Abstract
Nucleoside triphosphates (NTPs) diffuse to the active center of RNA polymerase II through a funnel-shaped opening that narrows to a negatively charged pore. Computer simulation shows that the funnel and pore reduce the rate of diffusion by a factor of ≈2 × 10–7. The resulting limitation on the rate of RNA synthesis under conditions of low NTP concentration may be overcome by NTP binding to an entry site adjacent to the active center. Binding to the entry site greatly enhances the lifetime of an NTP in the active center region, and it prevents “backtracking” and the consequent occlusion of the active site.
Keywords: computation, electrostatics, transcription
The RNA polymerases responsible for cellular gene transcription are large multiprotein complexes. Structure determination has revealed a core devoted to nucleic acid polymerization and an elaborate superstructure for the recognition of diverse promoter sequences and also for regulation (1–3). The proportion of the structure devoted to these different tasks may be gauged by comparison with the single-subunit viral RNA polymerases (4, 5), which are approximately one-fifth of the mass of the multisubunit enzymes and transcribe only one or a small number of promoters, with rudimentary requirements for control.
Among the many structural accoutrements of the multisubunit enzymes, one that has attracted recent attention is an undercarriage that serves an important purpose in transcript elongation but that also imposes an apparent limitation on the enzyme mechanism. This undercarriage harbors a funnel-shaped opening, narrowing to a pore beneath the active center (Fig. 1) through which the 3′ end of the RNA is extruded during “backtracking” (retrograde motion of the enzyme on the DNA template) and through which substrate nucleoside triphosphates (NTPs) must enter for addition to the 3′ end during RNA synthesis. Backtracking is crucial for recovery from pausing, for exposing DNA damage for repair, and possibly for proofreading the nascent transcript (reviewed in ref. 6). Pausing is of particular interest for its regulatory role. Recovery from pausing generally requires transcript cleavage in the polymerase active center, induced by the general transcription factors SII (TFIIS) in eukaryotes and GreA/B in bacteria. Defects in pausing and recovery underlie human diseases such as Cockayne syndrome, Wolf–Hirschhorn syndrome, and von Hippel–Lindau cancer-predisposition syndrome.
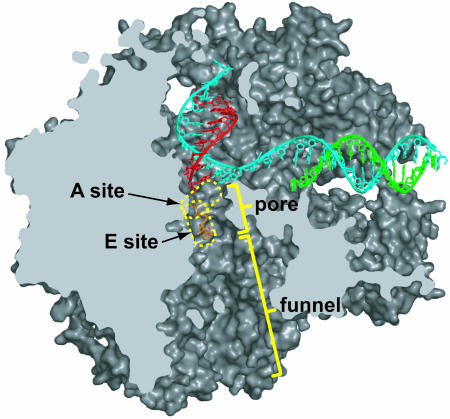
Fig. 1.
Path of NTP entry to the active center of a pol II transcribing complex. The structure of a transcribing complex (PDB ID code 1R9T) is shown in space-filling representation, sectioned along the length of the NTP entry path, and viewed en face (as in figure 2C of ref. 16). Backbone models of the DNA (template strand in blue, and nontemplate strand in green) and RNA (red) are shown. The nucleotide A and E sites are circled in yellow. An NTP bound to the E site is shown in orange.
The polymerase funnel and pore facilitate pausing and recovery. A backtracked transcript is sequestered, possibly with its 3′ end bound to a site of nucleotide interaction in the funnel at approximately nine residues from the active center (7). The transcript is protected from nucleases, accessible only to TFIIS or GreA/B, which bind to the surface of the funnel and possess a long, slender protrusion that is capable of reaching up through the pore to the active center (8, 9). The transcript and protrusion can occupy the pore and funnel simultaneously. The protrusion is thought to supply a Mg ion that is needed for transcript cleavage at the polymerase active center (10).
While protecting the nascent transcript and controlling its cleavage, the funnel and pore unavoidably restrict diffusion of NTPs to the active center. In this study, we have investigated the nature of this restriction by computer simulation, taking into account all relevant factors of funnel and pore topography, electrostatics, and NTP interaction. We have explored the effect of the recently described entry site (E site) (7, 11) from which NTPs are delivered to the nucleotide addition site (A site) at the 3′ end of the transcript (Fig. 1). All NTPs bind to the E site, whereas only an NTP able to base pair with the DNA template can bind the A site. We have asked whether binding at the E site, which has no base specificity and a much lower affinity for a matched NTP than the A site, can nevertheless enhance the rate of transcription.
Materials and Methods
Computation of Electrostatic Potential. The electrostatic potential was computed for the vicinity of RNA polymerase II (pol II) with a 1-Å grid spacing by using apbs software (12), which solves the Poisson–Boltzmann equation by the multilevel finite-element method. For this calculation, an ionic strength of 150 mM, a solvent dielectric constant of 78.54, and a protein dielectric constant of 1 were used.
Simulation Parameters. The position of the diffusing NTP evolves according to the following Langevin stochastic differential equation (13):
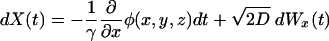 |
[1] |
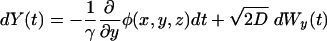 |
[2] |
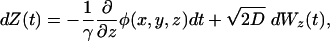 |
[3] |
where Wx(t) represents a one-dimensional standard Brownian motion, φ(x, y, z) is the electrostatic potential, D is the diffusion coefficient, and γ = kT/D (where k is the Boltzmann constant and T is the temperature). For computation of trajectories, a time step of 50 ps and a diffusion coefficient of 75 μm2/s were used, and simulations were performed for 0.1 ms. Because the potential was computed on a grid size of 1 Å, polynomial interpolation was used to determine the potential at nonlattice points.
Results
The computer simulation of NTP diffusion performed in this study was realistic, taking into account the protein structure and aqueous solution conditions. The surface topography of the funnel and pore were derived from the 2.3-Å structure of yeast pol II (7). The electrostatic potential within the funnel and pore was computed from the distribution of all amino acids in the protein structure in the presence of 150 mM counterions in water. An NTP was treated as a sphere of 7 Å in diameter with a net charge of –2, which is appropriate for a Mg–NTP complex under physiological conditions. The actual shape of the NTP as it deviates from spherical symmetry should have little effect on diffusion.
Simulations started with an NTP entering the funnel at 30 Å from the A site. Trajectories of Brownian motion were computed with steps at 50-ps intervals, typically terminating within ≈1 ns because of escape of the NTP from the funnel into solution. A sufficient number of trajectories was calculated to give a total simulation time of 0.1 ms.
Restriction of NTP Diffusion by the Funnel and Pore. Trajectories were computed in the absence of an electrostatic potential or E site to reveal the effect of funnel and pore topography on diffusion. Many trajectories were summed and normalized to obtain the probability of an NTP at any location in the funnel or pore (Fig. 2B, blue line). Of the NTPs introduced at the entrance of the funnel, ≈1/600 reached the A site. The probability increased with distance from the A site because of the increase in volume of the funnel along its length. Indeed, the increase corresponded with the simple quadratic dependence expected for the volume of a cone, and a similar curve was obtained by computing the diffusion of NTPs in a cone (Fig. 2B, green line). Deviations from a smooth distribution reflected irregularities in the surface contours of the funnel and, thus, variation in volume along the length. There was a peak at 30 Å from the A site because all trajectories started at this point and also because the opening of the funnel lay just beyond, so NTPs at a greater distance from the A site were likely to diffuse away from the polymerase into solution.
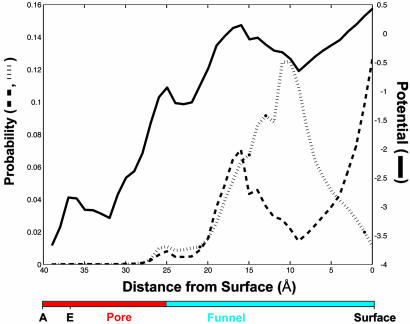
Fig. 2.
Computer simulations of NTP diffusion. (A) Selected trajectories of NTP diffusion in the funnel and pore in the presence of electrostatic potential and in the absence (Upper) or presence (Lower) of the E site. Each trajectory begins near the entrance to the funnel (lower boundary of the plot, as indicated by the colored bar on the left). The entrance to the pore is indicated by the dashed line and color change of the bar. Locations of A and E sites are marked on the bar. (B) Normalized sum of many trajectories for the case of no electrostatic potential and no E site (blue line), electrostatic potential but no E site (dashed line), or both electrostatic potential and E site (solid black line). The probability (average across a 1-Å-thick section of the funnel or pore) of an NTP at a particular position (distance from the A site) is shown. The orientation is with the A site at the left and pol II surface at the right, as indicated on the colored bar beneath the plot. The average trajectory computed for a simple conical opening is also shown (green line).
Effect of Electrostatic Potential on NTP Diffusion. The surface of the funnel contains approximately equal numbers of positively and negatively charged amino acids, whereas the surface of the pore is strongly negative. Because the pore is so narrow, a negative electrostatic potential persists to the center (Fig. 3). The average value of the potential from one side of the funnel or pore to the other is slightly negative and roughly constant along the funnel and then drops sharply at the entrance to the pore (Fig. 4, solid curve). As a result, an anion such as Mg–NTP will experience an electrical force repelling it from the pore. Indeed, most trajectories of NTPs diffusing in the presence of the electrostatic potential failed to enter the pore (Fig. 2 A Upper). The average trajectory showed a pronounced reduction in probability in the pore (Fig. 2B, dashed line). The probability of an NTP in the E site (2–5 Å from the A site) was 1/300 of that in the absence of the electrostatic potential.
Fig. 3.
Electrostatic potential in the funnel and pore. The value of the electrostatic potential in planes through the funnel and pore (bar on the left) in the orientations indicated (Inset) is displayed on a scale from –3 to +3 (red to blue, respectively, as shown in the bars beneath the images).
Fig. 4.
Equilibrium distribution of NTP in the funnel and pore calculated from the Boltzmann relation. The value of [NTP]funnel–pore/[NTP]solution is plotted (thick dashed line) as a function of position in the funnel and pore (indicated in the bar beneath the plot). The value of the electrostatic potential from which this ratio of NTP concentrations was derived (solid line) was computed by averaging over 1-Å-thick slabs of the solvent-accessible space region of the funnel and pore. For comparison, the normalized sum of trajectories for the case of no E site (dashed line in Fig. 2B) is reproduced here (thin dashed line).
The average of many trajectories would be expected to approximate the equilibrium distribution of NTP. Because of the electrical-potential gradient, the equilibrium concentration of NTP would be expected to decrease along the funnel and pore, reaching a minimum near the A site. The magnitude of this effect can be calculated from the following Boltzmann relation:
 |
[4] |
where z is the charge on the diffusing ion (–2 for MgNTP), Δφ is the potential difference between a point in the funnel and pore and a point in the solution beyond the pol II surface, k is the Boltzmann constant, and T is the temperature. The resulting probability distribution (Fig. 4, thick dashed curve) closely resembles the average of trajectories for NTP diffusion (Fig. 4, thin dashed curve) in the pore and also in the funnel as far as 25 Å from the A site (15 Å from the pol II surface). Further from the A site (closer to the pol II surface), the two curves diverge, reflecting the bias introduced by starting trajectories at 30 Å from the A site.
Effect of the E Site on NTP Diffusion. Binding of an NTP at the E site should increase the occupancy of the pore beneath the A site, to an extent depending on the lifetime of the bound NTP. This lifetime t1/2 is given by the following:
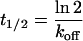 |
[5] |
with
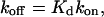 |
[6] |
where Kd is the dissociation constant for an NTP at the E site and kon is the rate constant for binding at that site. Kd is ≈1 mM (9), and kon for a diffusion-controlled reaction is, at most, 1011s–1·M–1. Thus, t1/2 is minimally 10 ns, and slower on-rates will lead to slower off-rates and larger values of t1/2. Trajectories for NTP diffusion, computed with an even more conservative value of 1 ns for t1/2, were characterized by a prolonged occupancy at the level of the E site (Fig. 2 A Lower). The average trajectory showed a pronounced peak in this position (Fig. 2B, solid black line).
Discussion
Diffusion of NTPs to the pol II active center is restricted by the opening of the funnel at the pol II surface and by the walls of the funnel and pore. The effect of the opening can be estimated from the ratio of the surface area of the opening to that of the sphere around the active center in which it lies; for an opening with a diameter of ≈30 Å at a distance of ≈40 Å from the active center, this ratio is ≈1/28. The wall effect is shown by the probability distribution of NTPs diffusing in the absence of an electrostatic potential or E site (Fig. 2B). The fraction of NTPs reaching the E site is ≈1/600. Therefore, the total reduction in diffusion rate due to steric factors alone is ≈1/16,800.
The diffusion rate is affected further by the negative electrostatic potential in much of the funnel and adjacent region of the pore (Fig. 3). The repulsive effect of this electrostatic potential diminishes the number of NTPs reaching the E site by a factor of ≈1/300. Thus, the overall effect of the funnel and pore is a reduction in the diffusion rate of NTPs to the pol II active center by a factor of ≈2 × 10–7.
The rate of diffusion of NTPs may be compared with the rate of synthesis of RNA to gauge the effect of the funnel and pore on transcription. The rate of encounters between molecules the size of pol II and an NTP in solution at 25°C is estimated from collision theory to be ≈1012s–1·M–1. This rate is reduced by the funnel and pore to ≈2 × 105s–1·M–1. The rate of collisions resulting in binding may be further reduced by one or even two orders of magnitude by the steric requirements for binding. At the concentration of NTPs normally occurring in vivo, ≈1 mM (14), NTPs will arrive at the pol II active center at a rate of ≈200 per s, and the number of binding events will be ≤20 per s. The rate of RNA synthesis by pol II in vivo is ≈10 nucleotides per s (15). Therefore, delivery of NTPs by diffusion may be just sufficient to maintain the rate of RNA synthesis. Under conditions of diminished NTP concentration, diffusion could become rate-limiting.
NTP binding at the E site can enhance the rate of NTP binding at the A site in two ways. First, binding at the E site is almost entirely ionic, involving little more than chelation of the Mg ion by a set of aspartate side chains. There are fewer steric requirements than for binding at the A site, where base stacking and base pairing also come into play. NTP binding could be an order of magnitude more rapid at the E site than at the A site, for this reason.
The second advantage of NTP binding at the E site relates to backtracking. Pol II is believed to oscillate between forward translocation and backtracking (reverse translocation) at every step of transcription. Forward translocation clears the A site for entry of the next NTP. Backtracking returns the nucleotide that was just added to the RNA back to the A site. One or more additional steps of backtracking result in a stalled transcription complex, which may persist for many minutes or even be irreversible. As mentioned above, regulatory factors may intervene to restart transcription, or the polymerase may be ubiquitinated and destroyed, to avoid gene inactivation and cell death. Therefore, maintenance of the transcription rate requires the entry of an NTP in the A site immediately after forward translocation and before backtracking can occur and lead to stalling. Entry of an NTP in the E site will suffice for this purpose because the E site overlaps the A site (7). NTP binding at the E site increases the barrier height for backtracking. The strong negative electrostatic potential in the vicinity of the E site may increase the barrier height as well. Thus, the E site and its associated features may be crucial for the overall purpose of the polymerase undercarriage in the pausing and restoration of transcription.
Acknowledgments
K.D.W. was supported by the Medical Scientist Training Program. This work was supported by National Institutes of Health Grants GM49985 (to R.D.K.) and GM63817 (to M.L.).
Abbreviations: A site, addition site; E site, entry site; NTP, nucleoside triphosphate; pol II, RNA polymerase II.
References
- 1.Zhang, G., Campbell, E. A., Minakhin, L., Richter, C., Severinov, K. & Darst, S. A. (1999) Cell 98, 811–824. [DOI] [PubMed] [Google Scholar]
- 2.Cramer, P., Bushnell, D. A., Fu, J., Gnatt, A. L., Maier-Davis, B., Thompson, N. E., Burgess, R. R., Edwards, A. M., David, P. R. & Kornberg, R. D. (2000) Science 288, 640–649. [DOI] [PubMed] [Google Scholar]
- 3.Bushnell, D. A., Westover, K. D., Davis, R. E. & Kornberg, R. D. (2004) Science 303, 983–988. [DOI] [PubMed] [Google Scholar]
- 4.Temiakov, D., Patlan, V., Anikin, M., McAllister, W. T., Yokoyama, S. & Vassylyev, D. G. (2004) Cell 116, 381–391. [DOI] [PubMed] [Google Scholar]
- 5.Yin, Y. W. & Steitz, T. A. (2004) Cell 116, 393–404. [DOI] [PubMed] [Google Scholar]
- 6.Shilatifard, A., Conaway, R. C. & Conaway, J. W. (2003) Annu. Rev. Biochem. 72, 693–715. [DOI] [PubMed] [Google Scholar]
- 7.Westover, K. D., Bushnell, D. A. & Kornberg, R. D. (2004) Cell 120, 481–489. [DOI] [PubMed] [Google Scholar]
- 8.Opalka, N., Chlenov, M., Chacon, P., Rice, W. J., Wriggers, W. & Darst, S. A. (2003) Cell 114, 335–345. [DOI] [PubMed] [Google Scholar]
- 9.Kettenberger, H., Armache, K. J. & Cramer, P. (2003) Cell 114, 347–357. [DOI] [PubMed] [Google Scholar]
- 10.Sosunova, E., Sosunov, V., Kozlov, M., Nikiforov, V., Goldfarb, A. & Mustaev, A. (2003) Proc. Natl. Acad. Sci. USA 100, 15469–15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sosunov, V., Sosunova, E., Mustaev, A., Bass, I., Nikiforov, V. & Goldfarb, A. (2003) EMBO J. 22, 2234–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. (2001) Proc. Natl. Acad. Sci. USA 98, 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardiner, C. W. (1997) Handbook of Stochastic Methods: For Physics, Chemistry, and the Natural Sciences (Springer, Berlin).
- 14.Bochner, B. R. & Ames, B. N. (1982) J. Biol. Chem. 257, 9759–9769. [PubMed] [Google Scholar]
- 15.Wang, M. D., Schnitzer, M. J., Yin, H., Landick, R., Gelles, J. & Block, S. M. (1998) Science 282, 902–907. [DOI] [PubMed] [Google Scholar]
- 16.Gnatt, A. L., Cramer, P., Fu, J., Bushnell, D. A. & Kornberg, R. D. (2001) Science 292, 1876–1882. [DOI] [PubMed] [Google Scholar]