Abstract
Background
Artificial nutrition support is central to the care of critically ill patients and is primarily provided enterally (EN). There are circumstances when parenteral nutrition (PN) is considered necessary. We are uncertain how each of these approaches confer clinical benefits beyond simply providing calories. We sought to better understand how each of these techniques influence metabolism in critically-ill patients using a broad-based metabolomics approach. Metabolic responses to EN and PN may differ in ways that could help us understand how to optimize use of these therapies.
Methods
We prospectively enrolled subjects over 7 months in 2015 at an urban, level-one trauma center. Subjects were included prior to starting either EN or PN during their inpatient admission. Plasma samples were obtained between 1–12 hours before initiation of artificial nutrition, and 3 and 7 days later. All samples were analyzed with liquid chromatography / mass-spectrometry-based metabolomics. Differences in metabolite concentrations were assessed via principal component analyses and multiple linear regression.
Results
We enrolled 30 subjects. Among the critically-ill subjects, 10 received EN and 10 received PN. In subjects receiving EN, amino acid and urea cycle metabolites (citrulline, p=0.04; ornithine, p=0.05) increased, as did ribonucleic acid metabolites (uridine, p=0.04; cysteine, 0=0.05; oxypurinol, p=0.04). Oxidative stress decreased over time. (increased betaine, p=0.05; decreased 4-pyridoxic acid, p=0.04). In subjects receiving PN amino acid concentrations increased over time (taurine, p=0.04; phenylalanine, p=0.05); omega 6 and omega 3 fatty acid concentrations decreased over time (p=0.05 and 0.03, respectively).
Conclusion
EN was associated with amino-acid repletion, urea cycle upregulation, restoration of antioxidants, and increasing RNA synthesis. Parenteral nutrition was associated with increased amino acid concentrations, but did not influence protein metabolism or antioxidant repletion. This suggests that parenteral amino acids are utilized less effectively than those given enterally. The biomarkers reported in this study may be useful in guiding nutrition therapy for critically-ill patients.
Level of Evidence
III, Study Type: Diagnostic Tests or Criteria
Keywords: Enteral Nutrition, Parenteral Nutrition, Metabolomics, Metabolism, Critical-illness
BACKGROUND
Nutrition therapy is important in the care of critically-ill surgical patients. Early enteral nutrition has a favorable impact on clinical outcomes such as nosocomial infections, duration of mechanical ventilation, length-of-stay, and mortality.1–3 However, published studies have led to varying recommendations for the optimal amount, type, route and timing of nutritional support.1,3–9 In particular, there is continuing controversy regarding the ideal clinical context for use of parenteral nutrition (PN).1 Enteral nutrition (EN) is generally the preferred form of artificial nutritional therapy in surgical critically-ill patients, but PN may be of some benefit in certain circumstances.9 Biologic mechanisms for differences in patient response to PN and EN remain largely unclear.
Ongoing debates in surgical nutrition science may stem, in part, from our inability to reliably characterize metabolism and to precisely measure responses to nutrition therapy in critically-ill patients.10,11 Although calorimetry and nitrogen-balance studies can provide some guidance, these methods are resource- and time- intensive, making them impractical for daily clinical use.10
Metabolomics, the study of small molecules involved in metabolism, may provide a rapid and comprehensive ‘snapshot’ of physiology in critically-ill patients.12 In this study, we aimed to understand how EN and PN influence metabolic pathways in critically-ill patients using a broad-based metabolomics approach. We hypothesized that metabolism of amino acids after initiation of EN and PN would be quantifiably different. Insights into these metabolic differences may help us understand how to optimize use of these therapies, thereby minimizing complications and improving outcomes.
METHODS
Study design, subjects and setting
Critically-ill trauma and surgical patients admitted to Harborview Medical Center from May 2015 to November 2015 were consented and enrolled in this prospective cohort study. Inclusion criteria were: a) admission to the surgical or trauma ICU, b) impending initiation of nutrition support, with ability to donate a blood sample 1–12 hours before initiation and c) age between 18 and 65 years. Ten healthy volunteers were also enrolled from among the hospital staff. These volunteers fasted for 12 hours, but were not hospitalized and were not on artificial nutrition. This group was included to help determine whether a given metabolite in a hospitalized patient had a higher or lower concentration than a ‘normal’ non-hospitalized subject. However, no direct statistical comparisons were made using these non-hospitalized volunteers. Any subject was excluded if she/he had a history of cancer, endocrine disorders, or chronic organ dysfunction, or if they were pregnant, obese (body mass index ≥ 35 m/kg2), had an active infection requiring antibiotic treatment, or had undergone an abdominal/orthopedic operation within 24 hours of sampling. These exclusion criteria were specified to reduce confounding factors, consistent with established procedures in other metabolomics studies.12,13 Patients who undergo exploratory laparotomy, pelvic fixation or open long-bone fixation develop a postoperative inflammation response which is expected to be much more pronounced compared to other surgical procedures.13 Therefore, postoperative patients from these groups were specifically excluded. All study procedures were approved by the University of Washington institutional review board.
Sample collection and processing
Venous blood samples from critically-ill subjects were obtained between 1–12 hours prior to nutrition initiation in 5ml K2 EDTA Vacutainer tubes (Franklin Lakes, NJ), and on day three and day seven after starting nutrition. Blood samples from healthy volunteers were obtained the morning after at least 12 hours of fasting. Samples were placed on ice and centrifuged at 2500g for 10 min. The plasma supernatants were then frozen at −80 °C.
Metabolite measurements
Mass-spectrometry based analysis occurred for all plasma samples after thawing. Our detailed protocol for sample processing and metabolomics analysis has been previously reported.11 Briefly, after metabolite extraction, samples underwent targeted liquid chromatography (LC) and quadrupole MS (Sciex 5500 Qtrap), and metabolites were identified by comparison with known standards to obtain relative concentrations. Quality control samples derived from the pooled plasma sample were included in all processing. We measured 214 previously identified metabolites from over 25 different metabolic pathways (Supplemental Table 1).
Treatment and outcomes
The primary exposures of interest were EN or PN. Main outcomes were concentrations of plasma metabolites. The majority of metabolites were quantified via relative concentrations. Absolute quantification (micro-molar, µM) was possible for some amino acids because isotope-labeled internal standards for these compounds have been developed.14,15 Of note, we did not compare metabolic differences between EN and PN directly; rather, our focus in this study was to compare before and after the initiation of each therapy.
Statistical analysis and data presentation
Continuous data are represented as medians and interquartile ranges. Categorical data are represented as counts and percentages.
Subjects were divided into groups based on receipt of EN versus PN for purposes of analysis. Broad metabolic comparisons before (day 0) and after (day 3 & 7) nutrition support initiation were made using principal component analysis (PCA),12 with oblique rotation to allow for clustering of repeated measures within individuals over time. We focused primarily on these before and after comparisons because the two groups of study subjects were doubtless quite different from a metabolic standpoint.
We also compared the three groups (critically ill subjects prior to initiation of nutritional support) to understand how they differed metabolically. Trends over time were assessed using linear regression with robust standard errors, allowing for clustering of repeated measures within individuals. One model was constructed for subjects on EN and one model was constructed for subjects on PN. The exposure variable corresponded to the time of nutrition initiation (a categorical variable for day 0, day 3 and day 7 of nutrition). Outcome variables were changes in metabolite concentrations over time. The Benjamini-Hochberg false-discovery rate correction16 was applied to all p-values to account for multiple testing, and statistical significance was set at alpha ≤0.05. All statistical analyses were performed using Stata 12.1 (StataCorp., College Station, TX) and the R software environment, version 3.2.3.17 Heat-maps for metabolite concentrations were generated using ‘GENE-E’ software 3.0.2,18 with scaling of relative concentrations by row.
RESULTS
We enrolled 20 critically-ill subjects and 10 healthy volunteers. Demographic and clinical characteristics are shown in Table 1 and summarized here. All 10 subjects on EN were trauma patients, with a median injury severity score (ISS) of 29 (IQR 27–34), and 90% (n=9) were injured by a blunt mechanism. Among subjects on PN, 50% (n=5) were admitted for trauma with a median ISS of 34 (IQR33–41), and 50% (n=5) were admitted for a non-trauma related operations. The majority of subjects in the EN group started nutrition due to tracheal intubation, while the majority of those in the PN group started nutrition due to ileus/enteral intolerance (Table 1). Subjects in the EN group started nutrition earlier in the hospital course (median hospital day 2.5 (IQR 5–9)) compared to subjects in the PN group (median hospital day 7 (IQR 5–9)).
Table 1.
Demographic and clinical characteristics for subjects started on nutrition therapy at a level-one trauma hospital, and healthy volunteers. All subjects underwent plasma sampling for mass-spectrometry-based metabolomics.a
| Characteristic | Enteral (n=10) | Parenteral (n=10) | Volunteer (n=10) |
|---|---|---|---|
| Age | 42.5 (31–56) | 39 (25–52) | 36 (30–47) |
| Female sex | 4 (40) | 4 (40) | 5 (50) |
| BMI | 27 (21–32) | 26 (22–31) | 26 (25–27) |
| Admission base deficit | 5.8 (3.8–7.4) | 8.3 (4.8–10.8) | |
| Hospital day that nutrition was started | 2.5 (2–4) | 7 (5–9) | |
| Indications for admission | |||
| Blunt trauma | 9 (90) | 3 (30) | |
| Emergent non-trauma operation | 0 | 4 (40) | |
| Penetrating trauma | 1 (10) | 2 (20) | |
| Non-emergent general surgery operation | 0 | 1 (10) | |
| Mechanism of injury | |||
| MVC | 3 (30) | 1(10) | |
| MCC | 2 (20) | 1 (10) | |
| Fall | 4 (40) | 2 (20) | |
| GSW or stab | 1 (10) | 1 (10) | |
| ISS | 29 (27–34) | 34 (33–41) | |
| Severe injury(AIS >=3) | |||
| Head | 4 (40) | 2 (20) | |
| Chest | 4 (40) | 1 (10) | |
| Abdomen | 5 (50) | 4 (40) | |
| Indication for Nutrition | |||
| Ileus/enteral intolerance | 0 | 8 (80) | |
| esophageal, stomach, duodenal injury | 1 (10) | 2 (20) | |
| Intubation | 9 (90) | 0 | |
| Nutrition markers | |||
| Albumin (g/dL) | 3.0 (2.6–3.1) | 2.2 (2.1–3.2) | |
| Transthyretin (mg/dL) | 11.8 (8.9–12.4) | 13.7 (6.6–14.1) | |
| C-reactive protein (mg/dL) | 158 (123–173) | 148 (102–155) |
: Categorical data are presented as number (percentage) and continuous data are presented as median (inter-quartile range)
We collected and analyzed 70 plasma samples using mass-spectrometry. Of 214 total metabolites assayed, 102 were reliably identified and quantified. The measurement of the plasma metabolites had high technical reproducibility; the average coefficient of variance (CV) for relative concentrations was 6.3% and for isotope-labeled internal standards, the CV was 3.4%.
Metabolic profile changes after initiation of enteral or parenteral nutrition
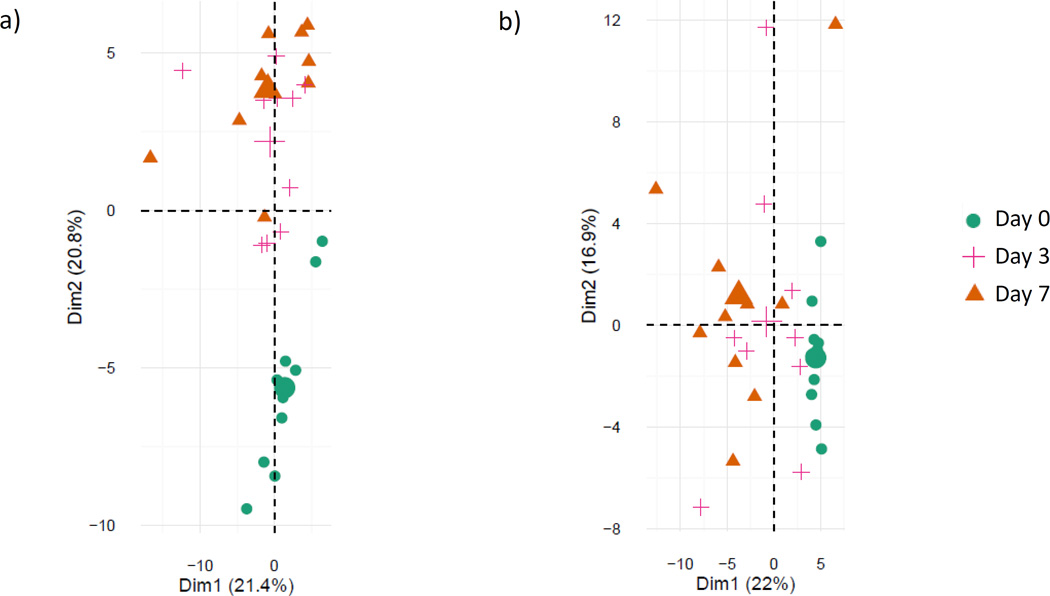
PCA is a method used to recognize statistical patterns in data, and selects metabolites which best explain the overall variation in the data.12 In both EN and PN, PCA demonstrated that plasma metabolic profiles at day 0 (before nutrition) were different from days 3 and 7 (after nutrition) (Figure 1a and 1b).
Figure 1.
Principal component analysis of plasma samples on day 0, day 3 and day 7 for a) subjects started on enteral nutrition and b) subjects started on parenteral nutrition.ϕ
ϕ Each data point represents an individual plasma sample. Points which are close together represent samples with similar metabolic phenotypes; points which are far away have dissimilar metabolic phenotypes. The large symbols represent the mean values of the sample scores for a given group.
For subjects receiving enteral nutrition, the first principal component accounted for 28% of the total variance among day zero, three and seven samples. Changes in N2-N2-dimethylguanosine, 1-methyladenosine, L-kyneurenine, N-acetylneuraminate, and deoxycarnitine contributed to the differences between time-points.
The second principal component accounted for an additional 17% of the variance and included leucine, isoleucine, asparagine, methionine, and arginine. The metabolites from these two components are involved in nucleotide, amino acid, and sugar metabolism.
For subjects receiving parenteral nutrition, changes in 1-methylguanosine, N2-N2-dimethylguanosine, glucoronate, inositol, and cystamine constituted the first principal component, which accounted for 25% of the total variance between days 0, 3 and 7. The second principal component accounted for 22% of the variance and included proline, alanine, glycine, threonine and pipecolate. The metabolites from these two components are all involved in amino acid, nucleotide, and lipid metabolism.
Metabolites which did not vary significantly among subjects over time included those involved with gut microflora metabolism and the pentose phosphate pathway.
Enteral nutrition is associated with increased amino acids, urea cycle products, antioxidants and RNA products
Next, we determined metabolite pathways which differed over time among subjects who started EN. After false-discovery-rate correction, 9 of 102 identified metabolites showed statistically-significant variation over the first week after starting enteral nutrition (Table 2). The initiation of enteral nutrition was associated with a gradual rise in plasma amino acids, urea cycle products and RNA synthetic products over the first week of nutrition (Figure 2a and 2b).
Table 2.
Plasma metabolites that change over time after exposure to a) enteral nutrition or b) parenteral nutrition, from day 0 to day 7.a
| day 0 | day 7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathway | metabolite | median concentrationb |
inter-quartile range | median concentrationb |
inter-quartile range | fold changec | p-value | BH-FDR p-valued | |||
| a) Enteral Nutrition | Citrulline | 5.52E+06 | 5.18E+06 | 8.21E+06 | 8.95E+06 | 7.25E+06 | 1.27E+07 | 0.62 | 0.003 | 0.042 | |
| Urea Cycle | Ornithine | 2.11E+06 | 1.37E+06 | 3.15E+06 | 2.33E+06 | 2.24E+06 | 4.82E+06 | 0.10 | 0.005 | 0.050 | |
| Uridine | 2.76E+04 | 1.85E+04 | 4.31E+04 | 7.49E+04 | 4.58E+04 | 7.73E+04 | 1.71 | <0.001 | 0.041 | ||
| Purine/Pyrimidine | Cystine | 2.08E+06 | 1.65E+06 | 2.90E+06 | 2.74E+06 | 2.15E+06 | 2.97E+06 | 0.31 | 0.003 | 0.051 | |
| Oxypurinol | 2.90E+05 | 2.50E+05 | 3.26E+05 | 3.02E+05 | 2.66E+05 | 4.25E+05 | 0.04 | 0.003 | 0.045 | ||
| Betaine | 2.51E+07 | 1.94E+07 | 3.47E+07 | 3.21E+07 | 2.72E+07 | 3.42E+07 | 0.28 | 0.001 | 0.054 | ||
| Oxidation | Biotin | 1.25E+05 | 9.90E+04 | 2.16E+05 | 3.42E+05 | 2.91E+05 | 4.12E+05 | 1.74 | 0.002 | 0.061 | |
| 4-Pyridoxicacid | 7.11E+05 | 6.34E+05 | 7.60E+05 | 6.33E+05 | 5.80E+05 | 6.68E+05 | −0.11 | 0.003 | 0.043 | ||
| Krebs Cycle | Alpha-Ketoglutaric Acid | 2.41E+06 | 2.19E+06 | 2.64E+06 | 2.27E+05 | 2.20E+06 | 2.81E+06 | −0.91 | 0.005 | 0.053 | |
| Essential Amino Acids | ShikimicAcid | 1.22E+07 | 8.89E+06 | 1.72E+07 | 7.15E+05 | 1.18E+06 | 2.35E+06 | −0.94 | 0.002 | 0.054 | |
| b) Parenteral Nutrition | Purine/Pyrimidine | Urate | 2.96E+07 | 2.72E+07 | 4.21E+07 | 2.76E+07 | 2.19E+07 | 3.28E+07 | −0.068 | 0.001 | 0.031 |
| LinoleicAcid | 4.58E+05 | 3.90E+05 | 5.48E+05 | 5.22E+05 | 2.74E+05 | 6.51E+05 | 0.139 | 0.001 | 0.051 | ||
| Fatty Acids | LinolenicAcid | 1.04E+06 | 9.00E+05 | 1.18E+06 | 6.68E+05 | 5.55E+05 | 7.34E+05 | −0.357 | 0.001 | 0.027 | |
| Carnitine | 1.81E+07 | 1.47E+07 | 1.88E+07 | 2.24E+07 | 2.12E+07 | 3.02E+07 | 0.238 | 0.003 | 0.055 | ||
| Taurine | 8.13E+05 | 3.19E+05 | 9.43E+05 | 8.97E+05 | 6.97E+05 | 1.11E+06 | 0.102 | 0.001 | 0.036 | ||
| Amino Acids | Phenylalanine | 3.59E+07 | 3.29E+07 | 3.65E+07 | 5.09E+07 | 3.48E+07 | 5.73E+07 | 0.418 | 0.003 | 0.053 | |
: Data generated using plasma mass-spectrometry-based metabolomics in subjects started on nutrition support at a level-one trauma center. Trends over time were assessed using linear regression with robust standard errors, allowing for clustering. Exposure: categorical time variable, corresponding to day 0, 3 and 7 after starting enteral nutrition; Outcome variables: metabolites listed above.
: Concentrations are unit-less and are relative to internal standards
: median fold change = median day 7 - median day 0 / median day 0
: Benjamini-Hochberg false discovery rate method for multiple testing
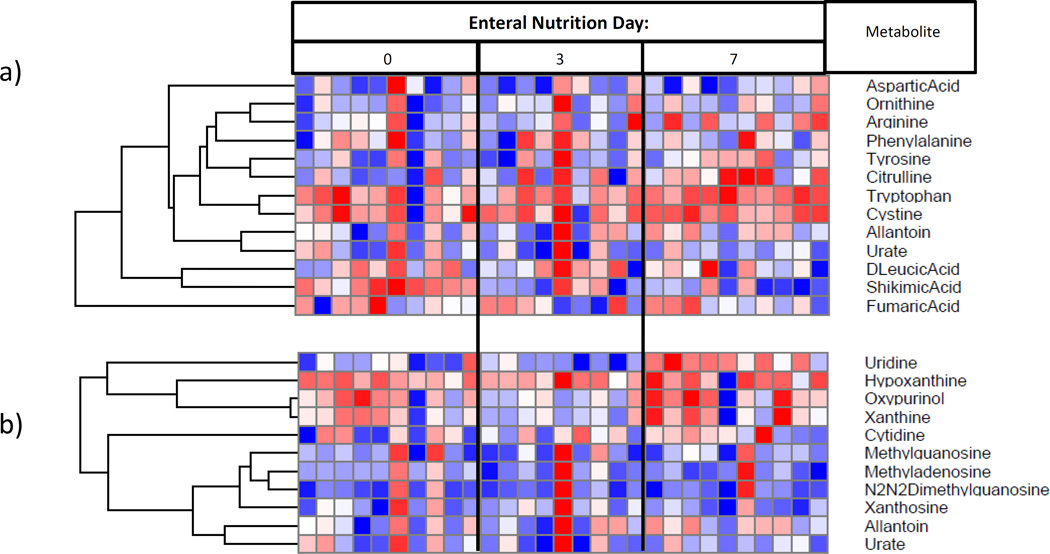
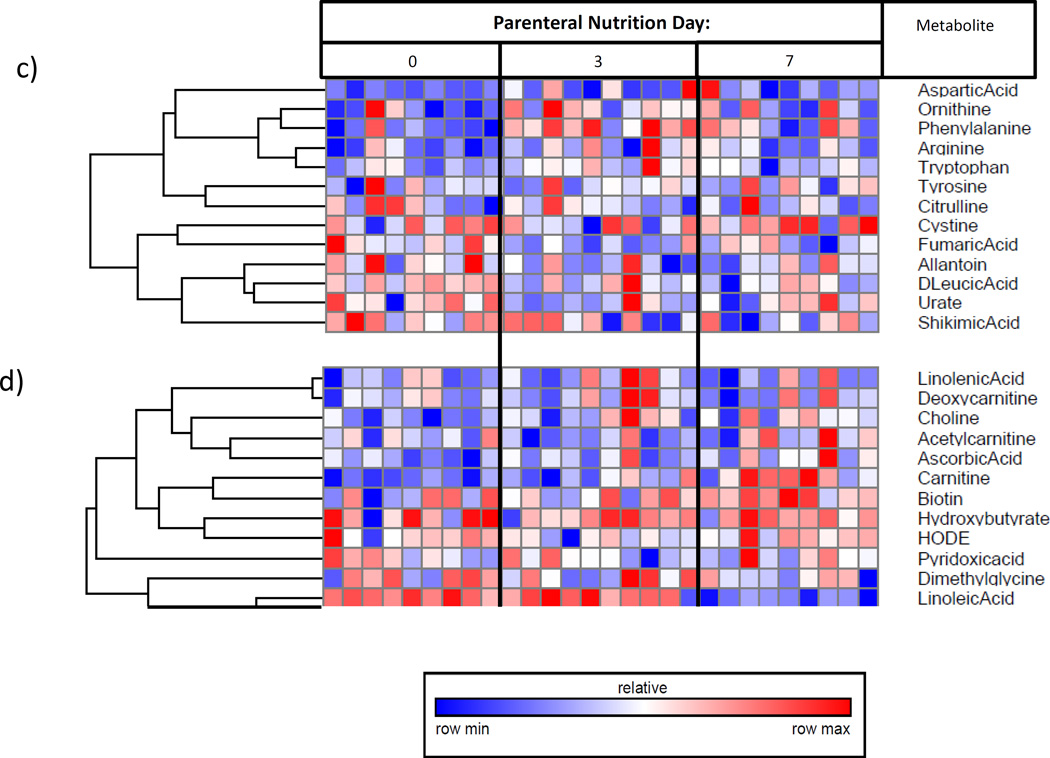
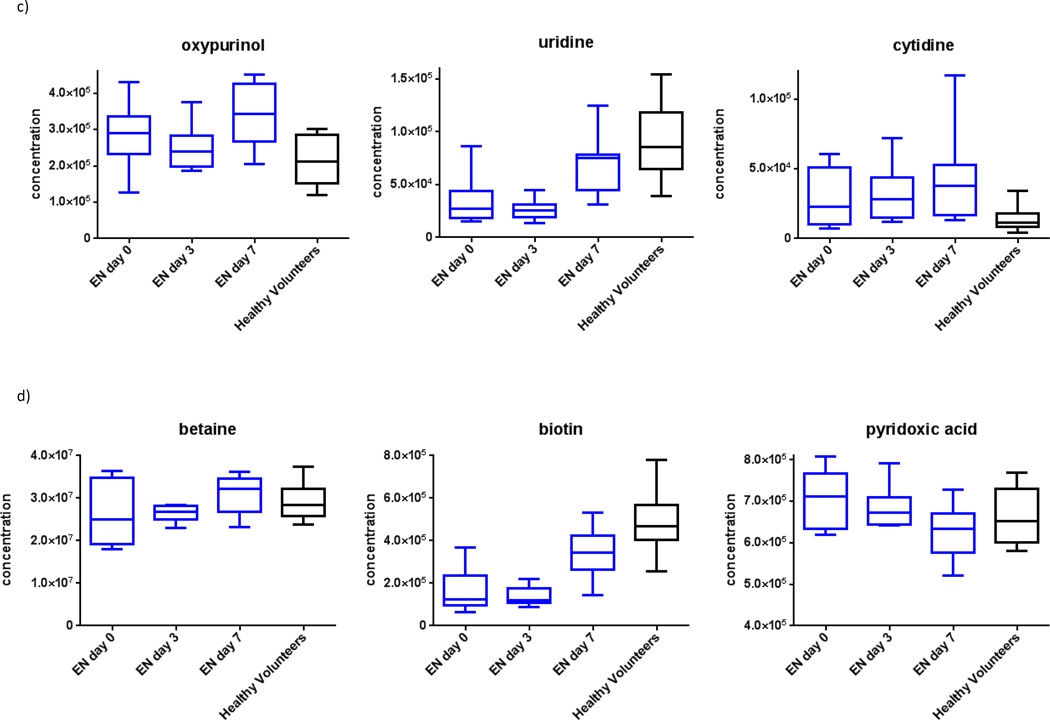
Figure 2.
Plasma metabolome heat-maps for: I) subjects on enteral nutrition over time, demonstrating a) increasing amino acid and urea cycle metabolites, and b) increasing RNA synthetic products; II) subjects on parenteral nutrition over time, demonstrating c) decreasing urea cycle metabolites, and d) decreasing essential fatty acids.ϕ
ϕ Generated using mass-spectrometry based metabolomics. Due to unmeasurably low levels of relevant metabolites, one sample was omitted from parts a) and b), and two samples were omitted from parts c) and d).
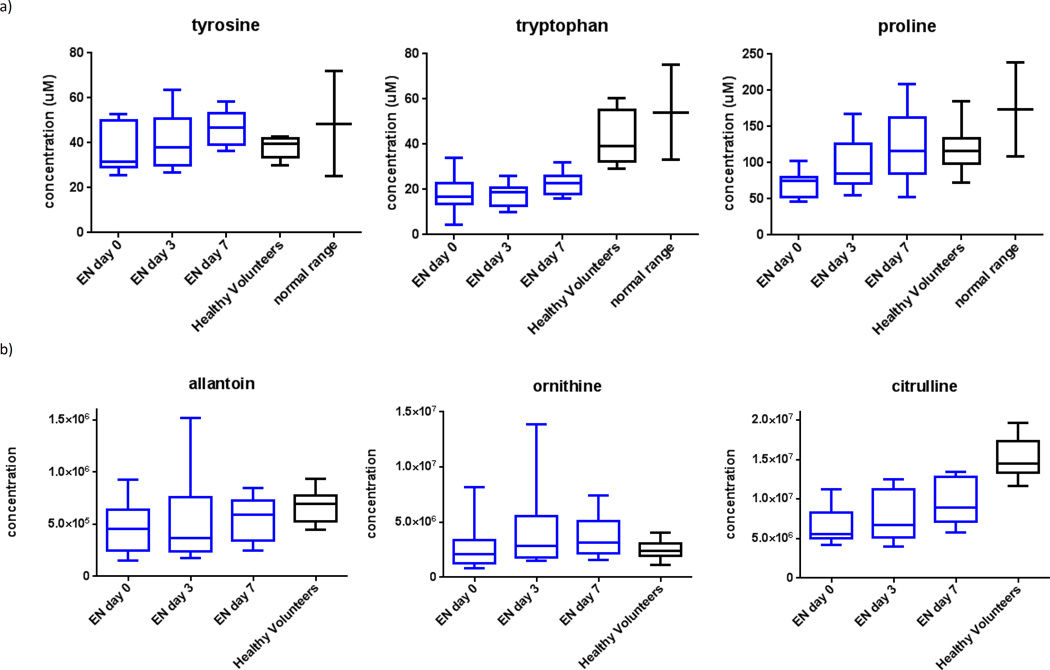
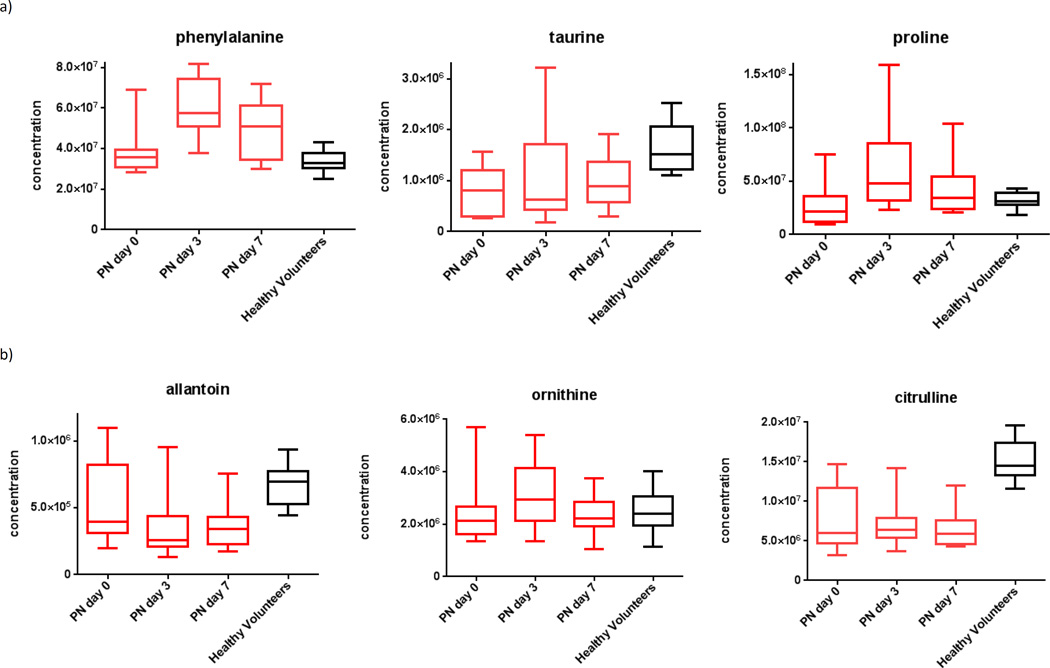
Relative to day zero, samples from day three and seven showed gradually increasing levels of both essential and non-essential amino acids (Figure 3a) and urea cycle metabolites (Figure 3b). Specifically, from day zero to seven, plasma concentrations for both citrulline and ornithine increased (p=0.04 and 0.05, respectively).
Figure 3.
Plasma metabolomics for subjects on enteral nutrition over time, demonstrating a) increasing amino acids, b) increasing urea cycle metabolites, c) increasing RNA synthetic metabolites, and d) restoration of antioxidant balance.ϕ
ϕ Generated using mass-spectrometry based metabolomics.
We also observed increased metabolites related to RNA synthesis (Figure 3c) and attenuation of oxidative stress (Figure 3d). Specific changes in ribonucleic acid (RNA) synthetic plasma metabolites included increases in uridine, cysteine and oxypurinol (p=0.04, 0.05 and 0.05, respectively). Specific changes in oxidation metabolites included increases in betaine and biotin, and a decrease in 4-pyridoxic acid (p=0.05, 0.06, and 0.04, respectively).
Parenteral nutrition is associated with increased amino acids, decreased urea cycle products and decreased essential fatty acids
We then determined metabolite pathways which differed over time among subjects who started PN. Overall, after false-discovery-rate correction, 5 of 102 identified metabolites showed statistically-significant variation over the first week after exposure to PN (Table 2). Generally, the initiation of PN was also associated with increased concentrations of plasma amino acids. However, subjects also showed decreased concentrations of urea cycle metabolites and essential fatty acids over time (Figure 2c and 2d).
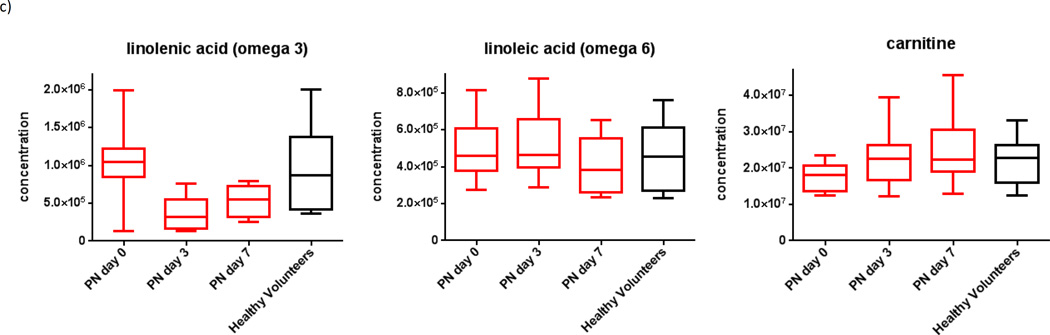
Relative to day zero, samples from days three and seven showed a gradual rise in both essential and non-essential amino acids (Figure 4a), decreased urea cycle metabolites (Figure 4b), and decreasing essential fatty-acids (Figure 4c). Specifically, from day zero to seven, subjects who started PN demonstrated rising plasma concentrations for taurine and phenylalanine (p=0.04 and 0.05, respectively), decreasing levels of urate (p=0.03), and decreasing omega-6 and omega-3 fatty acids (p=0.05 and 0.03, respectively).
Figure 4.
Plasma metabolomics for subjects on enteral nutrition over time, demonstrating a) increasing amino acids, b) low levels of urea cycle metabolites, and c) decreasing essential omega fatty-acids and increased endogenous fatty-acid transport (carnitine).ϕ
ϕ Generated using mass-spectrometry based metabolomics.
DISCUSSION
In this study of critically-ill surgical patients, we applied broad-based metabolomics in a novel way to determine the metabolic response to artificial nutritional support. The institution of EN was associated with a number of metabolic changes including amino-acid repletion, urea cycle upregulation, antioxidant restoration, and increased RNA synthesis, which, taken together, reflect anabolism. Subjects receiving parenteral nutrition had fewer changes in metabolic pathways. While there was evidence of amino acid repletion, this could simply be measurement of the amino acids that were infused as part of the parenteral formula. The reductions in circulating fatty acids perhaps reflect intermittent administration of lipids; of note, omega-6 fatty acids are absent in parenteral lipids. These observations suggest that parenteral nutrients do not promote urea cycling, antioxidant metabolism, or RNA transcription to a similar degree that enteral nutritional support does. This supports the notion that parenteral nutrients are not utilized as effectively as enteral nutrients.2
Initiation of EN was associated with restoration of antioxidant equilibrium over time. This was seen as progressively increasing levels of vitamin and antioxidant substrates (betaine and biotin), and diminishing levels of antioxidant catabolites (4-pyridoxic acid). Biotin is known as vitamin B7 and is a cofactor in carboxylase-based reactions in protein lysis.19 Betaine is an anti-oxidant and methyl-donor which is thought to protect against osmotic stress, modulate inflammation, and regulate lipid metabolism.20 Finally, 4-pyridoxic acid is a catabolite of vitamin B6 involved in nicotinamide metabolism.19 Restoration of these antioxidants with EN may be particularly relevant for trauma patients, who generally have ongoing depletion of antioxidant stores over the first week of injury.11
Of note, prior studies have found similar associations with EN and antioxidant repletion. For example, Windsor and colleagues studied 34 patients with acute pancreatitis who started on EN or PN, and found that the group on EN had lower markers of acute inflammation and an increased total antioxidant capacity.21 Other systemic reviews have also confirmed that EN appears to restore antioxidant balance more effectively than parenteral nutrition.22
Plasma amino acid concentrations and urea cycle products increased after starting enteral nutrition and approached levels measured in healthy volunteers. This suggests that EN is associated with restoration of circulating amino acids, coupled with processing of excess amino acids to their end-products in the urea cycle.19 This interpretation is consistent with prior literature showing that gut absorption of amino acids is tightly regulated to maintain a steady-state in the plasma, and any excess enteral amino acids are catabolized directly to urea cycle end-products in the liver.23,24 The increases in urea cycle products likely indicate that enteral amino acids are effectively utilized as a source for energy.
In contrast, subjects on PN showed plasma amino acid concentrations which were often higher than those in healthy volunteers, and urea-cycle products that were not clearly increased. These data are consistent with the fact that PN is not subject to the same hepatic ‘first-pass’ effect as EN,23 and therefore subjects on PN do not have tightly regulated plasma amino acid concentrations, or shunting of excess amino-acids directly to the urea cycle. Therefore, it appears that parenteral infusion can increase amino acid concentrations, but these amino acids are not efficiently metabolized. Our data are corroborated by a prior study of 49 trauma patients and 43 healthy volunteers, where PN initiation was associated with higher plasma levels of amino acids.25
As expected, subjects on PN showed down-trending levels of essential omega fatty-acids, which are not supplemented in our PN formulas. In addition, PN subjects showed increasing levels of carnitine, which is an amino acid involved in fatty acid transport from plasma to both skeletal and smooth muscle.19,26 A gradual increase in carnitine can be expected in subjects who receive regular intravenous lipid infusions, where continuous transport of lipid out of the intravascular space must occur.
As previously described,10,11,27 the evaluation of metabolic response to nutrition therapy in critically-ill patients is limited by time- and resource-intensive tools like calorimetry and nitrogen-balance studies. With recent innovations in mass-spectrometry-based metabolomics, the biomarkers reported in this study can now be obtained in approximately 3 hours.28 This makes bedside application of this tool a real possibility. In the near future, metabolomics could be used to identify nutritionally ‘high-risk’ patients, to quantify metabolic response to therapy, and to help guide titration of calories, protein, and micronutrients based on individual patient profiles.
Several limitations are relevant to the interpretation of this study and are related to the current capabilities of mass-spectrometry based metabolomics. First, all metabolite changes in this study should be interpreted with caution. Metabolites are often involved in multiple pathways, and change in a metabolite’s concentration could represent a change in utilization, or a change in production, or both. Therefore, individual metabolite changes should be interpreted in concert with other metabolites in the pathways of interest.29 Second, given the large quantity of data and multiple analyses in this study, there are more opportunities for random highly biased results (false positives). We have partly accounted for this fact using a false-discovery-rate correction in all our analyses, but our findings still need to be replicated in independent larger studies before any definitive conclusions are drawn. Third, observed effects may be due differences between the subjects in the two cohorts. For example, patients receiving parenteral nutrition were more likely to have an ileus, gastrointestinal tract dysfunction, and perhaps an altered microbiome; these may have influenced the metabolic response to nutrients, irrespective of the route of administration. However, we accounted for most other major confounding factors by excluding those subjects with cancer, chronic organ dysfunction, pregnancy, obesity, active infection, or a recent major operation. Fourth, it is possible that observed effects are due to differences in timing of nutrition initiation, which could lead to bias. However, we attempted to account for such individual variation by adjusting our analyses for clustering of serial observations within subjects,30 thereby accounting for potential bias due to individual factors like nutrition timing.
The metabolic response to enteral nutrition includes a cascade of events related to amino-acid metabolism, urea cycling, RNA synthesis, and antioxidant repletion. Parenteral nutrition appears to increase plasma amino acid concentrations, without concomitant increase in their metabolism. Also, fatty-acid concentrations dropped markedly. This suggests that parenteral nutrients are utilized less effectively than enteral nutrients. Biomarkers reported in this study may eventually become clinically useful in guiding nutrition therapy for critically-ill patients.
Supplementary Material
Acknowledgments
This article was prepared with financial support from the National Institute of Health grant 2T32 GM007037 (GEO and BP). The authors wish to thank Lauren Jacobson, MS, Peter Louras, MS and Laura Hennessey, RN for their contributions to sample collection for this study. We also wish to thank Sandra Navarro, PhD, for her contributions to the analysis of these data.
Footnotes
This study was presented at the 75th annual meeting of the American Association for the Surgery of Trauma, September 13–16, 2016, in Waikoloa, Hawaii.
Conflicts of interest and disclosures: There are no additional conflicts of interest declared by the authors.
AUTHOR CONTRIBUTION STATEMENT:
Dr. Brodie Parent and Dr. Grant O’Keefe contributed to literature search, study design, data collection, data analysis, data interpretation, writing and critical revision of the manuscript. Ms. Brittany Wheelock contributed to literature search, data collection, and critical revision of the manuscript. Dr. Max Seaton, Dr. Danijel Djukovic, Dr. Haiwei Gu, and Dr. Daniel Raftery contributed to study design, data collection, data analysis, data interpretation, and critical revision of the manuscript.
REFERENCES
- 1.Singer P, Pichard C. Reconciling divergent results of the latest parenteral nutrition studies in the ICU. Curr Op Clin Nutr. 2013;16(2):187–193. doi: 10.1097/MCO.0b013e32835c34be. [DOI] [PubMed] [Google Scholar]
- 2.Huynh D, Chapman MJ, Nguyen NQ. Nutrition support in the critically ill. Curr Op Gastroenterol. 2013;29(2):208–215. doi: 10.1097/MOG.0b013e32835c9c83. [DOI] [PubMed] [Google Scholar]
- 3.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer P, Anbar R, Cohen J, Sharpio H, Shalita-Chesner M, Lev S, Grozovski E, Theilla M, Frishman S, Madar Z. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Int Care Med. 2011;37(4):601–609. doi: 10.1007/s00134-011-2146-z. [DOI] [PubMed] [Google Scholar]
- 5.Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, Vale LD, Battison CG, Jenkinson DJ, Cook JA. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ. 2011;342:d1542. doi: 10.1136/bmj.d1542. [DOI] [PubMed] [Google Scholar]
- 6.Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, Davies AR, O'Leary M, Solano T, Peake S. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013;309(20):2130–2138. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 7.Choi EY, Park DA, Park J. Calorie Intake of Enteral Nutrition and Clinical Outcomes in Acutely Critically Ill Patients: A Meta-Analysis of Randomized Controlled Trials. JPEN. 2014 doi: 10.1177/0148607114544322. [DOI] [PubMed] [Google Scholar]
- 8.Chung CK, Whitney R, Thompson CM, Pham TN, Maier RV, O'Keefe GE. Experience with an enteral-based nutritional support regimen in critically ill trauma patients. J Am Coll Surgeons. 2013;217(6):1108–1117. doi: 10.1016/j.jamcollsurg.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Constanza MC, Thibault R, Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 10.Ferrie S, Allman-Farinelli M. Commonly used "nutrition" indicators do not predict outcome in the critically ill: a systematic review. Nutr Clin Practr. 2013;28(4):463–484. doi: 10.1177/0884533613486297. [DOI] [PubMed] [Google Scholar]
- 11.Parent BS, M. Sood RF, Gu H, Djukovic D, Raftery D, O'Keefe GE. Use of Metabolomics to Trend Recovery and Therapy After Injury in Critically-Ill Trauma Patients. JAMA Surg. 2016 doi: 10.1001/jamasurg.2016.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Rev Mol Diagn. 2008;8(5):617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao H, Wang H, Wang B, Liu X, Gao H, Xu M, Zhao H, Deng X, Lin D. Systemic metabolic changes of traumatic critically ill patients revealed by an NMR-based metabonomic approach. J Proteome Res. 2009;8(12):5423–5430. doi: 10.1021/pr900576y. [DOI] [PubMed] [Google Scholar]
- 14.Bueschl C, Krska R, Kluger B, Schuhmacher R. Isotopic labeling-assisted metabolomics using LC-MS. Anal Bioanal Chem. 2013;405(1):27–33. doi: 10.1007/s00216-012-6375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu H, Du J, Carnevale NF, Carroll PA, Turner SJ, Chiorean EG, Eisenman RN, Raftery D. Metabolomics method to comprehensively analyze amino acids in different domains. Analyst. 2015;140(8):2726–2734. doi: 10.1039/c4an02386b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 17.Team. RC. R Foundation for Statistical Computing. Vienna, Austria: 2014. R: A language and environment for statistical computing. URL http://www.R-project.org/ [Google Scholar]
- 18.Harvard TBIoMa. GENE-E. 2015 URL: http://www.broadinstitute.org/cancer/software/GENE-E/ Version 3.0.204. [Google Scholar]
- 19.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djombou Y, Mandal R, Aziat F, Dong E, et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acid Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010;43(9):732–744. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, Welsh F, Guilou PJ, Reynolds JV. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42(3):431–435. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29(12):2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Abumrad NN, Miller B. The physiologic and nutritional significance of plasma-free amino acid levels. JPEN. 1983;7(2):163–170. doi: 10.1177/0148607183007002163. [DOI] [PubMed] [Google Scholar]
- 24.Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128(8):1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- 25.Shaw JH, Wolfe RR. An integrated analysis of glucose, fat, and protein metabolism in severely traumatized patients. Studies in the basal state and the response to total parenteral nutrition. Ann Surg. 1989;209(1):63–72. doi: 10.1097/00000658-198901000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wachter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, Krahenbuhl S. Long-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta. 2002;318(1–2):51–61. doi: 10.1016/s0009-8981(01)00804-x. [DOI] [PubMed] [Google Scholar]
- 27.Schlein KM, Coulter SP. Best practices for determining resting energy expenditure in critically ill adults. Nutr Clin Pract. 2014;29(1):44–55. doi: 10.1177/0884533613515002. [DOI] [PubMed] [Google Scholar]
- 28.Rinehart D, Johnson CH, Nguyen T, Ivanisevic J, Benton HP, Lloyd J, Arkin AP, Deutschbauer AM, Patti GJ, Siuzdak G. Metabolomic data streaming for biology-dependent data acquisition. Nat Biotechnol. 2014;32(6):524–527. doi: 10.1038/nbt.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso A, Marsal S, Julia A. Analytical methods in untargeted metabolomics: state of the art in 2015. Front Biom. 2015;3:23. doi: 10.3389/fbioe.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.