Abstract
Background
Recipients of vascularized composite allografts require aggressive and lifelong immunosuppression, and because the surgery is usually performed in nonlife-threatening situations, the development of strategies to minimize immunosuppression is especially pertinent for this procedure. We investigated how complement affects acute graft injury, alloimmunity, and immunosuppressive therapy.
Methods
Vascularized composite allografts were transplanted from Balb/C to C57BL/6 mice that were complement deficient (C3 or double C3a Receptor (R)/C5aR), or treated with a targeted complement inhibitor (CR2-Crry). Allografts were analyzed for acute inflammation and injury, subacute T cell response, and survival in the absence and presence of cyclosporine A (CsA) therapy.
Results
Allografts in C3-deficient or CR2-Crry–treated recipients were protected from skin and muscle ischemia-reperfusion injury (IRI). C3aR/C5aR-deficient recipients were more modestly protected. IgM and C3d colocalized within allografts from wild type and C3aR/C5aR-deficient recipients indicating IgM-mediated complement activation, and C3d deposition was almost absent in allografts from C3-deficient and CR2-Crry–treated recipients. Inflammatory cell infiltration and P-selectin expression was also significantly reduced in C3-deficient and CR2-Crry–treated recipients. Acute treatment with CR2-Crry or with 3 mg/kg per day CsA modestly, but significantly increased median allograft survival from 5.8 to 7.4 and 7.2 days, respectively. However, combined acute CR2-Crry treatment and CsA therapy increased mean graft survival to 17.2 days. Protection was associated with significantly reduced T cell infiltration of allografts and Tc1 cells in recipient spleens.
Conclusions
Complement-mediated IRI augments graft allogenicity, and appropriate complement inhibition ameliorates IRI, decreases alloimmune priming and allows more immune-sparing CsA dosing.
After severe facial injury or limb loss, transplantation (Tx) is an accepted surgical approach for face or limb replacement, and Tx of composite tissue is required because such injuries involve multiple tissues. Reconstructive surgery involving vascularized composite (VC) allotransplantation (VCA) is an emerging field, with about 150 to 200 procedures having been performed since the first successful hand transplant in 1998.1 Restoring form and function has the potential to provide major psychological as well as physical benefits, and over 2 million people in the United States alone suffer from limb loss.2 VCA also represents a therapeutic option after tumor excision involving limb loss or large areas of tissue damage. However, due to the heterogenicity of tissues and the high immunogenicity of skin, VCA generates a strong immunological response and requires aggressive, and lifelong immunosuppression. Because VCA is usually performed in the context of nonlife threatening defects, an increased concern for this type of Tx is the toxicity of immunosuppressive drugs that can cause organ damage, metabolic dysfunction, cancer, and an increase in susceptibility to infection. An important goal in all Tx research, but particularly in VCA, which is typically not performed as a lifesaving procedure, is the development of strategies to minimize immunosuppression.
Relative to other areas of Tx, there are only a handful of studies reported on minimization/elimination of immunosuppression in this emerging field (for reviews, see Leonard et al3,4). Although it is clear that graft rejection is principally dependent on T cells, there are many other immune factors that can increase graft antigenicity and heighten alloresponsiveness leading to a strengthening of the rejection response. Of these other immune factors, ischemia-reperfusion injury (IRI) is thought to be a major risk factor for subsequent graft dysfunction and rejection. However, our knowledge on the influence of IRI in graft rejection has been gained in studies on solid organ Tx, and it has been proposed that composite tissue allografts are more susceptible to IRI than solid organs because of the heterogenous tissue types that all exhibit different immunogenicity.5 Little is known about how IRI modulates longer-term outcomes after VCA, but increased ischemia times are associated with more frequent and more severe acute rejections with increased antidonor lymphocyte proliferation after VCA.6–8 Although acute rejection episodes can usually be successfully controlled, the number and severity of these episodes are associated with chronic rejection.
The complement system has a documented role in posttransplant IRI of solid organs via promotion of inflammatory responses and the provision of an immunostimulatory environment, but no studies have addressed this important innate immune effector system in VCA in any depth. The few studies that have been performed have focused on acute antibody mediated rejection and have not investigated the role of complement in the context of ischemic injury or as an augmented therapy for the provision of immunosuppressive sparing regimes.9–11 In this study, we investigate the effect of C3 deficiency and complement inhibition on posttransplant IRI and inflammation in VC allografts. We used the complement inhibitor CR2-Crry, an inhibitor that blocks all complement pathways at the C3 activation step and that inhibits the generation of all biologically active complement activation products (C3 opsonins, C3a, C5a and the membrane attack complex). The fusion protein CR2-Crry is a targeted complement inhibitor that localizes to sites of complement activation via CR2-mediated recognition of deposited C3 opsonins.12 We have previously shown that this means targeting complement inhibition obviates the need for systemic inhibition,12 thus reducing any additional immunosuppressive effect that is of potential concern in a Tx setting where the patient is immunocompromised. We also investigate the specific role of the C3a and C5a activation products on posttransplant inflammation and IRI using mice deficient in both C3a and C5a receptors. Finally, using the clinically relevant paradigm of complement inhibition, we investigate the role of complement and IRI on VC allograft rejection and how this impacts the parameters of immunosuppressive treatment.
MATERIALS AND METHODS
Animals
Male Balb/c (H-2d) and C57BL/6 (B6; H-2b) mice (10-12 weeks) were obtained from Jackson laboratory (Bar Harbor, ME). Male C3aR−/−C5aR−/− and C3−/− C57BL/6 mice were provided by Dr. P Heeger from Icahn School of Medicine at Mount Sinai (New York) and in-house breeding colony, respectively. All mice weighed 25 to 30 g and were housed under specific pathogen-free conditions. All procedures were approved by the Medical University of South Carolina Committee for Animal Research in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Transplant Surgery
Balb/c mice were used as donors, and C57Bl/6, C3aR−/−C5aR−/− and C3−/− B6 mice were used as recipients. We used a heterotopic hindlimb Tx model as previously described by Cheng-Hung Lin et al.13 For IRI studies, animals were randomized into 4 groups: (1) Balb/c donors and wild type (WT) B6 recipients with phosphate-buffered saline (PBS) treatment, (2) Balb/c donors and C3aR−/−C5aR−/− B6 recipients, (3) Balb/c donors and C3−/− B6 recipients, (4) Balb/c donors and WT B6 recipients administered 2 doses of 0.25 mg CR2-Crry complement inhibitor immediately upon completion of surgery, and again 24 hours post-Tx. For transplant surgeries, mice were anesthetized with intraperitoneal injections of ketamine (75 mg/kg) and xylazine (16 mg/kg) diluted in sterile saline, and the cold ischemia time for all grafts was 2 hours. CR2-Crry was produced and purified as previously described.12 Dose was based on previously published data.12,14
Histopathology
For histological examination, VCAs were isolated and subsequently placed in 10% buffered formaldehyde solution for 48 hours before being embedded in paraffin. Four-micron graft sections were stained with hematoxylin-eosin and scored using a semiquantitative histology scoring system as described.15
Immunohistochemistry and Fluorescence Microscopy
Histology sections were stained using Abs directed against GR1, Mac-3, P-selectin (BD Pharmingen, San Jose, CA), IgM (Cappell Laboratories, Cochranville, PA), C3d (R&D Systems, Minneapolis, MN), and CD3 (Acris SM1754P, Hiddenhausen, Germany). Immunofluorescence double staining for IgM and C3d was performed using a FITC-labeled Ab directed against IgM (Millipore, Billerica, MA), and a Goat antimouse C3d Ab (R&D Systems) with subsequent visualization using Alexa 555 Rabbit antigoat secondary Ab (Invitrogen, Grand Island, NY). After staining, sections were coverslipped using Vecta fluorescent hard mount (Vector Laboratories, Burlingame, CA). Specificity of staining was assessed by omission of primary Ab and by use of isotype controls. Staining was scored semi-quantitatively by observers (C.A., P.Z.) blinded to specimens, as described previously.16 Intensity of the deposition and the distribution throughout the tissue was graded 0 (absent), 1 (weak staining in a few areas), 2 (moderate staining in several areas), or 3 (strong staining throughout the tissue).
Survival Time of Vascularized Composite Allografts
For allograft survival experiments, all WT B6 recipients implanted with Balb/c hindlimb grafts were randomized into 4 groups: (1) PBS control treatment group, (2) CR2-Crry group, which were administered 0.25 mg CR2-Crry at 0 hour and 24 hours postsurgery, (3) Low-dose cyclosporine A (CsA) group, which were administered CsA at 3 mg/kg per day, and 4. Dual therapy group, which were treated with 2 doses 0.25 mg CR2-Crry at 0 hour and 24 hours after surgery, supplemented with 3 mg/kg per day CsA. The CR2-Crry dose was based on dose response data obtained from other models of IRI and determined to be optimal,12 and the CsA dose chosen based on a preliminary study to determine a CsA dose that provided little or no therapeutic benefit alone. Complement inhibitor and CsA treatments were administered by intraperitoneal injection. Mice were either sacrificed at day 7 posttransplant or examined daily until complete rejection was observed. Grafts isolated at 7 days were analyzed for histomorphological evidence of rejection and T cell infiltration, and splenic T cell populations were analyzed by flow cytometry.
Flow Cytometry
Splenocytes were isolated from naïve mice, and from mice that had received Balb/c allografts from 4 groups; (1) B6 recipient, no treatment; (2) B6 recipient, treatment with 3 mg/kg per day CsA; (3) B6 recipient, treatment with CR2-Crry; and (4) B6 recipient, treatment with CR2-Crry + 3 mg/kg per day CsA. Spleens were isolated 7 days posttransplant. Isolated spleens were mechanical disrupted, suspensions passed through a series of nylon mesh strainers, and frozen in freezing media (Invitrogen) for later analysis. Splenocytes were thawed and stained for cell surface markers (CD3 eFluor450, CXCR3 FITC; eBioscience; CD4 APC Cy7, CD8 APC, CD44 PerCP Cy5.5, CD62L FITC, FoxP3 PE, BD Biosciences) and incubated in FACS buffer (PBS + 2% FBS) in the dark for 20 minutes at room temperature. Cells were then washed twice in FACS buffer and incubated in Fixation Buffer (BioLegend) for 10 minutes. After washing with FACS buffer, cells were run on a BDVerse (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistical Analysis
GraphPad Prism version 5.0 for Mac OS X (GraphPad, San Diego, CA) was used for statistical analysis. A student t test was used for the comparison of a normally distributed continuous variable between 2 groups. Differences between various groups were compared by the nonparametric Wilcoxon rank-sum or Mann-Whitney test because of the small sample sizes for some experiments. The Mantel Cox text was used to compare survival curves for different groups on univariate analyses. All analyses were 2 sided, and values of P less than 0.05 were considered to be statistically significant.
RESULTS
The Effect of Complement Deficiencies and Complement Inhibition on Vascularized Composite Allograft IRI and Inflammation
Vascularized composite allografts from Balb/c wt donors were transplanted into the following C57BL/6 recipients: (1) WT mice treated with PBS vehicle at 0 and 24 hours postreperfusion, (2) Double C3aR−/−/C5aR−/− mice, (3) C3−/− mice, (4) WT mice treated with 0.25 mg CR2-Crry at 0 and 24 hours postreperfusion. Skin and muscle tissue from allografts was isolated 48 hours after Tx and assessed for injury and cellular infiltration. Allografts isolated from PBS-treated WT recipients exhibited key features associated with IRI, including epidermal and muscle cell necrosis, and inflammatory cell infiltration of the epidermis localized to areas of muscle necrosis (Figure 1). All of these features were significantly reduced in the other 3 recipient groups, although there was less protection in allografts from C3aR−/−/C5aR−/− mice compared with C3-deficient or CR2-Crry–treated mice.
FIGURE 1.
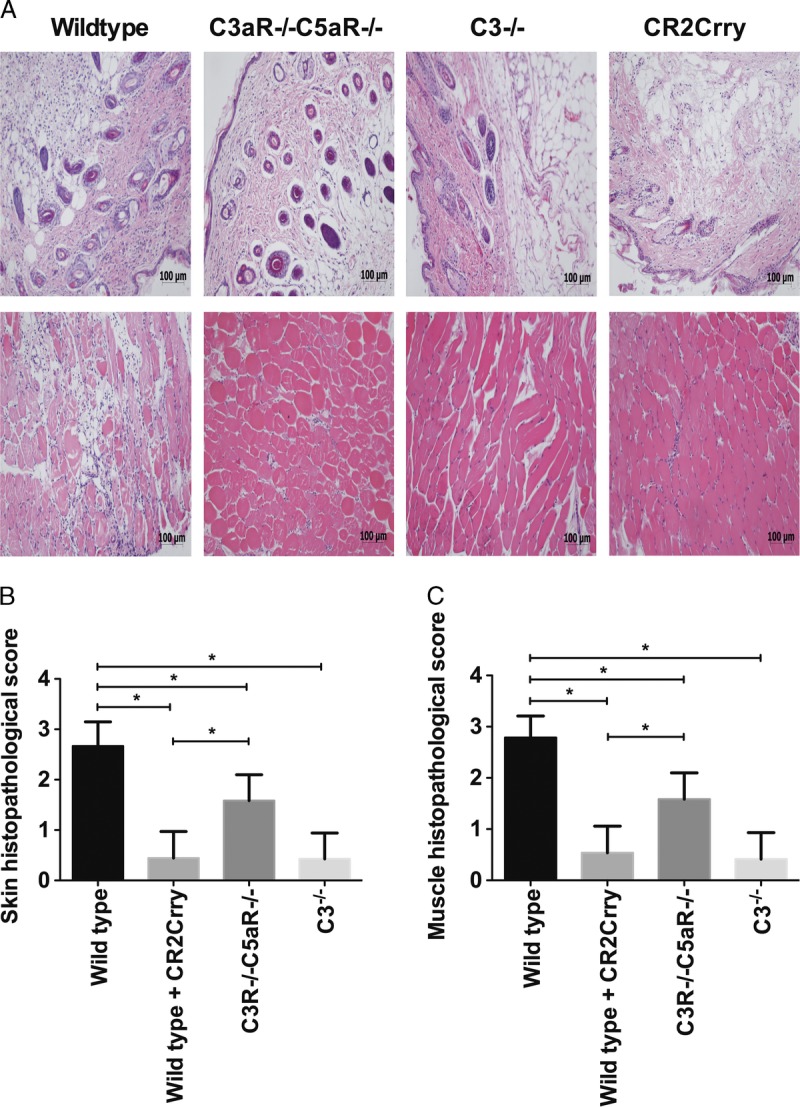
Assessment of IRI damage in vascularized composite allografts from different recipient mice or treated with CR2Crry. Upper panel shows representative H&E stained sections. Lower panel shows histological quantification of injury in grafts. The grafts were isolated at 48 hours posttransplant. Results are expressed as mean ± SD; n = 5-8 for all groups. *P < 0.05.
CR2-crry Ameliorates Complement Deposition in Composite Allografts
The paradigm for organ IRI is that after reperfusion, circulating self-reactive natural IgM Abs bind to ischemia-induced neoepitopes or danger-associated molecular patterns (DAMPs) and activate complement (reviewed in17). We therefore investigated binding of IgM and deposition of the C3d complement activation product in allografts. At 48 hours posttransplant, IgM staining was evident in both skin and muscle tissue, with a similar pattern and intensity in allografts from all recipient groups (Figures 2A and B). C3d deposition, on the other hand, was significantly lower in both the skin and muscle of allografts from C3-deficient and CR2-Crry–treated recipients when compared with allografts from WT and C3aR−/−/C5aR−/− mice (donor-derived complement can account for the low levels of C3d deposition observed in allografts from C3−/− recipients). Furthermore, IgM and C3d co-localized in allografts from WT mice, indicating IgM-mediated activation of complement (Figure 2C). These data indicate sequential IgM binding and complement activation, because neither complement deficiency nor inhibition affected IgM binding. The data further indicate that IgM binding is not in itself pathogenic. Also, although C3a/5a receptor deficiency in the recipient reduces IRI (and leukocyte infiltration, see below), it does not affect the level of complement activation as measured by C3d deposition.
FIGURE 2.
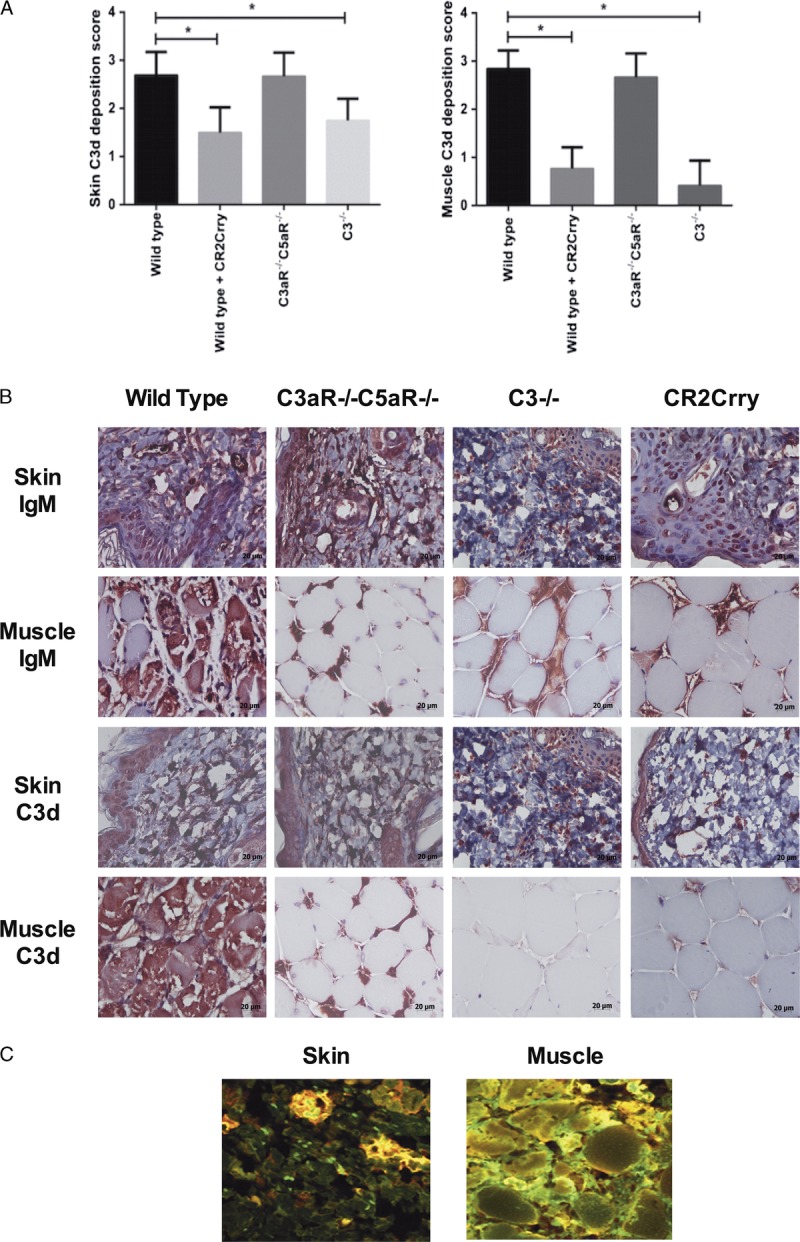
IgM and C3d deposition in vascularized allografts isolated from mice 48 hours after transplantation. A. At 48 hours post-Tx, similar levels of IgM deposition were seen in the skin of VC allografts from all groups, with some decreased deposition in muscle from deficient and inhibited recipients. Mean ± SD, n = 5. *P < 0.05. B. Representative immunohistochemistry images. C. IgM (green) and C3d (red) binding was assessed by immunohistochemistry. Representative immunofluorescence images of WT to WT allografts showing IgM (red) and C3d (green) with colocalization (yellow) n = 3.
Analysis of Inflammatory Cell Infiltration and Adhesion Molecule Expression in VC Allografts
The anaphylatoxins, in particular C5a, are known to play important roles in the recruitment of immune cells to sites of inflammation, and we investigated allograft infiltration of neutrophils and macrophages 48 hours after Tx. Immunohistological assessment demonstrated extensive infiltration of both cell types in allografts from control WT recipients. Neutrophils and macrophages were localized to the dermis in the skin and spread between myocytes in muscle tissue.
Compared with WT recipients, the number of infiltrating cells was significantly reduced in allografts from C3aR−/−/C5aR−/− recipients, with the exception of skin neutrophils. However, in C3-deficient and CR2-Crry–treated recipients there was an even greater reduction in the number of infiltrating neutrophils and macrophages in both the skin and muscle compartments, and in all cases infiltrating cell numbers were significantly lower compared with C3aR−/−/C5aR−/− recipients (Figure 3). Within each group, the relative distribution of each cell type in the skin and muscle was not significantly different. These data indicate that C3aR and/or C5aR expressed on leukocytes contribute to leukocyte infiltration, but also raise the possibility that other complement activation products, either directly or indirectly, play a role in leukocyte recruitment.
FIGURE 3.
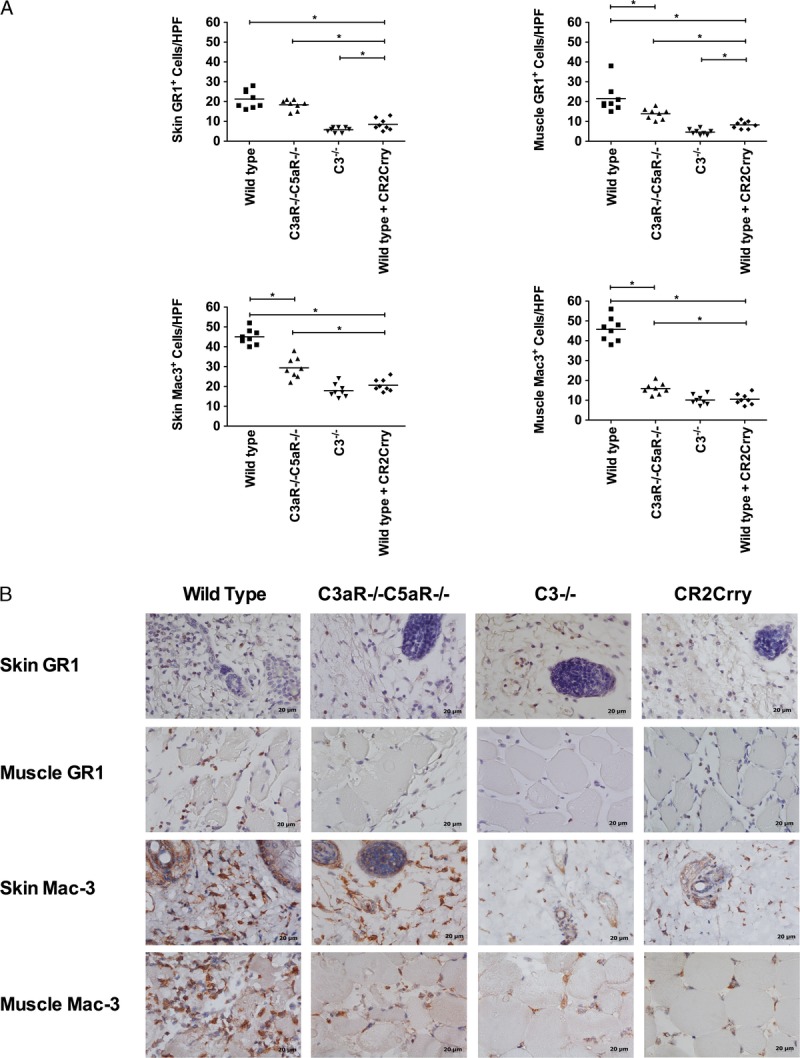
Effects of complement inhibition on the infiltration of neutrophils and macrophages into grafts. A, Neutrophils and macrophages were quantified on immunostained sections by counting 8 high-power fields. Grafts were isolated 48 h after transplant. Results are expressed as mean ± SD, n = 8 for all groups.*P < 0.05. B, Representative images showing immunohistochemical staining for neutrophils (GR1) and macrophages (Mac3). n = 8.
The adhesion molecule P-selectin is expressed on endothelial cells early after IR, and it is an important mediator of leukocyte infiltration and injury, at least in some organs and tissues. Adhesion molecules can also modulate the infiltration of lymphocytes involved in alloimmunity. Moreover, there is a dynamic between P-selectin expression and complement activation,18 and we therefore investigated the effect of complement deficiency and inhibition on P-selectin expression in VC allografts. Immunohistochemical analysis revealed significantly reduced levels of P-selectin expression in both the skin and muscle of allografts from C3-deficient and CR2-Crry–treated recipients compared with controls at 48 hours posttransplant. In vitro studies have implicated complement activation products in the expression of P-selectin,19–21 and the current data is consistent with a role for these products in the expression of P-selectin in both the skin and muscle of VC allografts (quantification, Figure 4). Expression of P-selectin in allografts from C3aR−/−/C5aR−/− recipients was not significantly different from that in allografts from WT control treated recipients, but donor tissue was not C3aR/C5aR-deficient. The reduced P-selectin expression in allografts from C3-deficient and CR2-Crry–treated recipients, but not in C3aR−/−/C5aR−/− recipients, provides a potential explanation for the increased leukocyte recruitment seen in C3aR−/−/C5aR−/− recipients relative to C3-deficient and inhibited recipients (see above).
FIGURE 4.
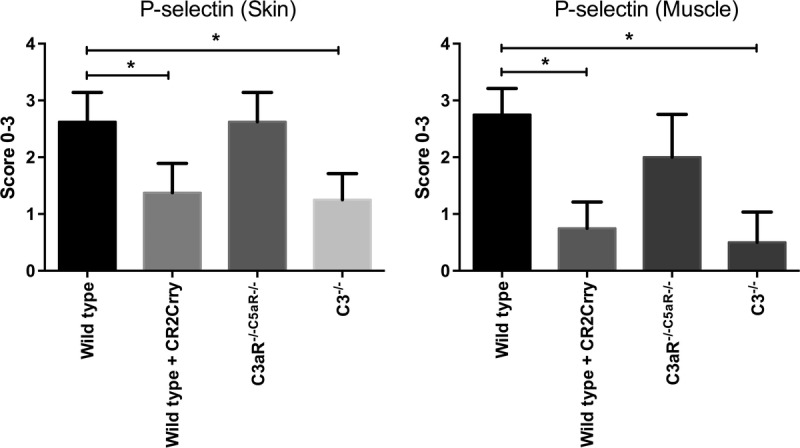
Effect of complement inhibition on the expression of P-selectin in grafts. All grafts were isolated from WT recipient mice with PBS (control) or CR2-crry treatment, C3aR−/−C5aR−/− or C3−/− recipient mice at 48 hours after Tx. Expression of P-selectin was examined by immunohistochemistry and scored on a scale of 0 to 3. Results are expressed as mean ± SD; n = 5 to 8 for all groups. *P < 0.05.
CR2-Crry Combined With a Subtherapeutic Dose of CsA Prolongs VC Allograft Survival and Reduces T Cell Infiltration
To investigate whether complement-dependent IRI modulates alloimmunity and VC allograft rejection in a clinically relevant paradigm, we determined the effect of acute posttransplant CR2-Crry treatment on allograft survival in the context of subtherapeutic immunosuppression with CsA. In PBS vehicle-treated C57BL/6 recipients, Balb/C allografts were rejected with a median survival time of 5.8 days posttransplant. Acute treatment of recipients with CR2-Crry (0 and 24 hours posttransplant) modestly, albeit still significantly, increased median survival of allografts to 7.4 days posttransplant. A similar 7.2 day median allograft survival time was observed in recipients treated with 3 mg/kg per day CsA. However, acute CR2-Crry treatment of recipients to protect against IRI, together with a subtherapeutic dose of 3 mg/kg/d CsA, significantly increased mean graft survival time to 17.2 days (Figure 5).
FIGURE 5.
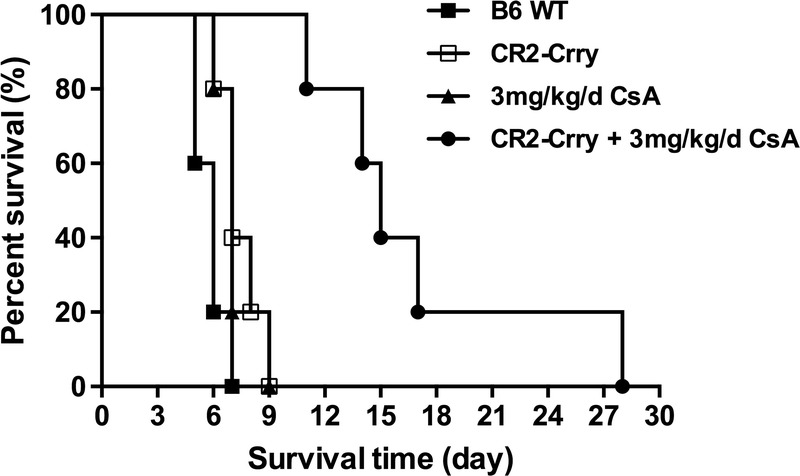
Effect of complement inhibition on the survival time of vascularized composite allografts. n = 5 for all groups.
Vascularized composite allograft rejection is primarily dependent on T cells, and published work indicates that complement activation within donor solid organs contributes to posttransplant ischemic injury and memory T cell infiltration and proliferation.22–24 We therefore determined how CR2-Crry treatment affected graft rejection and T cell infiltration into allografts when recipients were cotreated with a subtherapeutic 3 mg/kg per day dose of CsA. Graft rejection was evident macroscopically in untreated recipients and in recipients treated with a subtherapeutic dose of CsA alone at day 7 posttransplant (Figure 6A). Analysis of T cell infiltration by immunohistochemistry showed that compared with CsA alone, combined CR2-Crry + CsA treatment significantly reduced T cell infiltration (CD3+) into both the skin and muscle at 7 days posttransplant (Figures 6B and C). CR2-Crry treatment alone demonstrated that early inhibition of IRI with complement inhibitor led to a significant reduction in T cells as compared with control, although numbers were significantly elevated as compared with combined CR2-Crry + CsA (Figures 6B and C).
FIGURE 6.
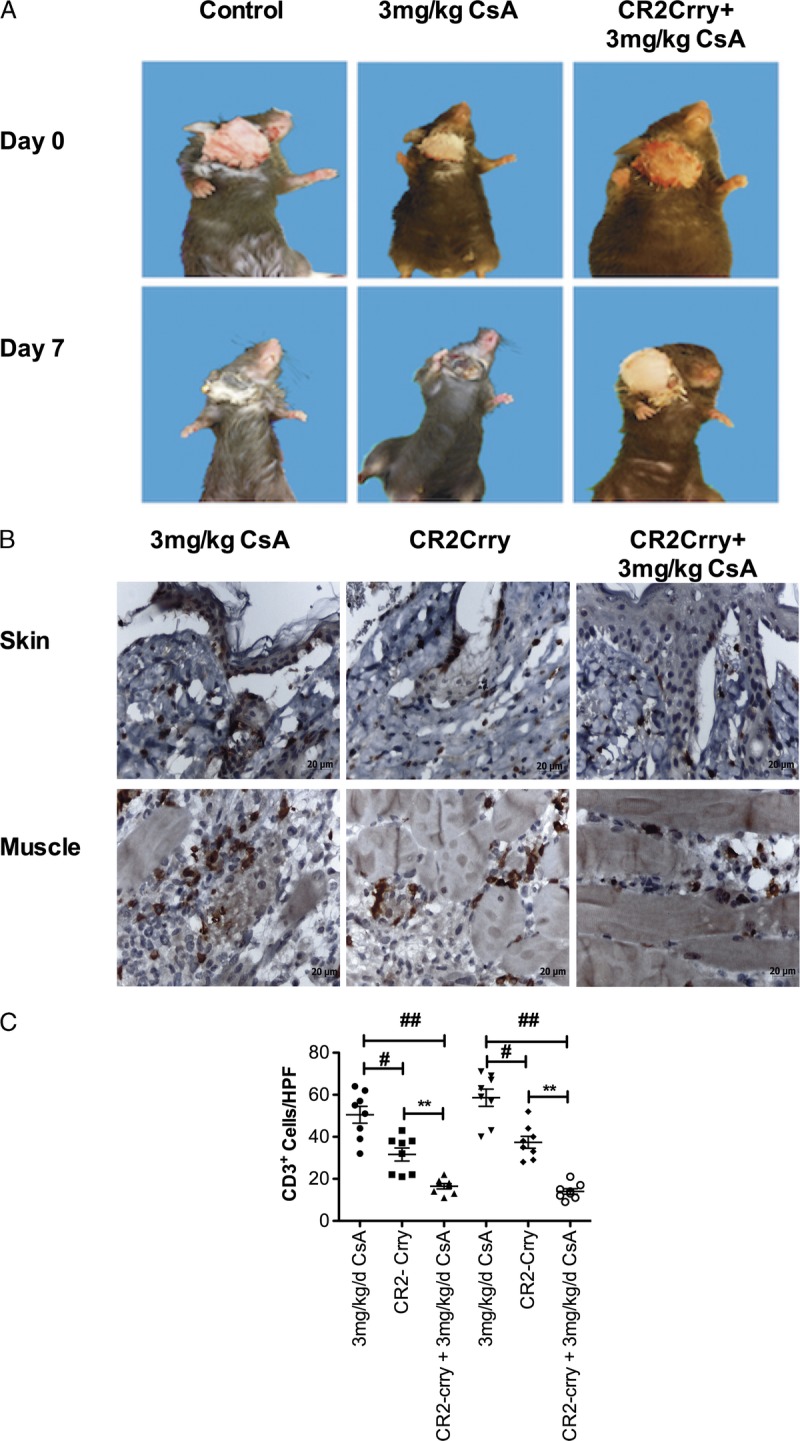
Combined CR2-Crry and subtherapeutic CsA treatment delays graft rejection and reduces T cell infiltration. A, Macroscopic assessment of vascularized composite allograft rejection. Note that rejection is absent in CR2-Crry + CsA–treated animals at day 7. Representative images of n = 8, B. Representative immunohistochemistry images of sections obtained from grafts 7 days post-Tx and stained for CD3. Note widespread diffuse T cell infiltrates in both the skin and muscle in CsA and CR2-Crry, as compared with CR2-Crry + CsA–treated animals. Images representative of n = 8. C, T cell infiltration quantified by counting 8 random high-power fields by observers blinded to groups. Mean, n = 6–8. ## CsA vs CsA + CR2-Crry, # CsA vs CR2-Crry, ** CR2-Crry vs CsA + CR2-Crry, P < 0.05.
Previous work has shown that complement activation is a key regulator of T cell immunity, and we therefore further analyzed the impact of combined CR2-Crry + CsA treatment on splenic T cell populations. Given that Tc1 and CD4 responses play important roles in VCA rejection, and that graft complement activation primes central memory expansion, we focused our effort on flow cytometric analysis of these factors.25–27 Total numbers of CD3, CD4, and CD8 were not significantly different between any analyzed groups (data not shown). Analysis of effector (CD3+CD44+CD62L−) and central memory (CD3+CD44−CD62L+) T cells showed no significant differences between any group (data not shown). Although C3a and C5a can switch the balance between T effector or T regulatory (Treg) cell development, and inhibition of C3a and C5a receptor signaling can promote Treg cell expansion, we found no significant difference in either the total percentage or total numbers of CD3+CD25+FoxP3+ or CD4+CD25+FoxP3+ Treg cells between any of the groups (data not shown). However, examination of CD3+CD8+CXCR3+ and CD3+CD4+CXCR3+Tc1 cells showed that combined therapy led to a significant reduction in the percentage and total number of Tc1 CD8 cells, and a trend in reduction in CD4 cells, as compared with untreated and CsA alone treated recipients (Figure 7).
FIGURE 7.
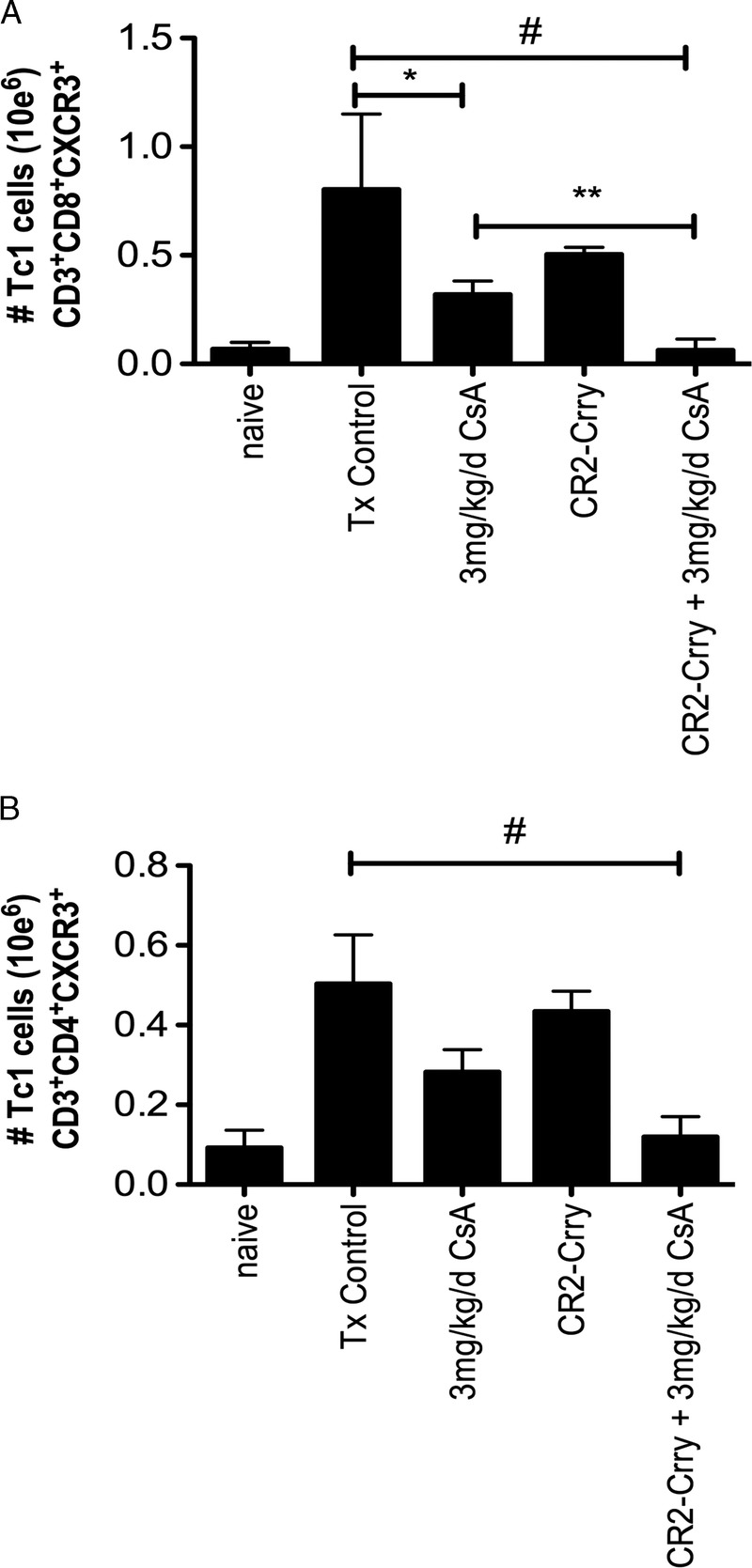
Combined CR2-Crry and CsA therapy reduces splenic Tc1 cell populations. Flow cytometric analysis of splenocytes for Tc1 cells CD3+CD8+ CXCR3+ (A) and CD3+CD4+CXCR3+ (B) demonstrate a significant reduction in overall number of CD8+ CXCR3+ cells in the combined therapy group, as compared with control and single therapy CR2-Crry and CsA groups. CD4+CXCR3+ T cells were significantly reduced in the combined group, as compared with Tx control. Although there was an overall trend to reduced total numbers between combined and CsA alone groups, this did not reach statistical significance. (# Tx Control vs CR2-Crry + CsA, p < 0.02, *Tx Controls vs CsA p < 0.03, **CsA vs CR2-Crry + CsA, p < 0.04. n = 3 in each group).
DISCUSSION
Although VCA surgical procedures have become highly successful, the high incidence of acute rejection within the first year after surgery28 and the requirement for long-term and high-dose immunosuppression28,29 remain as obstacles to its widespread application. Current immunosuppression strategies applied in VCA are extrapolated from solid organ Tx regimens,30 and because the toxicity of immunosuppressive therapies is a heightened concern in the context of a non-lifesaving procedure, an important goal in VCA research is the development of immune-sparing therapies.
There is strong evidence that IRI, an unavoidable complication in the process of Tx, can strongly impact graft quality,15 augment graft allogenicity,8 and strengthen the subsequent adaptive immune reaction.6–8 In this study, we first demonstrated that complement plays an important role in IRI to VC allografts. However, although C3aR/C5aR deficiency in the recipient was protective against IRI, C3 deficiency and C3 inhibition provided significantly better protection in both skin and muscle compartments. This indicates that whereas the anaphylatoxins may contribute to IRI, likely via the recruitment and/or activation of leukocytes, other operative mechanisms that are not affected by C3aR/C5aR deficiency, namely C3 opsonins and/or the MAC, play a prominent role in VCA IRI. Indeed, in some solid organs the MAC appears to be the primary mediator of IRI.31–34 In addition to being directly cytolytic, deposition of the MAC at sublytic concentrations can, as can the anaphylatoxins, result in the activation of endothelial cells, expression of adhesion molecules, and the recruitment and activation of leukocytes.35,36 Activated endothelial cells and recruited macrophages function as APCs, and C3 opsonization can facilitate cell/antigen uptake via complement receptor engagement. Complement-dependent injury will also result in the expression of DAMPs, such as heat shock proteins, reactive oxygen species and fibrinogen,5,37 that can play a role in the activation of APCs and the augmentation of effector T cell responses.5,37 In this context, our data also indicate IgM-mediated activation of complement occurs in composite allografts, and for cardiac grafts we have shown previously that DAMPs exposed after transplant are recognized by self-reactive natural IgM Abs that activate complement in the transplanted heart.38 Taken together, the activities of complement activation products provide a basis for how complement may augment graft allogenicity via IRI.5
To provide further support for this concept, we demonstrated that the level of injury in allografts from the different groups of complement-deficient or inhibited recipients correlated with the level of immune stimulation, as measured by leukocyte recruitment and endothelial cell activation. While there was a reduction in the number of neutrophils and macrophages in composite allografts from complement-deficient and inhibited mice compared with WT controls at 48 hours posttransplant, there was a more significant reduction in C3 deficient and CR2-Crry–treated mice compared with C3aR/C5aR-deficient mice. The same was true for expression of the endothelial adhesion molecule, P-selectin, and both leukocyte infiltration and P-selectin expression correlated with the level of complement activation as measured by C3 deposition (in both the skin and muscle). Thus, blockade of complement at the C3 activation step that inhibits all the major effector activation products of the complement cascade is more effective than anaphylatoxin blockade at ameliorating IRI and reducing the immunostimulatory environment of composite allografts. It should also be noted that signaling through C3a and C5a receptors expressed on APCs and T cells can stimulate differentiation, expansion and survival of effector T cells, and C3a/C5a receptor signaling can also reduce the suppressive activity of regulatory T cells, thus promoting T cell alloimmunity (reviewed in Kwan et al39). How the anaphylatoxins may modulate composite allograft alloimmunity via these more direct mechanisms, especially in the subacute posttransplant phase after IRI, is an area for further investigation.
Current therapeutic approaches in standard VCA protocols target primarily cell-mediated rejection.28 We therefore also investigated the effect of complement inhibition in the context of immunosuppressive therapy with CsA. We found that treatment of recipients with CR2-Crry alone, administered acutely after transplant, modestly but significantly prolonged allograft survival. Graft survival was similarly increased in recipients treated with only 3 mg/kg per day CsA. However acute treatment of recipients with CR2-Crry combined with 3 mg/kg per day CsA extended median graft survival from 7.4 to 17.2 days. In line with current evidence indicating a primary role for T cells in rejecting composite allografts, in recipients treated with a 3 mg/kg per day subtherapeutic dose of CsA, we found a high number of T cells infiltrating both the skin and muscle of rejecting allografts 7 days after Tx. However, at 7 days posttransplant, T cell infiltration was significantly reduced in allografts from recipients treated with both 3 mg/kg per day CsA and CR2-Crry. These data indicate that reduced IRI and the reduction in the acute immunostimulatory environment seen with CR2-Crry treatment, translates to reduced T cell infiltration in the subacute phase after Tx and to increased allograft survival. We further analyzed splenocyte populations in allograft recipients to gain insight into the shaping of the immune response. The role of Tc1 cells in VCA graft rejection is well established, and here we show that splenic populations of these effector cells are significantly reduced in recipients treated with combined CR2-Crry and subtherapeutic CsA therapy. This reduction in splenic numbers of Tc1 could be due to increased graft infiltration, but this is unlikely given that we were able to clearly demonstrate a reduction in intragraft T cells and a delay in macroscopic evidence of rejection. Taken together our data suggest that CR2-Crry acute therapy and amelioration of IRI decreases alloimmune priming, delaying the onset of rejection.
Complement inhibition has recently become a clinical reality, with the application of eculizumab for paroxysmal nocturnal hemoglobinuria, and has been further applied in small trials to presensitized kidney transplant recipients and for the therapy of acute antibody mediated rejection in solid organ Tx.40–42 These clinical studies, along with a number of promising small molecule and targeted complement therapeutics that are rapidly progressing along the development pipelines, may well present promise for the application of complement therapeutics in clinical VCA. In the studies reported here, we demonstrated that by layering acute post transplant complement inhibition together with sub-therapeutic doses of CsA, prolonged allograft survival can be achieved. This is likely an approach that could be adopted clinically, by augmenting standard of care triple immunosuppressive therapy with acute graft-targeted complement immunotherapy, and this may well present a strategy for introducing complement inhibition into the clinical practice of VCA. Importantly, similar to the CR2-mediated site-specific targeting used herein, a CR2-targeted inhibitor of the alternative complement pathway, TT30 (human CR2-fH), has completed human safety trials, and could potentially be used acutely to augment standard of care triple immunosuppression.
To conclude, the complement activation products C3a, C5a, C3 opsonins, and the MAC, have all been implicated in acute graft inflammation and injury after solid organ Tx, and organ IRI is implicated in promoting the development of graft rejection. CR2-Crry is a pan-complement inhibitor that inhibits the generation of all of the above complement activation products, and the data shown here indicate that a treatment directed at inhibiting complement-mediated IRI of VC allografts could be a promising therapeutic strategy. Furthermore, we have previously shown that CR2-mediated targeting of complement inhibition significantly increases bioavailability and efficacy and obviates the need for systemic inhibition that reduces any additional immunosuppressive effect, a potentially important consideration when the patient is immunocompromised. A humanized sister CR2-targeted inhibitor, CR2-fH (TT30), has been shown to be safe and well tolerated in a clinical trial for paroxysmal nocturnal hemoglobinuria.43
Footnotes
This work was supported by grants from the NIH (R56AI119026 and R01DK102912), Department of Veterans Affairs (Merit Award 1I01RX001141 and 1BX001218) and the Department of Defense (W81XWH-16-1-0783).
S.T. is an inventor on a licensed patent for CR2-targeted complement inhibition.
S.T. and C.A. designed the study and analyzed the data. P.Z. and B.L performed all surgeries, and prepared and analyzed grafts specimens. S.T, C.A. and P.Z. wrote the manuscript. S.R.B. and C.M.P. designed, performed and analyzed flow cytometry experiments.
Correspondence: Stephen Tomlinson, PhD, Department of Microbiology and Immunology, Medical University of South Carolina, 173 Ashley Avenue, Charleston, SC. (tomlinss@musc.edu). Carl Atkinson, PhD, Department of Microbiology and Immunology, and Surgery, Medical University of South Carolina, 173 Ashley Avenue, Charleston, SC. (atkinsoc@musc.edu).
A report on the impact of immunosuppression on complement mediated injury and immune responses in a vascularized composite allograft mouse model. The authors show that inhibition of posttransplant complement responses together with sub-therapeutic doses of CsA prolongs allograft survival.
REFERENCES
- 1.Dubernard JM, Owen E, Herzberg G. Human hand allograft: report on first 6 months Lancet 1999. 3531315–1320 [DOI] [PubMed] [Google Scholar]
- 2.Ziegler-Graham K, MacKenzie EJ, Ephraim PL. Estimating the prevalence of limb loss in the United States: 2005 to 2050 Arch Phys Med Rehabil 2008. 89422–429 [DOI] [PubMed] [Google Scholar]
- 3.Leonard DA, Gordon CR, Sachs DH. Immunobiology of face transplantation J Craniofac Surg 2012. 23268–271 [DOI] [PubMed] [Google Scholar]
- 4.Leonard DA, Cetrulo CL, McGrouther DA. Induction of tolerance of vascularized composite allografts Transplantation 2013. 95403–409 [DOI] [PubMed] [Google Scholar]
- 5.Caterson EJ, Lopez J, Medina M. Ischemia-reperfusion injury in vascularized composite allotransplantation J Craniofac Surg 2013. 2451–56 [DOI] [PubMed] [Google Scholar]
- 6.Xiao B, Xia W, Zhang J. Prolonged cold ischemic time results in increased acute rejection in a rat allotransplantation model J Surg Res 2010. 164e299–e304 [DOI] [PubMed] [Google Scholar]
- 7.Pradka SP, Ong YS, Zhang Y. Increased signs of acute rejection with ischemic time in a rat musculocutaneous allotransplant model Transplant Proc 2009. 41531–536 [DOI] [PubMed] [Google Scholar]
- 8.Shimizu F, Okamoto O, Katagiri K. Prolonged ischemia increases severity of rejection in skin flap allotransplantation in rats Microsurgery 2010. 30132–137 [DOI] [PubMed] [Google Scholar]
- 9.Kanitakis J, McGregor B, Badet L. Absence of c4d deposition in human composite tissue (hands and face) allograft biopsies: an immunoperoxidase study Transplantation 2007. 84265–267 [DOI] [PubMed] [Google Scholar]
- 10.Unadkat JV, Schneeberger S, Goldbach C. Investigation of antibody-mediated rejection in composite tissue allotransplantation in a rat limb transplant model Transplant Proc 2009. 41542–545 [DOI] [PubMed] [Google Scholar]
- 11.Gajanayake T, Olariu R, Leclère FM. A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft Sci Transl Med 2014. 6249ra–110 [DOI] [PubMed] [Google Scholar]
- 12.Atkinson C, Song H, Lu B. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection J Clin Invest 2005. 1152444–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CH, Sucher R, Shih YC. The neck as a preferred recipient site for vascularized composite allotransplantation in the mouse Plast Reconstr Surg 2014. 133133e–141e [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Qiao F, Atkinson C. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury J Immunol 2008. 1818068–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hautz T, Hickethier T, Blumer MJ. Histomorphometric evaluation of ischemia-reperfusion injury and the effect of preservation solutions histidine-tryptophan-ketoglutarate and University of Wisconsin in limb transplantation Transplantation 2014. 98713–720 [DOI] [PubMed] [Google Scholar]
- 16.Moseley EL, Atkinson C, Sharples LD. Deposition of C4d and C3d in cardiac transplants: a factor in the development of coronary artery vasculopathy J Heart Lung Transplant 2010. 29417–423 [DOI] [PubMed] [Google Scholar]
- 17.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage Clin Immunol 2011. 1413–14 [DOI] [PubMed] [Google Scholar]
- 18.Del Conde I, Cruz MA, Zhang H. Platelet activation leads to activation and propagation of the complement system J Exp Med 2005. 201871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozada C, Levin RI, Huie M. Identification of C1q as the heat-labile serum cofactor required for immune complexes to stimulate endothelial expression of the adhesion molecules E-selectin and intercellular and vascular cell adhesion molecules Proc Natl Acad Sci U S A 1995. 928378–8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori R, Hamilton KK, McEver RP. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface J Biol Chem 1989. 2649053–9060 [PubMed] [Google Scholar]
- 21.Foreman KE, Vaporciyan AA, Bonish BK. C5a-induced expression of P-selectin in endothelial cells J Clin Invest 1994. 941147–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson C, He S, Morris K. Targeted complement inhibitors protect against posttransplant cardiac ischemia and reperfusion injury and reveal an important role for the alternative pathway of complement activation J Immunol 2010. 1857007–7013 [DOI] [PubMed] [Google Scholar]
- 23.van der Touw W, Cravedi P, Kwan WH. Cutting edge: receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells J Immunol 2013. 1905921–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieyra M, Leisman S, Raedler H. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection Am J Pathol 2011. 179766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morelon E, Petruzzo P. Vascularized composite allotransplantation still remains an emerging field after 17 years Curr Opin Organ Transplant 2015. 20593–595 [DOI] [PubMed] [Google Scholar]
- 26.Kanitakis J, Petruzzo P, Badet L. Chronic rejection in human vascularized composite allotransplantation (hand and face recipients): an update Transplantation 2016. 1002053–2061 [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Benghiat FS, Charbonnier LM. CD8+ T-Cell depletion and rapamycin synergize with combined coreceptor/stimulation blockade to induce robust limb allograft tolerance in mice Am J Transplant 2008. 82527–2536 [DOI] [PubMed] [Google Scholar]
- 28.Fischer S, Lian CG, Kueckelhaus M. Acute rejection in vascularized composite allotransplantation Curr Opin Organ Transplant 2014. 19531–544 [DOI] [PubMed] [Google Scholar]
- 29.Napoli I, Noon LA, Ribeiro S. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo Neuron 2012. 73729–742 [DOI] [PubMed] [Google Scholar]
- 30.Petruzzo P, Lanzetta M, Dubernard JM. The international registry on hand and composite tissue transplantation Transplantation 2010. 901590–1594 [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Hu W, Xing W. The protective role of CD59 and pathogenic role of complement in hepatic ischemia and reperfusion injury Am J Pathol 2011. 1792876–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnberg D, Botto M, Lewis M. CD59a deficiency exacerbates ischemia-reperfusion injury in mice Am J Pathol 2004. 165825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall KM, He S, Zhong Z. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy J Exp Med 2014. 2111793–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austen WG, Kyriakides C, Favuzza J. Intestinal ischemia-reperfusion injury is mediated by the membrane attack complex Surgery 1999. 126343–348 [PubMed] [Google Scholar]
- 35.Morgan BP. The membrane attack complex as an inflammatory trigger Immunobiology 2016. 221747–51 [DOI] [PubMed] [Google Scholar]
- 36.Merle NS, Noe R, Halbwachs-Mecarelli L. Complement system part II: role in immunity. Front Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson HM, Murphy TJ, Purcell EJ. Injury primes the innate immune system for enhanced Toll-like receptor reactivity J Immunol 2003. 1711473–1483 [DOI] [PubMed] [Google Scholar]
- 38.Atkinson C, Qiao F, Yang X. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury Circulation 2015. 1311171–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwan WH, van der Touw W, Heeger PS. Complement regulation of T cell immunity Immunol Res 2012. 54247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nester C, Stewart Z, Myers D. Pre-emptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome Clin J Am Soc Nephrol 2011. 61488–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan SA, Al-Riyami D, Al-Mula Abed YW. Successful salvage treatment of resistant acute antibody-mediated kidney transplant rejection with eculizumab Sultan Qaboos Univ Med J 2016. 16e371–e374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran D, Boucher A, Collette S. Eculizumab for the treatment of severe antibody-mediated rejection: a case report and review of the literature. Case Rep Transplant. 2016;2016:9874261. doi: 10.1155/2016/9874261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storek M, Sahelijo L, Doyle M. Safety and pharmacokinetics of the complement inhibitor TT30 in a phase I trial for untreated PNH patients. Blood. 2015;126:2137. [Google Scholar]


