed] [Google Scholar]
- 35.Hyodo M, Hosoya Y, Hirashima Y, Haruta H, Kurashina K, Saito S, et al. Minimum leakage rate (0.5%) of stapled esophagojejunostomy with sacrifice of a small part of the jejunum after total gastrectomy in 390 consecutive patients. Digestive surgery. 2007;24(3):169–72. doi: 10.1159/000102100. [DOI] [PubMed] [Google Scholar]
- 36.Kanaji S, Ohyama M, Yasuda T, Sendo H, Suzuki S, Kawasaki K, et al. Can the intraoperative leak test prevent postoperative leakage of esophagojejunal anastomosis after total gastrectomy? Surgery today. 2015 doi: 10.1007/s00595-015-1243-y. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Pan Y, Cai JQ, Wu D, Yan JF, Chen DW, et al. Totally laparoscopic versus laparoscopic-assisted total gastrectomy for upper and middle gastric cancer: a single-unit experience of 253 cases with meta-analysis. World journal of surgical oncology. 2016;14(1):96. doi: 10.1186/s12957-016-0860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimoto S, Takahashi M, Endoh F, Takai M, Kobayashi K, Kiuchi S, et al. Stapled or manual suturing in esophagojejunostomy after total gastrectomy: a comparison of outcome in 379 patients. American journal of surgery. 1991;162(3):256–9. doi: 10.1016/0002-9610(91)90081-n. [DOI] [PubMed] [Google Scholar]
- 39.Seufert RM, Schmidt-Matthiesen A, Beyer A. Total gastrectomy and oesophagojejunostomy--a prospective randomized trial of hand-sutured versus mechanically stapled anastomoses. The British journal of surgery. 1990;77(1):50–2. doi: 10.1002/bjs.1800770118. [DOI] [PubMed] [Google Scholar]
- 40.Kunisaki C, Makino H, Oshima T, Fujii S, Kimura J, Takagawa R, et al. Application of the transorally inserted anvil (OrVil) after laparoscopy-assisted total gastrectomy. Surgical endoscopy. 2011;25(4):1300–5. doi: 10.1007/s00464-010-1367-5. [DOI] [PubMed] [Google Scholar]
- 41.Morimoto M, Kitagami H, Hayakawa T, Tanaka M, Matsuo Y, Takeyama H. The overlap method is a safe and feasible for esophagojejunostomy after laparoscopic-assisted total gastrectomy. World journal of surgical oncology. 2014;12:392. doi: 10.1186/1477-7819-12-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K, He Y, Cai JQ, Pan Y, Wu D, Chen DW, et al. Comparing the short-term outcomes of intracorporeal esophagojejunostomy with extracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer. BMC surgery. 2016;16:13. doi: 10.1186/s12893-016-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen K, Wu D, Pan Y, Cai JQ, Yan JF, Chen DW, et al. Totally laparoscopic gastrectomy using intracorporeally stapler or hand-sewn anastomosis for gastric cancer: a single-center experience of 478 consecutive cases and outcomes. World journal of surgical oncology. 2016;14(1):115. doi: 10.1186/s12957-016-0868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CB, Suh KW, Moon JI, Min JS. Roux-en-Y end-to-side esophagojejunostomy with stapler after total gastrectomy. Yonsei medical journal. 1993;34(4):334–9. doi: 10.3349/ymj.1993.34.4.334. [DOI] [PubMed] [Google Scholar]
- 45.Yoo CH, Sohn BH, Han WK, Pae WK. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer research and treatment : official journal of Korean Cancer Association. 2004;36(1):50–5. doi: 10.4143/crt.2004.36.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawamura Y, Satoh S, Suda K, Ishida Y, Kanaya S, Uyama I. Critical factors that influence the early outcome of laparoscopic total gastrectomy. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18(3):662–8. doi: 10.1007/s10120-014-0392-9. [DOI] [PubMed] [Google Scholar]
- 47.Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146(3):469–74. doi: 10.1016/j.surg.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 48.Son MW, Kim YJ, Jeong GA, Cho GS, Lee MS. Long-Term Outcomes of Proximal Gastrectomy versus Total Gastrectomy for Upper-Third Gastric Cancer. Journal of gastric cancer. 2014;14(4):246–51. doi: 10.5230/jgc.2014.14.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito H, Inoue H, Odaka N, Satodate H, Onimaru M, Ikeda H, et al. Evaluation of the safety and efficacy of esophagojejunostomy after totally laparoscopic total gastrectomy using a trans-orally inserted anvil: a single-center comparative study. Surgical endoscopy. 2014;28(6):1929–35. doi: 10.1007/s00464-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 50.Wada N, Kurokawa Y, Takiguchi S, Takahashi T, Yamasaki M, Miyata H, et al. Feasibility of laparoscopy-assisted total gastrectomy in patients with clinical stage I gastric cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2014;17(1):137–40. doi: 10.1007/s10120-013-0235-0. [DOI] [PubMed] [Google Scholar]
- 51.Nagai E, Ohuchida K, Nakata K, Miyasaka Y, Maeyama R, Toma H, et al. Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers. Surgery. 2013;153(5):732–8. doi: 10.1016/j.surg.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Nam BH, Ryu KW, Ryu SY, Park YK, Kim S, et al. Comparison of outcomes after laparoscopy-assisted and open total gastrectomy for early gastric cancer. The British journal of surgery. 2015;102(12):1500–5. doi: 10.1002/bjs.9902. [DOI] [PubMed] [Google Scholar]
- 53.Lee MS, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surgical endoscopy. 2013;27(7):2598–605. doi: 10.1007/s00464-013-2796-8. [DOI] [PubMed] [Google Scholar]
- 54.Jeong O, Ryu SY, Choi WY, Piao Z, Park YK. Risk factors and learning curve associated with postoperative morbidity of laparoscopic total gastrectomy for gastric carcinoma. Annals of surgical oncology. 2014;21(9):2994–3001. doi: 10.1245/s10434-014-3666-x. [DOI] [PubMed] [Google Scholar]
- 55.Kim DJ, Lee JH, Kim W. Comparison of the major postoperative complications between laparoscopic distal and total gastrectomies for gastric cancer using Clavien-Dindo classification. Surgical endoscopy. 2015;29(11):3196–204. doi: 10.1007/s00464-014-4053-1. [DOI] [PubMed] [Google Scholar]
- 56.Usui S, Tashiro M, Haruki S, Arita K, Ito K, Matsumoto A, et al. Spleen preservation versus splenectomy in laparoscopic total gastrectomy with D2 lymphadenectomy for gastric cancer: A comparison of short-term outcomes. Asian journal of endoscopic surgery. 2016;9(1):5–13. doi: 10.1111/ases.12255. [DOI] [PubMed] [Google Scholar]
- 57.Lee SR, Kim HO, Son BH, Shin JH, Yoo CH. Laparoscopic-assisted total gastrectomy versus open total gastrectomy for upper and middle gastric cancer in short-term and long-term outcomes. Surgical laparoscopy, endoscopy ' percutaneous techniques. 2014;24(3):277–82. doi: 10.1097/SLE.0b013e3182901290. [DOI] [PubMed] [Google Scholar]
- 58.Tsunoda S, Okabe H, Obama K, Tanaka E, Hisamori S, Kinjo Y, et al. Short-term outcomes of totally laparoscopic total gastrectomy: experience with the first consecutive 112 cases. World journal of surgery. 2014;38(10):2662–7. doi: 10.1007/s00268-014-2611-2. [DOI] [PubMed] [Google Scholar]
- 59.Chen K, Pan Y, Cai JQ, Xu XW, Wu D, Yan JF, et al. Intracorporeal esophagojejunostomy after totally laparoscopic total gastrectomy: A single-center 7-year experience. World journal of gastroenterology. 2016;22(12):3432–40. doi: 10.3748/wjg.v22.i12.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ichikawa D, Komatsu S, Kubota T, Okamoto K, Konishi H, Shiozaki A, et al. Evaluation of the safety and feasibility of laparoscopic total gastrectomy in clinical stage I gastric cancer patients. World journal of surgery. 2015;39(7):1782–8. doi: 10.1007/s00268-015-3008-6. [DOI] [PubMed] [Google Scholar]
- 61.Kim HS, Kim MG, Kim BS, Yook JH, Kim BS. Totally laparoscopic total gastrectomy using endoscopic linear stapler: early experiences at one institute. Journal of laparoendoscopic ' advanced surgical techniques Part A. 2012;22(9):889–97. doi: 10.1089/lap.2012.0238. [DOI] [PubMed] [Google Scholar]
- 62.Shim JH, Oh SI, Yoo HM, Jeon HM, Park CH, Song KY. Short-term outcomes of laparoscopic versus open total gastrectomy: a matched-cohort study. American journal of surgery. 2013;206(3):346–51. doi: 10.1016/j.amjsurg.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Song JH, Choi YY, An JY, Kim DW, Hyung WJ, Noh SH. Short-Term Outcomes of Laparoscopic Total Gastrectomy Performed by a Single Surgeon Experienced in Open Gastrectomy: Review of Initial Experience. Journal of gastric cancer. 2015;15(3):159–66. doi: 10.5230/jgc.2015.15.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang GT, Song YC, Zhang XD. Hand-assisted laparoscopic total gastrectomy with regional lymph node dissection for advanced gastric cancer. Surgical laparoscopy, endoscopy ' percutaneous techniques. 2014;24(3):e78–84. doi: 10.1097/SLE.0b013e31828fa6fd. [DOI] [PubMed] [Google Scholar]
- 65.Kim HS, Kim BS, Lee S, Lee IS, Yook JH, Kim BS. Reconstruction of esophagojejunostomies using endoscopic linear staplers in totally laparoscopic total gastrectomy: report of 139 cases in a large-volume center. Surgical laparoscopy, endoscopy ' percutaneous techniques. 2013;23(6):e209–16. doi: 10.1097/SLE.0b013e31828e3b79. [DOI] [PubMed] [Google Scholar]
- 66.Li P, Huang CM, Zheng CH, Xie JW, Wang JB, Lin JX, et al. Laparoscopic spleen-preserving splenic hilar lymphadenectomy in 108 consecutive patients with upper gastric cancer. World journal of gastroenterology. 2014;20(32):11376–83. doi: 10.3748/wjg.v20.i32.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]


