Abstract
CD74 mediates major histocompatibility complex class-II (MHC-II) antigenic peptide loading and presentation, and plays an important role in the pathogenesis of autoimmune diseases, including systemic lupus erythematosus (SLE). C57BL/6 Faslpr mice that develop spontaneous lupus-like autoimmunity and pathology showed elevated CD74 expression in the inflammatory cell infiltrates and the adjacent tubular epithelial cells (TECs) in kidneys affected by lupus nephritis, but negligible levels in kidneys from age-matched wild-type (WT) mice. The inflammatory cytokines IFN-g or IL6 induced CD74 expression in kidney TECs in vitro. The presence of kidney TECs from Faslpr mice, rather than from WT mice, produced significantly stronger histones, dsDNA, and ribonucleoprotein (RNP)-Smith antigen (Sm) complex RNP/Sm-induced CD4+ T-cell activation. Splenocytes from CD74-deficient FaslprCd74−/− mice had muted responses in a mixed lymphocyte reaction and to the autoantigen histones. Compared to FaslprCd74+/+ mice, FaslprCd74−/− mice had reduced kidney and spleen sizes, splenic activated T cells and B cells, serum IgG and autoantibodies, urine albumin to creatinine ratio, kidney PAS (Periodic acid-Schiff) score, IgG and C3 deposition, and reduced serum IL6 and IL17A but increased serum IL2 and TGF-β levels. Study of chronic graft-versus-host (cGVH) C57BL/6 mice that received donor splenocytes from bm12 mice and those that received syngeneic donor splenocytes yielded similar observations. CD74 deficiency reduced lupus-like autoimmunity and kidney pathology in cGVH mice. This investigation establishes the direct participation of CD74 in autoimmunity and highlights a potential role of CD74 in kidney TECs together with professional antigen-presenting cells in SLE.
Keywords: CD74, systemic lupus erythematosus, autoimmunity, tubular epithelial cell
INTRODUCTION
CD74, also called invariant chain, is a chaperon molecule expressed in antigen-presenting cells (APCs) and mediates loading of proteolytically processed antigenic peptide onto the groove of the major histocompatibility complex class-II (MHC-II) molecule (1, 2). The mouse long form of CD74, a 41-kDa (p41) polypeptide, which contains type I thyroglobulin domains (3), undergoes stepwise proteolysis to a 10-kDa (p10) fragment, which cysteine proteases further cleave, permitting its replacement by the antigenic peptide fragment (2). Besides assisting MHC-II antigen presentation, CD74 has several MHC-II-independent activities. It plays a role in B-cell maturation (4) and dendritic cell migration from skin to lymph nodes (5). CD74 also functions in thymic positive and negative selection (6). CD74 deficiency reduces thymic CD4+ T cells and attenuates superantigen responses (7). These activities determine its detrimental functions in human diseases, including atherosclerosis, Alzheimer’s disease, diabetes, cancer, and B-cell neoplasia (8). B-cell neoplasms, multiple myeloma, and renal and gastric cancers express superphysiologic amounts of CD74 (8). Targeting of CD74 with a humanized CD74 monoclonal antibody, milatuzumab, has undergone testing in several human trials in cancer patients (8). This antibody reduces naïve B-cell proliferation, migration, and adhesion molecule expression (9).
Increased expression of CD74 also occurred in B cells, kidneys, and brains from mice with induced or spontaneous systemic lupus erythematosus (SLE)-like autoimmunity and pathology (10). SLE, a prototypic systemic autoimmune disease, exhibits B-cell hyperreactivity and production of autoantibodies against nuclear proteins and nucleic acids (11, 12). CD74-mediated MHC-II peptide loading has a H-2 haplotype-restriction. Earlier studies demonstrated CD74-mediated antigen presentation in mice carrying the H-2b and H-2d (13, 14) but not the H-2k, H-2s, and H-2u haplotypes (15, 16). In human B cells and in splenocytes from H-2b mice, CD74 p10 formed a complex with human HLA-DR or mouse H-2b. In contrast, in splenocytes from DBA/1 mice (H-2q) or SJL/J mice (H-2s), the CD74 p10 peptide did not form complexes with H-2q or H-2s (17).
This study used CD74-deficient (Cd74−/−) mice and both the C57BL/6 (B6, H-2b) Faslpr mice and H-2b chronic graft-versus-host (cGVH) mice to test the direct participation of CD74 in lupus-like autoimmunity and pathological findings, and provide support for a novel CD74-dependent pathway of antigen presentation by kidney tubular epithelial cells (TECs) to infiltrating T cells.
METHODS
Mice
Faslpr mice (B6, N11, 000485, The Jackson Laboratory, Bar Harbor, ME) were crossbred with Cd74−/− mice (B6, N10) (18) to generate FaslprCd74+/− mice breeding pairs to produce female FaslprCd74−/− mice and their female FaslprCd74+/+ littermate control mice. Blood and urine samples were collected from experimental mice starting from week 12 for 12 weeks. Mice were harvested at week 24. To generate cGVH mice, splenocytes were prepared from both C57BL/6 (B6) wild-type (WT, 000664) mice and B6.C-H2bm12/KhEg (bm12) mice (001162, The Jackson Laboratory). Six-week-old B6 WT (Cd74+/+) and Cd74−/− recipient mice received intraperitoneal injection of 1×108 donor splenocytes in 200 μl HBSS (Hanks balanced salt solution). All mice were sacrificed 10 weeks post splenocyte grafting, and blood and urine samples were obtained from experimental mice on the day before transplant and biweekly thereafter. The mouse procedures conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Harvard Medical School Standing Committee on Animals.
Renal morphology
After sacrifice, mouse kidneys and spleens were weighed, as were the bodies. Mouse kidney pieces were fixed overnight in a 4% paraformaldehyde and embedded in paraffin. Serial sections (6 μm) were prepared for PAS (periodic acid Schiff) or H&E (hematoxylin and eosin) or processed for immunohistochemical studies by immunoperoxidase or alkaline phosphatase anti-alkaline phosphatase techniques (19). The renal disease was graded from 0 to 3 (from normal, mild, moderate, or severe) using the activity index described from human lupus nephritis (20). For each mouse, at least 15 glomerular, tubular, or interstitial areas were graded and evaluated for glomerular cellularity, infiltrating leukocytes, mesangial matrix expansion, crescent formation, interstitial mononuclear cell infiltrates in the medulla and cortex, hyaline deposits, fibrinoid necrosis, and tubular atrophy. Glomerulus or tubulointerstitial scores for each mouse were calculated as the mean of the summed individual scores for each image, with scores for necrosis and crescent formation weighted by a factor of 2.
For immunohistochemical analysis, primary antibodies included the following: rat anti–mouse macrophage (Mac-2, 1:1,00; CL8942AP, Cedarlane, Burlington, NC), hamster anti–mouse monocyte chemoattractant protein-1 (MCP-1) (1:50; 554440, BD Biosciences), and major histocompatibility complex class II (MHC-II, 1:250, 556999, BD Biosciences). Positive Mac-2+ cells were counted in 10 consecutive visual fields at the same magnification and presented as positive number per mm2. The MCP-1 and MHC-II were measured as immunostaining signal-positive area.
Kidney frozen sections (5 μm) were prepared for immunofluorescent staining using anti-human/mouse IgG (1:250, F2761, Invitrogen, Carlsbad, CA) and C3 antibodies (1:100, F020102-2, DAKO, Carpinteria, CA). Stained specimens were then observed under a fluorescent microscope. Fluorescence intensity was graded as 0 to 3 (from normal, mild, moderate, or bright).
ELISA
Serum autoantibodies were assessed by ELISA as described (21). NUNC maxisorp ELISA plates were pre-coated with ssDNA (100 μg/ml, D8899, Sigma, St. Louis, MO), dsDNA (100 μg/ml, D8515, Sigma), histones (20 μg/ml, 10223565001, Sigma), and ribonucleoprotein (RNP)-Smith antigen (Sm) complex RNP/Sm (20 μg/ml, SRC-3000, ImmunoVision, Springdale, AR) in PBS at 4°C overnight. Plates were blocked with 3% fetal calf serum for 1 hour at 37°C, washed, and incubated with 1/300~1/1000 dilutions of mouse sera for 1 hour at 37°C. Plates were washed, and specific antibodies were detected with a 1/1000 dilution of alkaline phosphatase-conjugated goat anti-mouse IgG (103605, Southern Biotech, Birmingham, AL) for 1 hour at 37°C, and developed with a phosphatase substrate for 30 min at 37°C. Mouse serum total IL6, IL2, transforming growth factor-β (TGF-β), IL17A, IgG, and IgG1 levels were determined according to the manufacturer’s instructions. The ELISA kits used in this study include: mouse IL6 DuoSet (DY406, R&D Systems, Minneapolis, MN), mouse IL2 DuoSet (DY402, R&D Systems), mouse TGF-β ELISA ready-SET-Go (88-8350, eBioscience, San Diego, CA), mouse IL17A Platinum ELISA (BMS6001, eBioscience), mouse IgG ELISA Quantitation Set (E90-131, Bethyl Laboratories, Inc., Montgomery, TX), and mouse IgG1 ELISA Quantitation Set (E90-105, Bethyl Laboratories, Inc). Urine albumin was determined using ELISA for mouse albumin (E90-134, Bethyl Laboratories, Inc.) according to the manufacturers’ instructions. Urine samples were also collected to determine creatinine concentration using a creatinine assay kit (500701, Cayman Chemical, Ann Arbor, Michigan).
Real-Time PCR and Western blot analysis
Total RNA was prepared from kidneys using the Qiagen mini kit (Qiagen Inc., Valencia, CA). RNA concentration and quality were evaluated using the Agilent 2100 bioanalyzer (Nano LabChip, Agilent Technologies, Santa Clara CA). After the cDNA synthesis, gene expression was quantified by real-time PCR in an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA) using TaqMan Gene Expression Master Mix and TaqMan Gene Expression Assays (Applied Biosystems) for mouse β-actin, CD74 and MHC-II. Each sample was run in duplicate and the mean value of each set of duplicates was normalized to β-actin and used to calculate relative gene expression by the ΔΔCt method. For immunoblot analysis, an equal amount of protein from each tissue preparation or cell lysate was separated on a 12% SDS-PAGE, blotted to a PVDF membrane (IPVH00010, EMD Millipore, Billerica, MA), and detected with different antibodies, including rat anti-mouse CD74 (p41, p10) (1:1,000, 28221D, BD Biosciences) and β-actin (1:3,000, sc-81178, Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Kidney TEC culture and autoantigen presentation
Primary cultures of kidney proximal TECs were prepared using established methods with modifications (22, 23). Briefly, the kidney cortex was dissected from medulla, diced, and then digested in a solution of collagenase type 2 (1 mg/ml) for 30 minutes at 37°C in a water bath rocker. The enzyme reaction was terminated with horse serum. Glomeruli and remaining tissue clumps were separated by decanting after gravity sedimentation (2 minutes). After washing 2 times in medium, tubules were resuspended in a tubule medium (DMEM/F-12 with transferrin, insulin, selenium, hydrocortisone, and EGF) and aliquoted into Matrigel–coated tissue culture grade dishes. Every second day, the medium was replaced with fresh medium. The TECs were used in assays between day 4 and day 5. To assess changes in CD74 expression, confluent cells (106 cells/ml) were treated with IL-6 (5 ng/ml, 300–327P, Gemini Bio-products, West Sacramento, CA), IFN-γ (10 ng/ml, 485-MI-100, R&D Systems), or complete medium alone for 48 hours. TECs (1×105) from both 8-week-old WT and Faslpr mice were also pre-treated with IFN-γ for 48 hours to stimulate CD74 expression, followed by incubation with CD4+ T cells (3×104) with and without histones (20 μg/ml), ssDNA (100 μg/ml), dsDNA (100 μg/ml), and RNP/Sm (20 μg/ml) for additional 48 hours to activate CD4+ T cells. T-cell activation was assessed by measuring culture media IL2 levels by ELISA.
Mixed lymphocyte reaction and in vitro autoantigen recall assay
Both the mixed lymphocyte reaction and autoantigen recall assays were performed based on our previously published protocols (13). Presenter splenocytes were prepared from FaslprCd74+/+ and FaslprCd74−/− mice and pre-treated with 2 μg/ml of mitomycin-C (M4287, Sigma) at 37 °C for 30 min. Different numbers of presenters (3×105 to 1×107 per 100 μl) were co-cultured with 5×105 bm12 splenocyte as responders. After 2 days, culture media IL2 levels were determined by ELISA. For the autoantigen recall assay, splenocytes were isolated from FaslprCd74+/+ and FaslprCd74−/− mice and incubated (5×106 well) with 50 μg/mL histones in 200 mL of complete RPMI 1640 for 2 days at 37 °C. Culture medium IL2 levels were determined by ELISA.
Statistical analysis
All data, including those from serum and urine samples were analyzed using non-parametric Mann-Whitney U test followed by Bonferroni corrections due to small sample sizes and often skewed data distribution. All data were presented as mean ± SEM. P<0.05 was considered statistical significant.
RESULTS
Increased CD74 expression in lupus-prone Faslpr mice
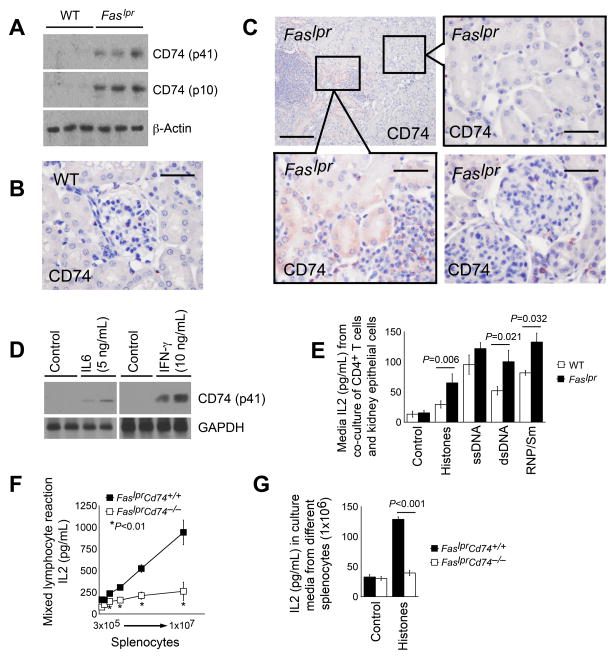
B6.Faslpr mice develop mild lupus-like manifestations after 4 months of age (24), and symptoms become significant when mice reached to 6 to 9 months of age (25, 26). 24-week-old Faslpr and WT control mice helped test whether development of lupus-like autoimmunity in Faslpr mice altered CD74 expression. Immunoblot analysis detected negligible CD74 expression in total kidney tissue extracts from WT mice but elevated expression of both the p41 and processed p10 fragments of CD74 in the kidney tissues of age-matched Faslpr mice (Fig. 1A). CD74 immunostaining detected few CD74-positive cells in the kidney tubulointerstitial space and the glomeruli from WT control mice (Fig. 1B). In kidneys from the Faslpr mice, however, many cells in the inflammatory infiltrate clusters expressed CD74. Interestingly, TECs adjacent to the inflammatory infiltrate clusters also expressed CD74, whereas the same cell type at a distance from the inflammatory infiltrate clusters remained CD74-negative (Fig. 1C). Compared to those from WT control mice, kidney glomeruli and tubulointerstitial space contained more CD74-positive cells, likely infiltrated inflammatory cells (Fig. 1C).
Figure 1.
CD74 expression and antigen presentation activity in Faslpr mice. A. Immunoblot detected CD74 expression, both the p41 full-length products and p10 processed fragments, in kidney extracts from 24-week-old WT and Faslpr mice. B. Immunostaining to detect CD74 (p41) expression in kidneys from 24-week-old WT mice. Scale: 50 μm. C. Immunostaining detected CD74 (p41) expression in kidneys from 24-week-old Faslpr mice. Scale: 200 μm and 50 μm, inset scale: 50 μm. D. CD74 (p41) expression in kidney TECs before and after stimulation with IL6 and IFN-γ. E. CD4+ T cell culture medium IL2 levels after activation with TECs from 24-week-old WT and Faslpr mice in the presence or absence of autoantigens as indicated. F. Mixed lymphocyte reactions of presenter splenocytes from 24-week-old FaslprCd74+/+ and FaslprCd74−/− mice and responder cells from bm12 mice. G. Autoantigen histone presentation assay. ELISA determined IL2 levels in splenocytes from 24-week-old FaslprCd74+/+ and FaslprCd74−/− mice treated with and without histone. Data in E–G are representative of three independent experiments.
Increased expression of CD74 in TECs next to the inflammatory infiltrate clusters in kidneys from Faslpr mice but not those at a distance or those in kidneys from normal mice suggested that cytokines derived from cells in those inflammatory infiltrates stimulated nearby TEC to express CD74. The isolation of mouse kidney TECs and stimulation of these cells with IL6 and IFN-γ, common inflammatory cytokines found in lupus nephritis kidneys (27), helped test this hypothesis. After 48 hours of stimulation, both cytokines greatly increased CD74 (p41) expression in these TECs (Fig. 1D). When kidney TECs from 24-week-old WT and lupus-prone Faslpr mice were pre-stimulated with IFN-γ for 48 hours to induce CD74 expression, followed by additional 48 hours of co-incubation with CD4+ T cells in the absence of IFN-γ, autoantigens histones, ssDNA, dsDNA, and RNP/Sm increased T-cell activation as determined by increased IL2 release. TECs from Faslpr mice also showed significantly higher activity than those from WT mice in T-cell activation after incubation with histones, dsDNA, and RNP/Sm (Fig. 1E).
As expected, splenocytes from FaslprCd74+/+ mice responded to allogeneic splenocytes from bm12 mice, but splenocytes from FaslprCd74−/− mice showed muted responses (Fig. 1F). Anti-histone autoantibodies are prevalent and are linked to kidney involvement in SLE patients (28). In lupus-prone mice, there are elevated numbers of histone-specific antibody-forming B cells from the splenocyte preparation and elevated serum anti-histone autoantibody titers (29), suggesting that immune cells from lupus-prone mice may have been pre-primed. Splenocytes from FaslprCd74+/+ mice responded to histone stimulation in a histone autoantigen recall assay, but those from FaslprCd74−/− mice did not respond to histones (Fig. 1G). Therefore, either TECs or professional APCs may present autoantigens and contribute to the autoimmunity in SLE-prone mice, a hypothesis previously investigated in intestinal epithelial cells (30).
CD74 deficiency mitigates SLE-like autoimmunity and pathological findings in Faslpr mice
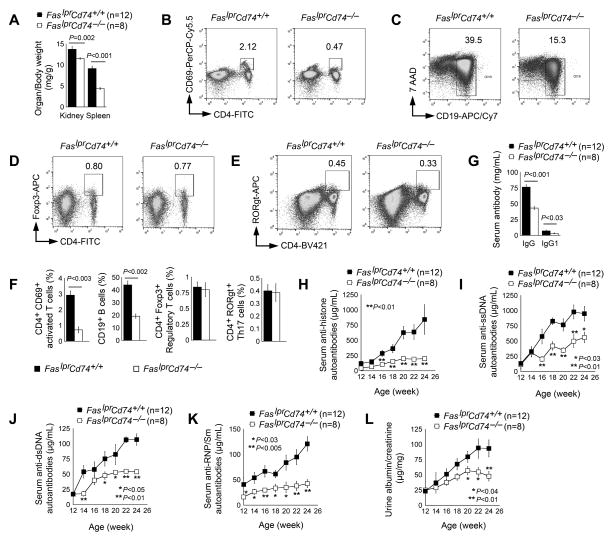
The generation of female FaslprCd74−/− mice and their FaslprCd74+/+ female littermates by crossbreeding the Cd74−/− mice with the B6-Faslpr mice, in which their H-2b permitted functional CD74 for autoantigen presentation (13–17, 31–33), permitted testing of a direct role of CD74 in a lupus-like disease. FaslprCd74−/− mice showed a reduction in both kidney and spleen weight ratio relative to body weight (Fig. 2A). CD74 mediates H-2 haplotype-restricted APC antigen presentation and is essential to T-cell activation (13–16). As we expected, splenocytes from H-2b B6-FaslprCd74−/− mice contained significantly lower numbers of CD4+CD69+ activated T cells and CD19+ B cells than those from B6-FaslprCd74+/+ control mice, although CD74 deficiency did not affect splenic regulatory T-cell (Treg) or Th17 cell numbers (Fig. 2B–2F). FaslprCd74−/− mice showed reduced serum total IgG and IgG1 levels as well (Fig. 2G). In addition to histones, ssDNA, dsDNA, and RNP/Sm are also common autoantigens from both SLE patients and lupus-like mice. Anti-ssDNA autoantibodies have been used for clinical diagnosis and follow-up of human SLE and are associated with the flare of symptoms (34–38). Plasma anti-ssDNA antibodies, along with anti-dsDNA, anti-histones, and anti-RNP/Sm antibodies are also increased in lupus-prone mice (39, 40). We showed that FaslprCd74−/− mice had significantly blunted serum autoantibodies against histones, ssDNA, dsDNA, and RNP/Sm, compared with those from the FaslprCd74+/+ mice (Fig. 2H–2K). FaslprCd74−/− mice also revealed significantly lower urine albumin-to-creatinine ratio than FaslprCd74+/+ mice (Fig. 2L).
Figure 2.
CD74 deficiency mitigates SLE-like autoimmunity in SLE-prone Faslpr mice. A. Kidney to body and spleen to body weight ratio in 24-week-old FaslprCd74+/+ and FaslprCd74−/− mice. FACS analysis determined CD4+CD69+ activated T cells (B), CD19+ B cells (C), CD4+Foxp3+ Treg cells (D), and CD4+RORgt+ Th17 cells (E) in splenocytes from 24-week-old FaslprCd74+/+ and FaslprCd74−/− mice. F. Composite FACS analysis of panels B to E. Data were from six mice per group. G. Serum IgG and IgG1 titers. Serum anti-histone autoantibodies (H), anti-ssDNA autoantibodies (I), anti-dsDNA autoantibodies (J), and anti-RNP/Sm autoantibodies (K), and urine albumin-to-creatinine ratios (L) from 24-week-old FaslprCd74+/+ and FaslprCd74−/− mice. The number of mice per group in panels A and G–L is indicated in each parenthesis.
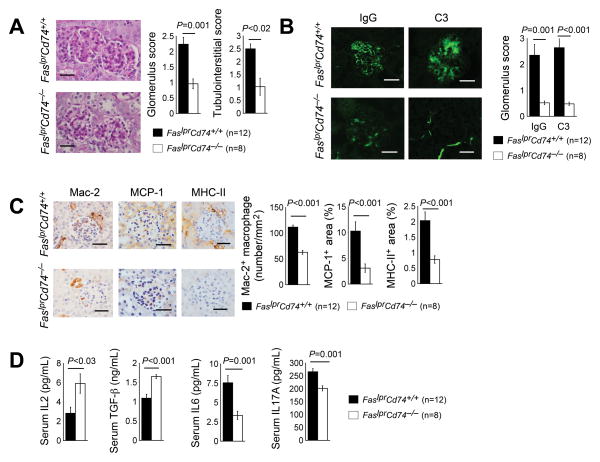
PAS staining revealed greatly improved kidney glomerular and tubulointerstitial scores in FaslprCd74−/− mice compared to FaslprCd74+/+ mice (Fig. 3A). FaslprCd74−/− mice showed a significantly greater reduction of the immunofluorescent intensity of kidney glomerulus IgG and complement C3 deposition than FaslprCd74+/+ mice (Fig. 3B). FaslprCd74−/− mice revealed reduced Mac-2+ macrophages within both the glomeruli and tubulointerstitial space, along with reduced local expression of chemokine MCP-1 inside the glomeruli and TECs and reduced inflammation (MHC-II) (Fig. 3C). TGF-β, IL6, IL17, and IL2 are common cytokines that associate with human and murine SLE development. TGF-β exerts broad anti-inflammatory and immunosuppressive effects. Its serum levels were lower in patients with SLE than those in healthy controls and correlated negatively with disease activity index (41, 42). In lupus-prone mice, TGF-β expression was low in lymphoid tissues and reduced TGF-β in immune cells predisposed to immune dysregulation and autoantibody production (43). IL-6 is a key cytokine that determines naïve T-cell differentiation to Treg cells or to Th17 cells (44). Serum IL6 levels were found increased in patients with active SLE (45, 46) and correlated with disease activity, B-cell hyperactivity, and autoantibody production (45–47). In lupus-prone mice, serum IL6 was increased as well (48, 49). Serum IL17 levels were also raised in SLE patients and correlated with disease activity (50). Lupus-prone mice also had increased serum IL17 and splenic IL17-producing cells (51, 52). In lupus-prone mice however, serum IL2 levels were reduced (53–55), although T cells were the main IL2-producing cells (56–58). IL2 is required for thymic development, homeostatic maintenance, and immunosuppressive activity of Treg cells (59, 60). Low levels of IL-2 enhanced IL6 production (45, 61) and favored Th17 cell development (62), thereby promoting autoimmune disorders (63, 64). Consistent with reduced kidney inflammation in FaslprCd74−/− mice, absence of CD74 in these mice reduced serum levels of pro-inflammatory cytokines IL6 and IL17A, but increased IL2 and TGF-β, compared with those with sufficient CD74 expression (Fig. 3D).
Figure 3.
CD74 deficiency mitigates SLE-like pathology in SLE-prone Faslpr mice. A. Kidney PAS staining and glomerulus and tubulointerstitial scores; B. Kidney IgG and C3 immunofluorescent staining and glomerulus scores. C. Kidney Mac-2, MCP-1, and MHC-II immunostaining. D. ELISA determined serum IL6, IL17A, IL2, and TGF-β levels from 24-week-old FaslprCd74+/+ and FaslprCd74−/− mice. The number of mice per group is indicated in each parenthesis. Representative graphs for A–C are shown to the left of each panel. Scale: 50 μm.
CD74 deficiency also mitigates SLE-like autoimmunity and pathology in cGVH mice
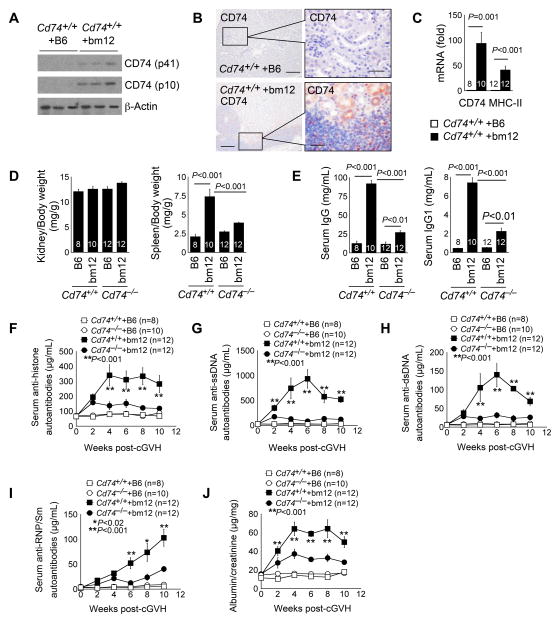
Like the Faslpr mice, B6 mice injected intraperitoneally with bm12 splenocytes develop cGVH manifested with elevated serum autoantibodies against dsDNA, ssDNA, histones, and chromatin and severe lupus nephritis (65). Ten weeks after receiving the bm12 splenocytes, the kidneys of B6 WT mice showed elevated expression of both CD74 fragments p41 and p10, as determined by immunoblot analysis (Fig. 4A). Immunostaining revealed CD74 expression in both the interstitial inflammatory cell infiltrates and nearby tubular epithelial cells in Cd74+/+ mice received bm12 splenocyte intraperitoneal administration, whereas kidneys from the same type of mice received B6 splenocytes showed negligible CD74 expression (Fig. 4B), similar to what we detected in kidneys from Faslpr mice with lupus-like manifestations and healthy WT mice (Fig. 1B, 1C). RT-PCR also revealed increased expression of both CD74 and MHC-II in those kidneys (Fig. 4C). Although the kidney weights relative to the body weights did not differ between the Cd74+/+ and Cd74−/− mice after receiving B6 splenocytes or bm12 splenocytes, Cd74−/− mice showed a reduction of increased spleen sizes after receiving bm12 splenocytes (Fig. 4D). Cd74−/− mice also showed reduced serum IgG and IgG1, compared with those from the Cd74+/+ control mice after receiving bm12 splenocytes (Fig. 4E). CD74 deficiency reduced serum autoantibodies against histone, ssDNA, dsDNA, and RNP/Sm (Fig. 4F–4I) and urine albumin-to-creatinine ratio (Fig. 4J) in bm12 splenocyte-treated Cd74−/− mice, compared with those in bm12 splenocyte-treated Cd74+/+ mice.
Figure 4.
CD74 deficiency improves cGVH-induced SLE-like autoimmunity in mice. Immunoblot analysis to detect kidney CD74 (both p41 and p10) expression (A), immunostaining to detect CD74 expression in kidneys (B, scale: 200 μm, inset scale: 50 μm), and RT-PCR to detect kidney CD74 and MHC-II mRNA levels (C) from B6-Cd74+/+ mice at 10 weeks after intraperitoneal transfer of splenocytes from B6 or bm12 mice. Kidney to body and spleen to body weight ratio (D), serum IgG and IgG1 (E), serum anti-histone autoantibody titers (F), serum anti-ssDNA autoantibody titers (G), serum anti-dsDNA autoantibody titers (H), serum anti-RNP/Sm autoantibody titers (I), and urine albumin-to-creatinine ratios (J) in both Cd74+/+ and Cd74−/− mice (both on B6 background) at 10 weeks after intraperitoneal transfer of splenocytes from B6 or bm12 mice. The number of mice per group is indicated in each parenthesis or bar.
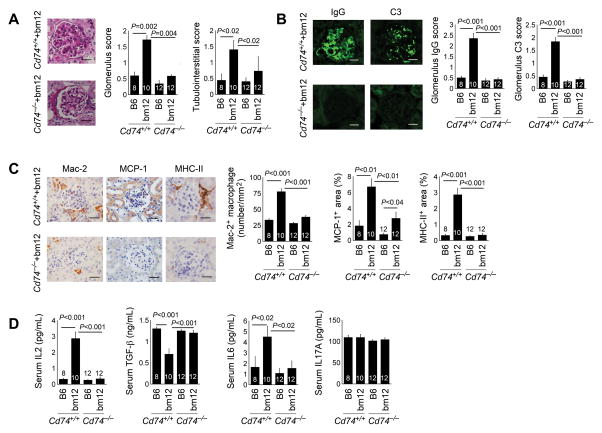
Kidney pathological findings also improved in bm12 splenocyte-treated Cd74−/− mice. PAS staining revealed that these mice had significantly reduced glomerulus and tubulointerstitial scores (Fig. 5A). Immunofluorescent examination demonstrated substantially reduced IgG and C3 deposition in the glomeruli of bm12 splenocyte-treated Cd74−/− mice (Fig. 5B). Similar to the FaslprCd74−/− mice, bm12 splenocyte-treated Cd74−/− mice revealed blunted kidney Mac-2+ macrophage accumulation in both the glomeruli and tubulointerstitial space, chemokine MCP-1 expression in the glomeruli and TECs, and kidney MHC-II levels, compared with those from bm12 splenocyte-treated Cd74+/+ mice (Fig. 5C). Similar to FaslprCd74−/− mice, bm12 splenocyte-treated Cd74−/− mice also had reduced serum IL6 and increased serum TGF-β. Yet, differing from FaslprCd74−/− mice, CD74 deficiency did not affect serum IL17A but reduced serum IL2, compared with those from bm12 splenocyte-treated Cd74+/+ mice (Fig. 5D).
Figure 5.
CD74 deficiency improves cGVH-induced SLE-like pathology in mice. A. Kidney PAS staining and glomerulus and tubulointerstitial scores. B. Kidney IgG and C3 immunoflorescent staining and glomerulus scores. C. Kidney Mac-2, MCP-1, and MHC-II immunostaining. D. ELISA determined serum IL-2, IL-6, IL-17A, and TGF-β levels in both Cd74+/+ and Cd74−/− mice (both on B6 background) at 10 weeks after intraperitoneal transfer of splenocytes from B6 or bm12 mice. The number of mice per group is indicated in each bar. Representative figures in panels A to C are shown to the left. Scale: 50 μm.
DISCUSSION
Previous studies have targeted CD74 as a treatment for autoimmune diseases (31). APCs, such as B cells and dendritic cells, participate in the immunopathogenesis of SLE by presenting autoantigens and activating T cells (66–68). Depletion or targeting of these cells mitigates SLE in humans and animals (69, 70). This study establishes a direct role for CD74 in the development of lupus-like autoimmunity and pathological findings in B6-Faslpr and in B6 mice developing cGVH after the injection of bm12 splenocytes. However, the involvement of CD74 in antigen presentation and autoimmune diseases can be restricted to certain types of H-2 haplotypes. In this study, all mice were on a H-2b genetic background, which remains essential for CD74 to assist MHC-II antigenic peptide loading (13–17, 31–33). Several mouse models have been used to study lupus-like manifestations and pathologies, among which MRL-Faslpr and (NZBxNZW)F1 mice develop more severe spontaneous lupus-like autoimmunity and at earlier ages than the B6-Faslpr and B6-cGVH mice that we used in this study. MRL-Faslpr mice start developing lupus-like manifestations at 12 weeks of age, whereas (NZBxNZW)F1 mice start developing severe lupus-like autoimmunity around 20 weeks of age (39). From our unpublished data, 8~10 weeks old MRL-Faslpr mice show similar plasma autoantibody levels (against histone, ssDNA, dsDNA, and RNP/Sm) to those of 24 weeks old B6-Faslpr mice. In this study, we did not use MRL-Faslpr mice or (NZBxNZW)F1 mice to test a role of CD74 in lupus because of their different genetic backgrounds. MRL-Faslpr mice are on a H-2k haplotype whereas (NZBxNZW)F1 mice are on a H-2d and H-2z mixed background. Antigen presentation activities of APCs from H-2k mice are CD74-independent (15, 71, 72), although there is no study to test a direct involvement of CD74 in antigen presentation in APCs from H-2Z mice. Therefore, changes in lupus-like flare of symptoms if any from CD74-deficient MRL-Faslpr mice or (NZBxNZW)F1 mice may be associated with different mechanisms other than antigen presentation, such as migration inhibitory factor (MIF)-mediated actions.
MIF is a pro-inflammatory innate and adaptive cytokine that has been implicated in several inflammatory diseases, including SLE. Serum MIF levels are increased in SLE patients and are correlated with disease severity (73). In both H-2k MRL-Faslpr mice and H-2d and H-2z mixed background (NZBxNZW)F1 mice, MIF expression is increased in serum and renal tissues (74, 75). Prior study established a role of MIF in mediating monocyte and T-cell retention and chemotaxis by binding to a cell surface complex that contained both C-X-C chemokine receptor type 2 (CXCR2) and CD74 (76). Genetic deficiency of MIF or its pharmacological inhibition by a MIF antagonist ISO-1 (4,5-Dihydro-3-(4-hydroxyphenyl)-5-isoxazoleacetic acid methyl ester) or anti-MIF antibody in MRL-Faslpr and (NZBxNZW)F1 mice prolonged mouse survival and reduced urine proteinuria, serum BUN (blood urea nitrogen) levels, skin lesion prevalence, and kidney glomerulonephritis, including glomerular scores, interstitial and glomerular inflammatory cell infiltration, kidney inflammatory cytokine expression. However, either MIF genetic deficiency or inhibition with ISO-1 or anti-MIF antibody did not affect autoantibody production and T-cell and B-cell activation in none of these lupus-prone mice (74, 75). These observations suggest an antigen presentation-independent role of CD74 in MRL-Faslpr and (NZBxNZW)F1 mouse lupus-like pathogenesis by mediating MIF actions, although none of these two studies tested whether these MIF actions were solely CD74-dependent. Therefore, we conjecture that CD74 deficiency in MRL-Faslpr and (NZBxNZW)F1 mice resemble the phenotypes from MIF-deficient and ISO-1- and anti-MIF antibody-treated MRL-Faslpr and (NZBxNZW)F1 mice, if MIF binding to CD74 is essential to MIF activities in lupus-like manifestation development in mice.
Epithelial cells also act as APCs, depending on the expression of surface CD74 (77–79). Quiescent intestinal epithelial cells express negligible CD74. Under inflammatory conditions, such as inflammatory bowel diseases, these cells may have increased CD74 expression after exposure to inflammatory cytokines, such as IFN-γ (30). IFN-γ-treated epithelial cells exhibit antigen presentation activity indistinguishable from conventional APCs (30). In the stomach, gastric epithelial cells also expressed negligible CD74, but showed increased expression of CD74 after Helicobacter pylori infection (80). In normal kidneys, TECs also did not express CD74. After inflammatory cell infiltration during the pathogenesis of SLE, these inflammatory cells may produce IFN-γ and IL6 or other untested cytokines (27) to stimulate CD74 expression from these cells. Increased expression of TEC CD74 may increase the autoimmunity and promote lupus-like kidney nephritis in B6-Faslpr mice or in bm12 splenocyte-treated cGVH mice, boosting or complementing responses evoked by traditional APCs. Therefore, in addition to revealing a direct participation of CD74 in experimental SLE, this study suggested a potentially important and concordant role of TECs together with “professional” APCs in autoantigen presentation and autoimmunity in human and experimental SLE.
Improved lupus-like manifestations, including serum autoantibodies and kidney glomerulonephritis from CD74-deficient B6-Faslpr mice and from bm12 splenocyte-treated cGVH mice suggest that pharmacological inactivation of CD74 has clinical implications, although we did not test this hypothesis in this study. Milatuzumab is a humanized anti-CD74 monoclonal antibody. It binds to CD74+ B cells and dendritic cells, reduces B-cell and dendritic cell numbers, enhances cell migration, and reduces cell surface adhesion molecule expression (81), suggesting its potential efficacy for autoimmune disease therapy by targeting these CD74+ cells. In human/mouse xenogeneic severe combined immunodeficiency (SCID) acute graft versus host disease (GVHD) mice, in which GVHD was induced by engrafting human CD4+ T cells and dendritic cells, milatuzumab prevented acute GVHD onset and manifestations (82). Indeed, milatuzumab is currently under the evaluation in a phase Ib trial for patients with active SLE (150 mg and 250 mg subcutaneous administration once a week for 4 weeks) (ClinicalTrials.gov identifier: NCT01845740, Immunomedics, Inc., Morris Plains, NJ), although the outcomes of this trial will be ready by this coming spring.
In addition to binding to CD74 and affecting the biology of CD74+ cells (e.g. B cells and dendritic cells), milatuzumab has been broadly modified to enhance its activities on targeting cells or to reduce the off-target risks during the drug delivery process. Incorporation of milatuzumab with immunoliposome increased antibody cytotoxicity to cultured chronic lymphocytic leukemia (CLL) cells (83). Milatuzumab-conjugated liposomes also acted as carrier to deliver corticosteroid to CD74+ B-cell malignancies in cultured primary CLL cells and in a SCID xenograft model, by promoting CD74+ cell killing and enhancing therapeutic efficacy (84). Milatuzumab-conjugated irinotecan showed improved responses to solid tumor xenografts, and milatuzumab-conjugated doxorubicin increased the drug efficacy against mouse lymphoma (85), likely by enhancing the drug delivery accuracy to CD74+ cells. Combined therapy also increased antibody efficacy. Anti-CD20 antibody rituximab alone showed modest activity on mantle cell lymphoma (MCL) cell death. Combined therapy of milatuzumab with rituximab (86), or with immunosuppressive agent FTY720 (fingolimod, a synthetic analog of sphingosine) (87) expedited MCL cell death, supporting clinical evaluation of combined milatuzumab therapy in MCL.
Human trials also suggested that modified milatuzumab may exert better efficacy than naked milatuzumab. In a phase I/II multicenter dose-escalation trial of 25 patients with relapsed or refractory multiple myeloma, milatuzumab treatment modestly decreased B-cell levels. Milatuzumab was rapidly cleared from the circulation with little serum accumulation (ClinicalTrials.gov Identifier: NCT00421525, Immunomedics, Inc.) (88). Another phase I trial of 22 patients with previously treated B-cell lymphoma (both non-Hedgkin lymphoma and CLL), milatuzumab treatment showed no clear evidence of tumor targeting and serum milatuzumab half-life was only 2 hours (89), suggesting that modified milatuzumab, including liposomal formulation (84), antibody-drug conjugates (85), and bispecific antibodies (90), is a better regimen for the purpose of increased therapeutic efficacy. Indeed, in a phase I/II trial of 35 patients with relapsed or refractory non-Hedgkin lymphoma, combined treatment with milatuzumab together with anti-CD20 antibody veltuzumab demonstrated greatly improved efficacy (91).
Together, studies from cultured primary cancer cells, mouse tumor models, and human trials all point to the hypothesis that modified milatuzumab may exert improved efficacy than naked milatuzumab. It is possible that milatuzumab-immunoliposome conjugation or milatuzumab conjugation with known SLE immunosuppressors, or combined administration of milatuzumab with SLE immunosuppressors may increase the efficacy of milatuzumab (ClinicalTrials.gov identifier: NCT01845740) or SLE immunosuppressors, a hypothesis that merits future investigation.
Acknowledgments
The authors thank Ms. Eugenia Shvartz for her technical assistance and Ms. Chelsea Swallom for her editorial assistance.
Financial support: This study is supported by grants from the National Institutes of Health (HL81090, HL60942, HL123568 to G.P.S.; and HL34636, HL80472, to P.L.).
Footnotes
Disclosures: The authors have no financial conflict of interest.
References
- 1.Castellino F, Zhong G, Germain RN. Antigen presentation by MHC class II molecules: invariant chain function, protein trafficking, and the molecular basis of diverse determinant capture. Hum Immunol. 1997;54:159–169. doi: 10.1016/s0198-8859(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 2.Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev. 2005;207:229–241. doi: 10.1111/j.0105-2896.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 3.Koch N, Lauer W, Habicht J, Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987;6:1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matza D, Lantner F, Bogoch Y, Flaishon L, Hershkoviz R, Shachar I. Invariant chain induces B cell maturation in a process that is independent of its chaperonic activity. Proc Natl Acad Sci U S A. 2002;99:3018–3023. doi: 10.1073/pnas.052703299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faure-Andre G, Vargas P, Yuseff MI, Heuze M, Diaz J, Lankar D, Steri V, Manry J, Hugues S, Vascotto F, Boulanger J, Raposo G, Bono MR, Rosemblatt M, Piel M, Lennon-Dumenil AM. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322:1705–1710. doi: 10.1126/science.1159894. [DOI] [PubMed] [Google Scholar]
- 6.Wright RJ, Bikoff EK, Stockinger B. The Ii41 isoform of invariant chain mediates both positive and negative selection events in T-cell receptor transgenic mice. Immunology. 1998;95:309–313. doi: 10.1046/j.1365-2567.1998.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong P, Rudensky AY. Phenotype and function of CD4+ T cells in mice lacking invariant chain. J Immunol. 1996;156:2133–2142. [PubMed] [Google Scholar]
- 8.Borghese F, Clanchy FI. CD74: an emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert Opin Ther Targets. 2011;15:237–251. doi: 10.1517/14728222.2011.550879. [DOI] [PubMed] [Google Scholar]
- 9.Frolich D, Blassfeld D, Reiter K, Giesecke C, Daridon C, Mei HE, Burmester GR, Goldenberg DM, Salama A, Dorner T. The anti-CD74 humanized monoclonal antibody, milatuzumab, which targets the invariant chain of MHC II complexes, alters B-cell proliferation, migration, and adhesion molecule expression. Arthritis Res Ther. 2012;14:R54. doi: 10.1186/ar3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapter S, Ben-David H, Sharabi A, Zinger H, Telerman A, Gordin M, Leng L, Bucala R, Shachar I, Mozes E. A role for the B-cell CD74/macrophage migration inhibitory factor pathway in the immunomodulation of systemic lupus erythematosus by a therapeutic tolerogenic peptide. Immunology. 2011;132:87–95. doi: 10.1111/j.1365-2567.2010.03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 12.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 13.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, Riese R, Ploegh HL, Chapman HA. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 14.Kala M, Chen CR, McLachlan SM, Rapoport B, Aliesky H, Chapman HA. Cathepsin S is not crucial to TSHR processing and presentation in a murine model of Graves’ disease. Immunology. 2005;116:532–540. doi: 10.1111/j.1365-2567.2005.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riese RJ, Shi GP, Villadangos J, Stetson D, Driessen C, Lennon-Dumenil AM, Chu CL, Naumov Y, Behar SM, Ploegh H, Locksley R, Chapman HA. Regulation of CD1 function and NK1.1(+) T cell selection and maturation by cathepsin S. Immunity. 2001;15:909–919. doi: 10.1016/s1074-7613(01)00247-3. [DOI] [PubMed] [Google Scholar]
- 16.Villadangos JA, Riese RJ, Peters C, Chapman HA, Ploegh HL. Degradation of mouse invariant chain: roles of cathepsins S and D and the influence of major histocompatibility complex polymorphism. J Exp Med. 1997;186:549–560. doi: 10.1084/jem.186.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Podolin PL, Bolognese BJ, Carpenter DC, Davis TG, Johanson RA, Fox JH, Long E, 3rd, Dong X, Marquis RW, Locastro SM, Terfloth GJ, Kurali E, Peterson JJ, Smith BR, McQueney MS, Yamashita DS, Capper-Spudich EA. Inhibition of invariant chain processing, antigen-induced proliferative responses, and the development of collagen-induced arthritis and experimental autoimmune encephalomyelitis by a small molecule cysteine protease inhibitor. J Immunol. 2008;180:7989–8003. doi: 10.4049/jimmunol.180.12.7989. [DOI] [PubMed] [Google Scholar]
- 18.Maehr R, Kraus M, Ploegh HL. Mice deficient in invariant-chain and MHC class II exhibit a normal mature B2 cell compartment. Eur J Immunol. 2004;34:2230–2236. doi: 10.1002/eji.200425246. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer L, Raslik I, Grone HJ, Schonherr E, Macakova K, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J. 2001;15:559–561. doi: 10.1096/fj.00-0493fje. [DOI] [PubMed] [Google Scholar]
- 20.Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni OP, Sayyed SG, Kantner C, Ryu M, Schnurr M, Sardy M, Leban J, Jankowsky R, Ammendola A, Doblhofer R, Anders HJ. 4SC-101, a novel small molecule dihydroorotate dehydrogenase inhibitor, suppresses systemic lupus erythematosus in MRL-(Fas)lpr mice. Am J Pathol. 2010;176:2840–2847. doi: 10.2353/ajpath.2010.091227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheridan AM, Schwartz JH, Kroshian VM, Tercyak AM, Laraia J, Masino S, Lieberthal W. Renal mouse proximal tubular cells are more susceptible than MDCK cells to chemical anoxia. Am J Physiol. 1993;265:F342–350. doi: 10.1152/ajprenal.1993.265.3.F342. [DOI] [PubMed] [Google Scholar]
- 24.Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 25.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Chen H, Liu L, Yu X, Sukhova GK, Yang M, Kyttaris VC, Stillman IE, Gelb B, Libby P, Tsokos GC, Shi G-P. Cathepsin K deficiency ameliorates systemic lupus erythematosus-like manifestations in Faslpr mice. J Immunol. 2017 doi: 10.4049/jimmunol.1501145. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean GS, Tyrrell-Price J, Crawley E, Isenberg DA. Cytokines and systemic lupus erythematosus. Ann Rheum Dis. 2000;59:243–251. doi: 10.1136/ard.59.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Xu Z, Sui M, Han J, Sun L, Jia X, Zhang H, Han C, Jin X, Gao F, Liu Y, Li Y, Cao J, Ling H, Zhang F, Ren H. Co-Positivity for Anti-dsDNA, -Nucleosome and -Histone Antibodies in Lupus Nephritis Is Indicative of High Serum Levels and Severe Nephropathy. PLoS One. 2015;10:e0140441. doi: 10.1371/journal.pone.0140441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong EB, Khan TN, Mohan C, Rahman ZS. The lupus-prone NZM2410/NZW strain-derived Sle1b sublocus alters the germinal center checkpoint in female mice in a B cell-intrinsic manner. J Immunol. 2012;189:5667–5681. doi: 10.4049/jimmunol.1201661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershberg RM, Framson PE, Cho DH, Lee LY, Kovats S, Beitz J, Blum JS, Nepom GT. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204–215. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rupanagudi KV, Kulkarni OP, Lichtnekert J, Darisipudi MN, Mulay SR, Schott B, Gruner S, Haap W, Hartmann G, Anders HJ. Cathepsin S inhibition suppresses systemic lupus erythematosus and lupus nephritis because cathepsin S is essential for MHC class II-mediated CD4 T cell and B cell priming. Ann Rheum Dis. 2015;74:452–463. doi: 10.1136/annrheumdis-2013-203717. [DOI] [PubMed] [Google Scholar]
- 32.Sette A, Southwood S, Miller J, Appella E. Binding of major histocompatibility complex class II to the invariant chain-derived peptide, CLIP, is regulated by allelic polymorphism in class II. J Exp Med. 1995;181:677–683. doi: 10.1084/jem.181.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avva RR, Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994;1:763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 34.Teodorescu M. Clinical value of anti-ssDNA (denatured DNA) autoantibody test: beauty is in the eyes of the beholder. Clin Appl Immunol Rev. 2002;2:115–128. [Google Scholar]
- 35.Kaburaki J, Kuwana M, Ogasawara T, Takano M, Funatsu Y, Tojo T. Specificity of antibodies to single-stranded (ss) DNA in SLE patients with anti-phospholipid syndrome. Keio J Med. 1992;41:10–15. doi: 10.2302/kjm.41.10. [DOI] [PubMed] [Google Scholar]
- 36.Pavlovic M, Kats A, Cavallo M, Chen R, Hartmann JX, Shoenfeld Y. Pathogenic and Epiphenomenal Anti-DNA Antibodies in SLE. Autoimmune Dis. 2010;2011:462841. doi: 10.4061/2010/462841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cozzani E, Drosera M, Gasparini G, Parodi A. Serology of Lupus Erythematosus: Correlation between Immunopathological Features and Clinical Aspects. Autoimmune Dis. 2014;2014:321359. doi: 10.1155/2014/321359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alba P, Bento L, Cuadrado MJ, Karim Y, Tungekar MF, Abbs I, Khamashta MA, D’Cruz D, Hughes GR. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann Rheum Dis. 2003;62:556–560. doi: 10.1136/ard.62.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards HB, Satoh M, Jennette JC, Croker BP, Yoshida H, Reeves WH. Interferon-gamma is required for lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 41.Manolova I, Gerenova J, Ivanova M. Serum levels of transforming growth factor-beta1 (TGF-beta1) in patients with systemic lupus erythematosus and Hashimoto’s thyroiditis. Eur Cytokine Netw. 2013;24:69–74. doi: 10.1684/ecn.2013.0331. [DOI] [PubMed] [Google Scholar]
- 42.Hammad AM, Youssef HM, El-Arman MM. Transforming growth factor beta 1 in children with systemic lupus erythematosus: a possible relation with clinical presentation of lupus nephritis. Lupus. 2006;15:608–612. doi: 10.1177/0961203306071873. [DOI] [PubMed] [Google Scholar]
- 43.Saxena V, Lienesch DW, Zhou M, Bommireddy R, Azhar M, Doetschman T, Singh RR. Dual roles of immunoregulatory cytokine TGF-beta in the pathogenesis of autoimmunity-mediated organ damage. J Immunol. 2008;180:1903–1912. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto M, Serada S, Mihara M, Uchiyama Y, Yoshida H, Koike N, Ohsugi Y, Nishikawa T, Ripley B, Kimura A, Kishimoto T, Naka T. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–3719. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 45.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 46.Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18:565–570. [PubMed] [Google Scholar]
- 47.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki H, Yasukawa K, Saito T, Narazaki M, Hasegawa A, Taga T, Kishimoto T. Serum soluble interleukin-6 receptor in MRL/lpr mice is elevated with age and mediates the interleukin-6 signal. Eur J Immunol. 1993;23:1078–1082. doi: 10.1002/eji.1830230515. [DOI] [PubMed] [Google Scholar]
- 49.Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991;3:273–278. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- 50.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, V, Kyttaris C, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 53.Altman A, Theofilopoulos AN, Weiner R, Katz DH, Dixon FJ. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981;154:791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wofsy D, Murphy ED, Roths JB, Dauphinee MJ, Kipper SB, Talal N. Deficient interleukin 2 activity in MRL/Mp and C57BL/6J mice bearing the lpr gene. J Exp Med. 1981;154:1671–1680. doi: 10.1084/jem.154.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davignon JL, Cohen PL, Eisenberg RA. Rapid T cell receptor modulation accompanies lack of in vitro mitogenic responsiveness of double negative T cells to anti-CD3 monoclonal antibody in MRL/Mp-lpr mice. J Immunol. 1988;141:1848–1854. [PubMed] [Google Scholar]
- 56.Cheng LE, Ohlen C, Nelson BH, Greenberg PD. Enhanced signaling through the IL-2 receptor in CD8+ T cells regulated by antigen recognition results in preferential proliferation and expansion of responding CD8+ T cells rather than promotion of cell death. Proc Natl Acad Sci U S A. 2002;99:3001–3006. doi: 10.1073/pnas.052676899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tenbrock K, Tsokos GC. Transcriptional regulation of interleukin 2 in SLE T cells. Int Rev Immunol. 2004;23:333–345. doi: 10.1080/08830180490452558. [DOI] [PubMed] [Google Scholar]
- 58.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol. 2001;166:4216–4222. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- 59.Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, Fleisher T, Balow JE, Lipsky PE. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T, Orinska Z, Bulfone-Paus S, Scheffold A. IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38:1643–1653. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Pan HF, Cen H, Tian J, Ma Y, Tao JH, Ye DQ. Interleukin-21 as a potential therapeutic target for systemic lupus erythematosus. Mol Biol Rep. 2011;38:4077–4081. doi: 10.1007/s11033-010-0527-y. [DOI] [PubMed] [Google Scholar]
- 62.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O’Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 64.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179:7949–7958. doi: 10.4049/jimmunol.179.11.7949. [DOI] [PubMed] [Google Scholar]
- 66.Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fransen JH, van der Vlag J, Ruben J, Adema GJ, Berden JH, Hilbrands LB. The role of dendritic cells in the pathogenesis of systemic lupus erythematosus. Arthritis Res Ther. 2010;12:207. doi: 10.1186/ar2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol. 2006;177:5878–5889. doi: 10.4049/jimmunol.177.9.5878. [DOI] [PubMed] [Google Scholar]
- 69.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 70.Chan VS, Tsang HH, Tam RC, Lu L, Lau CS. B-cell-targeted therapies in systemic lupus erythematosus. Cell Mol Immunol. 2013;10:133–142. doi: 10.1038/cmi.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rovere P, V, Zimmermann S, Forquet F, Demandolx D, Trucy J, Ricciardi-Castagnoli P, Davoust J. Dendritic cell maturation and antigen presentation in the absence of invariant chain. Proc Natl Acad Sci U S A. 1998;95:1067–1072. doi: 10.1073/pnas.95.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koonce CH, Wutz G, Robertson EJ, Vogt AB, Kropshofer H, Bikoff EK. DM loss in k haplotype mice reveals isotype-specific chaperone requirements. J Immunol. 2003;170:3751–3761. doi: 10.4049/jimmunol.170.7.3751. [DOI] [PubMed] [Google Scholar]
- 73.Foote A, Briganti EM, Kipen Y, Santos L, Leech M, Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol. 2004;31:268–273. [PubMed] [Google Scholar]
- 74.Leng L, Chen L, Fan J, Greven D, Arjona A, Du X, Austin D, Kashgarian M, Yin Z, Huang XR, Lan HY, Lolis E, Nikolic-Paterson D, Bucala R. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J Immunol. 2011;186:527–538. doi: 10.4049/jimmunol.1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoi AY, Hickey MJ, Hall P, Yamana J, O’Sullivan KM, Santos LL, James WG, Kitching AR, Morand EF. Macrophage migration inhibitory factor deficiency attenuates macrophage recruitment, glomerulonephritis, and lethality in MRL/lpr mice. J Immunol. 2006;177:5687–5696. doi: 10.4049/jimmunol.177.8.5687. [DOI] [PubMed] [Google Scholar]
- 76.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 77.Beers C, Burich A, Kleijmeer MJ, Griffith JM, Wong P, Rudensky AY. Cathepsin S controls MHC class II-mediated antigen presentation by epithelial cells in vivo. J Immunol. 2005;174:1205–1212. doi: 10.4049/jimmunol.174.3.1205. [DOI] [PubMed] [Google Scholar]
- 78.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells - polarity and complexity. Immunol Today. 2000;21:123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 79.Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM. Expression of MHC class II-associated invariant chain (Ii;CD74) in thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2000;8:210–215. doi: 10.1097/00129039-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Barrera CA, Beswick EJ, Sierra JC, Bland D, Espejo R, Mifflin R, Adegboyega P, Crowe SE, Ernst PB, Reyes VE. Polarized expression of CD74 by gastric epithelial cells. J Histochem Cytochem. 2005;53:1481–1489. doi: 10.1369/jhc.4A6552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frolich D, Blassfeld D, Reiter K, Giesecke C, Daridon C, Mei HE, Burmester GR, Goldenberg DM, Salama A, Dorner T. The anti-CD74 humanized monoclonal antibody, milatuzumab, which targets the invariant chain of MHC II complexes, alters B-cell proliferation, migration, and adhesion molecule expression. Arthritis Res Ther. 2012;14:R54. doi: 10.1186/ar3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Chang CH, Stein R, Cardillo TM, Gold DV, Goldenberg DM. Prevention of acute graft-versus-host disease in a xenogeneic SCID mouse model by the humanized anti-CD74 antagonistic antibody milatuzumab. Biol Blood Marrow Transplant. 2013;19:28–39. doi: 10.1016/j.bbmt.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, Lucas DM, Muthusamy N, Goldenberg DM, Lee RJ, Byrd JC. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood. 2010;116:2554–2558. doi: 10.1182/blood-2009-11-253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao Y, Triantafillou G, Hertlein E, Towns W, Stefanovski M, Mo X, Jarjoura D, Phelps M, Marcucci G, Lee LJ, Goldenberg DM, Lee RJ, Byrd JC, Muthusamy N. Milatuzumab-conjugated liposomes as targeted dexamethasone carriers for therapeutic delivery in CD74+ B-cell malignancies. Clin Cancer Res. 2013;19:347–356. doi: 10.1158/1078-0432.CCR-12-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Govindan SV, Cardillo TM, Sharkey RM, Tat F, Gold DV, Goldenberg DM. Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol Cancer Ther. 2013;12:968–978. doi: 10.1158/1535-7163.MCT-12-1170. [DOI] [PubMed] [Google Scholar]
- 86.Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, Hertlein E, Lustberg ME, Quinion C, Zhang X, Lozanski G, Muthusamy N, Praetorius-Ibba M, O’Connor OA, Goldenberg DM, Byrd JC, Blum KA, Baiocchi RA. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. 2011;117:4530–4541. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, Mani R, Mao Y, Yu B, Quinion C, Towns WH, Chen CS, Goldenberg DM, Blum KA, Byrd JC, Muthusamy N, Praetorius-Ibba M, Baiocchi RA. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893–6903. doi: 10.1182/blood-2011-06-363879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaufman JL, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H, Wegener WA, Goldenberg DM. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br J Haematol. 2013;163:478–486. doi: 10.1111/bjh.12565. [DOI] [PubMed] [Google Scholar]
- 89.Martin P, Furman RR, Rutherford S, Ruan J, Ely S, Greenberg J, Coleman M, Goldsmith SJ, Leonard JP. Phase I study of the anti-CD74 monoclonal antibody milatuzumab (hLL1) in patients with previously treated B-cell lymphomas. Leuk Lymphoma. 2015;56:3065–3070. doi: 10.3109/10428194.2015.1028052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta P, Goldenberg DM, Rossi EA, Cardillo TM, Byrd JC, Muthusamy N, Furman RR, Chang CH. Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas. Blood. 2012;119:3767–3778. doi: 10.1182/blood-2011-09-381988. [DOI] [PubMed] [Google Scholar]
- 91.Christian BA, Poi M, Jones JA, Porcu P, Maddocks K, Flynn JM, Benson DM, Jr, Phelps MA, Wei L, Byrd JC, Wegener WA, Goldenberg DM, Baiocchi RA, Blum KA. The combination of milatuzumab, a humanized anti-CD74 antibody, and veltuzumab, a humanized anti-CD20 antibody, demonstrates activity in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Br J Haematol. 2015;169:701–710. doi: 10.1111/bjh.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]