Abstract
Increased expression of Th22 cytokine IL-22 is a characteristic finding in atopic dermatitis (AD). However, the specific role of IL-22 in the pathogenesis of AD in vivo has yet to be elucidated. Consistent with observations in human AD, IL-22 was significantly increased in the AD skin of mice after epicutaneous sensitization to house dust mite allergen. Utilizing a skin-specific inducible transgenic system, we show here that expression of IL-22 in the skin of mice caused an AD-like phenotype characterized by chronic pruritic dermatitis associated with Th2-biased local and systemic immune responses, down-regulation of Epidermal Differentiation Complex genes and enhanced dermatitis upon epicutaneous allergen exposure. IL-22 potently induced the expression of gastrin-releasing peptide (GRP), a neuropeptide pruritogen, in dermal immune cells and sensory afferents and in their skin-innervating sensory neurons. IL-22 also differentially up-regulated the expression of GRP receptor (GRPR) on keratinocytes of AD skin. The number of GRP+ cells in the skin correlated with the AD severity and the intensity of pruritus. IL-22 directly upregulated the expression of epithelial-derived type 2 cytokines (TSLP and IL-33) and GRP in primary keratinocytes. Furthermore, GRP not only strongly induced TSLP but also increased the expression IL-33 and GRPR synergistically with IL-22. Importantly, we found that the expression of GRP was strikingly increased in the skin of patients with AD. These results indicate that IL-22 plays important pathogenic roles in the initiation and development of AD, in part through inducing keratinocyte production of type 2 cytokines and activation of the GRP/GRPR pathway.
Introduction
Atopic dermatitis (AD) is the most common chronic, pruritic, inflammatory skin disease and it affects 15–20% of children living in the cities worldwide (1). Recent studies show that epidermal barrier defects and immune dysregulation of Th2 (IL-4 and IL-13) and Th22 (IL-22) are the key features in the pathogenesis of in AD (2). Chronic pruritus and chronic colonization of superantigen-producing Staphylococcal aureus (S. aureus) on AD skin are also important features of the disease (3, 4). However, the factors that influence the expression, regulation, and function of IL-22 and the relationship between dysregulation of Th22, Th2, the skin barrier defects, and chronic itch are still not fully understood.
IL-22 belongs to the IL-10 cytokine family and it signals through a heterodimeric receptor (IL-22R) consisting of IL-22 receptor 1 (IL-22R1), which is only expressed in tissue cells, and IL-10R2. In the skin, IL-22 acts on keratinocytes through binding to IL-22R expressed on these cells (5). IL-22 expression has been found to be highly up-regulated in the AD skin compared to psoriasis skin lesions and normal skin (6). Robust progressive activation of Th2 and Th22 characterizes the nature of dysregulated immunity in both acute and chronic AD (4, 6–12) and alterations in Th2 and Th22 cytokines correlate positively with the AD disease severity (13). The role of IL-22 in the development and maintenance of AD has not been specifically explored. We hypothesized that IL-22 plays an important role in the development of AD by impairing skin barrier function, immune dysregulation and pruritus. To further understand the mechanisms by which IL-22 induces dermatitis, we looked into the potential mediators and pathways of itch and the interaction of IL-22 with allergen induced Th2 biased allergic dermatitis.
Herein we show that that epicutaneous sensitization to allergen HDM induced up-regulation of IL-22 in the skin and that overexpression of IL-22 in the skin caused a chronic AD phenotype characterized by pruritus, heightened dermal and systemic Th2 immunity, a “leaky” skin barrier with down-regulation of Epidermal Differentiation Complex (EDC) genes and enhanced epidermal colonization of S. aureus. In addition, IL-22 potently induced the expression of GRP in dermal cells, dermal afferents fibers and skin-innervating ganglion neurons that positively correlates with the intensity of itching and scratching behaviors. Consistent with these, the expression of GRP is strikingly enhanced in the lesional skin of patients with AD. These findings revealed previously unrecognized pathological effects of IL-22 in the pathogenesis of AD.
Materials and Methods
Animals
All procedures performed on mice were in accordance with the NIH guidelines for humane treatment of animals and were approved by the Institutional Animal Care and Use Committee of Yale University. Mice were housed in cages with microfilters in a specific pathogen-free environment. Wild type mice and transgenic lines were all on the C57BL/6 genetic background.
Generation of skin-specific inducible IL-22 transgenic mice and induction of IL-22 expression
To study the effector functions of IL-22 in the skin we first generated a TRE-Tight-IL-22 transgenic mouse line as we previously described (14, 15). Mouse IL-22 cDNA was PCR amplified from a previous IL-22 transgenic construct using primers: 5′-GCGAATTCCCCCTTCACCGC-3′ and 5′-CGCGGATCCTTCCAGTTTAAT-3′ containing restriction enzyme sites for EcoRI/BamHI and a Kozak consensus sequence. Following EcoRI/BamHI enzyme digestion, the product was inserted into the multiple cloning sites located downstream of the TRE-Tight promoter of the pTRE-Tight vector (Clontech, Mountain View, CA). The fragment containing the TRE-Tight promoter, IL-22 cDNA, and the SV40 polyadenylation signal sequence was excised by Xhol, purified, and microinjected into pronuclei as described previously (15). To express IL-22 specifically and inducibly in the skin, we crossbred the TRE-Tight-IL-22 mouse line with the K5-tTA mouse line and obtained double-transgenic K5-tTA-IL-22 mice. The generation and use of the K5-tTA mouse line has been described previously (16). The genotypes of the mice were determined by PCR using specific primers for K5-tTA and TRE-Tight-IL-22. Starting at breeding, doxycycline (Dox) was added to the drinking water (1 mg/mL) to suppress tTA and to keep the IL-22 transgene off until the K5-tTA-IL-22 mice were 6 weeks old. Experiments were initiated by withdrawing Dox from the drinking water. In all experiments, Tg(−) littermate controls received the same amount of Dox or no Dox for the same length of time.
Clinical observation and itch/scratching scores
The K5-tTA-IL-22 Tg(+) mice and Tg(−) littermate controls were examined for skin lesions 3 times per week. The clinical manifestation of itch/scratching behavior of the mice was videotaped for 15 minutes each session. The scratching behavior was assessed in a blind fashion as described previously with slight modification (17).
The in situ skin permeability assay
The in situ skin permeability assay with toluidine blue was performed as described previously (18, 19). In brief, anesthetized wild type mice and Tg(+) mice prior to developing clinical AD lesions were shaved and rinsed in PBS. The whole body except the head of the mouse was immersed successively in 25%, 50%, 75% and 100% methanol for 1 minute each. The skin was then rehydrated in PBS and stained in 0.1% toluidine blue for 10 minutes at room temperature. After a brief wash in PBS, the mice were immediately photographed.
S. aureus colonization on the skin
Colonization of S. aureus was determined by isolating the bacteria from the dorsal skin of the mice using DD checker Seiken mannitol salt agar with egg yolk (MSEY) plates (Denka Seiken, Tokyo, Japan). For sampling, the plates were applied to be in contact with the skin for 10 seconds. Then the plates were incubated at 37°C for 24 hrs. After incubation, the number of colonies was counted and identification of the bacteria was done according to the manufacturer’s instructions (20). Serum samples for SEB-specific IgE were measured using ELISA Plates coated with SEB (1 μg/ml, Toxin Technology, Inc.) in 0.1 M carbonate-bicarbonate buffer (pH 9.5) overnight at 4°C, as described previously (21).
Epicutaneous allergen sensitization
For experiments shown in Supplemental Figure 1, 6–8-week old (male and female) WT mice on C57BL/6 genetic background were used. The mice were epicutaneously sensitized (EC) with allergen house dust mite (HDM) extract (10 μg in 50 μl of PBS) (Greer Laboratories, Lenoir, North Carolina) or PBS as vehicle control. For the experiments shown in Figure 5, transgenic IL-22 mice and their Tg(−) littermates, kept under specific pathogen-free condition, were used. Two weeks after their transgene IL-22 being activated and before the onset of clinical AD, Tg(+) mice and their Tg(−) littermates (both male and female mice) were epicutaneously sensitized (EC) with Ovalbumin (Ova), 100 μg in 50 μL of PBS, (Grade V; Sigma, St. Louis, MO) or PBS as control. The same patch protocol was used for the experiments in both Supplemental Figure 1 and Figure 5 as previously described (21, 22). In brief, the back area of anesthetized mice was shaved with a razor and tape-stripped four times using adhesive tapes to introduce skin abrasion. Allergen (HDM or Ova) was placed on a patch of sterile gauze (1×1 cm), which was then secured to the skin with a transparent bio-occlusive dressing. Each mouse received a total of three one-week exposures to the patch, separated by 2-week intervals. Experimental procedures were in accordance with the Animal Care and Use Committee at Yale University.
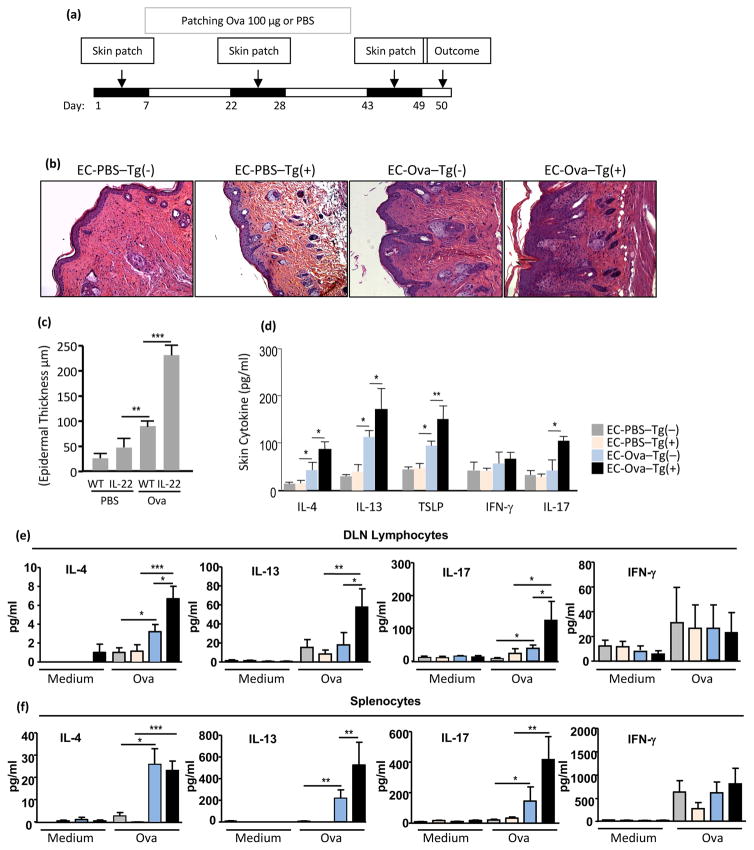
Figure 5. IL-22 promotes allergen sensitization, local and systemic Th2 and Th17 immune responses.
(a) Epicutaneous sensitization protocol. Before onset of AD, IL-22 Tg(+) mice and Tg(−) littermates were epicutaneously (EC) sensitized with PBS or Ova (100 μg) on a sterile patch. Skin pathology and immune responses were examined at day 50. (b) H&E of skin sections at magnification 20x. Marked dermal Inflammation and (c) Epidermal thickness (μm). (d) ELISA quantification of cytokines in the skin; (e) Ova-stimulated lymphocytes of DLNs; and (f) splenocytes. Data are representative of two experiments with similar results, Mean±SEM (n = 5–7 mice per group; *p<0.05, **p<0.01, ***p<0.001).
Histology and immunohistochemistry (IHC) evaluation of dermatitis
After the mice were sacrificed, lesional and non-lesional skin was excised and fixed in neutral-buffered formalin at 4°C overnight, embedded in paraffin, sectioned at 5 μm thickness, and stained with H&E, toluidine blue or IHC as previously described (16, 21). Specific antibodies used in this study included rat anti-mouse major basic protein (MBP) monoclonal antibody (a kind gift from Drs. Nancy and James J. Lee, Mayo Clinic, Scottsdale, AZ) for eosinophils; rat anti-CD4 mAb for CD4+ cells, (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); rat anti-F4/80 antibody (Pan macrophage marker clone: BM8, 14-4801; eBioscience, San Diego, CA) for activated Langerhans cells and macrophages; goat polyclonal anti-mouse IL-22 antibody for IL-22 (R&D Systems, Minneapolis, MN); rabbit anti-GRP (ImmunoStar,1:1000) (23); goat polyclonal IgG anti-langerin, goat polyclonal IgG anti-neutrophil elastase, and mouse monoclonal IgG2a anti-CD8-α (Santa Cruz Biotechnology) for Langerhans cells, neutrophils, and CD8+ cells, respectively. Rat anti-mouse CD11b (BD Pharmingen, 553312). Vector M.O.M. Immunodetection Kit was used to reduce the background staining. Appropriate ABC Staining Systems were used to visualize the target protein staining in the tissues (Santa Cruz Biotechnology). Skin inflammatory cells were quantified by counting 8–10 high power fields (HPF at magnification x200) per mouse (5–7 mice per group). Evaluation of epidermal thickness was performed as previously described (16, 21).
Culture and stimulation of primary human keratinocytes
Neonatal foreskin epidermal keratinocytes were obtained from discarded and de-identified neonatal foreskin tissues. The research study was approved by the Institutional Review Board of Yale University. Epidermal keratinocytes were cultured in CnT-07 medium (CELLnTEC, Bern, Switzerland) containing penicillin 100U/mL and streptomycin sulfate 100 U/mL at 37°C in air containing 5% CO2. Cells of passages 2–5 were used for all in vitro experiments. The cells at 75–80% of confluency were stimulated with freshly made IL-13 (20 ng/mL), IL-4 (20 ng/mL), IL-22 (20 ng/mL), INF-γ (20 ng/mL) and GRP 10−7M for 24 hrs. The supernatant was collected for cytokine measurement and the cells were collected for real-time PCR and Western blot.
RNA isolation and qPCR analysis
Skin samples collected from the same anatomical locations, including epidermis and dermis, were used and compared in the experiments. After excision, skin tissues were immediately placed in RNAlater solution (Ambion, Austin, TX), stored at −80°C and used later. To extract RNA, skin samples were placed in liquid nitrogen and ground with a mortar and pestle. Total RNA was then isolated with Trizol reagent (Invitrogen, Carlsbad, CA) and further purified according to the manufacturer’s instructions. Reverse transcription was performed using 2 μg total RNA for first strand cDNA synthesis with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) in a total volume of 25 μL. A portion of the resulted reverse-transcription product (2 μL) was used for PCR amplification of specific genes. For primary human keratinocytes, total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen). Extracted RNA was used as templates for RT-PCR and qPCR. For RT-PCR, the reverse transcription, initial activating, denaturing, annealing and extension condition of each cycle were 50°C for 30 min, 95°C for 15 min, 94°C for 15 sec, 60°C for 30 sec, 72°C for 30 sec, respectively. The relative expression levels for specific genes was analyzed usng qPCR and the results were calculated using the ΔΔCt method. The mRNA level of each target gene was normalized to the level of GAPDH. The primer sequences for the specific genes are listed in the Supplemental Table 1.
Skin protein extracts and measurement of cytokines
Protein extracts from the skin samples were prepared as described previously (16, 21). Briefly, frozen skin tissues were weighed, placed in liquid nitrogen, and crushed with a mortar and pestle. Triton X-100 0.25% (wt/vol) in phosphate-buffered saline was added to the skin powder. The homogenate was stirred at 4°C overnight and then centrifuged at 3000xg for 15 minutes to remove debris. Supernatants were collected and stored in small aliquots at −80°C until assayed by ELISA or Western blot. All samples were normalized to the weight of the skin samples.
Assessment of cytokine production by lymphocytes from draining lymph nodes and spleen
Lymphocytes from draining lymph nodes (inguinal, axillaries and cervical) and spleen were isolated and cultured in complete RPMI1640 medium containing 5% FBS and activated using 96-well anti-CD3 coated plate (BD Bioscience, San Jose, CA) plus anti-CD28 (5 μg/mL) for 72 hrs. Supernatants were collected and examined for cytokine production (17, 21, 24).
Measurement of cytokines and immunoglobulins
Cytokines in the skin samples were measured using ELISA kits according to the manufacturer’s instruction (R&D Systems, Minneapolis, MN). Serum samples in duplicates were analyzed using ELISA for total IgE (BD Biosciences), IgG1 and IgG2a (Southern Biotechnology Associates, Birmingham, AL), according to the manufacturer’s instructions.
Western blot
Proteins were extracted by RIPA buffer from primary keratinocytes after stimulation with IL-22 (20 ng/mL) in the presence or absence of GRP 10−7M and Th2 and Th1 cytokines, separated by 10% SDS-PAGE, and electro-transferred onto a PVDF membrane (Bio-Rad). After blocking with 5% non-fat dry milk for 1 hr at room temperature, the membranes were incubated at 4°C overnight with the following primary antibodies: anti-STAT3 (Cell signaling), anti-p-STAT3 (Cell signaling) and anti-GAPDH (Santa Cruz). The membranes were incubated with appropriate HRP-conjugated secondary antibodies for 2 hr at room temperature. Immunoreactive bands were detected by SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific).
Human skin biopsy samples and IHC
De-identified skin biopsy samples from AD patients and healthy subjects were described previously (17, 25). After explanation of the nature of research and obtaining informed consent from patients, 3- to 5-mm punch biopsies were taken from the forearms of healthy individuals (n = 4) and from lesional or non-lesional skin of AD patients (n = 3). Skin samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis. The study was approved by the local ethics committee. Cryosectioned human skin samples were analyzed with immunofluorescence staining as described earlier. Acetone-fixed frozen sections were blocked with 10% donkey serum (Sigma-Aldrich) for 1 hr and incubated with goat anti-PGP9.5 (sc-23852; Santa Cruz Biotechnology) and rabbit anti-GRP antibody (ImmunoStar,1:1000) (the antibody reacts with human and mouse) (23, 26) at 4°C overnight. The slides were rinsed and incubated with Alexa Fluor 594-conjugated donkey anti-goat IgG, Alexa Fluor 488-conjugated donkey anti-rabbit IgG, and/or Alexa Fluor 594-conjugated donkey anti-mouse IgG (Invitrogen). Cell nuclei were stained with DAPI for 10 min. Images were obtained using a Nikon Eclipse Ti fluorescence microscope.
Statistical analysis
Two-tailed Student’s t-test was used to determine the significance of difference between two groups or one-way ANOVA was used to determine statistical differences between multiple groups. Differences between samples in comparison with p values smaller than 0.05 were considered statistically significant. Unless otherwise specified, all data were presented as Mean ± SEM.
Results
Epicutaneous (EC) allergen (HDM) sensitization-induced AD-like dermatitis and enhanced IL-22 expression in AD lesions
To determine whether Th22 cytokine IL-22 could be induced by epicutaneous exposure to allergens, C57BL/6 WT mice were subjected to three cycles of EC sensitization with house dust mite (HDM), an important allergen for patients suffering from AD (27), or saline control using the protocol as previously described (21, 22). EC sensitization of WT mice with HDM caused epidermal and dermal thickening as previously reported (27) and increased itch-evoked scratching, marked IL-22 and Th2 cytokines in the AD skin lesions and elevated serum IgE (Supplemental Figure 1a–e). These data indicate that IL-22 can be induced in the AD lesions in response to allergen sensitization and IL-22 is highly relevant in epidermal HDM allergen induced AD lesions.
Generation of externally inducible skin-specific IL-22 transgenic mice (K5-tTA-IL-22) and induction of IL-22 expression
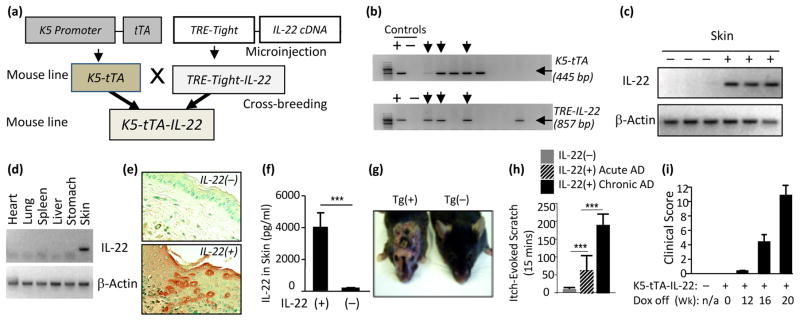
To explore whether chronic expression of IL-22 in the skin could cause pruritic dermatitis, transgenic constructs were made and microinjected to generate TRE-Tight-IL-22 mice. These mice were then cross-bred with K5-tTA mice to generate K5-tTA-IL-22 mice (Figure 1a), as we previously described (16). As shown in Figure 1b, the cross-breeding resulted in transgenic mice that were positive for both K5-tTA and TRE-Tight-IL-22 transgenes, using primers specific for K5-tTA and TRE-Tight -IL-22 (arrows). We then tested if expression of IL-22 could be controlled by doxycycline (Dox). The keratin 5 (K5) promoter controlled tTA is inactive in the presence of Dox and active in the absence of Dox. To express IL-22 only in the adult mice, we gave Dox (1 mg/mL with 4% sucrose) in the drinking water, starting at breeding and continuously to K5-tTA-IL-22 double positive mice and their Tg(−) littermates until they were at 6 weeks of age. In the beginning of each experiment, Dox was withdrawn and the induction of the IL-22 transgene was initiated. Tg(−) littermate controls received the same amount of Dox or no Dox for the same length of time. After initiation of transgene induction for 6 weeks, IL-22 mRNA was only detected in the skin of IL-22 transgenic mice, not in the skin of Tg(−) mice (Figure 1c). Furthermore, IL-22 mRNA expression in the skin and other tissues of Tg(+) and Tg(−) littermate mice was determined by RT–PCR. IL-22 mRNA was only detected in the skin, not in other tissues, of Tg(+) mice, indicating that IL-22 was selectively expressed in the skin (Figure 1d). In all cases, IL-22 mRNA was appropriately translated since IL-22 protein was easily detected immunologically and biologically in the skin extracts of Tg(+) animals, but not in the skin of Tg(−) animals (Figure 1e, 1f). All subsequent experiments were performed to compare Tg(+) mice and Tg(−) littermate controls after transgene induction with Dox withdrawal, starting from 6 weeks of age. These results showed that using the K5-tTA system, the IL-22 transgene was specifically targeted to the skin in an inducible fashion.
Figure 1. Generation of inducible skin-specific IL-22 transgenic mice (K5-tTA-IL-22) and IL-22 expression in the skin caused a chronic pruritic atopic dermatitis (AD)-like phenotype.
(a) Schematic transgenic constructs and generation of K5-tTA-IL-22 mouse line. (b) Genotyping of IL-22 Tg mice. Arrows point to mice positive for K5-tTA, TRE-Tight-IL-22 or both transgenes. (c, d) Skin specificity of IL-22 expression in Tg(+) mice as determined by RT–PCR 6 weeks after induction of the transgene by Dox withdrawal. (e) Expression of IL-22 in the epidermis of Tg(+) mice by IHC, light brown color, using anti-IL-22 antibody (40x). (f) IL-22 protein levels in skin samples by ELISA. (g) IL-22 Tg(+) mice exhibit chronic itchy eczematous dermatitis on the face, ears, neck and back, 4 months after the IL-22 transgene was activated in vivo, but not in Tg(−) mice. Shown is a representative pair, Tg(+) mouse on the left and Tg(−) mouse on the right. (h) Numbers of itch-evoked scratching and (i) Clinical scores of dermatitis: the progression of AD in a representative group of Tg(+) mice after induction of IL-22 transgene for varying time periods. Ten Tg(+) mice and 5–6 Tg(−) mice per group for e, f, h and i. (*** p<0.001).
Expression of IL-22 in the skin caused chronic pruritic dermatitis
All K5-tTA-IL-22 transgenic mice developed impressive pruritic dermatitis after the IL-22 transgene was activated for 12–14 weeks (withdrawal of Dox from drinking water). The Tg(+) mice began to develop pruritus as indicated by constant scratching of the affected areas and the itch/scratching behavior was recorded and quantitated as described previously (16, 17, 28). In addition, other signs of dermatitis, including loss of hair, erythema, crusting, excoriation, and erosions in the skin, involving the areas of the neck, ear, face and abdomen were observed in these mice. Dry lichenified skin lesions were noted when the lesions became chronic. In contrast to Tg(+) mice, their Tg(−) littermates showed no skin abnormalities either with or without Dox, indicating that Dox had no effect on the skin (Figure 1g). Tg(+) mice given Dox did not exhibit any signs of abnormality of the skin because no IL-22 was expressed under this condition (data not shown). Chronic pruritus, a cardinal feature of AD, was assessed three times per week. The number of itch-evoked scratching increases as the AD (disease score) progressed from acute AD (onset < 2 weeks) to chronic AD (longer than 2 weeks) (Figure 1g, 1h and 1i). As the AD progressed, the body weight of the Tg(+) mice was significantly lower than that of the Tg(−) littermates (data not shown).
IL-22-induced AD was characterized by Th2 biased immune responses
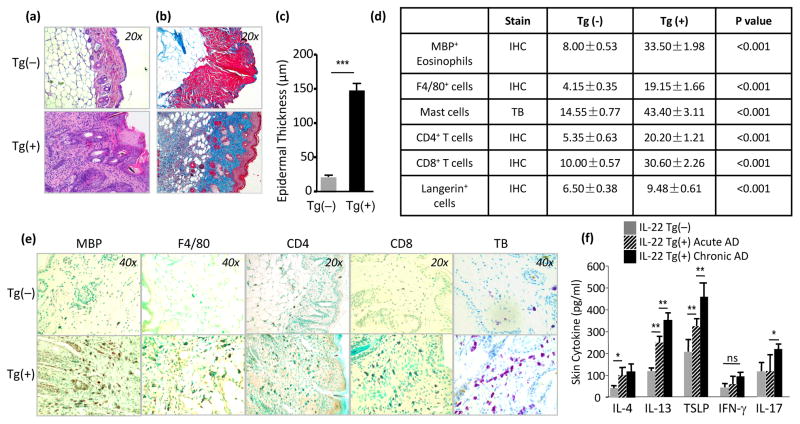
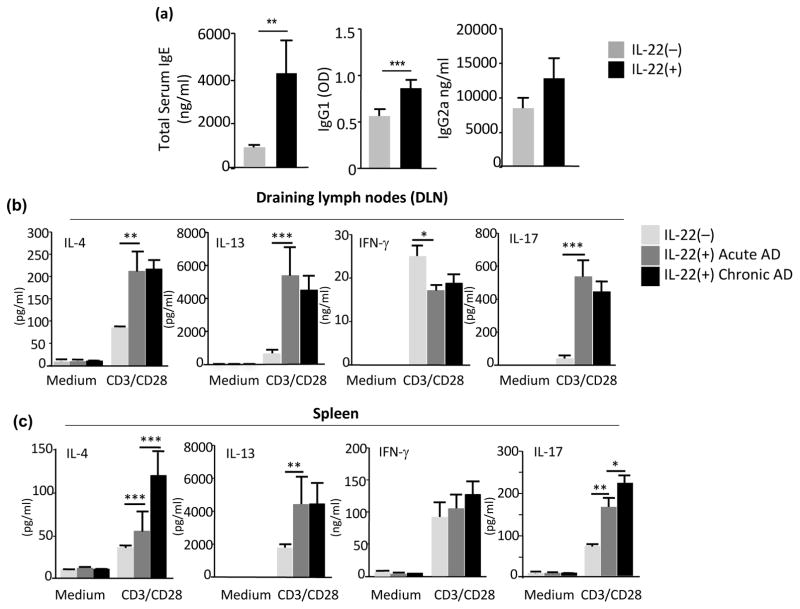
Histologically, in comparison to the Tg(−) littermates, the lesional skin of Tg(+) mice exhibited marked thickening of the epidermis and dermis, spongiosis, hyperkeratosis and an impressive inflammatory cell infiltration and dermal collagen accumulation (Figure 2a–c). Many cell types, including eosinophils, CD4+ cells, CD8+ cells, F4/80+ activated macrophages and Langerhans cells, and mast cells are involved in the inflammatory responses in human AD. Using antibodies to specific cellular markers in a series of IHC staining we examined the skin samples from IL-22 Tg(+) mice and Tg(−) littermate controls. The IHC stains demonstrated significantly increased numbers of MBP+ eosinophils, F4/80+ macrophages/Langerhans cells, CD4+ T cells, CD8+ T cells and toluidine blue (TB) stain showed mast cells in the sub-epidermal, intradermal, and perivascular spaces of the skin samples from Tg(+) mice, whereas neutrophils were rarely seen (Figure 2d and 2e). Furthermore, the immune responses in AD skin were marked by a dominant Th2 response with increased IL-4 and IL-13, indicating that IL-22 is a potent stimulator of Th2 responses in both the acute and chronic AD lesions. Epithelial-derived TSLP, a critical cytokine for promoting type 2 immune responses, was also significantly increased in the AD skin of Tg(+) mice compared to Tg(−) mice. IL-17 was mildly increased in chronic AD lesions, suggesting that IL-17 may play a role in chronic lesions. On the other hand, the level of IFN-γ was not significantly altered compared to Tg(−) skin (Figure 2f). Comparing to Tg(−) mice, the serum levels of total IgE and IgG1, but not IgG2a, were significantly increased in Tg(+) animals (Figure 3a). In addition, anti-CD3/CD28 TCR activation of lymphocytes from draining lymph nodes (DLN) of Tg(+) animals produced significantly higher levels of Th2 cytokines (IL-4 and IL-13) and Th17 cytokine IL-17A. In contrast, decreased levels of IFN-γ were noted (Figure 3b). Stimulated splenocytes from Tg(+) mice showed an early surge of Th2 cytokines and the heightened Th2 response was sustained in chronic AD and IL-17A was also elevated (Figure 3c). IFN-γ was not significantly altered compared to activated splenocytes of Tg(−) mice. These results indicate that IL-22-induced AD-like dermatitis promotes a strong Th2-biased systemic immune response.
Figure 2. IL-22 caused chronic pruritic dermatitis characterized by increased thickness of the epidermis, spongiosis, epidermal hyperplasia, and striking dermal inflammation (biased Th2 responses) and dermal fibrosis.
(a, c) Increased thickness and qualification of the epidermis (H&E, 20x). (b) Collagen accumulation in Tg(+) dermal region (Trichrome staining, Blue, 20x). (d, e) Inflammatory cell differential (Stain with IHC or TB: toluidine blue). (f) Skin cytokine profile (markedly increased IL-13, IL-4, TSLP and IL-17), but not IFN-γ by ELISA (n=8 mice each group, *p<0.05, **p<0.01, and ***p<0.001); ns, not significant).
Figure 3. IL-22-induced chronic AD associated with enhanced systemic Th2 responses.
(a) Increased total serum IgE and IgG1, but not IgG2a in Tg(+) mice; (b) Cytokine profiles of IL-4, IL-13 and IL-17 and IFN-γ produced by lymphocytes from cervical, axillary, inguinal draining lymph nodes (DLNs) and (c) from spleen from Tg(+) mice compared to Tg(−) mice. Cells were stimulated with anti-CD3/CD28 for 72 hrs and cytokines in the supernatant were measured by ELISA. Data are representative of three experiments with similar results. Data are Mean±SEM (n=7–8 mice each group; *p<0.05, **p<0.01, and ***p<0.001).
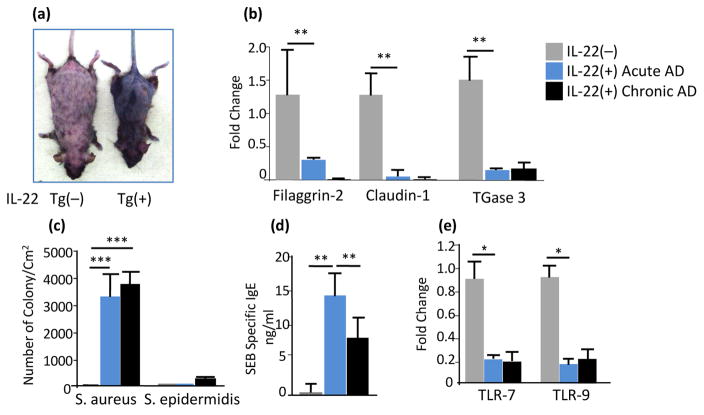
IL-22 induced atopic dermatitis exhibited skin barrier defects and increased susceptibility to colonization of S. aureus
Abnormalities in both skin barrier structures (stratum corneum and tight junctions), a robust Th2 response to environmental antigens, and defects in innate immunity may occur as a consequence of epidermal dysfunction (29). To assess the skin barrier functions, IL-22 Tg(+) mice without visible clinical dermatitis were immersed in toluidine blue solution using an in situ skin permeability assay (19). The skin of Tg(−) mice was not stained, indicating that the skin of these mice was not permeable to toluidine blue (Figure 4a left). In contrast, the entire skin of IL-22 Tg(+) mice stained dark blue (Figure 4a. right), indicating significantly increased skin permeability. To further determine the cause of the skin barrier dysfunction in IL-22 Tg(+) mice, several genes encoding the stratum corneum and tight junctions that are critical for normal keratinization of the skin, including Filaggrin-2 (FLG2), Claudin-1, and epidermal transglutaminase 3 (TGase 3), were measured. Compared to Tg(−) mice, these genes were significantly down-regulated in the skin of Tg(+) mice (Figure 4b). Chronic colonization of superantigen-producing S. aureus is a feature of human AD (30, 31). We examined whether IL-22 Tg(+) mice were more susceptible to S. aureus colonization. Strikingly high numbers of colonized superantigen-producing S. aureus, not S. epidermidis, were found on acute AD skin and persisted on chronic AD skin of Tg(+) mice and the number of the colonies was positively correlated with the AD disease severity. Conversely, a few colonies were seen on the skin of Tg(−) littermates (Figure 4c). Systemic responses of specific SEB sensitization by epidermal superantigen (sAg) SEB-producing S. aureus sensitization was reflected by markedly elevated serum levels of specific SEB IgE (Figure 4d). Furthermore, skin expression of genes encoding TLR-7 and TLR-9, critical for innate immune defense against bacterial and viral infections were markedly down-regulated (Figure 4e). These results indicate that IL-22 expression in the skin impairs skin barrier integrity and down-regulates TLR expression which could create a portal easily allowing superantigen-producing S. aureus to further perpetuate and exacerbate inflammation and barrier impairment in the skin.
Figure 4. IL-22 Tg(+) mice exhibited marked impairment of the epidermal barrier function, down-regulation of epidermal barrier genes and alternation of the expression of TLRs and heavy colonization of superantigen (SEB)-producing S. aureus on the skin.
(a) Using a skin permeability assay in situ, anesthetized Tg(−) and IL-22 Tg(+) mice without visible AD were immersed in toluidine blue, the entire skin of IL-22 Tg(+) mice was stained dark blue (right). In contrast, the skin of IL-22 Tg(−) mice was not permeable to toluidine blue (left). (b) Diminished expression of the genes encoding the Epidermal Differentiation Complex (EDC) including filaggrin-2, Claudin-1 and TGase 3 by qPCR. (c) Increased colonization of S. aureus, but not S. epidermidis, on AD skin of Tg(+) mice and (d) Associated specific IgE to S. aureus derived SEB. (e) Down-regulation of genes encoding TLR-7 and TLR-9 in IL-22 Tg(+) mouse skin by qPCR. Data represent Mean±SEM (n=7–8 mice each group; *p<0.05, **p<0.01, and ***p<0.001).
IL-22 transgenic mice exhibited enhanced dermatitis upon epidermal allergen exposure
Next we examined whether IL-22 Tg(+) mice were more susceptible to chronic antigen sensitization. Studies have shown that epicutaneous sensitization with protein antigen (Ag) in mice induces localized allergic dermatitis (21, 31). The effects of IL-22 on epidermal antigen sensitization in Tg(+) mice before they developed visible AD was tested in an Ova epicutaneous sensitization AD model as described previously by our group and others (21, 31, 32) (Figure 5a). After Dox withdrawal for 2 weeks, IL-22 Tg(+) mice and Tg(−) littermate controls were randomly assigned to receive epicutaneous (EC) Ova sensitization (EC-Ova group) or PBS (EC-PBS group). One day after the third cycle of Ag sensitization, samples of skin, blood, DLNs, and spleen were collected for evaluation. The skin of Tg(+) mice sensitized to Ova (EC-Ova-Tg(+) group) exhibited increased epidermal thickness with hyperplasia and increased inflammatory responses (Figure 5b and 5c) as compared to the EC-Ova-Tg(−) group. In line with other studies, in the skin of PBS sensitized mice (EC-PBS-Tg(+), and EC-PBS-Tg(−) groups, a few inflammatory cells were seen in the dermis, whereas in Ova sensitized skin there was significantly increased dermal inflammation. Furthermore, strong Th2 cytokines (IL-4, IL-13 and TSLP) and Th17 cytokine (IL-17A) were seen in the skin of EC-Ova-Tg(+) comparing to EC-Ova-Tg(−) mice. However, the levels of IFN-γ were comparable between the groups (Figure 5d). The levels of IL-4 and IL-13 produced by Ova-stimulated lymphocytes of DLNs (Figure 5e) and spleen (Figure 5f) from EC-Ova-Tg(+) mice were significantly higher than EC-Ova-Tg(−) mice. In addition, an elevation of IL-17A produced by cells of DLNs and spleen of EC-Ova-Tg(+) mice was seen but to a lesser degree. However, IFN-γ levels were not altered in all groups.
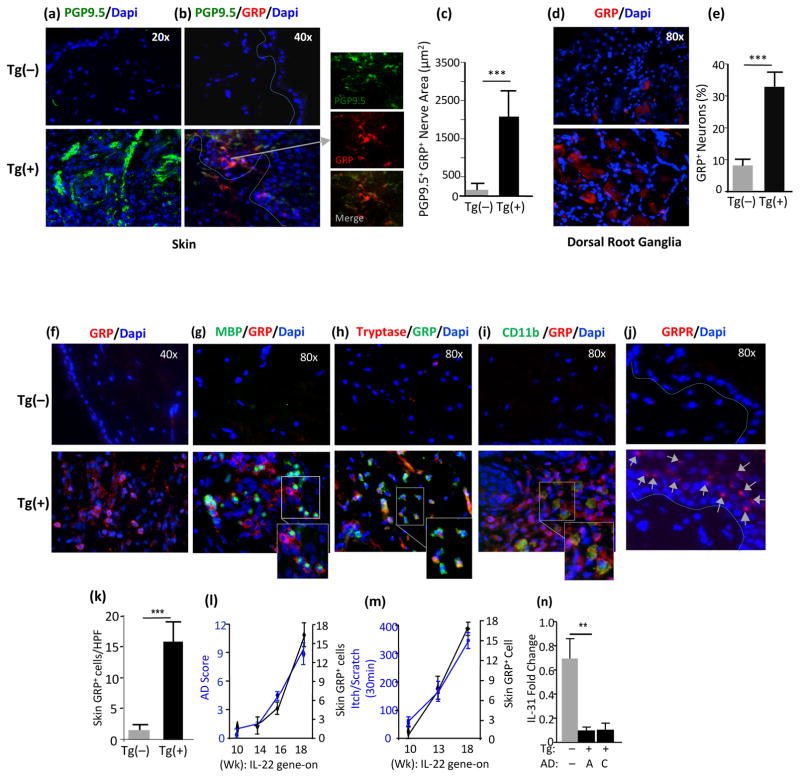
Chronic pruritus and robust expression of GRP in dermal afferents and skin-innervating dorsal root ganglia (DRG) of IL-22 mice
The mechanisms of chronic itch in human AD are poorly understood. Recently, GRP and its receptor (GRPR) have been shown to play key roles in itch transmission, but not in nociception (26, 33). When GRP was injected intradermally, it elicited scratching through mast cell degranulation (34). IL-22 mice demonstrated chronic itch that evoked intense scratching (Figure 1h). To ascertain whether or not the expression of GRP and GRPR was altered in IL-22 Tg(+) mice, lesional AD skin and DRG isolated from Tg(+) mice and Tg(−) littermate controls were investigated. Using immunofluorescence, we found that the dermis was highly innervated by PGP9.5+ afferent fibers co-expressing GRP with marked ramifications to the epidermis (Figure 6a, 6b, and 6c). In addition, GRP+ skin-innervating neurons in Dorsal Root Ganglia (DRG) were significantly increased in Tg(+) mice compared to Tg(−) littermates (Figure 6d, 6e), which may reflect a peripheral role of GRP in AD. Marked increases in GRP+ cells co-expressing markers for tryptase+ mast cells, MBP+ eosinophils, CD11b+ DCs and macrophages were readily seen in AD lesional dermal region of Tg(+) mice, but rarely seen in the skin of Tg(−) littermates (Figure 6f, 6g, 6h, 6i, 6k). Furthermore, the expression of GRP receptor was highly induced in the keratinocytes of IL-22 Tg(+) skin, but not in the keratinocytes of Tg(−) mice (Figure 6j). Clinically, the expression of GRP was progressively enhanced in the Tg(+) skin correlating with the frequency of itch-evoked behavior and the disease severity of AD (Figure 6l and 6m). Interestingly, IL-31, a pruritic cytokine in AD was markedly down-regulated in both acute and chronic IL-22 AD lesion samples (Figure 6n). These data implicate that the GRP/GRPR pathway may have an important role in the pathogenesis of IL-22–induced pruritus and inflammation in AD, which may act on keratinocytes to regulate the functions of these cells.
Figure 6. IL-22 induced enhanced expression of GRP and GRPR in the dermal immune cells, afferent nerves and neurons in skin-innervating dorsal root ganglia (DRG).
(a, b, c) Expression and quantification of enhanced PGP9.5+ sensory nerves (green) co-expressing GRP (red) in the epidermis; and (d, e) Expression and quantification of GRP+ (red) neurons in Tg(+) DRG by IF. (f, k) Increased expression and numbers of GRP+ cells (red) in the dermis of Tg(+) skin; (g) GRP+ cells (red) co-expressing MBP+ eosinophils (green), (h) GRP+ cells (green) co-expressing Tryptase+ mast cells (red), and (i) GRP+ cells (red) co-expressing CD11b+ DCs/Macrophages (green) by IF. (j) GRP Receptor (GRPR) (red) inducibly expressed on Tg(+) keratinocytes, arrows, not on Tg(−) keratinocytes by IF. (l) Increased GRP+ cells in Tg(+) AD correlated with AD severity and (m) with chronic pruritus in Tg(+) mice. (n) Expression of IL-31 in the skin by qPCR, in acute AD (A) and chronic AD (C). (n=7–8 mice each group; *p<0.05, **p<0.01, and ***p<0.001. HPF: high power field).
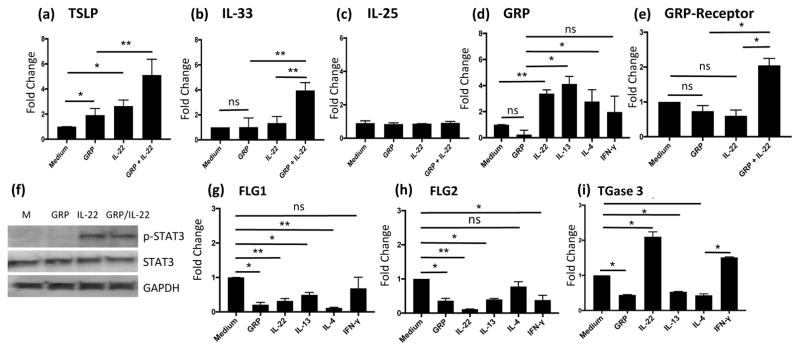
IL-22 directly upregulated the expression of GRP and GRP receptor in human primary keratinocytes and GRP synergistically enhanced IL-22-induced epithelial-keratinocyte innate type 2 cytokines
To understand the mechanism by which exaggerated Th2 responses were induced in the transgenic IL-22-induced AD skin and systemic Th2 responses and to determine whether IL-22 could regulate the expression GRP and GRP receptor, the expression of epithelial-innate type 2 cytokines (TSLP, IL-33 and IL-25) by KCs when stimulated with IL-22 in the presence and absence of GRP was evaluated by qPCR. TSLP expression in both IL-22-stimulated and GRP-stimulated KCs was significantly increased. Synergistically enhanced expression of TSLP was seen in IL-22 and GRP co-stimulated KCs (Figure 7a). Similarly, IL-22/GRP co-stimulation of KCs caused a synergistic upregulation of IL-33, although either IL-22 or GRP alone did not (Figure 7b). However, IL-22 and GRP did not alter the expression of IL-25 (Figure 7c). IL-22 and Th2 cytokines (IL-4 and IL-13) potently upregulated the expression of GRP (Figure 7d), but not by IFN-γ. GRP acted in concert with IL-22 to enhance the expression its receptor GRPR (Figure 7e). Furthermore, the expression of the genes encoding FLG1, FLG2 and TGase 3, important for epithelial differentiation and skin barrier function were significantly reduced in both GRP treated and IL-22 treated KCs and in Th2 cytokine-stimulated KCs (Figure 7g. 7h. 7i). Co-stimulation of KCs with IL-22 and GRP did not alter the activation/phosphorylation of STAT3 induced (Figure 7f). These data strongly suggested that IL-22 initiates Th2 responses in AD in part, by differentially inducing keratinocyte derived Th2 inflammation promoting cytokines (TSLP and IL-33), but not IL-25, and by working in concert with GRP to promote these Th2 inflammation and activation of the GRP/GRPR pathway. Also, these Data suggest that in addition to the ability of GRP to enhance IL-22-indeuced epithelial-keratinocyte innate type 2 cytokines and GRP receptor, GRP also has a role in down-regulation of the expression of FLG1, FLG2 and TGase 3 to contribute to the pathogenesis of AD.
Figure 7. Upregulation of epithelial keratinocyte-derived Type 2 cytokines, GRP and GRPR by IL-22 and GRP.
After stimulation with various cytokines and GRP for 24 hrs, total RNA was purified from primary human keratinocytes (KCs). Quantitative PCR was used to evaluate the expression of genes of interest. (a, b, c) mRNA expression of epithelial TSLP, IL-33 and IL-25 relative to medium-treated KCs. (d, e) Enhanced GRP and GRPR expression. (f) Activation of STAT3 in KCs by IL-22 +/− GRP for 24 hrs. (g, h, i) Regulation of mRNA expression of FLG1, FLG2 and TGase 3 by GRP and cytokines. Similar results were obtained in two other independent experiments. Data are Mean±SEM (*p<0.05; **p<0.01; ***p<0.001; ns, not significant).
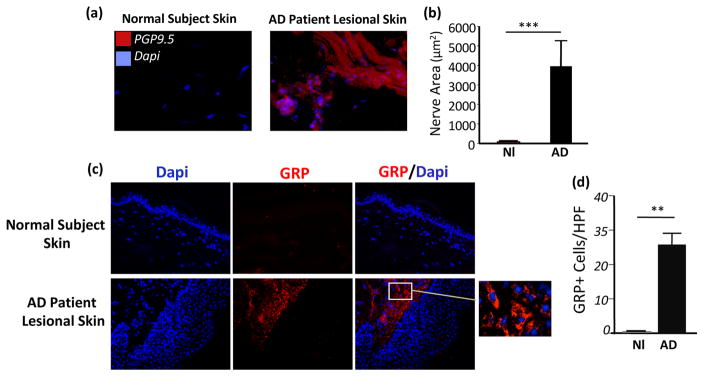
Highly increased expression of GRP in dermal cells and dermal afferent nerves in human AD lesions
It has been shown that the serum levels of GRP in patients with AD correlated with the disease severity (35). However, whether GRP and GRPR are expressed in human AD skin is unknown. We compared the expression of GRP in the skin biopsy samples from patients with AD and normal subjects. Importantly, in line with the findings of expression of GRP in IL-22 Tg(+) mice, we found strikingly high levels of expression of GRP in dermal cells and in dermal and epidermal sensory afferents in the AD skin biopsies from patients with AD; however, minimal or no expression of GRP was seen in the skin biopsies from normal subjects (Figure 8a–d). These previous unrecognized findings suggest that the GRP/GRPR pathway may have a role in the pathogenesis of human AD.
Figure 8. Significant increase of dermal PGP9.5+ sensory nerves and enhanced expression of GRP in human AD lesions.
(a, b) IHC and quantification of dermal PGP9.5+ afferents. (c) Increased GRP+ dermal cells in AD (Normal subject skin (Nl) and lesional skin (AD) from patients), (**p<0.01, and ***p<0.001; HPF: high power field).
Discussion
AD is a heterogeneous disorder with different clinical courses. It is poorly understood why some of the children with AD enter the path of the atopic march, progressing from AD to asthma, while others have a mild disease course or spontaneous remission. It is certain that multiple immunological mechanisms are involved in the pathogenesis of AD. Studies of various immunological factors and pathways in human AD and in animal models of AD are necessary to improve our understanding of the disease. Mouse models of atopic dermatitis and other chronic dermatitis have shed lights on the pathogenic mechanisms of chronic dermatological diseases. For instance, transgenic kallikrein 5 (KLK5) mice reproduced the major features of Netherton Syndrome associated with Th2/Th17/Th22 T cell responses (36). Skin-specific expression of IL-4, IL-13, TSLP, and IL-33 elicited pruritic AD-like dermatitis (16, 37–40). More recently, a study showed that transgenic expression of ΔNp63 resulted in keratinocyte activation and an AD-like phenotype (41). Several studies, including our own, have showed that chronic epicutaneous antigen exposure caused AD-like dermatitis and enhanced airway inflammation and airway obstruction after allergen inhalation challenge (21, 42). Further, skin-derived TSLP has been shown to trigger progression from epidermal-barrier defects to asthma (43). We showed that IL-13-induced AD and airway obstruction and lung inflammation were largely dependent on TSLP (44).
Recent studies established the presence and activation of Th22/IL-22 in both acute and chronic skin lesions in patients with AD (45, 46), a positive correlation of IL-22 with the AD disease activity (SCORAD score) and a negative correlation with expression of epidermal terminal differentiation markers (5, 7, 47–50). Evidence for the effector function and immunopathological mechanisms of IL-22 in skin diseases is accumulating. Th22 cells express chemokine receptor (CCR4), a skin homing receptor, indicating a prominent role of IL-22 in the skin (51). IL-22R is abundantly expressed on keratinocytes and some fibroblasts, but not on immune cells. Depending on the cytokine milieu and tissue in which it is expressed, IL-22 can regulate the expression of genes encoding molecules associated with inflammation, repair or chemotaxis or the expression of antimicrobial peptides (52). IL-22 suppresses major epidermal terminal differentiation proteins (i.e., filaggrin and loricrin) (6, 53) and induces pro-inflammatory gene expression of the S100 family of calcium-binding proteins in human epidermal keratinocytes (53). Local delivery of exogenous IL-22 is sufficient to promote acute phase responses (e.g. in liver) and IL-22 deficiency decreases IL-23-induced ear skin acanthosis and inflammation (54, 55). A Phase II clinical trial testing an anti-IL-22 antibody as a therapy for AD has been initiated (ClinicalTrials.gov Identifier: NCT01941537). However, many aspects of IL-22 in the pathogenesis of AD have not been elucidated, such as the interactions between IL-22 and Th2 immune responses and the effect of IL-22 on itch in AD.
In HDM allergen induced AD model, we found that IL-22, as well as Th2 cytokines, was markedly increased in the lesion skin of mice with AD. To gain insight into the role that IL-22 might play in atopic dermatitis, we took the transgenic approach. It has been reported that over-expression of IL-22 under the control of the lymphoid EμLCK promoter or rat insulin II promoter in mice resulted in skin alterations including acanthosis and hypogranularity but neonatal mortality (56). We utilized the K5-tTA and TRE-Tight-IL-22 skin specific inducible transgenic system and obtained live mice that allowed us to analyze the biological effects of IL-22 in a controlled fashion (Figure 1). When selectively expressed in the skin, IL-22 caused chronic pruritic dermatitis with many key clinical, immunopathological and molecular features resembling those in human AD. These include chronic pruritus, skin barrier impairment, local and systemic biased Th2 responses, increased susceptibilities to S. aureus colonization, and enhanced antigen-sensitization, highlighting IL-22 as an important link between skin barrier and adaptive immunity.
IL-22 impairs skin barrier function in Tg(+) mice by down-regulating Filaggrin-2, Claudin-1 and TGase 3 critical for the formation of the epidermal differentiation (Figure 4). It has been shown that T cells can be induced by S. aureus derived enterotoxins to produce IL-17 and IL-22 (15, 21, 57). The role of IL-22 in allergen sensitization in AD models has not been determined. In the present study IL-22 Tg(+) mice exhibited marked skin barrier dysfunction even before the onset of clinical dermatitis (Figure 4). When these mice were epicutaneously exposed to Ova antigen before the onset of visible AD, exaggerated Th2 responses and Th17 responses were seen as compare to Ova-sensitized Tg(−) littermates, suggesting that a biased Th22 milieu facilitates Ag sensitization and associated dermal and systemic Th2/Th17 inflammatory responses (Figure 5). In this chronic AD model, IL-22 may serve as an important link between the skin barrier defect with suppression of EDC differentiation and Th2 polarization, possibly in part by enhancing allergen penetration through damaged epidermis and by increased production of epithelial TSLP.
Patients with AD have a unique predisposition to colonization or infection by S. aureus (3). The skin of 90% of AD patients is colonized with S. aureus whereas it occurs in only 10% of healthy individuals (58, 59). Exacerbation of AD can be provoked by exposure to allergens (60, 61) and superantigen-producing S. aureus (62). Our group and others have shown that repeat epicutaneous (EC) allergen sensitization with ovalbumin (Ova) and HDM extract induced AD-like dermatitis (21, 31, 32) and SEB synergistically enhances allergen induced dermatitis in mice (21, 63). Although the diverse roles of SEB and antigen in skin inflammation of AD are well appreciated, their role in itch in AD is still poorly understood. Strikingly high numbers of colonies of SEB-producing S. aureus on AD skin of IL-22 mice, not on Tg(−) skin implicate a role for IL-22 in increased susceptibility to S. aureus, possibly in part via inhibiting TLRs (Figure 4e).
Chronic itch is a cardinal feature of human AD. Little is known about how dermal itch sensory nerves (C fibers) interact with dermal immune cells and keratinocytes in the initiation or aggravation of itch. GRP has been implicated as an itch-specific neurotransmitter required for non-histaminergic pruritoceptive transmission (26, 64). The pathophysiology of chronic itch (pruritoception) in AD involves a complex network of cutaneous and neuronal cells, immune cells, mediators and impaired skin barrier and the associated transdermal water loss (65). Antihistamines are ineffective in treating chronic itch in AD, pointing to the existence of distinct pruritogens and histamine-independent itch pathways (66, 67). A study using GRPR−/− mice showed that the GRP/GRPR system is specifically involved in itch but not in pain (33). The role of GRP in specific transmission of chronic itch in primates was demonstrated by over-expressing GRP in cutaneous nerve fibers and its receptor on spinal cord (68).
It has been shown that the nerve endings (dermal afferents) of dorsal root ganglion neurons have a variety of sensory receptors that are activated by mechanical, inflammation and noxious stimuli. It is clear that both sensory neurons and the peripheral afferents are capable of both elaborating and responding to cytokines (e.g. TNF-α) and producing neuropeptides and cytokines (IL-6 and IL-1β), suggesting a complex network of inter-dependent signaling exist between these various cellular elements of sensory nerves in the skin (69–73). In IL-22 induced AD, we showed strikingly high expression of GRP in AD lesional afferents and increased numbers of the skin innervating sensory neurons in dorsal root ganglia (DRG) (Figure 6a–e) as well as in GRP+ cells that co-expressed MBP (eosinophils), tryptase (mast cells), and CD11b (DCs/Macrophages cells) (Figure 6f–i), suggesting complex bidirectional interactions between the peripheral nerve system (skin GRP+ afferents and GRP+ sensory neurons of these afferents) and dermal inflammatory and immune cells/cytokine stimulation, which orchestrates a strong Th2/Th22 biased inflammation in AD skin. Further, GRP enhanced the effects of IL-22 on GRP receptor expression on human epidermal keratinocytes (Figure 7e), in agreement with the finding of differential expression of GRP receptor in epidermal keratinocytes of transgenic IL-22 AD mice (Figure 6j).
Together these studies suggest that GRP may be involved in neurogenic inflammation and chronic itch. GRP could be released by GRP+ neurons/afferents. Physical close interaction between GPR+ afferents with the GRP receptor highly expressed on KCs could be important in initiating and sustaining the skin neuro-inflammation and pruritus in IL-22-induced AD. On the other hand, the expression of IL-31, a pruritic cytokine involved in chronic AD, was significantly down-regulated by IL-22 (Figure 6n), suggesting that complex mechanisms are involved in the pruritogenesis of chronic itch in AD. Consistently, GRP expression was also significantly increased in skin lesions in patients with AD, but rarely seen in normal skin of control subjects (Figure 8). In keeping with studies on the mechanisms of chronic itch in AD, we have recently shown that TRPA1 is important in chronic itch in IL-13-induced AD via a mast cell dependent mechanism (17) and others have shown TRPA1 is essential for the signaling pathways that promote histamine-independent itch (17, 74). Further studies will need to characterize the molecular pathways connecting the cellular interactions between inflammatory cells, keratinocytes, and nerves that are important in the pathogenesis and in chronic itch in AD.
The importance of epithelial cells in regulating type-2 cell-mediated chronic inflammatory disorders, such as asthma and atopic dermatitis, have been highlighted (75, 76). Epithelial cell derived IL-25, IL-33 and TSLP promote type 2 cytokine responses either directly by Th2 cytokine expressing cells (ILC2s, basophils and others) or indirectly via dendritic cell polarization (10, 25, 77–84). IL-22 produced by activated T cells directly regulates the function of barrier epithelial cells through binding to IL-22 receptor (IL-22R) (52, 85). To understand how IL-22 induced atopic dermatitis with prominent Th2 inflammation and Th2 immunity, we tested the effects of IL-22 on keratinocytes, with or without GRP. The results showed that IL-22 alone or in concert with GRP can directly act on keratinocytes to enhance the expression of TSLP and IL-33 (Figure 7). Interestingly, GRP, an itch-specific neuropeptide, not only stimulated KCs to produce TSLP but also collaborated with IL-22 in upregulating epithelial GRPR expression, suggesting a direct role of IL-22 in promoting the epidermal GRP/GRPR pathway, in agreement with strong expression of GRP in IL-22-induced AD lesions, the afferents and associated skin-innervating sensory neurons in DRG and GRP receptor. IL-22 exerts its function on keratinocytes by activating STAT3 pathway. However, co-stimulation with GRP on these cells did not alter STAT3 phosphorylation. An up-regulation of GRP/GRPR has been suggested as a feature of the development of chronic itch (26). Together, these findings highlighted a possible contributing role of GRP/GRPR and IL-22 in chronic pruritus and neurogenic inflammation in AD.
In conclusion, our studies have demonstrated an important pathological role of IL-22 in an AD-like disorder when IL-22 is selectively expressed in the mouse skin. This presents with chronic pruritus, skin barrier impairment, biased local and systemic Th2 responses, and chronic colonization of superantigen-producing S. aureus that mimic important pathologic and immunologic features of human atopic dermatitis. Mechanistic studies showed that IL-22 acts in part by stimulating keratinocyte production of innate epithelial Th2-promoting cytokines, by down-regulating skin barrier and tight junction genes, and by stimulating the GRP/GRPR pathway, which in turn is able to stimulate keratinocytes. These are previously unrecognized effects of IL-22 and its interactions with the Th2 immunity and with a neuropeptide signaling pathway in the pathogenesis of AD.
Supplementary Material
Acknowledgments
This work was supported by NIH AI07502 grant and the Milstein Medical Asian American Partnership (MMAAP) Foundation grant to TZ and NIH P01HL107151 to ZZ.
References
- 1.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, Group IPTS. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258. e1223. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Guttman-Yassky E, Dhingra N, Leung DY. New era of biologic therapeutics in atopic dermatitis. Expert Opin Biol Ther. 2013;13:549–561. doi: 10.1517/14712598.2013.758708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125:4–13. doi: 10.1016/j.jaci.2009.11.027. quiz 14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371–379. doi: 10.1016/j.jaip.2014.03.006. quiz 380–371. [DOI] [PubMed] [Google Scholar]
- 5.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252 e1242. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung DYM, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J Allergy Clin Immun. 2014;134:769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souwer Y, Szegedi K, Kapsenberg ML, de Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22:821–826. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–1432. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011:2. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szegedi K, Kremer AE, Kezic S, Teunissen MB, Bos JD, Luiten RM, Res PC, Middelkamp-Hup MA. Increased frequencies of IL-31-producing T cells are found in chronic atopic dermatitis skin. Exp Dermatol. 2012;21:431–436. doi: 10.1111/j.1600-0625.2012.01487.x. [DOI] [PubMed] [Google Scholar]
- 12.Teraki Y, Sakurai A, Izaki S. IL-13/IL-22-coproducing T cells, a novel subset, are increased in atopic dermatitis. J Allergy Clin Immunol. 2013;132:971–974. doi: 10.1016/j.jaci.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA, Clark RA. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. J Invest Dermatol. 2013;133:973–979. doi: 10.1038/jid.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Jr, Chapman HA, Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang P, Zhou L, Zhou Y, Kolls JK, Zheng T, Zhu Z. Immune modulatory effects of IL-22 on allergen-induced pulmonary inflammation. PLoS One. 2014;9:e107454. doi: 10.1371/journal.pone.0107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129:742–751. doi: 10.1038/jid.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, Zheng T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. Journal of immunology. 2013;191:5371–5382. doi: 10.4049/jimmunol.1300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa N, Yamamoto M, Imai Y, Sakaguchi Y, Takizawa T, Ohta N, Yagi N, Hatta I, Hitomi K, Takizawa T, Takeda J, Tsuda T, Matsuki M, Yamanishi K. Knocking-in the R142C mutation in transglutaminase 1 disrupts the stratum corneum barrier and postnatal survival of mice. J Dermatol Sci. 2012;65:196–206. doi: 10.1016/j.jdermsci.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Kambara T, Aihara M, Matsukura S, Sato I, Kubota Y, Hirasawa T, Ikezawa Z. Effects of photocatalytic agent on DS-Nh mice, developing atopic dermatitis-like eruption with an increase of Staphylococcus aureus. Int Arch Allergy Immunol. 2006;141:151–157. doi: 10.1159/000094717. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Oh MH, Park JU, Myers AC, Dong C, Zhu Z, Zheng T. Epicutaneous exposure to staphylococcal superantigen enterotoxin B enhances allergic lung inflammation via an IL-17A dependent mechanism. PLoS One. 2012;7:e39032. doi: 10.1371/journal.pone.0039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyoshi MK, He R, Kanaoka Y, ElKhal A, Kawamoto S, Lewis CN, Austen KF, Geha RS. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc Natl Acad Sci U S A. 2012;109:4992–4997. doi: 10.1073/pnas.1203127109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, Liu YJ, Zhu Z, Zheng T. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. Journal of immunology. 2011;186:7232–7242. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature immunology. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 26.Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, Liu XY, Barry DM, Wan L, Liu ZC, Li H, Turkoz A, Ma K, Cornelius LA, Kopan R, Battey JF, Jr, Zhong J, Chen ZF. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest. 2013;123:4769–4780. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Benedictis FM, Franceschini F, Hill D, Naspitz C, Simons FE, Wahn U, Warner JO, de Longueville M. The allergic sensitization in infants with atopic eczema from different countries. Allergy. 2009;64:295–303. doi: 10.1111/j.1398-9995.2008.01779.x. [DOI] [PubMed] [Google Scholar]
- 28.Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, Kietzmann M, Baumer W. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp Dermatol. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 29.Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151–161. doi: 10.2332/allergolint.13-RAI-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui K, Nishikawa A, Suto H, Tsuboi R, Ogawa H. Comparative study of Staphylococcus aureus isolated from lesional and non-lesional skin of atopic dermatitis patients. Microbiol Immunol. 2000;44:945–947. doi: 10.1111/j.1348-0421.2000.tb02587.x. [DOI] [PubMed] [Google Scholar]
- 31.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 34.Andoh T, Kuwazono T, Lee JB, Kuraishi Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides. 2011;32:2098–2103. doi: 10.1016/j.peptides.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Kagami S, Sugaya M, Suga H, Morimura S, Kai H, Ohmatsu H, Fujita H, Tsunemi Y, Sato S. Serum Gastrin-Releasing Peptide Levels Correlate with Pruritus in Patients with Atopic Dermatitis. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.38. [DOI] [PubMed] [Google Scholar]
- 36.Furio L, de Veer S, Jaillet M, Briot A, Robin A, Deraison C, Hovnanian A. Transgenic kallikrein 5 mice reproduce major cutaneous and systemic hallmarks of Netherton syndrome. J Exp Med. 2014;211:499–513. doi: 10.1084/jem.20131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001;117:977–983. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 38.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci U S A. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizzo JM, Oyelakin A, Min S, Smalley K, Bard J, Luo W, Nyquist J, Guttman-Yassky E, Yoshida T, De Benedetto A, Beck LA, Sinha S, Romano RA. DeltaNp63 regulates IL-33 and IL-31 signaling in atopic dermatitis. Cell Death Differ. 2016;23:1073–1085. doi: 10.1038/cdd.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci U S A. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Z, Oh MH, Yu J, Liu YJ, Zheng T. The Role of TSLP in IL-13-Induced Atopic March. Sci Rep. 2011;1:23. doi: 10.1038/srep00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, Mitsui H, Cardinale I, de Guzman Strong C, Krueger JG, Guttman-Yassky E. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavani A, Pennino D, Eyerich K. Th17 and Th22 in skin allergy. Chem Immunol Allergy. 2012;96:39–44. doi: 10.1159/000331870. [DOI] [PubMed] [Google Scholar]
- 47.Tintle S, Shemer A, Suarez-Farinas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, Johnson-Huang L, Chiricozzi A, Cardinale I, Duan S, Bowcock A, Krueger JG, Guttman-Yassky E. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583–593. e581–584. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rebane A, Zimmermann M, Aab A, Baurecht H, Koreck A, Karelson M, Abram K, Metsalu T, Pihlap M, Meyer N, Folster-Holst R, Nagy N, Kemeny L, Kingo K, Vilo J, Illig T, Akdis M, Franke A, Novak N, Weidinger S, Akdis CA. Mechanisms of IFN-gamma-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:1297–1306. doi: 10.1016/j.jaci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, Hansen KC, Leung DY. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–2258. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 50.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 51.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nature immunology. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 52.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nature immunology. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 53.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. Journal of immunology. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 54.Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, Fouser LA. IL-22 induces an acute-phase response. Journal of immunology. 2010;185:5531–5538. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 56.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, Sabat R. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 57.Niebuhr M, Scharonow H, Gathmann M, Mamerow D, Werfel T. Staphylococcal exotoxins are strong inducers of IL-22: A potential role in atopic dermatitis. J Allergy Clin Immunol. 2010;126:1176–1183 e1174. doi: 10.1016/j.jaci.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 58.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. The British journal of dermatology. 1974;90:525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 59.Breuer K, Kapp A, Werfel T. Bacterial infections and atopic dermatitis. Allergy. 2001;56:1034–1041. doi: 10.1034/j.1398-9995.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- 60.Maeda K, Yamamoto K, Tanaka Y, Anan S, Yoshida H. House dust mite (HDM) antigen in naturally occurring lesions of atopic dermatitis (AD): the relationship between HDM antigen in the skin and HDM antigen-specific IgE antibody. J Dermatol Sci. 1992;3:73–77. doi: 10.1016/0923-1811(92)90038-d. [DOI] [PubMed] [Google Scholar]
- 61.Kimura M, Tsuruta S, Yoshida T. Correlation of house dust mite-specific lymphocyte proliferation with IL-5 production, eosinophilia, and the severity of symptoms in infants with atopic dermatitis. J Allergy Clin Immunol. 1998;101:84–89. doi: 10.1016/S0091-6749(98)70197-6. [DOI] [PubMed] [Google Scholar]
- 62.Zollner TM, Wichelhaus TA, Hartung A, Von Mallinckrodt C, Wagner TO, Brade V, Kaufmann R. Colonization with superantigen-producing Staphylococcus aureus is associated with increased severity of atopic dermatitis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2000;30:994–1000. doi: 10.1046/j.1365-2222.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- 63.Savinko T, Lauerma A, Lehtimaki S, Gombert M, Majuri ML, Fyhrquist-Vanni N, Dieu-Nosjean MC, Kemeny L, Wolff H, Homey B, Alenius H. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, a mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. Journal of immunology. 2005;175:8320–8326. doi: 10.4049/jimmunol.175.12.8320. [DOI] [PubMed] [Google Scholar]
- 64.Zhao ZQ, Liu XY, Jeffry J, Karunarathne WK, Li JL, Munanairi A, Zhou XY, Li H, Sun YG, Wan L, Wu ZY, Kim S, Huo FQ, Mo P, Barry DM, Zhang CK, Kim JY, Gautam N, Renner KJ, Li YQ, Chen ZF. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron. 2014;84:821–834. doi: 10.1016/j.neuron.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin Rev Allergy Immunol. 2015 doi: 10.1007/s12016-015-8488-5. [DOI] [PubMed] [Google Scholar]
- 66.Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617–622. doi: 10.2165/00128071-200304090-00004. [DOI] [PubMed] [Google Scholar]
- 67.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 68.Nattkemper LA, Zhao ZQ, Nichols AJ, Papoiu AD, Shively CA, Chen ZF, Yosipovitch G. Overexpression of the gastrin-releasing peptide in cutaneous nerve fibers and its receptor in the spinal cord in primates with chronic itch. J Invest Dermatol. 2013;133:2489–2492. doi: 10.1038/jid.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976) 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 71.Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Gadient RA, Otten U. Postnatal expression of interleukin-6 (IL-6) and IL-6 receptor (IL-6R) mRNAs in rat sympathetic and sensory ganglia. Brain Res. 1996;724:41–46. doi: 10.1016/0006-8993(96)00264-8. [DOI] [PubMed] [Google Scholar]
- 73.Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. Journal of neurochemistry. 1999;73:2206–2213. [PubMed] [Google Scholar]
- 74.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nature medicine. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 77.Gilliet M, Soumelis V, Watanabe N, Hanabuchi S, Antonenko S, de Waal-Malefyt R, Liu YJ. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197:1059–1063. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, Ziegler SF, Comeau MR. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. Journal of immunology. 2008;181:4311–4319. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 81.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 85.Wolk K, Witte E, Reineke U, Witte K, Friedrich M, Sterry W, Asadullah K, Volk HD, Sabat R. Is there an interaction between interleukin-10 and interleukin-22? Genes Immun. 2005;6:8–18. doi: 10.1038/sj.gene.6364144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.