Abstract
Relatively little is known about factors that initiate immunosuppression in tumors and act at the interface between tumor cells and host cells. In this article, we report novel immunosuppressive properties of the ribosomal protein S19 (RPS19), which is upregulated in human breast and ovarian cancer cells and released from apoptotic tumor cells, whereupon it interacts with the complement C5a receptor 1 (C5aR1) expressed on tumor infiltrating myeloid-derived suppressor cells (MDSC). This interaction promotes tumor growth by facilitating recruitment of these cells to tumors. RPS19 also induces the production of immunosuppressive cytokines including TGF-β by MDSC in tumor draining lymph nodes, leading to T cell responses skewed toward TH2 phenotypes. RPS19 promotes generation of T regulatory cells while reducing infiltration of CD8+T cells into tumors. Reducing RPS19 in tumor cells or blocking the C5aR1-RPS19 interaction decreases RPS19-mediated immunosuppression, impairs tumor growth, and delays the development of tumors in a transgenic model of breast cancer. This work provides an initial preclinical evidence for targeting RPS19 for anticancer therapy enhancing antitumor T cell responses.
Introduction
The vast majority of processes mediated through the complement C5aR1 is thought to be initiated by the interaction of C5aR1 with its best-studied ligand C5a (1). RPS19 is the only other endogenous ligand of eukaryotic-origin known to bind to this receptor (2, 3). RPS19 released from apoptotic cells was shown to have chemotactic activity toward leukocytes in vitro through interaction with C5aR1 (4). Interestingly, unlike C5a, RPS19 differentially regulates monocytes vs. neutrophils during inflammation (5). It selectively enhances the recruitment of monocytes that give rise to macrophages involved in clearance of dying cells and, simultaneously, halts the migration of neutrophils to sites of inflammation (5). Therefore, RPS19 has been postulated to protect tissues from neutrophil-induced damage and to facilitate the clearance of dying cells in inflammation (5). Since cell death resulting from hypoxia is present in virtually all malignancies, even in early stages of tumor growth (6), and C5aR1 is known to activate and recruit MDSC to tumors (7, 8), we hypothesize that RPS19 released from dying tumor cells is involved in the initiation of immunosuppression in the tumor microenvironment through its interaction with C5aR1 expressed on MDSC.
In support of this hypothesis, an early study has demonstrated upregulation of RPS19 expression (mRNA) in colon carcinoma compared to normal epithelial colon cells (9). In addition, small quantities of RPS19 have been found in feces of patients with colorectal cancer (10), and single nucleotide polymorphisms in the RPS19 gene have been found to be associated with an increased risk of cervical intraepithelial neoplasia and cervical cancer (11). However, the extracellular functions of RPS19 on the whole remain largely unknown. Moreover, knowledge of the involvement of RPS19 in pathogenesis of diseases is limited to a rare hematologic condition named Diamond-Blackfan anemia, with 25 % of patients having mutations in the RPS19 gene (12).
Materials and Methods
Mice, Cell lines, and human tissues
Eight to twelve week old FVB/N-Tg(MMTVneu) 202Mul/J (FVB/N Her2/neu transgenic), wild type FVB/NJ and BALB/c female mice from the Jackson Laboratory were used for this study with the approval of the Institutional Animal Care and Use Committee of the Texas Tech University Health Sciences Center (TTUHSC), according to guidelines of the National Institutes of Health. NT-5 tumor cells derived from a spontaneously occurring mammary adenocarcinoma in a FVB/N Her2/neu-transgenic mouse expressing nontransforming rat Her2/neu (13) were subcutaneously (s.c.) injected (1×106) into the rear flanks of mice. 4T1 cells were injected into the mammary fat pad to generate breast tumors, as previously described (14). Tumors were measured with calipers and the volume of tumors was calculated with the formula: volume = (length x width x depth)/2. Mouse cell lines (RAW 264.7 and 4T1) and human cell lines (benign mammary epithelium: MCF-10A, breast cancer: MCF-7, MDA-MB-231, MDA-MB-453, and BT-20, ovarian adenocarcinoma: SKOV-3, colorectal cancer: COLO 205) were obtained from ATCC and maintained according to its recommendations except human 1520 melanoma and OCAR-5 ovarian carcinoma cells that were obtained from Dr. Jon Weidanz (TTUHSC). Cell lines were routinely tested for the absence of mycoplasma contamination (Aldevron, ND, USA). Human cell lines were authenticated by ATCC or providing investigator by Short Tandem Repeat profiling and used within 6 months after resuscitation. Breast cancer human tissues were procured from National Disease Research Interchange.
C5aR1 inhibition and C5a detection
For pharmacological blockade of C5aR1, mice were injected s.c. with a selective C5aR1 antagonist (C5aRA), the cyclic peptide – Ac-(2,6)-F[OP(D-Cha)WR]. This compound, acetyl-phenylalanine–[ornithine-proline-(D-cyclohexylalanine)-tryptophan-arginine], originally named 3D53 and also previously licensed as PMX53 (15), does not bind to the second receptor for C5a (C5L2) nor to the C3a receptor (16). It was synthesized and characterized as described (17), dissolved in PBS, and administered to mice at a dose of 1 mg per kg body weight, every 2–3 days beginning at day 3 or 4 after tumor cell injection (3.3 μmol per kg body weight per week), as previously described (7). Control mice were injected s.c. with PBS as placebo. C5a was detected in mouse plasma by ELISA (MyBiosource, CA) according to the manufacturer’s instructions.
Immunoprecipitation (IP) and Western blotting
Tumor cell suspension was obtained through the incubation of minced tumor fragments with 1mg/ml of collagenase P (Roche), 0.4mg/ml of DNase I (Roche), and 100ug/ml of trypsin inhibitor (Sigma) dissolved in RPMI on a shaker at 37°C for 1 h. After two washes with PBS cells were incubated with 2mM cross-linking reagent DTSSP (Pierce) for 30 minutes at room temperature (RT), and the reaction was stopped by adding 10mM Tris-HCL (pH7.5) and incubating on a shaker at RT for 15 minutes. After centrifugation, the pellet was lysed with the IP lysis buffer (Thermo Fisher) containing protease and phosphatase inhibitors. Lysate was incubated with rabbit IgG (Santa Cruz, sc-2729,), RPS19 (Abcame, ab-155994) or C5aR1 (sc-25774) antibodies overnight at 4°C on a shaker. IP was carried out with Dynabeads (Thermo Fisher, 11203D) according to the manufacturer’s instructions. Reduced samples were electrophoresed, transferred onto nitrocellulose membrane that were probed for RPS19 (ab-155994) and C5aR1 (Biolegend, 135807) and then incubated with clean blot IP-HPR (Pierce, 21230).
Binding assay
C5a and RPS19 binding to C5aR1 expressed on murine RAW 264.7 macrophages and MDSC, isolated using magnetic beads from spleens of NT-5 tumor bearing mice, was determined by FACS. Approximately 95% purity of isolated cells was achieved (Suppl. Fig. 1). Cells were incubated with flurochrome labelled recombinant murine C5a (R&D systems) or recombinant human RPS19 (Abcam) at a concentration of 40nM for 40 min. Some cells were preincubated with 10μM C5aRA for 40 min. We used human RPS19 since there is 99.3% homology between human and mouse proteins.
Chemotaxis assay
The assay was performed using a Corning® HTS Transwell®-96 well permeable support (Sigma-Aldrich) according to the manufacturer’s instructions. Recombinant human RPS19 (15181, Abcam) and recombinant murine C5a (R&D Systems), at a concentration of 10nM, were used as chemoattractants. Purchased recombinant RPS19, in addition to containing the most abandon monomer form, contained also the RPS19 dimer (Suppl. Fig. 2A). For some experiments cells were preincubated with 10μM C5aRA.
RPS19 downregulation by shRNA
Stable NT-5 cell lines expressing RPS19 shRNA were generated by transducing parental NT-5 cells with the SMART Human Inducible Lentiviral Vector (Thermo Scientific VSM6405) carrying RPS19 shRNA. Control cells received the Lentiviral non-targeting vector (VSC6571). Lentivirus was added to the cells with Polybrene (8μg/ml) for maximum viral transduction. Stable cells were cultured in Doxycycline-free medium and selected using puromycin (2μg/ml). Gene knockdown was confirmed by Western blot analysis after doxycycline (1ug/ml) induction.
Tissue processing, cell isolation, and immunofluorescence
Portions of tumors and tumor draining lymph nodes (TDLN) were frozen in OCT at −70 °C and sectioned with cryostat for immunofluorescence or were used for cell isolation for subsequent FACS. The following monoclonal antibodies (mAb) were used for immunofluorescence in frozen sections: CD8a (53–6.7, BD Pharmingen), perforin (CB5.4, Abcam), CD11b (M1/70, BD Pharmingen), Gr-1 (RB6-8C5, BD Pharmingen), C3b/iC3b/C3c (2/11, Hycult Biotech) (18), RPS19 (ab-155994), CD33 (825601, Biolgend), CD11b (ab52478, Abcam) and C5aR1 (C85-2506, BD Pharmingen). Apoptotic cells were detected with Anexin V (sc-1929). Secondary antibodies included Alexa Fluor (AF) 488, Texas Red, and AF 633-conjugated goat anti-rat antibodies (Invitrogen). Perforin-expressing CD8+ T cells and MDSC (CD11b+Gr-1+) tumor infiltrates were quantified with Nikon Elements Advanced Research image-analysis software; cells were counted in entire tissue sections and mean values per 63x field were calculated.
FACS and functional assays
Fluorochrome-conjugated mAbs were purchased from Biolegend: CD45-FITC (30-F11), CD3-PE/Cy7 (17A2), PerCP/Cy5.5-CD4 (GK1.5), APC/Cy7-CD8a (53-6.7, RM4-5), APC/Cy7-CD25 (PC61), PE-CD80 (16-10A1), APC-CD86 (GL-1), PE/Cy7-F4/80 (BM8), APC/Cy7-CD11c (N418), Alexa Fluor (AF) 488-IA/IE (M5/114.15.2), AF700-CD11b (M1/70), APC/Cy7-CD25 (PC61), PerCP/Cy5.5-Gr-1 (RB6-8C5), PE-FOXP3 (150D), PE-IL-6, APC-IL-12/p40 (C15.6), PerCP/Cy5.5-TGFβ1, Pacific blue-IL-10 (JES5-16E3), PE-IFN-γ (XMG1.2), APC/Cy-7-IL-4 (11B11), and APC-IL-17a (TC11-18H10.1). PE-Texas Red-CD8a (53–6.7) was from Invitrogen. Her2 peptide (PDSLRDLSVF; 420–429) was obtained from EZBiolab, Inc. and its biotinylated-monomer from the NIH tetramer facility was tetramerized with streptavidin-APC (Molecular probes) and used for staining of Her2 specific CD8+ T cells.
For CD8+ T cell stimulation, cells were incubated with PDSLRDLSVF for 6 to 8 h with Golgi-inhibitors (brefeldin-A and monensin; BD Biosciences), and IFN-γ production was assessed with PE-IFN-γ antibody (XMG1.2). For myeloid cell function, cell preparations from TDLN were stimulated with 1 μg/ml of LPS for 8 to 12 h with Golgi-inhibitors and subsequently stained for cytokines.
For all FACS stainings, cells were pre-incubated with mAbs CD16-CD32 (Fc block; 2.4G2; BD Pharmingen). For enumerating regulatory T cells, cells were stained with surface markers and permeabilized before staining with FOXP3 antibodies. For all intracellular cytokine staining, cells were stained with surface markers prior to permeabilization with cytofix/cytoperm (BD Biosciences). For analysis of the cell surface expression of C5aR1, cells were sequentially incubated with rat mAb to mouse C5aR1 (20/70; Hycult Biotechnology; Cell Science) or rat isotype-matched control antibody (553928; BD Pharmingen), followed by fluorescein isothiocyanate–conjugated anti–rat IgG (81–9511; Zymed-Invitrogen).
For Her2/tumor-specific CD8+ T cells, viable lymphocytes were gated based on forward-(FSC) and side-scatter (SCC) followed by gating CD3+CD45+ and then CD8+Her2+ cells. The frequencies and numbers of regulatory-T cells were calculated by gating CD4+CD25+FOXP3+ cells, while MDSC were identified as CD11b+Gr-1+ MHC IIlow. Frequencies of DC were calculated based on MHCII and CD11c positivity whereas the expression CD80 and CD86 was determined as mean fluorescence intensity.
To study the impact of C5aR1 signaling in MDSCs on polarization of T cell responses, groups of tumor-bearing FVB/N Her2/neu transgenic mice were injected with C5aRA or PBS. On day 28 spleens of these mice were harvested for MDSCs isolation by magnetic columns (Miltenyi). Naïve CD4+ T cells were negatively sorted (Miltenyi) from splenocytes of non-tumor-bearing female FVB/N Her2/neu transgenic mice. Purity of cells was verified by FACS and found to be above 98%. CD4+ T cells and MDSC were co-cultured in 1:5 ratios in a 24-well plate coated with anti-CD3/CD28 antibodies. C5aRA was added to some wells at 10 nM concentrations. CD4+ T cell cultures were harvested at day 5 and intracellular staining for IFN-g, IL-4, and IL-17 was performed as described (14). TGF-β1 ELISA (Thermo-Fisher Scientific) was done according to manufacturer’s instructions. Data acquired on LSRFortessa were analyzed with FlowJo software (Tree Star).
Statistical analysis
Data were analyzed with un-paired t-test or non-parametric Mann-Whitney test, depending on results of the normality test (Kolmogorov-Smirnov), or One-Way ANOVA for more than two mean values. Tumor growth with time was analyzed by Two-Way-ANOVA. Bar graphs indicate mean+SEM. All statistical analysis was done with Graph Pad Prism 6 software.
Results
RPS19 is upregulated in human breast and ovarian cancer cells and interacts with C5aR1 expressed on MDSC
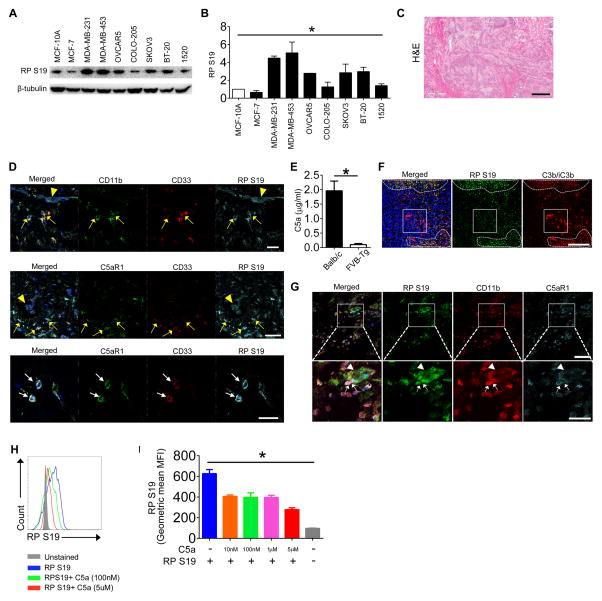
Ribosomal proteins and ribosomal biogenesis, which are linked to the MDM-p53 pathway, appear to have dual and sometimes contradictory functions in cancer development (19). Elevated levels of ribosomal biogenesis and protein translation have been linked to formation of tumors in multiple mouse models (20). On the other hand, a reduction of ribosomal biogenesis and translational capacity have been associated with a high incidence of cancer in humans (21). For example, Diamond-Blackfan anemia, which is associated with the mutations in the gene for RPS19 in 25% of patients, increases susceptibility to hematopoietic malignancies (19). Therefore, we tested human tumor cell lines for RPS19 expression and found that three out of four tested human breast and two out of two tested ovarian cancer cell lines overexpress RPS19 compared to normal epithelia from mammary gland (Fig. 1A, B). In addition, we found RPS19 on the surface of cells expressing CD11b and CD33, likely representing MDSC, in tumor sections from patients with ductal breast carcinoma (Fig. 1C and D-upper panel). RPS19 on cell surface co-localized with C5aR1 expressed on these cells, suggesting interaction of RPS19 with C5aR1 (Fig. 1 D-middle and lower panels). Since complement activation, which leads to generation of C5a, was reported to occur in breast cancer (22), these data suggest that RPS19 interacts with C5aR1, even in the presence of C5a in human tumors. To interrogate a further interrelationship between both ligands in tumor tissue, we stain mouse 4T1 tumors from BALB/c mice, which have C5 and generate C5a in the response to breast tumors, in contrast to FVB/N Her2/neu transgenic mice (Fig. 1E), for deposition of C3 cleavage fragments, which indicates complement activation and the subsequent generation of C5a in tissue. Importantly, we used anti-C3 antibody, which reacts exclusively with C3 cleavage products but not with intact C3 (18), therefore, this staining indicates areas of tumor where complement activation occurs. We found overlap of C3 fragments’ deposition with RPS19 accumulation (Fig. 1F-dashed lines’ outlines, yellow color), likely, because RP S19 is released from apoptotic cells (4) that also activate complement (23). This data suggest that both ligands potentially compete for C5aR1 binding. However, in the same tumor areas, we found RPS19 and C3 fragment’s deposition without apparent overlap (Fig. 1F-dashed line, green and red color). Moreover in some portions of tumor, the large areas of complement deposition were observed in some distance from RPS19 accumulation (Fig. 1F-square, red color). Despite the presence of complement activation and C5a generation, RPS19 co-localized with C5aR1 on the surface of myeloid (CD11b+) cells in proximity of larger RPS19 accumulation in tumor cells (Fig. 1G-square, arrows-myeloid cells, and arrowhead-tumor cells). Thus, we concluded that RPS19 interacts with C5aR1 even in the presence of C5a. This conclusion is supported by results of an in vitro competitive binding assay. We found that increasing concentrations of C5a reduced RPS19 binding in a dose dependent manner, however, even 125 fold greater concentration of C5a (5μM) has not entirely eliminated RPS19 binding (Fig. 1H, I).
Figure 1. RPS19 is overexpressed in breast and ovarian cancer cells and interacts with C5aR1 in the interface between tumor and MDSC.
(A) RPS19 (16kD) is overexpressed in breast (MDA-MB-231, 354 and BT-20) and ovarian (OVCAR5 and SKOV3) cancer-derived cell lines relative to normal mammary gland epithelium (MCF-10A) (B) Quantification of data from A normalized to β-tubulin. *P<0.0001 by One-Way ANOVA. (C) Hematoxylin & eosin (H&E) staining of the section of tumor from breast ductal adenocarcinoma patient. The entire section contains only tumor tissue. Scale bar-500μm (D) Confocal microscopy analysis of human MDSC (CD11b+ CD33+), RPS19 and C5aR1 in breast cancer section shown in C (arrowheads-tumor cells, yellow arrows-MDSC, white arrows C5aR1 and RPS19 colocalization on MDSC). Scale bars 50 (two upper panels) and 10μm (lower panel). (E) C5a in plasma of 4T1 tumor-bearing BALB/c and NT-5 tumor-bearing FVB/N Her2/neu transgenic (FVB-Tg) mice. *P=0.0006 by t-test (F) RPS19 and C3 cleavage fragments (C3b/iC3b) deposition in mouse 4T1 tumor. Dash lines-areas with abundant RPS19 accumulation. Square-prominent C3 fragments’ deposition that does not colocalize with RPS19. Yellow inside dash outlines in merged images-RPS19 and C3 fragments’ colocalization. Scale bar-200 μm (G) RPS19, CD11b and C5aR1 in 4T1 tumors. The square outlines area, which is magnified in a lower panel. Arrowheads-tumor cells. Arrows-CD11b+ myeloid cells expressing C5aR1, which colocalizes with RPS19. Scale bars 25 (lower) and 50 (upper image) μm (H) Binding of 40 nm recombinant RPS19 in the presence of increasing concentrations C5a by FACS, representative histograms, (I) Quantification of data from (H). *P<0.0001 by One-Way ANOVA. Data are representative of three independent experiments with three replicates for A, B, H and I, n=2 for C and D, and n=5 for E, F and G.
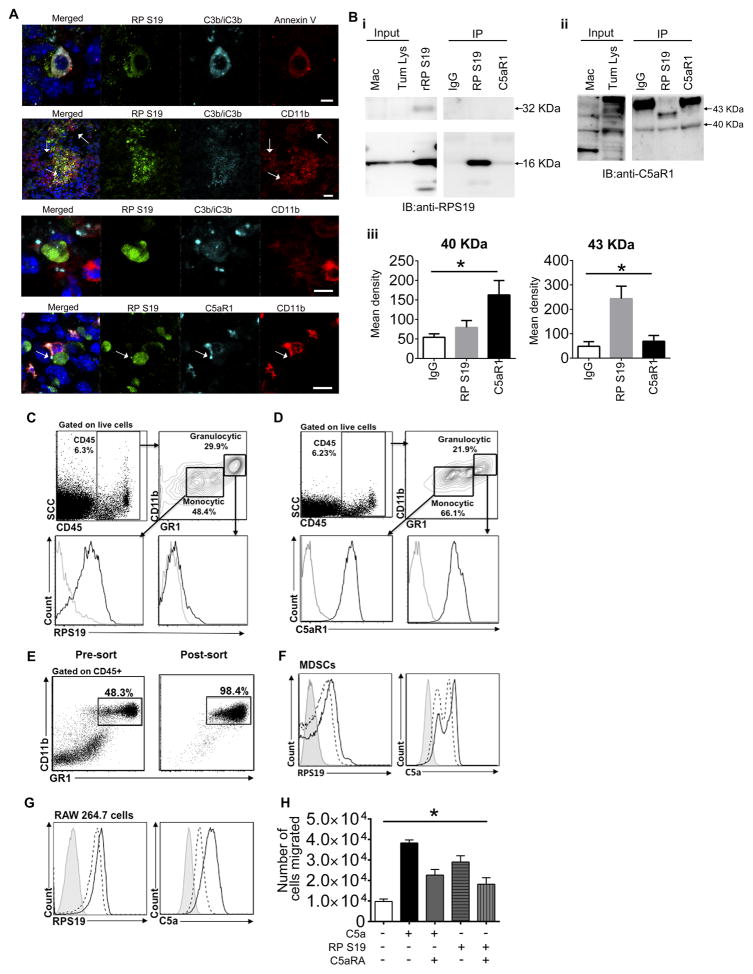
To confirm that RPS19 interacts with C5aR1 on MDSC we used Her2/neu transgenic mice (on FVB background) that in contrast to BALB/c mice do not produce C5 and C5a (Fig. 1E and https://www.jax.org/strain/002376). To expedite mechanistic studies these mice were injected with NT-5 cells that do not express C5aR1 (data not shown) and are derived from spontaneous breast carcinoma developing in these mice at older age (13). Tumor cells were injected prior to development of spontaneous tumors. The FVB/N Her2/neu mouse exhibits T cell tolerance to rat Her2/neu, which is a self-antigen in this mouse, and is, thus, a useful breast cancer model mimicking human cancer and suitable for testing the role of tolerance in anti-tumor immune responses (24). We chose to inject mice into the rear flank to avoid potential confusion of transplantable tumor with spontaneous breast carcinoma developing in these mice. We found that RPS19 accumulated in tumor cells undergoing apoptosis, indicated by binding of Annexin V to phosphatidylserine on the external surface of tumor cells (Fig. 2A-upper panel). Since apoptotic cells activate complement leading to coating of apoptotic cells with the cleavage products C3b and iC3b of the complement fragment C3 (25), we examined deposition of these fragments on apoptotic (“Annexin V+”) tumor cells and found that these cells were uniformly coated by C3b/iC3b (Fig. 2A-upper panel). These complement fragments interact with complement receptor 3 (CR3), which is composed of CD11b and CD18 (26). Therefore, as anticipated, CD11b expressing myeloid cells were present in close proximity to apoptotic tumor cells with abundant expression of RPS19 (Fig. 2A-middle upper panel, arrows) and, in some areas, CD11b positivity seemed to be adjacent to C3b/iC3b (Fig. 2A-middle lower panel), pointing to possible interaction between CR3 and iC3b in the interphase between tumor and host cells. Importantly, RPS19 staining in tumor cells co-localized with C5aR1 expressed on CD11b+ cells (Fig. 2A-lower panel, arrow), suggesting an interaction of RPS19 with C5aR1. This interaction is supported by results of IP of RPS19 protein from the tumor whole cell lysate by C5aR1 antibody (Fig. 2B-i). This IP resulted in the pulldown only a monomer (16kD) of RPS19, likely due to very low concentrations of dimerized form of RPS19 (32kD) in a mouse tumor. We have observed only low amounts of RPS19 dimer in the recombinant protein preparation used as a positive control, however, we failed to detect RPS19 dimer in the tumor whole cell lysate (2B-i-input). The lack of RPS19 dimer in tumors is consistent with Yamamoto’s and colleagues’ report indicating that although RPS19 dimer is formed in apoptotic cells, it is quickly inactivated by serine proteases released from the same cells (27). Interestingly, IP of C5aR1 with RPS19 antibody resulted in pulldown of phosphorylated receptor with a reported molecular size of 43kD (28) (Fig. 2B-ii and iii) whereas IP with C5aR1 antibody caused pulldown of mainly unphosphorylated form (40kD) with very limited contribution of phosphorylated receptor (2B-ii and iii). This data suggest that engaging of C5aR1 with interaction with RPS19 leads to receptor internalization/phosphorylation similar to observed for C5a (28). The dominance of unphosphorylated receptor in IP with C5aR1 antibody is likely associated the overabundance of C5aR1, which is not engaged with an interaction with a ligand.
Figure 2. RPS19 binds to C5aR1 expressed on MDSC and macrophages and triggers chemotaxis of macrophages.
(A) RPS19, C3b/iC3b, Anexin V, CD11b, and C5aR1 in murine tumors generated in FVB/N Her2/neu transgenic mouse by injecting NT-5 cells. Arrows: accumulation of CD11b+ cells (upper middle) and co-localization of RPS19 with C5aR1 (lowest panel), scale bars 5 μm except upper middle panel which is 25 μm. (B-i, ii) Immunoprecipitation of RPS19-C5aR1 complex from whole cell lysate (Tum. Lys.) of NT-5 tumors from Her2/neu transgenic mouse. Murine macrophages RAW 264.7 (Mac) were used a positive control for C5aR1 expression and recombinant RPS19 (rRP S19) as control for RPS19. (iii) Quantification of data from ii, *P=0.0364 (right) and *P=0.003 (left) by One-Way-ANOVA. FACS analysis of (C) RPS19 on the surface of monocytic (Gr-1int) vs. granulocytic (Gr-1high) MDSC and (D) C5aR1 expression on monocytic vs. granulocytic MDSC. Black lines: RPS19 and C5aR1 antibodies. Grey lines: isotype controls. (E) Pre- and post-sort FACS of spleen MDSC from NT-5 tumor-bearing mice. Biding of fluorescently labeled-RPS19 or C5a in the absence (continuous black lines) or presence (dashed lines) of C5aR1 antagonist (C5aRA) to (F) sorted MDSC, P<0.0001-RPS19, P=0.0001-C5a by One-Way ANOVA or (G) murine macrophages, P<0.0001-RPS19 and C5a by One-Way ANOVA. (H) Chemotaxis assay; y-axis number of murine macrophages (RAW 264.7) in lower chamber at the end of experiment, *P<0.0001 by One-Way ANOVA. Data are representative of one experiment with n=5 for A, three independent experiments for B–G, and four replicates for H.
To verify that MDSC are engaged in interaction with RPS19 we mechanically disintegrated NT-5 tumors. Through FACS we found RPS19 on the surface of MDSC (CD45+MHC IIlowCD11b+Gr-1+) with higher amount of RPS19 bound to monocytic (Gr-1int) vs. granulocytic (Gr-1high) MDSC (Fig. 2C), which is consistent with reported chemotactic activity of RPS19 toward monocytes/macrophages (4). C5aR1 expression on both MDSC populations has been previously reported (7). Consistent with this report, MDSC retrieved from NT-5 tumors were found to express C5aR1 (Fig. 2D). Next, we sorted MDSC from spleens of NT-5 tumor-bearing mice (Fig. 2E and Suppl. Fig. 1) and incubated with fluorescently-labeled C5aR1 ligands C5a or RPS19 in the absence or presence of C5aR1 inhibitor, which binds only to C5aR1 but not to C5L2. Preliminary studies established that binding of RPS19 or C5a to C5aR1 is optimal in 40nM ligand concentrations. We found that both C5a and RPS19 binds to sorted MDSC and preincubation with C5aR1 inhibitor reduces this bindings significantly (Fig 2F). Similar results were obtained for murine macrophage cell line (Fig. 2G), which was also utilized for testing chemotactic activity of the recombinant RPS19 in the transwell system. We found that the recombinant RPS19 preparation used for this assay contained a small amount of dimerized form of RPS19 (Supplemental Fig. 2A), which was reported to have chemotactic activity (5). We found that RPS19 was as potent as C5a as a chemoattractant for these cells (Fig. 2H). Both RPS19 and C5a chemotactic activities were mediated by the interaction of these ligands with C5aR1, as the preincubation of macrophages with the specific C5aR1 inhibitor attenuated C5a- and RPS19-mediated chemotaxis (Fig. 2H). These functional data suggest that the C5aR1 inhibitor used herein interferes with binding of RPS19 to C5aR1 and blocks the functions of RPS19 that are mediated through its interaction with C5aR1.
RPS19 downregulation in tumor cells correlates with slower tumor growth and enhanced antitumor immunity
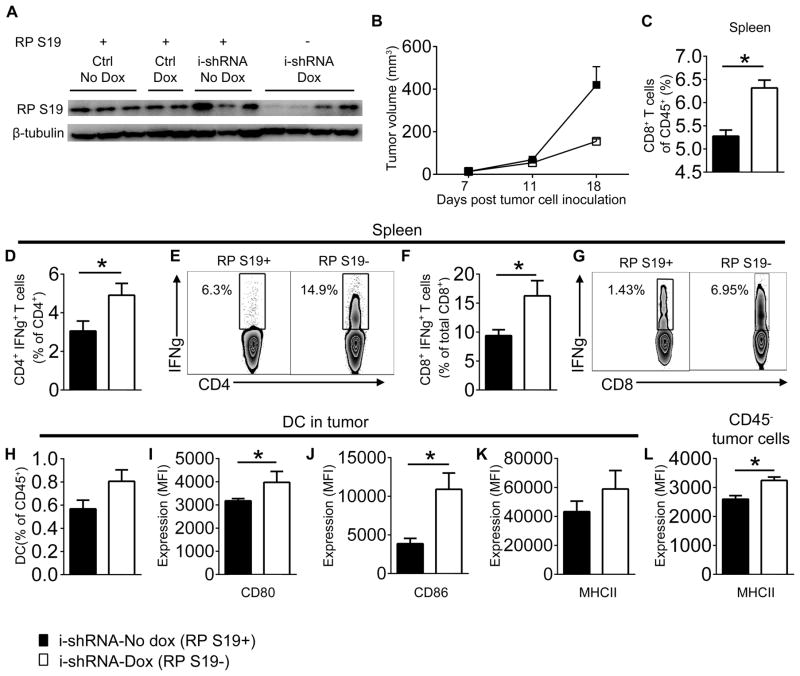
To investigate a role of RPS19 in tumor growth in vivo, we used a doxycycline inducible shRNA approach to downregulate RPS19 in NT-5 cell (Suppl. Fig. 2B, C). We injected FVB/N Her2/neu transgenic mice with NT-5 cells transduced with an empty lentiviral vector (control) or inducible lentiviral RPS19 shRNA prior to the development of spontaneous mammary tumors. Neither the transgenic mice nor NT-5 cells produce C5 and C5a. Consequently, RPS19 is the only known ligand to interact with C5aR1 in this experimental setting. The doxycycline-induced RPS19 shRNA expression led to the downregulation of RPS19 protein in the majority (but not all) NT-5 tumors (Fig. 3A, representative examples are shown) in vivo and reduced tumor growth (Fig. 3B). In contrast, doxycycline did not impact tumor growth in control mice that were injected with NT-5 cells transduced with empty lentivirus (data not shown). To avoid prolong exposure of mice to doxycycline the experiments were terminated when statistically significant differences in tumor growth were first observed.
Figure 3. The downregulation of RPS19 in NT-5 tumor cells leads to reduced tumor growth and improved immunity in Her2/neu transgenic mice.
(A) RPS19 in whole cell lysates of tumors composed of NT-5 cells transduced with an empty lenitiviral vector (Ctrl) or inducible lentiviral RPS19 shRNA (i-shRNA) from Her2/neu vehicle (PBS)-treated transgenic mice (No Dox) or mice treated with doxycycline (Dox) by western blot. (B) Volumes of tumors generated by injecting NT-5 cells, transduced with inducible lentiviral RPS19 shRNA, to Her2/neu transgenic mice treated with vehicle (black square-i-shRNA-No dox [RPS19+]) or doxycycline (white square-shRNA-Dox [RPS19−]), P=0.0201 by Two-Way ANOVA (C–L) Immunity in mice bearing RPS19+ vs. RPS19− tumors by FACS. (C) CD8+ T cells in spleens, *P=0.0081 by t-test. (D) IFN-γ producing CD4+ T cells in spleens, *P=0.0499 by t-test. (E) Representative plots for D. (F) IFN-γ producing CD8+ T cells in spleens, *P=0.04 by t-test. (G) Representative plots for F. (H) Dendritic cells (DC) in tumors. (I) CD80 on DC, MFI-median fluorescence intensity *P=0.0373 by t-test. (J) CD86 on DC, *P=0.0402 by t-test. (K) MHC II on DC and (L) CD45− tumor cells, *P=0.0193 by t-test. Legend described in B applies to all panels. Data are representative of two independent experiments with n=3–5.
Mice expressing lower amounts of RPS19 in tumor cells also have higher numbers of CD8+ T cells (Fig. 3C) and IFN-γ producing CD4+ (Fig. 3D, E) and CD8+ T (Fig. 3F, G) cells in their spleens, suggesting enhanced Th1 and cytotoxic T cell responses. Importantly, for IFN-γ intracellular staining in CD8+ T cells, splenocytes were stimulated ex vivo with peptide corresponding to the Her2/neu immunodominant epitope (29). Therefore, the evaluated CD8+ T cells responses are tumor-specific. We found no difference in the number of tumor infiltrating CD4+ T and CD8+ T cells (data not shown), however, dendritic cells [(DC), CD45+CD11c+MHCII+] show a trend to be more frequent (not statistically significant) in tumors with RPS19 downregulation (Fig. 3H) and they have better antigen presenting functions as demonstrated by the increased expression of CD80 (Fig. 3I) and CD86 (Fig. 3J). MHC II expression showed a trend for higher expression on DC from tumors with RPS19 downregulation (Fig. 3K). Interestingly, tumor cells identified by the lack of CD45 expressed more MHC II in the context of reduced RPS19 expression (Fig. 3L) rendering them more susceptible for immune recognition and destruction (30).
Pharmacological blockade of the interaction of RPS19 with C5aR1 delays the development of spontaneous breast tumors, reduces tumor growth and enhances antitumor immunity
Although we did not observe an impact of a moderate downregulation of RPS19 on NT-5 cell growth in vitro, as demonstrated by identical morphology and growth rate of the cultured cells treated with doxycycline (induced RPS19 shRNA) vs. non-treated cells (Suppl. Fig. 2D), there is concern that the downregulation of RPS19, which is involved in ribosomal biogenesis, may affect tumor cell proliferation and/or survival in vivo. Thus, it may affect tumor growth regardless of antitumor immunity. Therefore, we next used a pharmacological approach to block RPS19 interaction with C5aR1. We found that the C5aR1 inhibitor used in our study efficiently blocks functions of RPS19 that are mediated through C5aR1 receptor (Fig. 2H), therefore, we used this inhibitor in a FVB/N Her2/neu transgenic model of breast cancer or FVB wild-type mice. Due to lack of C5 and C5a in these mice, C5aR1 inhibitor blocks the interaction of C5aR1 with the only remaining known C5aR1 ligand RPS19.
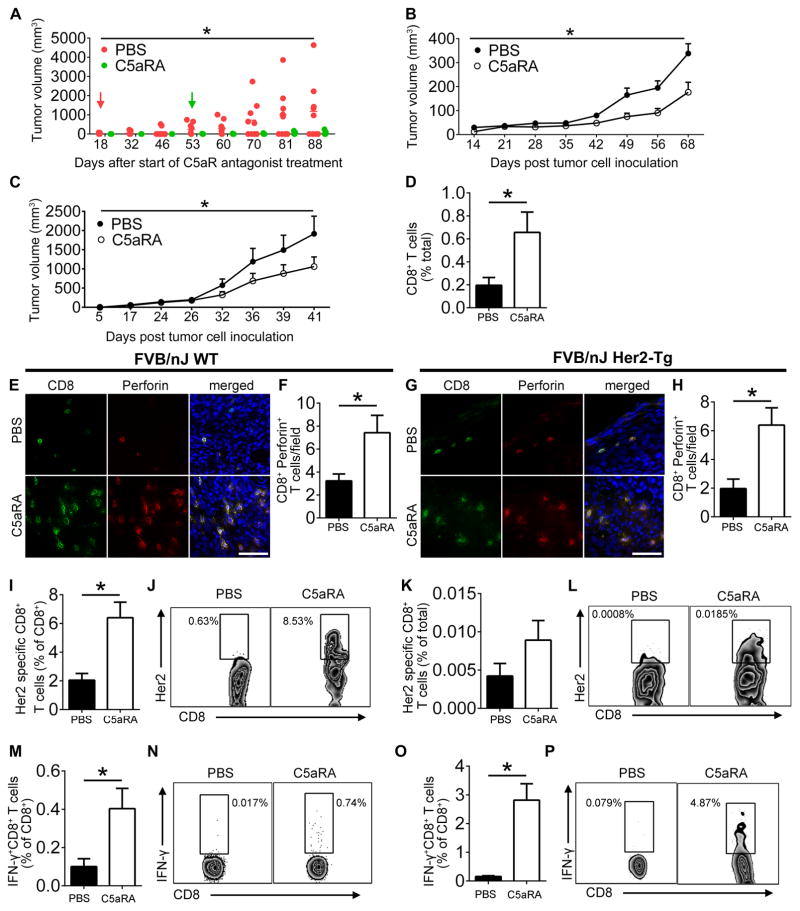
Since hypoxia and associated tumor cell death occur early during tumor development and we found that RPS19 is released from apoptotic tumor cells, we hypothesize that RPS19 may be one of the factors involved in the initiation of tumor associated immunosuppression, which is pivotal for the escape of tumors from immune surveillance (31). To test this hypothesis, we treated FVB/N Her2/neu transgenic mice with C5aR1 inhibitor prior to the development of spontaneous tumors. Focal mammary tumors first appear in these mice at 4 months, with a median incidence at 205 days (https://www.jax.org/strain/002376). Therefore, we used randomized 6 month old female mice without palpable breast tumors for these studies. C5aR1 antagonism delayed the development and reduced the growth of spontaneous tumors in these mice (Fig. 4A).
Figure 4. The pharmacological disruption of RPS19-C5aR1 interaction delays the development and growth of tumors and enhances antitumor immunity.
(A) Volume of spontaneous mammary tumors in Her2/neu transgenic mice treated with C5aR1 antagonist (C5aRA) or vehicle (PBS). Red and green arrows indicate the onset of clinically detectable tumors in vehicle or C5aRA-treated mice, respectively. *P=0.0373 by Two-Way ANOVA. (B) Volumes of NT-5 tumors in FVB wild-type mice that were treated with C5aRA or vehicle, once palpable tumors were present, *P=0.0063 by Two-Way ANOVA. (C) Volumes of NT-5 tumors in transgenic Her2/neu mice treated as in B, *P=0.064 by Two-Way ANOVA. (D) CD8+ T cells in NT-5 tumors of FVB wild-type mice treated as in B by FACS, *P=0.0511, Confidence Interval = −0.9229–0.0029. (E) Tumor CD8+Perforin+T cells from mice shown in B. (F) Quantification of E, *P=0.0419. (G) CD8+Perforin+T cells from mice shown C. (H) Quantification of G, *P=0.0125. (I) Her2-specific CD8+ T cells from NT-5 tumors of FVB wild-type mice treated as in B, *P=0.0014 (J) FACS plots for I. (K) Her2-specific CD8+ T cells from NT-5 tumors of Her2/neu transgenic mice treated as in B. (L) FACS plots for K. (M) IFN-γ producing CD8+ T cells from NT-5 tumors of FVB wild-type mice treated as in B, *P=0.037. (N) FACS plots for M. (O) IFN-γ producing CD8+ T cells from NT-5 tumors of Her2/neu transgenic mice treated as in B, *P=0.0006. (P) FACS plots for O. All statistics for D–P by t-test. Scale bars 50 μm. Data are representative of at least three independent experiments with n=4–10.
To expedite the next studies, FVB wild type mice were injected s.c. with syngeneic NT-5 cells, expressing normal level of RPS19 and not expressing C5aR1 (data not shown) and treated with C5aR1 antagonist when flank tumors were palpable. C5aR1 antagonism reduced tumor growth (Fig. 4B). To determine impact of C5aR1 blockade on tumor growth in mice that are tolerant to tumor-associated antigens (Her2/neu), NT-5 cells, which also overexpress Her2/neu, were injected s.c. to rear flanks of FVB/N Her2/neu transgenic mice prior the development of spontaneous breast tumors. Similar to FVB wild-type mice, C5aR1 antagonism reduced growth of syngeneic tumors in transgenic mice (Fig. 4C), although a rate of tumor growth was more rapid in transgenic mice as a result of poorer immunosurveillance.
The reduced tumor growth in wild-type and transgenic mice treated with C5aR1 inhibitor was associated with the increased infiltration of tumors by cytotoxic CD8+ T cells (Fig. 4D, FACS data from wild-type mice) that expressed high level of perforin, indicating their tumoricidal capacity (Fig. 4E–H). Importantly, tumor (Her2/neu)-specific T cells were increased in tumors from wild-type and transgenic mice treated with C5aR1 antagonist (Fig. 4I–L). In addition, C5aR1 inhibitor improved CD8+ T cell functions, as indicated by the increased production of IFN-γ by these cells stimulated with peptide corresponding to Her2/neu immunodominant epitope (Fig. 4M–P).
Tumor-promoting RPS19 regulates tumor-associated immunosuppression
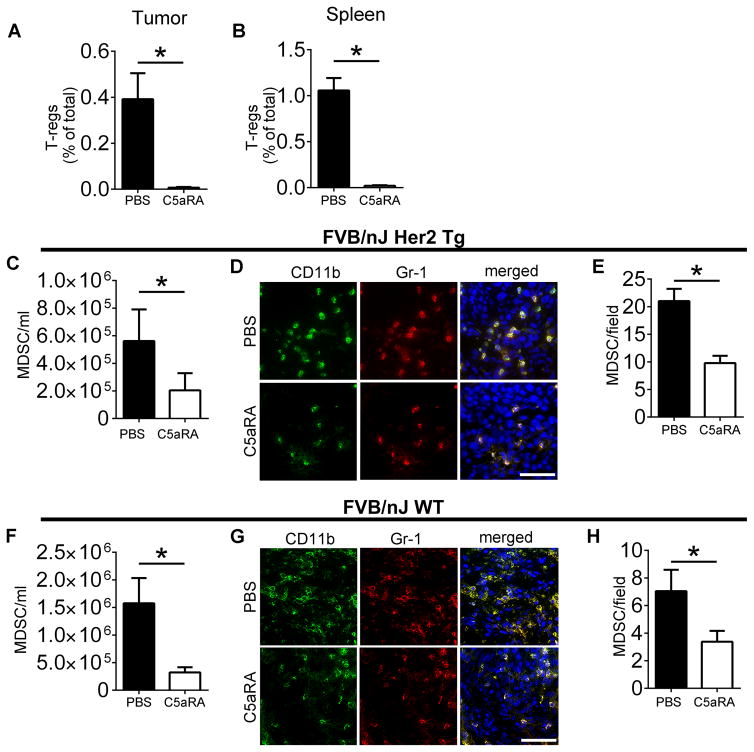
Since the C5aR1/C5a axis is thought to regulate immunosuppression in the tumor microenvironment (7), we hypothesized that RPS19 has similar immunosuppressive functions through its interaction with C5aR1. Therefore, we examined immunosuppressive cells using a similar experimental setting as for Fig. 4, in which C5/C5a are absent. C5aR1 inhibition led to the reduction of T regulatory (Treg) cells in mouse tumors (Fig. 5A) and spleens (Fig. 5B).
Figure 5. The pharmacological disruption of RPS19-C5aR1 interaction reduces Tregs and MDSC in tumors and periphery.
T reg cells in (A) tumors, *P= 0.0052, and (B) spleen, *P<0.0001, of Her2/neu transgenic NT-5 tumor-bearing mice treated with PBS or C5aR1 antagonist (C5aRA). MDSC in blood of NT-5 tumor-bearing (C) Her2/neu, *P=0.04 and (F) FVB wild-type mice, *P=0.0361 treated as in A, B. MDSC (CD11b+GR-1+) in tumors of (D) Her2/neu transgenic and (G) FVB wild-type mice treated as in A, B. Quantification of (E) D, *P=0.0025 and (H) G, *P=0.04. All statistical analyses by t-test. Scale bars 50 μm. Data are representative of at least three independent experiments with n=4–10.
Treg generation is thought to be controlled by MDSC (32) and C5aR1 has been demonstrated to facilitate the recruitment of these cells to tumors (7). In addition, our studies reported here have demonstrated interaction of RPS19 with C5aR1 expressed on human and murine MDSC (Fig. 1 and 2). Therefore, we examined MDSC by FACS and immunofluorescence. Using FACS, we found a reduction in the absolute number of MDSC in peripheral blood of FVB/N Her2/neu transgenic (Fig. 5C) and FVB wild-type mice (Fig. 5F) bearing tumors after treating with C5aR1 inhibitor. FACS failed to demonstrate statistically significant differences in tumor MDSC (data not shown), however, immunofluorescence indicated a reduction in tumor infiltrating MDSC in both FVB/N Her2/neu transgenic (Fig. 5D, E) and FVB wild-type mice (Fig. 5G, H) that were treated with C5aR1 antagonist. This discrepancy between FACS and immunofluorescence studies maybe related to difficulties in obtaining MDSC embedded in tumor stroma into single cell suspension.
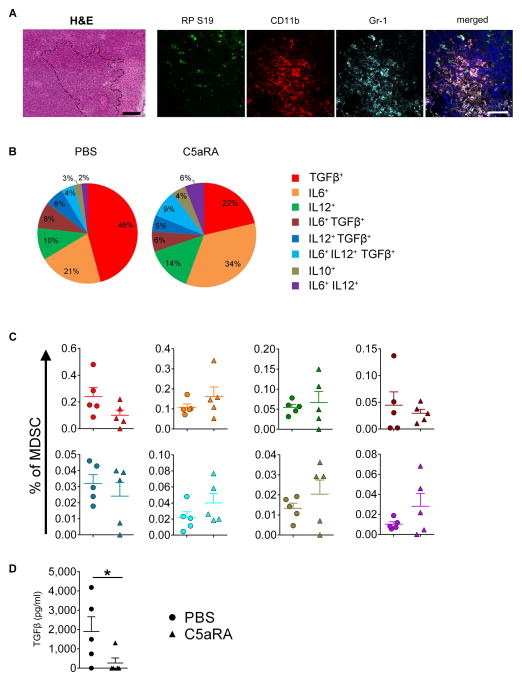
The expansion of Tregs is controlled by several cytokines, such as TGF-β and IL-10, produced by cells of myeloid origin (33). In addition, the crosstalk between macrophages and MDSCs, producing IL-6 and IL-10, which enhance immunosuppressive properties of MDSC and reduce IL-12 production in macrophages, plays an important role in this process (32). Furthermore, TGF-β is considered to be a major immunosuppressive cytokine inhibiting effector T cell responses, except Th17, which is favored by TGF-β in concert with IL-6 (34). Therefore, we examined production of TGF-β1, IL-6, IL-10, or IL-12 individually or in combination in MDSC (CD11b+Gr-1+). We focus on tumor draining lymph nodes (TDLNs), as they have been predicted as preferred sites for inducible (i)Treg generation (35, 36) and it has been suggested that the acquisition of Treg lineage and phenotypes requires antigenic stimulation of naïve T cells in the presence of TGF-β (37, 38). In addition, we found large quantities of extracellular RPS19 colocalized with MDSC (CD11b+Gr-1+) in TDLNs (Fig. 6A). We found that in tumor-bearing PBS-injected control mice the majority of MDSCs, expressing at least one of the measured cytokines (3% of total MDSCs), produced only TGF-β1, with lower fractions of cells producing only IL-6 or IL-12 alone (Fig. 6B). The fractions of multifunctional cells producing more than one of the measured cytokines were low. However, we observed distinct subpopulations of cells that coproduced IL-6 and TFG-β1 or IL-12 and TGF-β1, or the combination of all three IL-6, IL-12 and TGF-β1. Cells producing IL-10 alone or coproducing IL-6 and IL-12 were rare (Fig 6B). Fractions of cells producing all four cytokines (IL-6+ IL-12+ IL-12+ TGF-β1+) were negligible. The well-established immunosuppressive properties of TGF-β, together with the high proportions of cells producing this cytokine alone or in combination with other cytokines, suggests a key role of TGF-β in augmenting immunosuppressive mechanisms in TDLNs. In contrast to the tumor microenvironment, the role of IL-10 in TDLNs, was less significant based on these data. C5aR1 blockade reduced fractions and frequencies of TGF-β1 producing MDCS, including single TGF-β1 producers and cells that coproduced TGF-β1 with IL-12 or IL-6 (Fig 6B, C), although these differences did not reach statistical significance in this experimental setting. However, the significant impact of C5aR1 signaling on TGF-β1 production was confirmed by the reduction of TGF-β1 levels in plasma of tumor-bearing mice treated with C5aR1 inhibitor (Fig. 6D). In contrast, targeting C5aR1 modestly increased fractions and frequencies of cells that produced IL-6 (Fig 6B, C). Although IL-6 in the tumor microenvironment is reported to impart immunosuppression by regulating IL-10 production in MDSCs, other studies have found that IL-6 can counteract TGF-β1 inhibition of CD3 cell activation (39). In addition, IL-6 is a pleiotropic cytokine that has been found to favor priming, generation and survival of antigen-specific CD8+ T cells (40, 41). Thus, we hypothesize that an increase in IL-6 production promotes induction and survival of tumor-specific T-cells upon C5aR1 blockade. Furthermore, with simultaneous decrease in the TGF-β production, IL-6 can inhibit induction of Treg cells and promote other T cell lineages (42).
Figure 6. RPS19-C5aR1 interaction controls the cytokine milieu in TDLN.
(A) H&E of TDLN (dash lines surrounds cells with neutrophil-like MDSC morphology) and immunofluorescent detection of RPS19 in TDLN in proximity of MDSC (CD11b+Gr-1+), scale bars 100 μm for H&E and 50 μm for immunofluorescence. (B) Fractions of MDSC that produced TGF-β1, IL-6, IL-10 and/or IL-12 alone or in various combinations after ex vivo stimulation with LPS obtained from TDLN of control (PBS) and C5aR1 antagonist (C5aRA) NT-5 tumor-bearing Her2/neu transgenic mice. (C) Frequencies of MDSC producing various cytokines as in B. Legend in B applies to both B and C. (D) TGF-β1 levels in plasma of mice as in B, *P<0.0001. All statistics by t-test. Each symbol represents an individual mouse. Horizontal lines represent mean + S.E.M. Data are representative of one experiment with n=5.
Inhibiting C5aR1/RPS19 signaling favors Th1 and Th17
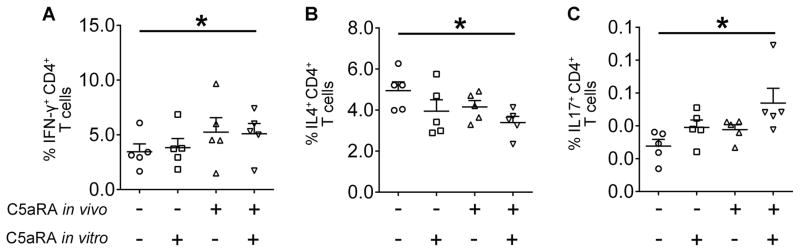
To confirm that C5aR1 signaling in MDSCs can impact the polarization of T cell responses, naïve CD4+ T cells not expressing C5aR1 (43) were isolated from the spleens of tumor-free FVB/N Her2/neu transgenic mice and were stimulated with CD3/CD28 in the presence of MDSCs from control (PBS injected) or C5aR1 inhibitor treated tumor-bearing bearing mice with or without addition of C5aR1 inhibitor to the culture. We found that C5aR1 blockade increased frequencies of CD4+ T cells producing IFN-γ (Fig. 7A) and IL-17 (Fig. 7C), indicating Th1 and Th17 polarization. In contrast, C5aR1 antagonism reduced CD4+ T cells producing IL-4 (Fig. 7B), which suggests a reduction of Th2 responses. These changes in polarization of T cells correspond to alterations in the TGF-β to IL-6 ratio resulting from C5aR1 inhibition and to established functions of these cytokines in regulating adaptive antitumor immune responses (42).
Figure 7. Inhibition of C5aR1 signaling in MDSCs favors Th1 and Th17 responses.
Percentage of CD4+ T cells producing (A) IFN-γ, *P=0.0203 or (B) IL-4, *P=0.0174 or (C) IL-17, *P=0.0296; stimulated with CD3/CD28, and co-cultured in the absence (C5aRA in vitro −) or the presence (C5aRA in vitro +) of C5aR antagonist with MDSCs from tumor-bearing mice injected with PBS (C5aRA in vivo −) or C5aR antagonist (C5aRA in vivo +). All statistics by One-Way ANOVA. Each symbol represents an individual mouse. Horizontal lines represent mean + S.E.M. Data are representative of one experiment with n=5.
Discussion
The complement system and, in particular, C5aR1 have been repeatedly demonstrated to promote tumor growth and immunosuppression in primary tumors (7, 44) and at metastatic sites (14, 45). C5aR1-mediated tumor promoting and immunosuppressive functions have been attributed to the well-characterized C5aR1 ligand C5a (7, 8), which is generated through the proteolytic cleavage of C5 by the C5 convertases and perhaps other serine proteases (46). Therefore, complement-mediated immunosuppression and tumor promoting functions have been unequivocally associated with the activation of the complement cascade and generation of complement effector proteins such as C3a and C5a (47). In fact several studies have demonstrated complement activation leading to generation of C5a in human (47, 48) and experimental mouse malignancies (7). In addition, deficiency in the complement protein C3, which is a pivotal junction, upstream of C5a, for multiple arms of the complement activation cascade, and is required for complement functions, is associated with reduced tumor growth (7, 44). Therefore, it appears that complement activation and complement effectors promote tumor development and play important roles in suppressing antitumor immunity.
This study identifies a novel additional control mechanism linked to complement-mediated regulation of tumor growth and antitumor immunity, as we discovered that C5aR1 is also actively engaged in immunosuppression through its interaction with RPS19, even in the absence of C5 and C5a. Therefore, it appears that complement activation is not mandatory for complement-mediated immunosuppression. Interrupting or altering the RPS19-C5aR1 interaction either through downregulation of RPS19 in tumor cells or pharmacological blockade of C5aR1 by C5aRA reduced this immunosuppression, led to the generation of tumor specific T cell responses and slower tumor growth, pointing to the likely importance of a direct RPS19-C5aR1 interaction for immunosuppression. Furthermore, the delay in the development of clinically overt mammary tumors in C5 deficient FVB/N Her2/neu transgenic mice treated with C5aRA suggests the participation of a C5aR1-RPS19 axis in the initiation of immunosuppression during early stages of tumor development when tumors start to escape immune surveillance (31). RPS19 is an abundant intracellular protein expressed by virtually all cells in the body and its extracellular functions, including interaction with C5aR1, are activated upon its release from dying cells (5). It is possible therefore that the immunosuppressive actions of RPS19 occur not only in tumors but also in other cells undergoing apoptosis. This notion is supported by the accumulation of RPS19 in TDLN, where circulating immunosuppressive cells including MDSC are undergoing apoptosis (Fig. 6A). Since inflammatory cells also undergo rapid apoptosis, it is conceivable that a C5aR1-RPS19 interaction contributes to tumor-promoting properties of chronic inflammatory conditions (49).
Although the pharmacological blockade of C5aR1 in mice on FVB background (lacking C5/C5a) may theoretically block the interaction of C5aR1 with other, as yet unidentified, ligands in addition to blocking C5aR1-RPS19 interactions, the concordance between data from these studies and from those utilizing RPS19 shRNA supports important contributions of RPS19 to suppression of antitumor immunity. The less robust phenotype observed in the studies utilizing shRNA approaches likely results from moderate downregulation of RPS19 in only a portion of the mice in the study. However, significant downregulation of RPS19 does not appear to be feasible, without impacting cell survival and proliferation, given the pivotal role of RPS19 for protein translation.
We report here original findings on a new player RPS19 that interacts with the complement system in the tumor microenvironment and suppresses antitumor immunity. These findings further highlight the importance of the complement receptor C5aR1 in cancer and the need to better understand its interactions with endogenous ligands that can regulate immunosuppression and tumorigenicity. The current study demonstrates the high redundancy of mechanisms involved in tumor-associated immunosuppression and underscores the need to target multiple immunosuppressive mechanisms for successful cancer immunotherapy.
Supplementary Material
Footnotes
Grant Support
This research was supported the Australian NHMRC (456060, 1027369) and ARC (DP150104609, DP160104442, CE140100011) grants to D. F, CPRIT RP 120168 and DOD TS 140010 grants to M. K. and by NIH 1R01CA190209-01A1 grant to M.M.M.
References
- 1.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiura H, Shibuya Y, Matsubara S, Tanase S, Kambara T, Yamamoto T. Monocyte chemotactic factor in rheumatoid arthritis synovial tissue. Probably a cross-linked derivative of S19 ribosomal protein. J Biol Chem. 1996;271:878–882. doi: 10.1074/jbc.271.2.878. [DOI] [PubMed] [Google Scholar]
- 3.Nishiura H, Shibuya Y, Yamamoto T. S19 ribosomal protein cross-linked dimer causes monocyte-predominant infiltration by means of molecular mimicry to complement C5a. Lab Invest. 1998;78:1615–1623. [PubMed] [Google Scholar]
- 4.Nishimura T, Horino K, Nishiura H, Shibuya Y, Hiraoka T, Tanase S, Yamamoto T. Apoptotic cells of an epithelial cell line, AsPC-1, release monocyte chemotactic S19 ribosomal protein dimer. J Biochem. 2001;129:445–454. doi: 10.1093/oxfordjournals.jbchem.a002876. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T. Roles of the ribosomal protein S19 dimer and the C5a receptor in pathophysiological functions of phagocytic leukocytes. Pathol Int. 2007;57:1–11. doi: 10.1111/j.1440-1827.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 6.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 7.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markiewski MM, Lambris JD. Unwelcome complement. Cancer Res. 2009;69:6367–6370. doi: 10.1158/0008-5472.CAN-09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondoh N, Schweinfest CW, Henderson KW, Papas TS. Differential expression of S19 ribosomal protein, laminin-binding protein, and human lymphocyte antigen class I messenger RNAs associated with colon carcinoma progression and differentiation. Cancer Res. 1992;52:791–796. [PubMed] [Google Scholar]
- 10.Chien CC, Tu TC, Huang CJ, Yang SH, Lee CL. Lowly expressed ribosomal protein s19 in the feces of patients with colorectal cancer. ISRN gastroenterology. 2012;2012:394545. doi: 10.5402/2012/394545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safaeian M, Hildesheim A, Gonzalez P, Yu K, Porras C, Li Q, Rodriguez AC, Sherman ME, Schiffman M, Wacholder S, Burk R, Herrero R, Burdette L, Chanock SJ, Wang SS. Single nucleotide polymorphisms in the PRDX3 and RPS19 and risk of HPV persistence and cervical precancer/cancer. PloS one. 2012;7:e33619. doi: 10.1371/journal.pone.0033619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, Ball S, Tchernia G, Klar J, Matsson H, Tentler D, Mohandas N, Carlsson B, Dahl N. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet. 1999;21:169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- 13.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, Muller WJ, Dixon KH, Jaffee EM. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–3576. [PubMed] [Google Scholar]
- 14.Vadrevu SK, Chintala NK, Sharma SK, Sharma P, Cleveland C, Riediger L, Manne S, Fairlie DP, Gorczyca W, Almanza O, Karbowniczek M, Markiewski MM. Complement c5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Res. 2014;74:3454–3465. doi: 10.1158/0008-5472.CAN-14-0157. [DOI] [PubMed] [Google Scholar]
- 15.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J, Iyer A, Suen JY, Seow V, Reid RC, Brown L, Fairlie DP. C5aR and C3aR antagonists each inhibit diet-induced obesity, metabolic dysfunction, and adipocyte and macrophage signaling. FASEB J. 2013;27:822–831. doi: 10.1096/fj.12-220582. [DOI] [PubMed] [Google Scholar]
- 17.Reid RC, Abbenante G, Taylor SM, Fairlie DP. A convergent solution-phase synthesis of the macrocycle Ac-Phe-[Orn-Pro-D-Cha-Trp-Arg], a potent new antiinflammatory drug. J Org Chem. 2003;68:4464–4471. doi: 10.1021/jo034228r. [DOI] [PubMed] [Google Scholar]
- 18.Mastellos D, Prechl J, Laszlo G, Papp K, Olah E, Argyropoulos E, Franchini S, Tudoran R, Markiewski M, Lambris JD, Erdei A. Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol Immunol. 2004;40:1213–1221. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16:369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai MS, Lu H. Crosstalk between c-Myc and ribosome in ribosomal biogenesis and cancer. J Cell Biochem. 2008;105:670–677. doi: 10.1002/jcb.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nature Reviews Cancer. 2003;3:179–192. doi: 10.1038/nrc1015. [DOI] [PubMed] [Google Scholar]
- 22.Niculescu F, Rus HG, Retegan M, Vlaicu R. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992;140:1039–1043. [PMC free article] [PubMed] [Google Scholar]
- 23.Fishelson Z, Attali G, Mevorach D. Complement and apoptosis. Mol Immunol. 2001;38:207–219. doi: 10.1016/s0161-5890(01)00055-4. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Paterson Y. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol Immunother. 2007;56:927–938. doi: 10.1007/s00262-006-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 27.Horino K, Nishiura H, Ohsako T, Shibuya Y, Hiraoka T, Kitamura N, Yamamoto T. A monocyte chemotactic factor, S19 ribosomal protein dimer, in phagocytic clearance of apoptotic cells. Lab Invest. 1998;78:603–617. [PubMed] [Google Scholar]
- 28.Naik N, Giannini E, Brouchon L, Boulay F. Internalization and recycling of the C5a anaphylatoxin receptor: evidence that the agonist-mediated internalization is modulated by phosphorylation of the C-terminal domain. J Cell Sci. 1997;110(Pt 19):2381–2390. doi: 10.1242/jcs.110.19.2381. [DOI] [PubMed] [Google Scholar]
- 29.Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, Jaffee EM. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003;170:4273–4280. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 30.Qi L, Rojas JM, Ostrand-Rosenberg S. Tumor cells present MHC class II-restricted nuclear and mitochondrial antigens and are the predominant antigen presenting cells in vivo. J Immunol. 2000;165:5451–5461. doi: 10.4049/jimmunol.165.10.5451. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 32.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–281. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 34.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 35.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 36.Fransen MF, Arens R, Melief CJ. Local targets for immune therapy to cancer: tumor draining lymph nodes and tumor microenvironment. Int J Cancer. 2013;132:1971–1976. doi: 10.1002/ijc.27755. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao YW, Liao KW, Hung SW, Chu RM. Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity. J Immunol. 2004;172:1508–1514. doi: 10.4049/jimmunol.172.3.1508. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 41.Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J Exp Med. 2000;191:915–926. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.Karsten CM, Laumonnier Y, Eurich B, Ender F, Broker K, Roy S, Czabanska A, Vollbrandt T, Figge J, Kohl J. Monitoring and cell-specific deletion of C5aR1 using a novel floxed GFP-C5aR1 reporter knock-in mouse. J Immunol. 2015;194:1841–1855. doi: 10.4049/jimmunol.1401401. [DOI] [PubMed] [Google Scholar]
- 44.Nunez-Cruz S, Gimotty PA, Guerra MW, Connolly DC, Wu YQ, DeAngelis RA, Lambris JD, Coukos G, Scholler N. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14:994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma SK, Chintala NK, Vadrevu SK, Patel J, Karbowniczek M, Markiewski MM. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194:5529–5538. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 46.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pio R, Ajona D, Lambris JD. Complement inhibition in cancer therapy. Semin Immunol. 2013;25:54–64. doi: 10.1016/j.smim.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markiewski MM, Lambris JD. Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends in immunology. 2009;30:286–292. doi: 10.1016/j.it.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.