Abstract
Introduction
Physical activity (PA) has been linked to a lower risk of developing and dying of cancer, yet many cancer survivors do not exercise. We evaluated the impact of the LIVESTRONG at the YMCA exercise program, available at YMCAs across the United States, on PA, fitness, quality of life (QOL), fatigue, body composition, serum biomarkers, and program safety in cancer survivors.
Methods
Cancer survivors were recruited through the Yale Cancer Center and the Dana-Farber Cancer Institute and randomized to a 12-week twice-weekly LIVESTRONG at the YMCA exercise program at YMCAs in CT or MA or to a control group. Questionnaires, DEXA scans, 6-Minute Walk Tests (6MWT) and a fasting blood draw were completed at baseline and 12-weeks. Intervention effects were evaluated using mixed model repeated measures analysis, with change at 12-weeks in PA and 6MWT as primary endpoints.
Results
186 participants were randomized (95 to exercise and 91 to control). The majority of patients were diagnosed with stage I–II cancer and 53% had breast cancer. Participants randomized to the LIVESTRONG at the YMCA program experienced increases in PA (71% exercising at 150+ minutes/week vs. 26% of controls, p < .05), and improvements in the 6MWT (group difference: 28.9 meters (95% CI: 0.3–49.0), p=.004) and QOL (group difference: 2.6 (95% CI: 0.1–5.0), p=.04). No adverse events were reported.
Conclusions
This program has the potential to impact thousands of survivors across the YMCA network and could lead to improvement in disease and psychosocial outcomes in the growing population of cancer survivors.
Keywords: physical activity, obesity, fatigue, quality of life, cancer
INTRODUCTION
Physical activity (PA) has been linked to a lower risk of both developing and dying of cancer (1–4). Studies show that people who are physically active are 20–40% less likely to develop cancers of the breast, prostate and colon, and growing research suggests that the risk of cancer-related death could be up to 50% lower in individuals who exercise regularly after a cancer diagnosis (1). Although there are no randomized trials testing the impact of increased PA upon the risk of cancer recurrence or mortality, many studies have evaluated the benefits of exercise interventions in cancer survivors, showing that exercise improves quality of life, fatigue, fitness, body composition, mood, and biomarkers linked to cancer risk and outcomes (1,5–8).
Given the benefits of exercise in individuals with cancer, guidelines from the American Cancer Society (ACS) and the American College of Sports Medicine (ACSM) recommend that cancer survivors engage in 150 minutes/week of moderate-intensity aerobic exercise (or 75 minutes/week of vigorous-intensity aerobic exercise) and two strength training sessions/week, as well as decreased sedentary time (5,6). Despite these recommendations, fewer than 30% of individuals diagnosed with cancer engage in recommended levels of PA (9,10). In order to provide an opportunity for cancer survivors to experience the many benefits of exercise after a cancer diagnosis, programs are needed to help cancer survivors exercise in a safe setting that provides support to meet the unique needs of cancer survivors.
Although numerous studies have been conducted to date, the vast majority of trials have involved supervised exercise programs administered at a health care facility, typically enrolling fewer than 100 patients. Although these studies have generally reported significant increases in PA as a result of program participation, this model is difficult to scale for the large population of cancer survivors currently living in the USA, especially to those who live in less populated rural areas, without easy access to a health care facility.
Currently, 80% of U.S. households are within 5 miles of a YMCA; the YMCA serves more than 22 million members each year in more than 10,000 communities (11). This broad network provides the unique ability to disseminate healthy lifestyle programs across the Unites States. In 2008, the LIVESTRONG Foundation partnered with YMCA-USA to develop and offer safe and effective PA options for those living with, through and beyond cancer (11). The LIVESTRONG at the YMCA exercise program is a 12-week, small group-based exercise program comprised of twice-weekly supervised exercise sessions led by a YMCA trainer. Participating YMCAs must demonstrate their capacity and willingness to develop and sustain LIVESTRONG at the YMCA, offering the program at little or no cost to participants.
The LIVESTRONG at the YMCA exercise program is currently offered in 478 (18%) YMCA branches across the country and has served more than 37,000 cancer survivors since its creation in 2008. However, the safety and effectiveness of this program has yet to be tested. Thus, the purpose of our study was to provide a comprehensive evaluation of the physical and psychological impact, as well as the safety, of the LIVESTRONG at the YMCA exercise program for cancer survivors, to determine if the impact of this community-based program was similar to the benefits seen in increasing PA, fitness and quality of life in hospital-based exercise studies in cancer survivors.
METHODS
Participants and Recruitment
Eligibility criteria for our study were similar to the eligibility requirements of the LIVESTRONG at the YMCA exercise program, i.e., individuals with a prior cancer diagnosis who received medical clearance from a health care provider to participate in the program. Other study-related eligibility criteria included: ability to complete forms and understand instructions in English, willingness to be randomized and to attend an exercise program at a participating YMCA, and to attend baseline and 3-month clinic visits.
Cancer survivors were recruited between April 2013 and October 2014 from Smilow Cancer Hospital at Yale (New Haven, CT) and Dana-Farber Cancer Institute (DFCI, Boston, MA) using multiple strategies including review of patient lists and tumor registries. Interested subjects were screened by study staff either in person or by phone and if eligible, they were then scheduled for a baseline visit.
After baseline data collection, participants were randomized to immediate participation in the LIVESTRONG at the YMCA exercise program or to a wait-list control group using a random permuted block design of varying block size in a 1:1 ratio. Patients were stratified on study site and current use of chemotherapy (yes vs. no). The Yale School of Medicine Human Investigation Committee and Dana-Farber/Harvard Cancer Center Institutional Review Board approved all procedures, including written informed consent.
LIVESTRONG at the YMCA Exercise Program
Participants were randomized to the LIVESTRONG at the YMCA exercise program at one of the participating YMCAs in CT or MA (11). Study participants were incorporated into standard, ongoing regular LIVESTRONG at the YMCA exercise program sessions with sessions led by two YMCA fitness instructors, trained in the basics of cancer etiology, treatment and survivorship. Trainers worked with each participant to fit the program to their individual needs based on the participant’s age, cancer type, and any comorbidities, with types of exercise and progression of duration or intensity modified accordingly. Two sessions were held each week, and each session was 90 minutes in duration. Sessions followed the recommendations of the American College of Sports Medicine (6) for cancer survivors and began with a warm-up, followed by aerobic exercise, resistance training exercises, and a cool-down period. The trainers recorded participant attendance, and participants recorded their exercise during the sessions as well as any exercise done outside of the sessions in a physical activity log book provided by the study.
Wait-List Control Group
Participants randomized to the wait-list control group were able to start the LIVESTRONG at the YMCA exercise program after their 3-month visit. They were allowed to exercise as much as they wished during the waiting period, but were not given instructions or support regarding an exercise program during this time.
Outcome and Covariate Measures
The following measures were collected from study participants during clinic visits at Yale or DFCI at baseline and 3-months.
Demographics and Medical History
Self-report questionnaires were used to determine cancer type, date of diagnosis, disease stage, surgery, radiotherapy, chemotherapy and endocrine therapy.
Weight/Height
Participants were weighed and measured in light indoor clothing, without shoes, rounding up to the nearest 0.1 kg for weight and to the nearest 0.1 cm for height. All measurements were performed and recorded twice in succession, and averaged for analyses.
Physical Activity
Participants completed an interview-administered physical activity questionnaire, assessing the past 3 months of physical activity including the type, frequency, and duration of 20 activities (12).
Six Minute Walk Test (6MWT)
The 6MWT is considered a valid measure of cardiorespiratory fitness, with a r=0.67 between the 6MWT and VO2peak (13). Participants wore walking shoes and walked indoors along a long, flat, straight, enclosed corridor, on a flat surface for 6 minutes (13). They were instructed to walk as fast as they could continuously along an area at least 15 meters in length, back and forth, for 6 minutes.
Quality of Life and Fatigue
Quality of life was measured using the Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire version 3 (Cronbach alpha coefficient = 0.89) (14), and fatigue was assessed through the FACT-Fatigue Scale (test-retest r ranging from 0.84–0.90) (15). Both measures have been found to be valid instruments for use in cancer survivors (14,15).
Dual Energy X-Ray Absorptiometry (DEXA) Scans
DEXA scans were performed to assess body fat, lean body mass (LBM) and total bone mineral density (BMD) with a Hologic Discovery scanner version 3.3.0.1 (CV = 1.07). All scans were evaluated by a Radiologic Technician Certified in Bone Density blinded to randomization group.
Blood Draw and Serum Biomarkers
Blood was collected after a 12 hour fast and was stored at −80°C until assayed. Upon completion of all data collection, DFCI shipped their frozen samples to Yale, where all blood analyses were conducted. Serum concentrations of insulin were measured using radioimmunoassay kits (EMD Millipore, Catalog HI-14K); and C-Reactive Protein (CRP) was measured using an automated chemistry analyzer (Alfa Wassermann Alera analyzer; Raichem Cliniqa hsCRP Wide Range Reagent Catalogue number 85550). Baseline and 3-month specimens were assayed simultaneously, and participants from both randomization groups were included in each batch of assays. Samples were measured in duplicate with coefficients of variation for all samples under 10%. Laboratory technicians were blinded to treatment assignment.
Safety Evaluation/Injury Questionnaire
Study participants completed a safety evaluation/injury questionnaire at 3 months, which was reviewed in person at the 3-month clinic visit. The questionnaire asked about any falls, muscle or joint injuries, or other injuries, and whether they were a result of the exercise program or not.
Norman Lymphedema Questionnaire
Breast cancer survivors completed the validated Norman Lymphedema Questionnaire (16).
Statistical Analyses
The planned sample size was 200 participants, providing an evaluable sample of 90 participants per group, assuming a 10% attrition rate. This sample size would provide 80% power to detect a difference of 105 minutes/week of exercise between groups with type 1 error at 0.05, based on a priori estimate of baseline exercise of 2.0 ± 4.0 hr/week. Participants were grouped according to the intention-to-treat principle. Intervention effects were evaluated by the differences in the mean baseline to 3-month changes between the intervention and control groups using the mixed model repeated measure analysis to account for the longitudinal nature of the data and the ability to use all available data to provide a robust estimate based on the assumption of missing at random. We also adjusted for study site (Yale or DFCI) and baseline value of the outcome of interest, and explored a priori effect modification by gender, cancer site, currently receiving chemotherapy (yes/no), baseline physical activity level, body mass index (BMI), and time from diagnosis. Also for exercisers only, we examined changes in these endpoints based upon attendance to the LIVESTRONG at the YMCA exercise program. Analyses were performed using SAS version 9.4 (Cary, NC). Statistical significance was set at p < 0.05 using 2-sided tests.
RESULTS
Baseline Characteristics
A total of 186 men and women diagnosed with cancer were enrolled into the study (Figure 1). Baseline characteristics were similar for both groups (Table 1). Participants were, on average, 59.3 ± 10.4 years old, 80% non-Hispanic white, 3.8 ± 3.1 years from diagnosis, with a BMI of 29.0 ± 5.7 kg/m2, with 53% diagnosed with breast cancer.
Figure 1.
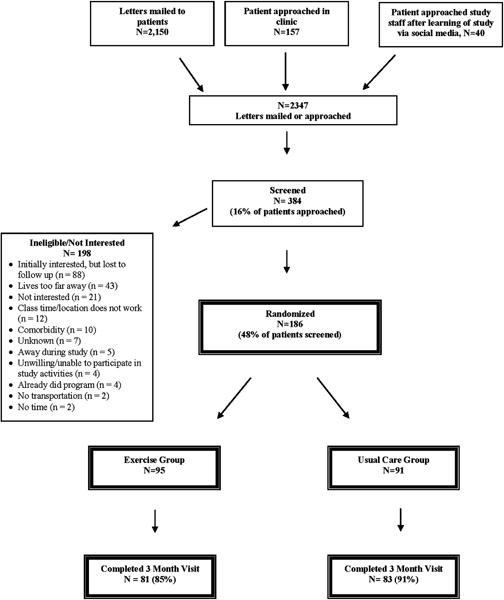
Flow of participants through the LIVESTRONG at the YMCA Study
Table 1.
Baseline characteristics of randomized participants in the LIVESTRONG Study (N=186).
| Exercisers Mean (SD) or % |
Controls Mean (SD) or % |
p-value | |
|---|---|---|---|
| N | 95 | 91 | |
| Site | 0.98 | ||
| Yale | 54% | 54% | |
| Dana-Farber | 46% | 46% | |
| Gender (% Female) | 70% | 81% | 0.06 |
| Age (years) | 58.7 (11.2) | 59.9 (9.5) | 0.42 |
| Education (%) | 0.45 | ||
| High School | 10% | 12% | |
| College + | 90% | 88% | |
| Race (%) | 0.82 | ||
| White | 87% | 84% | |
| Black | 11% | 12% | |
| Asian | 1% | 2% | |
| Unknown | 1% | 2% | |
| Ethnicity (%) | 0.40 | ||
| Non-Hispanic | 92% | 96% | |
| Hispanic | 4% | 1% | |
| Unknown | 4% | 3% | |
| Time Since Diagnosis (years) | 3.8 (2.8) | 4.7 (3.4) | 0.07 |
| Cancer Type (%) | 0.14 | ||
| Breast | 52% | 55% | |
| Colon | 13% | 7% | |
| Rectum | 0% | 2% | |
| Lung | 4% | 5% | |
| Endometrial | 3% | 8% | |
| Prostate | 9% | 9% | |
| Lymphoma | 14% | 4% | |
| Other | 5% | 10% | |
| Self-Reported Disease Stage (%) | 0.84 | ||
| Stage I | 35% | 30% | |
| Stage II | 23% | 29% | |
| Stage III | 17% | 14% | |
| Stage IV | 4% | 3% | |
| Don’t Know | 21 | 24 | |
| Adjuvant Therapy (%) | 0.79 | ||
| Radiotherapy | |||
| No Radiotherapy | 45% | 44% | |
| Currently Receiving Radiotherapy | 1% | 1% | |
| Completed Radiotherapy | 54% | 55% | |
| Chemotherapy | |||
| No Chemotherapy | 43% | 42% | |
| Currently Receiving Chemotherapy | 5% | 5% | |
| Completed Chemotherapy | 52% | 53% |
A total of 164 participants of the 186 enrolled completed the study (88%). Study site (3% dropouts from Yale and 22% dropouts from DFCI, p < .0001) and race (9% White and 29% Black dropped out of the study, p =.04) were different between participants who dropped out of the study vs. those who completed the study.
Changes in Physical Activity and Fitness
A majority of participants were inactive at baseline, with 34% reporting at least the recommended level of 150 minutes/week of PA (Figure 2). Participants randomized to the LIVESTRONG at the YMCA program who began the exercise program (N = 81) attended on average 83% of the 24 sessions over the 12-week program. At the end of the intervention period, 71% of LIVESTRONG at the YMCA participants reported at least 150 minutes of exercise per week vs. 26% of controls (p < .05) (Figure 2). Intervention participants increased their exercise by an average 127.0 minutes/week compared to a decrease of 5.8 minutes/week in the control group (p = 0.0001; Table 2). Participation in the intervention increased exercise both in participants who were more and less active at baseline (Table 2).
Figure 2.
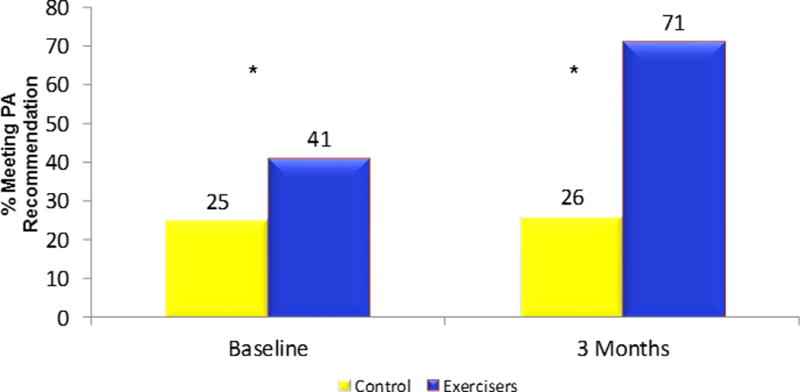
Percent meeting physical activity recommendations at baseline and 3 months
*p<.05
Table 2.
Effect of the exercise program vs. usual care on study endpoints, adjusted for study site and baseline value, mean (95% CI).
| Outcomes | Month | Exercise | Control | p-value |
|---|---|---|---|---|
| Physical Activity (min/wk) | ||||
| Overall (N=188) | ||||
| Baseline | 140.2 (107.0, 173.3) | 108.5 (77.7, 139.3) | 0.18 | |
| Change at 3 months | +127.0 (93.0, 160.0) | −5.8 (−39.0, 27.3) | < .0001 | |
| Subgroup: Baseline Physical Activity < 150 min/wk, N = 124 | ||||
| Baseline | 33.5 (20.7, 46.2) | 35.0 (23.0, 47.1) | 0.86 | |
| Change at 3 months | +165.2 (121.6, 208.8) | +23.9 (−13.9, 61.7) | <0.0001 | |
| Subgroup: Baseline Physical Activity ≥ 150 min/wk, N = 62 | ||||
| Baseline | 293.4 (246.1, 340.7) | 325.8 (271.3, 380.3) | 0.38 | |
| Change at 3 months | +43.2 (−6.2, 92.7) | −54.4 (−116.8, 8.0) | 0.01 | |
| Six Minute Walk Test (6MWT) (meters) | ||||
| Baseline | 485 (469, 501) | 481 (459, 503) | 0.75 | |
| Change at 3 months | +33.8 (19.6, 47.9) | +4.9 (−9.1, 18.8) | 0.004 | |
| YMCA Sessions | 20.1 (3.3) | |||
| FACT-General | ||||
| Baseline | 90.4 (87.5, 93.4) | 88.3 (85.7, 91.0) | 0.29 | |
| Change at 3 months | +1.6 (−0.2, 3.3) | −1.0 (−2.8, 0.8) | 0.039 | |
| FACT-Fatigue | ||||
| Baseline | 42.0 (40.0, 43.9) | 40.4 (38.1, 42.5) | 0.38 | |
| Change at 3 months | +1.6 (0.1, 3.0) | +0.8 (−0.7, 2.2) | 0.42 | |
| BMI (kg/m2) | ||||
| Baseline | 28.9 (27.7, 30.0) | 29.2 (28.0, 30.5) | 0.66 | |
| Change at 3 months | −0.1 (−0.3, 0.1) | −0.1 (−0.3, 0.1) | 0.84 | |
| % Body Fat | ||||
| Baseline | 39.1 (37.4, 40.9) | 40.2 (38.4, 42.0) | 0.39 | |
| Change at 3 months | −0.3 (−0.8, 0.2) | −0.2 (−0.7, 0.4) | 0.66 | |
| Lean Mass (kg) | ||||
| Baseline | 44.25 (42.06, 46.45) | 43.87 (41.72, 46.03) | 0.81 | |
| Change at 3 months | −0.282 (0, 0.454) | +0.015 (−0.745, 0.775) | 0.58 | |
| Bone Mineral Density (g/cm2) | ||||
| Baseline | 1.171 (1.141, 1.201) | 1.163 (1.128, 1.199) | 0.73 | |
| Change at 3 months | +0.009 (0.003, 0.016) | +0.001 (−0.005, 0.008) | 0.09 | |
| Insulin (uU/mL) | ||||
| Baseline | 15.87 (12.71, 19.03) | 15.19 (13.37, 17.00) | 0.71 | |
| Change at 3 months | −0.38 (−1.90, 1.14) | −0.90 (−2.41, 0.62) | 0.62 | |
| CRP (mg/L) | ||||
| Baseline | 4.53 (3.18, 5.87) | 5.49 (3.56, 7.42) | 0.42 | |
| Change at 3 months | −1.36 (−2.41, −0.31) | −1.27 (−2.32, −0.23) | 0.88 |
Participation in the exercise program also led to significant improvements in fitness as measured by the 6MWT. Intervention participants increased the distance that they could walk in 6 minutes by 33.8 meters vs. 4.9 meters in the control group, p = .004. Additionally, improvements in fitness as a result of the exercise program were seen in both participants who were inactive and active at baseline, with participants who engaged in less than 150 minutes of exercise/week at baseline increasing their distance by 27.7 meters vs. 3.4 meters in controls (p < .05), and among those exercising more than 150 minutes of exercise/week at baseline increasing their distance by 39.6 meters vs. 10.1 meters in controls (p <.05).
Changes in Quality of Life and Fatigue
The exercise program improved QOL, assessed via the FACT-G, by 1.6 units vs. a decrease in quality of life of 1.0 units in the control group, p = .039 (Table 2). Although there was no significant impact of the program on fatigue in the population as a whole, among participants diagnosed with cancer within the past 3.5 years (median cutpoint), cancer-related fatigue improved by 7.5% in exercisers vs. a slight worsening in controls, p = .03 (Table 3). A dose-response effect of higher attendance to the exercise program was also associated with greater and statistically significant improvements in quality of life and fatigue (Figure 3). Baseline characteristics including gender, cancer site (breast cancer vs. other) and currently receiving chemotherapy (yes/no) did not modify the effect of exercise on quality of life and fatigue.
Table 3.
Changes in FACT-General and FACT-F scores by time since diagnosis, adjusted for study site and baseline value of the outcome variable.
| Outcome | Month | Exercise | Control | Exercise Effect | P-value |
|---|---|---|---|---|---|
| FACT-General | |||||
| < 3.6 Years | |||||
| N | 54 | 39 | |||
| Baseline | 90.9 (87.0, 94.8) |
86.2 (82.2, 90.1) |
0.10 | ||
| Change at 3 months | +2.3 (−0.2, 4.6) |
−1.8 (−4.7, 1.0) |
4.1 (0.5, 7.7) |
0.03 | |
| ≥ 3.6 Years | |||||
| N | 40 | 50 | |||
| Baseline | 89.9 (85.2, 94.6) |
90.0 (86.3, 93.6) |
0.99 | ||
| Change at 3 months | +0.7 (−1.9, 3.4) |
−0.5 (−2.8, 1.8) |
1.2 (−2.2, 4.6) |
0.48 | |
| Difference (Effect modification) |
2.9 (−2.1, 7.8) |
0.26 | |||
|
| |||||
| FACT-Fatigue | |||||
| < 3.6 Years | |||||
| N | 53 | 37 | |||
| Baseline | 41.4 (38.5, 44.3) |
38.6 (34.6, 45.2) |
0.24 | ||
| Change at 3 months | 3.1 (1.1, 5.0) |
−0.4 (−2.3, 2.2) |
3.1 (0.26, 6.0) |
0.03 | |
| ≥ 3.6 Years | |||||
| N | 41 | 50 | |||
| Baseline | 42.7 (40.0, 45.3) |
41.5 (39.0, 44.1) |
0.54 | ||
| Change at 3 months | −0.1 (−2.3, 2.0) |
1.2 (−0.6, 3.1) |
−1.4 (−4.0, 1.3) |
0.32 | |
| Difference (Effect modification) |
4.5 (0.54,8.4) |
0.03 | |||
Figure 3.
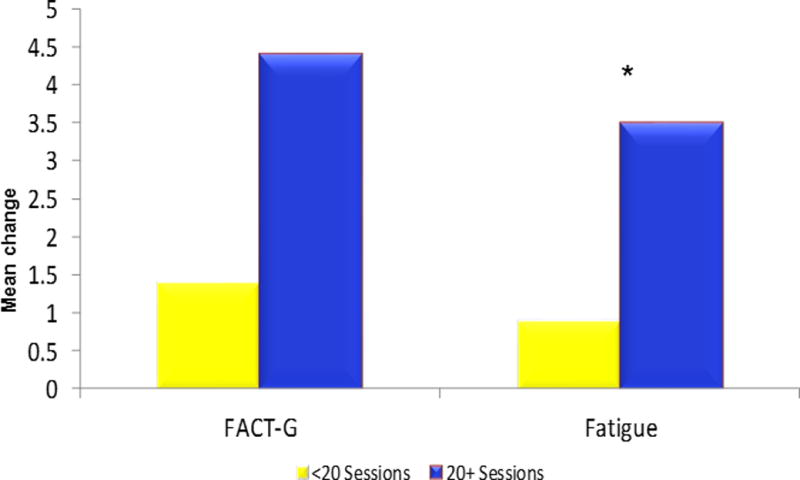
Change in FACT-G and Fatigue from baseline to 3 months by attendance to LIVESTRONG at the YMCA Sessions
*p<0.05
**N=27 completed < 20 sessions; N=54 completed ≥ 20 sessions
Changes in Body Composition and Serum Biomarkers
No between group differences were observed for baseline to 3-month changes in body composition, insulin or CRP levels (Table 2). While baseline PA levels did not modify the effect of exercise on body composition or serum biomarkers, more favorable changes in body weight, % body fat, LBM, insulin and CRP were observed among participants randomized to the LIVESTRONG at the YMCA exercise program who had baseline PA levels > 150 min/wk compared with participants randomized to control who were active or inactive at baseline, as well as participants randomized to the LIVESTRONG at the YMCA exercise program who were inactive at baseline.
Safety Evaluation
No injuries were reported over the duration of the study based on an interview-administered survey of musculoskeletal injuries, falls or general injuries. In the subgroup of patients with breast cancer, 20 of 99 participants (10 of 49 in the exercise group and 10 of 50 in usual care group) reported upper body lymphedema at baseline. At 3 months, among the exercise group, three of the 10 women reported an improvement in lymphedema, with the other seven women reporting no worsening of symptoms. Among women randomized to control, five of the 10 women reported an improvement in lymphedema, with the other five women reporting no worsening of symptoms. Among women without lymphedema at baseline, two women in the control group and none in the exercise group reported lymphedema at 3 months.
DISCUSSION
The LIVESTRONG at the YMCA program was effective in improving PA, fitness, QOL and cancer-related fatigue in a mixed group of cancer patients, including long-term cancer survivors, newly-diagnosed patients receiving adjuvant therapy, and individuals living with advanced disease. Benefits were seen both in patients who were more and less active at baseline. Interest in study participation was high, with 186 participants enrolled within 15 months from two centers. Forty-eight percent of survivors who were screened for the study were ultimately randomized, a rate higher than that seen in many health care facility-based exercise studies in healthy adults and cancer survivors (17,18).
Previous trials have shown a benefit of exercise in cancer survivors, including improvements in quality of life, fatigue, fitness, body composition and biomarkers linked to cancer risk and outcomes (6–8). Our findings of an average 33 m or 6.7% increase in fitness, assessed via the 6MWT, is considered clinically meaningful (19). In a recently published meta-analysis of 16 exercise trials in cancer survivors (8), exercise significantly improved QOL in comparison to usual care in only eight trials (50%), with a mean between-group difference of 5.55, 95% CI (3.19 to 7.90), p<0.001. Possible reasons for the greater improvement in QOL in those eight exercise trials as compared with the improvement in the LIVESTRONG at the YMCA exercise program is because of differences in frequency of exercise sessions. In the meta-analysis, exercising more frequently than two times a week was associated with greater benefit in QOL and fatigue. Timing of the intervention was also important, as exercise improved QOL and fatigue significantly more often in studies during cancer treatment, as compared to studies post-treatment. This is most probably explainable by the negative impact of radiotherapy and chemotherapy on fatigue and the positive effect of exercise on this factor. We also observed a greater benefit of exercise on QOL and fatigue among participants in the LIVESTRONG at the YMCA exercise program who recently completed adjuvant treatment and were diagnosed in the past 3.5 years compared with participants diagnosed greater than 3.5 years and therefore further out from completing adjuvant treatment. We found a clinically meaningful 4.1 and 3.5 between-group difference in QOL and fatigue, respectively, among participants diagnosed within the past 3.5 years compared with a 1.2 and 1.3 between-group difference in QOL and fatigue, respectively among participants diagnosed greater than 3.5 years.
Although previous studies provide an important rationale for the inclusion of exercise as an important part of the care of cancer survivors, they do not provide much information regarding effective means of implementing exercise programs in large groups of survivors in the community. The LIVESTRONG at the YMCA exercise program provides significant benefits for cancer survivors through a community-based exercise program that is available in hundreds of communities across the Unites States, providing a novel mechanism to disseminate high-quality exercise programing to a diverse group of cancer survivors.
Our study also evaluated the safety of the LIVESTRONG at the YMCA exercise program. There has been some controversy regarding the optimal setting in which a cancer patient or survivor should initiate an exercise program. The American College of Sports Medicine exercise guidelines do not recommend a medical assessment for cancer survivors prior to initiating an exercise program due to the increased barriers this may pose to beginning an exercise program, as well as the high benefit/low risk of exercising (6). However, the LIVESTRONG at the YMCA exercise program requires physician consent prior to beginning their program in an attempt to further minimize any risks associated with exercise. In our study, no injuries were reported in either group. Additionally, more than 50% of our study participants were women with breast cancer, and there were no additional cases of lymphedema in patients without this condition at baseline or new cases of lymphedema in women who already had this condition as a result of participating in the LIVESTRONG at the YMCA exercise program. Similar to the LIVESTRONG at the YMCA exercise program, none of the 16 exercise trials included in the recent meta-analysis (8) reported adverse effects of exercise.
A number of weaknesses of our study should be noted. Our study population was comprised primarily of non-Hispanic white, highly educated breast cancer survivors. We also focused on the immediate impact of the intervention upon PA and other outcomes, and do not have longer-term data regarding the maintenance of changes in physical activity and other measures over time. Lastly, there was no effect of the LIVESTRONG at the YMCA exercise program on body composition or serum biomarkers. Our study enrolled both physically active and inactive participants, with a range of BMI levels and different cancer types, which increases the generalizability of the study findings, yet may confound our ability to determine an effect of exercise on body composition, insulin and CRP. Future studies are needed to evaluate the dose-response effect of the LIVESTRONG at the YMCA program and whether additional sessions per week or higher intensity of exercise is necessary. Additional studies evaluating maintenance of physical activity over time, and to how to improve attendance to the exercise sessions and associated predictors of attendance. Lastly, it is important to examine the effectiveness of the LIVESTRONG at the YMCA exercise program by geography, race/ethnicity and socio-economic status.
Evaluation of other YMCA chronic disease prevention programs may also assist in future evaluation and effectiveness of the LIVESTRONG at the YMCA program. Since 2005, YMCA has worked to become a leading provider of chronic disease prevention programs. In 2010, Y-USA began working with the Diabetes Prevention and Control Alliance to spread and sustain the YMCA’s Diabetes Prevention Program (DPP) through claims-based reimbursements from insurers and employers. Y-USA then worked closely with the American Medical Association to obtain a Current Procedural Terminology code for diabetes prevention programming and helped the local YMCAs secure national provider IDs that would allow them to bill health plans for program delivery. In March 2016, the U.S Department of Health and Human Services announced that a successful four-year demonstration project that served nearly 8,000 Medicare beneficiaries with the YMCA’s DPP produced significant cost savings for Medicare, thus creating a path for Medicare coverage of the program in 2018. Building on these successes, Y-USA is charting a course to achieve similar successes with LIVESTRONG at the YMCA by establishing stronger clinical partnerships, pursuing commercial payors to ensure program accessibility and sustainability, and exploring how YMCAs (and other community-based organizations) can help clinical teams produce health improvements and cost savings under alternative payment models like the oncology care model.
In summary, our findings provide important data regarding the physical and psychological benefits, as well as the safety, of the LIVESTRONG at the YMCA exercise program in cancer survivors, and may support the continued implementation of the LIVESTRONG at the YMCA program in YMCA facilities across the country. Given that increasing PA carries a tremendous potential to improve both the length and quality of survival, and prevent or control comorbidities and side effects associated with cancer or its treatment, the LIVESTRONG at the YMCA exercise program is an attractive option for cancer survivors throughout the Unites States and can serve as model for the development of exercise programs for cancer survivors around the world.
Acknowledgments
Funding/Support: Supported by the LIVESTRONG Foundation and in part by a grant from the Breast Cancer Research Foundation. Also supported in part by the Yale Cancer Center Support Grant (CCSG) P30 CA016359 and the CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH).
Role of the Funder/Sponsor: The LIVESTRONG Foundation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT02112149
Author contributions: Drs. Irwin and Ligibel had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and Design: Irwin, Ligibel
Acquisition, analysis or interpretation of data: Irwin, Cartmel, Harrigan, Li, Shockro, O’Connor, Campbell, Ligibel
Drafting of the manuscript: Irwin, Li, Ligibel
Critical revision of the manuscript for important intellectual content: Irwin, Cartmel, Harrigan, Li, Sanft, Shockro, O’Connor, Campbell, Tolaney, Mayer, Jung, Freedman, Partridge, Ligibel
Statistical analysis: Li
Obtained Funding: Irwin, Ligibel
Administrative, technical or material support: Irwin, Cartmel, Harrigan, Shockro, O’Connor, Campbell, Ligibel
Study Supervision: Irwin, Ligibel
Conflict of Interest Disclosures: The authors have no conflicts of interest.
Additional Contributions: We thank Bridget Winterhalter, Norbert Hootsmans, Celeste Wong and Meghan Hughes for their assistance. We thank Smilow Cancer Hospital at Yale-New Haven and all the clinicians who consented or referred their patients to our study. Most importantly, we thank the YMCAs (Hockomock Area YMCA: Foxborough, Franklin and North Attleborough branches; MetroWest YMCA: Framingham branch; Southington-Cheshire Community YMCAs; Wallingford Family YMCA; YMCA of Central Massachusetts; YMCA of Greater Boston: Charles River and West Roxbury branches) that participated in the study and all the study participants for their dedication and time to the study.
References
- 1.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012 Jun 6;104(11):815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA. 2003 Sep 10;290(10):1331–6. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 3.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA: the journal of the American Medical Association. 2005 May 25;293(20):2479–86. doi: 10.1001/jama.293.20.2479. Epub 2005/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 4.Irwin ML, McTiernan A, Manson J, et al. Physical activity and survival in women diagnosed with breast cancer: Results from the Women’s Health Initiative. Cancer Prevention Research. 2011;4(4):522–9. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012 Jul-Aug;62(4):243–74. doi: 10.3322/caac.21142. Epub 2012/04/28. eng. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz K, Courneya K, Matthews C, et al. American College of Sports Medicine Guidelines on Exercise Testing and Prescription in Cancer Survivors. Medicine and Science in Sports and Exercise. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 7.Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010 Jun;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 8.Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2015 Dec 30; doi: 10.1136/bjsports-2015-094787. pii: bjsports-2015-094787. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Mason C, Alfano CM, Smith AW, et al. A Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013 Jun;22(6):1153–61. doi: 10.1158/1055-9965.EPI-13-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchard CM, Courneya KS, Stein K. Cancer Survivors’ Adherence to Lifestyle Behavior Recommendations and Associations With Health-Related Quality of Life: Results From the American Cancer Society’s SCS-II. Journal of Clinical Oncology. 2008 May 1;26(13):2198–204. doi: 10.1200/JCO.2007.14.6217. 2008. [DOI] [PubMed] [Google Scholar]
- 11.Heston AH, Schwartz AL, Justice-Gardiner H, Hohman KH. Addressing physical activity needs of survivors by developing a community-based exercise program: LIVESTRONG® at the YMCA. Clin J Oncol Nurs. 2015 Apr;19(2):213–7. doi: 10.1188/15.CJON.213-217. [DOI] [PubMed] [Google Scholar]
- 12.Kriska A, Knowler W, LaPorte R, et al. Development of a questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt K, Vogt L, Thiel C, et al. Validity of the six minute walk test in cancer patients. Int J Sports Med. 2013;34(7):631–6. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 14.Cella D. FACIT Manual: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Scales. Evanston, IL: Center on Outcomes, Research, and Education (CORE); 1997. [Google Scholar]
- 15.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81:1192–1205. [PubMed] [Google Scholar]
- 17.Tworoger SS, Yasui Y, Ulrich CM, et al. Mailing strategies and recruitment into an intervention trial of the effect of exercise on breast cancer biomarkers. Cancer Epidemiol Biomarkers Prev. 2002 Jan;11(1):73–7. [PubMed] [Google Scholar]
- 18.Irwin ML, Cadmus L, Alvarez-Reeves M, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: The Yale Exercise and Survivorship Study. Cancer. 2008;112(S11):2593–2606. doi: 10.1002/cncr.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91:221–225. doi: 10.1016/j.apmr.2009.10.017. [DOI] [PubMed] [Google Scholar]


