Abstract
We have investigated the potential for contrast-enhanced near-infrared fluorescence imaging of tissue on a mobile phone platform. CCD- and phone-based cameras were used to image molded and 3D-printed tissue phantoms, and an ex vivo animal model. Quantitative and qualitative evaluations of image quality demonstrate the viability of this approach and elucidate variations in performance due to wavelength, pixel color and image processing.
Index Terms: Biomedical optical imaging, cellular phone, fluorescence, near-infrared, phantoms, telemedicine
I. Introduction
Many of the features that have led to the commercial success of mobile phones – portability, rapid data transmission and ever-improving capabilities for computation, communication and imaging – also make them an attractive platform for medical diagnostics. As a result, interest in adapting biomedical optical imaging techniques to this platform has been growing rapidly in recent years [1]. Initial successes have been achieved in white-light imaging for ophthalmology [2] and otolaryngology [3]. More advanced mobile biophotonic approaches are also under investigation, including photoplethysmography [4] and visible-wavelength fluorescence imaging for fluorescein fundus photography [5], skin cancer detection [6], monitoring of photodynamic therapy [7], and in vitro diagnostics [8], [9].
Phone cameras employ CMOS detectors that are sensitive to both visible and near-infrared (NIR) light. Typically, short-pass filters are incorporated into these cameras to block NIR wavelengths for consumer photography. We are aware of only one study – on pupil size measurement [10] – that has exploited this NIR detection capability for a medical purpose. By removing the blocking filter, phone cameras have the potential to be used for near-infrared fluorescence (NIRF) imaging or other biophotonic techniques that take advantage of the “optical window” in biological tissue where light penetration is high (and autofluorescence is low).
Clinical NIRF procedures typically involve the use of indocyanine green (ICG) fluorescent dye for visualization of tissue perfusion and discrete vasculature, in surgical guidance [11] and retinal diagnostic [12] applications. NIRF-based approaches have also shown promise for assessing burn depth due to thermal injury and chemical warfare agents [13]. In recent years, revolutionary molecular imaging techniques based on NIRF dyes (e.g., IR800) and nanoparticles have been developed that enable targeted clinical imaging of cancer biomarkers for early detection and surgery [14].
The confluence of NIR biophotonic imaging and mobile phone technology has the potential to vastly improve in vivo (and in vitro) screening and diagnosis. However, ensuring quality in emerging portable, low-cost technologies is critical to public health. Therefore, the purpose of this study was to establish a proof of principle for mobile-phone-based NIRF imaging in biological tissue, and provide quantitative insights into image quality with respect to a scientific CCD. Preliminary versions of this work has been reported [15], [16].
II. Methods
A. Experimental Setup
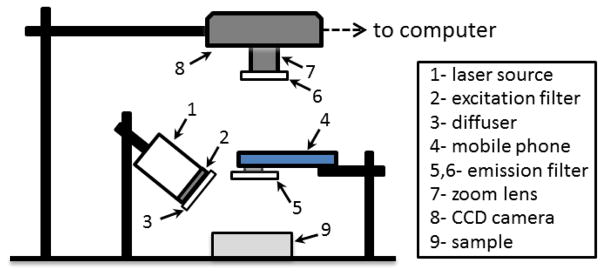
The current study investigates an approach for mobile phone-based NIRF imaging involving an external light source, rather than a fully self-contained imaging system (Fig. 1). The source was a light emitting diode (M780L3, Thorlabs, Inc., Newton, NJ) with 780 nm center wavelength, 30 nm bandwidth. Irradiance at the sample surface was 2 mW/cm2. An 800 nm short-pass filter (84-729, Edmund Optics, Barrington, NJ) was used to reduce the potential for detection of excitation light. A convex lens and diffuser were used to achieve uniform illumination. The phone camera was based on an 8-bit CMOS sensor (3264×2448 pixels) with an f/2.4 aperture lens and the NIR blocking filter removed (Eigen Imaging, Inc., San Diego, CA). The field of view (FOV) was adjusted to 10×10 cm. A long-pass emission filter with a cut-off wavelength at 825 nm (86-078, Edmund Optics, Barrington, NJ) was secured to the camera. The phone was attached to a stable platform and images acquired with manufacturer provided auto-exposure software (“App #1”), as well as an application enabling measurement of unprocessed data with fixed exposure parameters (Camera FV-5, Flavio Gonzalez App-Entwicklung, Stuttgart, Germany) (“App #2”). We present mean image results (n = 5), as averaging improved SNR over a single acquisition by up to 30%. Imaging was also performed using a 16-bit monochrome scientific CCD (1200×1600 pixels, Alta U2000, Apogee Imaging Systems, Roseville, CA) with a zoom lens (75 mm focal length, f/3.9, Tamron, Commack, NY) and the aforementioned emission filter. The FOV was adjusted to 7×10 cm. Key acquisition parameters – exposure duration (τ) and gain (ISO) – are noted in figure captions.
Fig. 1.
Diagram of the NIRF imaging system setup.
The primary fluorophore in this study was ICG (Pulsion Medical Inc., Powell, OH), due to its common clinical use. We verified the NIR absorption and fluorescence properties of a solution containing 2.5 μg/ml ICG and 5.0 g/dl human serum albumin (Sigma-Aldrich Co., St. Louis, MO) with a spectrophotometer (Lambda 1050, Perkin Elmer Inc., Waltham, MA) and a spectrofluorometer (QuantaMaster QM4, Photon Technologies Int., Inc., Birmingham, NJ) [15].
B. Performance Testing
Camera phone spectral sensitivity in the 700–1100 nm range was determined from measurements of a Spectralon® diffuse reflectance standard (Labsphere, Inc., North Sutton, NH). Light from a tungsten-halogen source (Oriel Instruments, Stratford, CT) was delivered through a 7–10 nm bandwidth liquid crystal tunable filter (VariSpec SNIR-10-20, CRI, Inc., Waltham, MA). Irradiance at the sample surface was measured with a power meter (1918-C and 818-ST-UV, Newport Corp., Irvine, CA). Images were separated into red, green and blue components and the mean signal was calculated for a 150 ×150 pixel region in the center of the standard. The signal for each image was then normalized by the irradiance to determine sensitivity.
Spatial resolution was evaluated using a 3×3 inch, chrome-on-glass, negative USAF 1951 resolution target (36–408, Edmund Optics, Inc., Barrington, NJ). We fabricated a high-turbidity, ICG-doped phantom (25 μg/ml of ICG) to provide a homogeneous background for the target. This phantom also contained ethanol (0.5%) and TiO2 (12 mg/ml) in an epoxy matrix, which was cured for three days. Images were acquired with the resolution test target placed on top of the phantom.
Sensitivity and linearity was assessed using a black, 96-well plate in which eight wells were filled with bovine blood solution containing concentrations of ICG (0.02 to 2.5 μg/ml), and a separate reference well contained bovine blood alone. SNR was calculated from CCD and phone camera NIRF images as follows: SNR = (I-μ)/σ, where I is the mean signal from a well, and μ and σ are the mean and standard deviation of the signal from the reference well.
C. Tissue Phantom and Ex Vivo Imaging
Three-dimensional (3D) printing was used to fabricate turbid, biomimetic phantoms for preliminary qualitative testing [17]. A stereolithography 3D printer was used along with a proprietary material (Form 1+ and white photopolymer, FormLabs, Somerville, MA) that was determined to have absorption and reduced scattering coefficients of 0.1 cm−1 and 8 cm−1, respectively at 800 nm [18]. Printed samples exhibited no detectable NIR fluorescence based on spectrofluorometer and CCD measurements. The phantom was printed with a semi-planar network of channels derived from a segmented clinical image of retinal vasculature [18]. Channels were 0.75 mm in diameter, with the top edge located 0.75 mm below the surface. The phantom’s overall dimensions were 45×45×13 mm. An aqueous ICG-bovine blood solution (2.5 μg/ml of ICG) was injected into the channels for imaging.
To provide validation in biological tissue, ex vivo measurements were performed in a Lewis rat model. The rodent cadaver had been perfused with a solution of phosphate buffered saline (PBS) and 4% paraformaldehyde. Using a peristaltic pump (MPII, Harvard Apparatus Inc., Holliston, MA), the paraformaldehyde was replaced with PBS. The cadaver was subsequently perfused with an aqueous ICG-bovine blood solution (2.5 μg/ml of ICG) before NIRF imaging with CCD and phone camera systems.
III. Results
A. Spectral Sensitivity
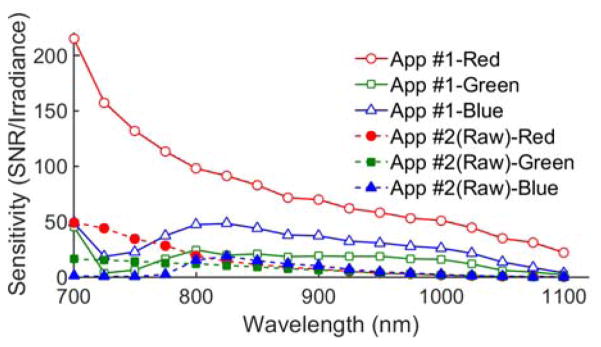
The sensitivity of each color channel in the phone camera (without emission or NIR blocking filters) varied strongly with NIR wavelength (Fig. 2). For App #1, red pixels showed the greatest change, with sensitivity decreasing by 80% across the range, whereas blue and green pixels tended to peak near 825 nm, followed by a slow decay. In the 800–900 nm range, App #1 red pixel signals were twice as sensitive as blue and four times as sensitive as green pixels. It is also notable that raw red pixel sensitivity at 650 nm was only 3.7 times greater than that at 800 nm (data not shown). Green pixel images maintained SNR levels of about 20 across much of the NIR. For wavelengths over 800 nm, all raw signal channels in App #2 showed lower SNR than green pixels in App #1 and decayed monotonically with wavelength.
Fig. 2.
Mobile phone camera NIR spectral sensitivity, shown as irradiance-normalized SNR, based on 1 μW/cm2 (App#1: τ = 1/20-1/9s, ISO 1113-2476; App#2: τ = 1/15s, ISO 400).
B. Contrast Sensitivity and Linearity
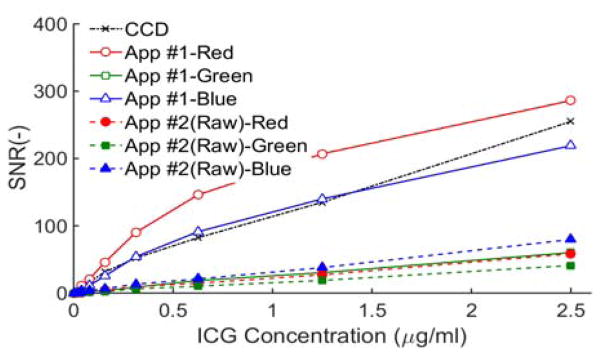
Measurements of the sensitivity of mobile phone and CCD systems to varying concentrations of ICG are presented in Fig. 3. Overall, variations with pixel color and software are similar to what was shown for the 800–900 nm band in Fig. 2. CCD results indicate similar sensitivity levels as the red and blue channels with App #1, however the former shows strong linearity for all intensities whereas the latter is linear up to an SNR of 100. Green pixel images from App #1 and all three channels in App #2 showed better linearity than red and blue pixels with App #1. While one prior mobile phone visible-wavelength fluorescence study demonstrated a highly linear sensitivity curve [7], this curve only covered a range of pixel intensities up to 50 “AU”, and it did not intersect the origin.
Fig. 3.
Mobile phone and CCD contrast sensitivity, expressed in SNR (CCD: τ = 1s, phone - App#1: τ = 1/9s, ISO 2476; App #2: τ = 1/8s, ISO 3200).
C. Spatial Resolution
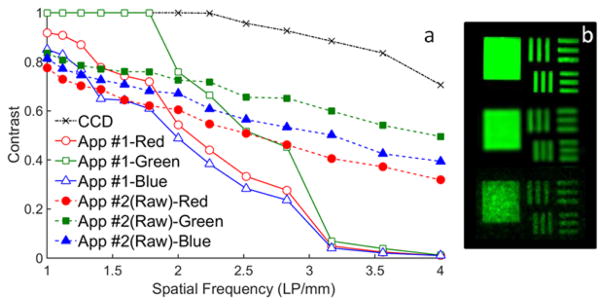
Both quantitative determination of contrast transfer functions (CTFs) and subjective evaluation of USAF 1951 target images (Fig. 4) were used to analyze variations in spatial resolution. The CCD CTF curve shows much higher contrast than the mobile phone for all but the lowest spatial frequencies. Using App #1, CTF results show better contrast at all spatial frequencies for green pixel images than red and blue pixels. Raw data acquired with App #2 indicate worse contrast than App #1 for low spatial frequencies, but better contrast at high spatial frequencies. Images of part of the resolution target illustrate the high quality of CCD results, the high noise level in raw phone camera images, and the brighter, smoother, but soft-edged features from processed images (App #1).
Fig. 4.
(a) CTFs for CCD and phone camera using Apps #1 and #2 (raw); (b) images of a square and bars with spatial frequencies of 2 and 2.4 LP/mm from CCD (top) and green channel phone images with App #1 (middle) and App #2 (bottom) (CCD: τ = 1s; phone - App #1: τ = 1/10 s, ISO 2667; App #2: τ = 1/8s, ISO 1600).
D. 3D-printed Phantoms
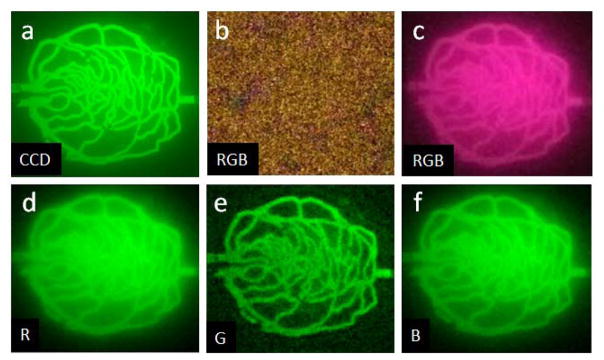
Phone camera images of biomimetic phantoms and animals were acquired using App #1, due to its strong image quality testing results; quantitative fluorescence measurements that would require unprocessed signals were not necessary to achieve the goals of this study. Images of the biomimetic vascular phantom (Fig. 5) illustrate the high resolution and low noise of the CCD. Signals seen in non-vessel regions are attributable to a minor amount of diffuse fluorescence. Green pixel images (Fig 5e) exhibit noticeable noise, yet delineate all major vessels seen in the CCD image, except in the highest density regions. Red and blue pixel images display less noise, but softening of vessel edges and greater background signal in non-vessel regions are present. A color phone camera NIRF image of the phantom without ICG (Fig. 5b) shows a low intensity (<4% of phantom with ICG), noisy minimal background fluorescence and light leakage.
Fig. 5.
Intensity-normalized NIRF images of 3D-printed phantom from (a) CCD (with ICG), and mobile App#1: (b) RGB without ICG, as well as (c) RGB (d) red, (e) green and (f) blue pixel images with ICG. (CCD: τ = 1 s, App #1: τ = 1/9s, ISO 2461). Maximum intensity in (b) is <4% of that in (c).
E. Ex Vivo Rodent Model
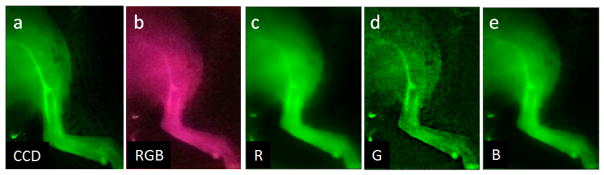
NIRF images acquired in the ex vivo ICG-perfused rodent model (Fig. 6) provide transdermal visualization of a right femoral artery bifurcation (no skin was removed). Vascular fluorescence is apparent in all images, as is background signal due to microcapillaries and light diffusion. The CCD image provides clear delineation of major vessels with high fluorescence levels from the lower intensity signals in surrounding regions. RGB, red and blue pixel images acquired with mobile phone App #1 exhibit degraded vessel contrast. The optimal phone-based visualization of vessels was provided by the green pixel image, although noise levels were much greater than for the CCD.
Fig. 6.
Normalized ex vivo NIRF images of the femoral region of an ICG-perfused Lewis rat, including an arterial bifurcation. Results are presented for (a) the CCD and (b–e) mobile App #1, including (b) RGB and (c) red, (d) green, and (e) blue pixel images. (CCD: τ = 7.5s, phone: τ = 1/9s, ISO 2461).
IV. Discussion
Imaging of targets, phantoms and an ex vivo rodent model have provided significant insight into the potential for performing biological NIRF imaging with a slightly modified mobile phone. This capability is most clearly evidenced by images of ICG-filled rodent femoral vasculature (Fig 5e, 6d), and artificial vessel-simulating structures in a 3D-printed tissue phantom. Quantitative test results indicated that phone-based imaging may approach the sensitivity of a scientific CCD, although with inferior spatial resolution, SNR and signal linearity. Furthermore, spectral sensitivity data suggests that phone-based cameras may be useful for applications across the NIR range.
Imaging performance of the phone-based system varied by pixel type and software. With App #1 the green channel provided good resolution and linearity, albeit with relatively low SNR. Resolution target images for this channel (Fig. 4) showed background signal near zero, resulting in higher CTF values than other channels. Green pixels also produced sharper edges in target, phantom and ex vivo images than red or blue pixels, possibly because green pixels are twice as common in Bayer filters. Red and blue pixel images with App #1 showed SNR levels on par with the CCD and much better than green pixels (Fig. 3). However, these images also displayed minimal vessel contrast, likely a result of signal nonlinearity. Based on comparisons with raw data, App #1 color-specific differences in resolution, noise and sensitivity appear to be due to tradeoffs made during image processing. The high sensitivity and low noise of red and blue pixels may be useful for tumor detection in a dark field, whereas green pixels may be more suited to delineating complex heterogeneous structures. Additionally, while App #2 results showed much higher noise and lower sensitivity than App #1, the strong NIR signal linearity seen for all three pixel types indicates potential suitability for quantitative imaging and the strong response at higher spatial frequencies (Fig 4a) indicates high resolution.
Target- and phantom-based test methods provided objective, quantitative insights into key image quality characteristics, including spectral sensitivity, contrast sensitivity, signal linearity and spatial resolution. With its biomimetic morphology and non-fluorescent turbid matrix, the 3D-printed phantom also proved a practical tool for qualitative bench testing. Insights gained from both types of testing approaches were useful in explaining biological tissue imaging results.
In conclusion, quantitative bench testing and ex vivo animal model results provided strong evidence of the utility of a mobile phone platform for NIRF tissue imaging, albeit at reduced image quality from a scientific CCD. Additionally, phantom-based test methods were shown to be effective in elucidating device performance. Clinical translation of phone-based NIRF imaging systems for retinal imaging, early cancer detection and other diagnostic tasks will require development of novel compact optical components (e.g., light source attachments) and NIRF image processing software. As these challenges are met, innovative mobile-phone-based NIR imaging devices may lead to improvements in a wide variety of point-of-care, global health, and first response applications.
Acknowledgments
This work was supported in part by the NSF/FDA Scholar-in-Residence Program (CBET-1445701) and FDA’s Critical Path Initiative (CP-11).
The authors would like to thank Drs. Srikanth Vasudevan Jessica Ramella-Roman, Scott Mathews, and Stanley Wang for their contributions to this project.
Footnotes
Disclaimer: The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services
Contributor Information
Pejhman Ghassemi, Center for Devices and Radiological Health, Food and Drug Administration, Silver Spring, MD 20815, USA.
Bohan Wang, Department of Electrical and Computer Engineering, University of Maryland, College Park, MD 20742 USA.
Jianting Wang, Center for Devices and Radiological Health, Food and Drug Administration, Silver Spring, MD 20815, USA.
Quanzeng Wang, Center for Devices and Radiological Health, Food and Drug Administration, Silver Spring, MD 20815, USA.
Yu Chen, Fischell Department of Bioengineering, University of Maryland, College Park, MD 20742 USA.
T. Joshua Pfefer, Center for Devices and Radiological Health, Food and Drug Administration, Silver Spring, MD 20815, USA.
References
- 1.Xie QM, Liu J. Mobile phone based biomedical imaging technology: A newly emerging area. Recent Pat Biomed Eng. 2010;3:41–53. [Google Scholar]
- 2.Maamari RN, et al. A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol. 2014;98:438–441. doi: 10.1136/bjophthalmol-2013-303797. [DOI] [PubMed] [Google Scholar]
- 3.Richards JR, et al. Comparison of traditional otoscope to iPhone otoscope in the pediatric ED. Am J Emerg Med. 2015;33:1089–1092. doi: 10.1016/j.ajem.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 4.Scully CG, et al. Physiological parameter monitoring from optical recordings with a mobile phone. IEEE Trans Biomed Eng. 2012;59:303–306. doi: 10.1109/TBME.2011.2163157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suto S, et al. Fluorescein fundus angiography with smartphone. Retina. 2014;34:203–205. doi: 10.1097/IAE.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 6.Lihachev A, et al. Autofluorescence imaging of basal cell carcinoma by smartphone RGB camera. J Biomed Opt. 2015;20:120502. doi: 10.1117/1.JBO.20.12.120502. [DOI] [PubMed] [Google Scholar]
- 7.Hempstead J, et al. Low-cost photodynamic therapy devices for global health settings: Characterization of LED performance and smartphone imaging in 3D tumor models. Sci Rep. 2015;5:10093. doi: 10.1038/srep10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coskun AF, Ozcan A. Computational imaging, sensing and diagnostics for global health. Curr Opin Biotech. 2014;25:8–16. doi: 10.1016/j.copbio.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skandarajah A, et al. Quantitative imaging with a mobile phone microscope. PLoS One. 2014;9:e96906. doi: 10.1371/journal.pone.0096906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim T, Youn J. Development of a smartphone-based pupillometer. J Opt Soc Korea. 2013;17:249–254. [Google Scholar]
- 11.Marshall MV, et al. Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg Oncol J. 2010;2:12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yannuzzi LA, et al. Ophthalmic fundus imaging: today and beyond. Am J Ophth. 2004;137:511–524. doi: 10.1016/j.ajo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Braue EH, et al. Noninvasive methods for determining lesion depth from vesicant exposure. J Burn Care Res. 2007;28:275–85. doi: 10.1097/BCR.0B013E318031A1A8. [DOI] [PubMed] [Google Scholar]
- 14.Tichauer KM, et al. Tumor endothelial marker imaging in melanomas using dual-tracer fluorescence molecular imaging. Mol Imaging Biol. 2014;16:372–382. doi: 10.1007/s11307-013-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B, et al. Near-infrared fluorescence imaging on a mobile phone platform: Image quality assessment based on targets and 3D-printed phantoms. presented at the NIH-IEEE Strategic Conference on Healthcare Innovation and Point-of-Care Technologies for Precision Medicine; Bethesda, MD. Nov. 9–10, 2015. [Google Scholar]
- 16.Wang B, et al. Performance evaluation of CCD- and mobile-phone-based near-infrared fluorescence imaging systems with molded and 3D-printed phantoms. Proc. SPIE BIOS; San Francisco, CA. 2016. pp. 9700–06. [Google Scholar]
- 17.Wang J, et al. Three-dimensional printing of tissue phantoms for biophotonic imaging. Opt Lett. 2014;39(10):3010–3013. doi: 10.1364/OL.39.003010. [DOI] [PubMed] [Google Scholar]
- 18.Ghassemi P, et al. Rapid prototyping of biomimetic vascular phantoms for hyperspectral reflectance imaging. J Biomed Opt. 2015;20(12):121312, 1–10. doi: 10.1117/1.JBO.20.12.121312. [DOI] [PMC free article] [PubMed] [Google Scholar]