Although accumulation of triglyceride in the liver is part of normal energy storage physiology for species such as migratory birds, it is usually considered pathologic in humans. Nonalcoholic fatty liver disease (NAFLD), defined as the accumulation of excess intrahepatic triglyceride (IHTG), has become a major health burden as it is associated with increased cardiovascular, metabolic and liver-related morbidity and mortality. However, how one defines excess is not clear in this context.
One of the challenges in defining excess is to distinguish norms in the entire population from norms in healthy cohorts. The population approach is widely used for many medical tests (for example to define the reference range of the components of complete blood counts) where affected individuals are readily excluded or disease prevalence is low. However, when affected individuals are not excluded and disease prevalence is relatively high, the reference range can be skewed by selecting the reference population without regard to possible coexisting disease, as was shown for serum alanine aminotransferase (ALT) levels(1). With careful selection of subjects having a normal BMI, normal blood glucose and lipids, a healthy upper reference range of 19 U/L for women and 30 U/L for men(2) was suggested and similar low values have been incorporated into recent clinical guidelines(3).
Similarly, efforts have been made to define the norm for the amount of liver fat by using a cohort of people who lack risk factors for NAFLD. In a paper from the Dallas Heart Study(4), 1H-magnetic resonance spectroscopy (MRS) was used to measure liver fat content in a selected cohort of 345 subjects with normal glucose levels, normal body mass index (BMI), low alcohol consumption and no known liver disease. In this study, the 95th percentile of liver fat content was found to be 5.56%. Remarkably, this is very similar to data from a study performed 35 years earlier, where the hepatic triglyceride concentration in 12 autopsy samples with normal liver histology was measured biochemically at 19.4±15.9 mg/g, with a predicted 95th percentile (mean+2*SD) of 51.2 mg/g or 5.12%(5). Thus these two studies define a normal range based on an apparently healthy population; however, these studies do not establish the threshold at which the prevalence of comorbidities begins to increase.
For some biomarkers, defining health-based reference ranges by association with clinical outcomes, not by the distribution in healthy cohorts, may be more appropriate. For example, the fasting blood glucose upper limit of 126 mg/dL is used to diagnose type 2 diabetes based on the threshold for developing microvascular complications(6). An association with an absence of clinical outcomes or concomitant disease processes is especially important to avoid a drift in reference ranges due to changes over time in the underlying population; thus the rising incidence of obesity, diabetes and NAFLD should not drive a change in the normal ranges for BMI, glucose and ALT, respectively.
To establish if there is a threshold level for steatosis at which comorbidities begin to occur, Bril et al (reference) carefully evaluated a cohort of 352 adult subjects that was enriched for obesity and diabetes and present their results in this issue. They quantified IHTG by MRS and performed extensive metabolic testing. Most importantly, in a subset of 144 subjects, a hyperinsulinemic euglycemic clamp, the gold standard for assessing insulin sensitivity, was performed. Although similar studies have been performed before(7, 8), the large number of subjects across a wide range of liver fat content and a quantile-based analysis allowed the authors to identify thresholds of IHTG levels that are associated with specific metabolic abnormalities.
Hepatic fat content was correlated with adipose tissue insulin resistance (IR) along the whole range of IHTG levels. In contrast, fasting plasma triglyceride levels, reflecting VLDL secretion from the liver, are only associated with liver fat up to IHTG of 8.1%, after which no further increase is seen. This is consistent with previous data(9) showing a plateau in liver VLDL secretion in subjects with NAFLD, ascribed to inability to increase apo-B100 production. Surprisingly, hepatic insulin sensitivity (assessed as suppression of endogenous glucose production during the clamp) was not different across the whole spectrum of IHTG, except for marginally better sensitivity in subjects with lowest quantile of liver fat (< 1.5%) compared to all other quantiles. Consistent with most studies, the data showed that insulin levels, and thus HOMA-IR values, are typically higher in NAFLD but surprisingly this was shown to be related to impaired hepatic insulin clearance rather than impaired insulin suppression of hepatic glucose production. This is a very important observation because the HOMA is commonly interpreted to be a measure of hepatic insulin resistance, with higher numbers thought to reflect more severe hepatic insulin resistance. From data in this study, it appears that the elevated HOMA levels in patients with NAFLD reflect impaired insulin clearance and not impaired hepatic insulin responsiveness. Muscle insulin sensitivity, on the other hand, appeared to be very sensitive to the dysmetabolic state and reached maximal impairement with liver triglyceride of 4.2% without further impairment associated with higher amounts of liver fat.
Taken together, these data suggest a complex association between IHTG and energy metabolism. Adipose insulin resistance, leading to excess post-prandial delivery of fatty acids to the liver from unsuppressed lipolysis is likely the major driver of the increased liver fat content(10). On the other hand, muscle insulin resistance and hepatic VLDL secretion deteriorate progressively with increasing IHTG at relatively low amounts and reach a plateau at a relatively low IHTG of roughly 6±2%, after which no further change occurs. These observations suggest that the healthy population-based threshold for normal liver fat content conforms to a pathophysiological set-point, possibly defined by the point at which the liver is unable to increase VLDL output to compensate for increasing fat accumulation. However, this threshold defines an already established metabolic abnormality (analogous to the threshold of blood glucose to define diabetes), whereas the threshold for identifying early pathophysiologic changes (analogous to the cutoff for pre-diabetes) probably occurs at much lower IHTG levels. This is further supported by the interesting observation that the insulin-mediated suppression of hepatic glucose production is already impaired when there are very low amounts of excess IHTG and does not worsen further with higher IHTG levels.
There are several important limitations to the conclusions that can be drawn from Bril et al's work. First, the racial makeup of the patient population is not specified. Given the known impact of race and ethnicity on the risk of developing fatty liver(11), it is possible that a different physiological IHTG threshold exists for them as well. Second, it is unclear whether the associations described are independent of obesity, and especially of visceral adiposity. Third, the study is cross-sectional and does not examine longitudinal effects; it should not be used to imply that an increase or decrease in IHTG within an individual will translate to a change in metabolic phenotype. Although this is likely true, data is needed to support this supposition. Finally, the data should not suggest that accumulation of excess liver triglyceride is directly pathogenic, and in fact, implies the opposite, suggesting that liver fat content is a very sensitive marker of metabolic abnormalities, not their cause. Substantial animal and cell culture data also supports this conclusion.
Bril et al clearly demonstrate that an IHTG greater than 4-8% is appropriate for the diagnosis of NAFLD with an emphasis on the D, pointing out that this is a reflection of a disease with an associated impact on comorbidities. The challenge remains to identify the underpinnings of insulin resistance in adipose tissue and how this is best addressed therapeutically. Work in this area is now focused on adipose inflammation and the central role of adipose macrophages in regulating metabolism(12). Thus the treatment of lipotoxic liver injury (i.e., NASH) may be best focused on adipose tissue inflammation, with the amount of fat in the liver serving as a highly sensitive biosensor of pathological processes outside of the liver the lead to the many consequences of the metabolic syndrome.
Figure.
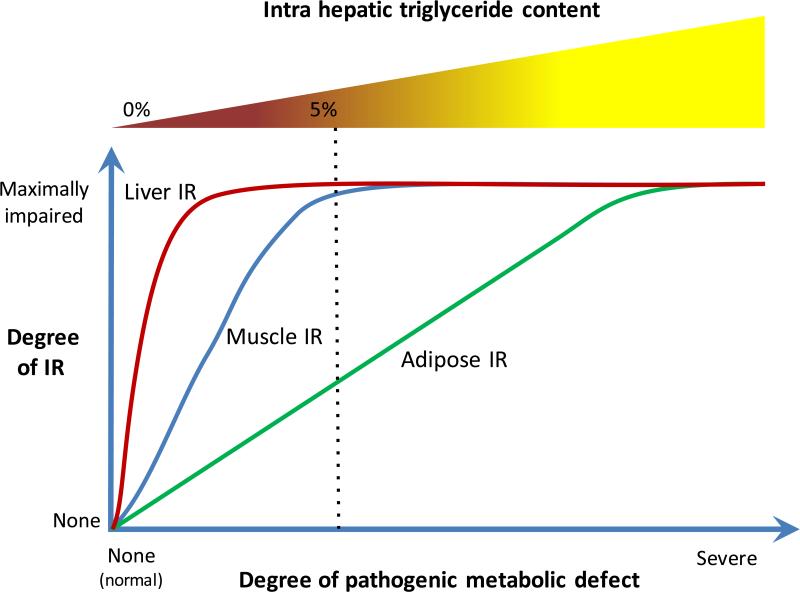
The data provided by Bril et al [reference] from a well-characterized cohort of adults with the full spectrum of NAFLD demonstrates that impairment of insulin responsiveness in the liver (measured by insulin-mediated suppression of endogenous glucose production), muscle (measured by insulin-stimulated glucose uptake), and adipose tissue (measure by insulin-mediated suppression of lipolysis) was markedly different in these three major targets of insulin signaling. One interpretation of their data is that there is an underlying metabolic defect responsible for these changes. Recent studies point to a role of stress activated kinases such as the c-Jun NH2-terminal kinases (JNKs) throughout the body and especially in adipose tissue in mediating these global effects. Using the data from Bril et al, the consequences of this global stress response can be envisioned as shown with liver insulin resistance occurring with minimal provocation, muscle being less easily impaired and adipose tissue the most resistant to being impaired. The accumulation of triglyceride in the liver appears to be a sensitive indicator of adipose insulin resistance and they are correlated over the spectrum of the underlying metabolic abnormality. Interestingly, their data also show that the amount of fat in the liver does not correlate with histological evidence of liver inflammation or hepatocellular injury. Thus the degree of adipose insulin resistance determines the severity of steatosis but it is not directly related to the severity of the indices of NASH.
Abbreviations
- BMI
Body mass index
- IHTG
Intrahepatic triglyceride
- IR
Insulin resistance
- MRS
Magnetic resonance spectroscopy
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
References
- 1.Neuschwander-Tetri BA, Unalp A, Creer MH, Nonalcoholic Steatohepatitis Clinical Research N. Influence of local reference populations on upper limits of normal for serum alanine aminotransferase levels. Arch Intern Med. 2008;168:663–666. doi: 10.1001/archinternmed.2007.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2016 doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 4.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 5.Kwiterovich PO, Jr., Sloan HR, Fredrickson DS. Glycolipids and other lipid constituents of normal human liver. J Lipid Res. 1970;11:322–330. [PubMed] [Google Scholar]
- 6.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 7.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087–1093. doi: 10.1002/hep.23116. [DOI] [PubMed] [Google Scholar]
- 11.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 12.Pal M, Febbraio MA, Lancaster GI. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. J Physiol. 2016;594:267–279. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]