Abstract
Background
Activity limitation stages based on activities of daily living (ADLs) and instrumental activities of daily living (IADLs) are associated with 3-year mortality in elderly Medicare beneficiaries, but their associations with hospitalization risk in this population have not been studied.
Objective
To examine the independent association of activity limitation stages with risk of hospitalization within a year among Medicare beneficiaries aged 65 years and older.
Design
Cohort study.
Setting
Community.
Participants
A total of 9447 community-dwelling elderly Medicare beneficiaries from the Medicare Current Beneficiary Survey (MCBS) for years 2005–2009.
Methods
Stages were derived for activities of daily living (ADLs) and instrumental activities of daily living (IADLs) separately. Associations of stages with time to first hospitalization and time to recurrent hospitalizations within a year were assessed with Cox proportional hazards model, accounting for baseline sociodemographics, smoking status, comorbidities, and the year of survey entry.
Main Outcomes
Time to first hospitalization and time to recurrent hospitalizations within one year.
Principle Findings
The adjusted risk of first hospitalization increased with higher activity limitation stages (except stage III). The hazard ratios (95% confidence intervals) for ADL stages I-IV compared to stage 0 (no limitations) were 1.49 (1.36–1.63), 1.61 (1.44–1.8), 1.54 (1.35–1.76), and 2.06 (1.61–2.63), respectively. The pattern for IADL stages was similar. For recurrent hospitalizations, activity limitation stages were associated with the risk of the first hospitalization, but not with subsequent hospitalizations.
Conclusion
Activity limitation stages are associated with risk of first hospitalization in the subsequent year among elderly Medicare beneficiaries. Stages capture clinically interpretable profiles of ADL and IADL functionality and describe preserved functions and activity limitation in an aggregated measure. Stage can inform interventions to ameliorate disability and thus reduce the risk of a subsequent hospitalization in this population.
Limitation in activities of daily living (ADLs) or instrumental activities of daily living (IADLs) is a known risk factor for hospitalization 1–3 and consequently excess health care costs. 2 This phenomenon is especially accentuated in older adults because of their high prevalence of ADL and IADL limitations and greater background risk for hospitalization. ADL/IADL limitation refers to difficulty in performing ADL/IADL tasks, whether or not human assistance is required. About 41% of U.S. individuals aged 65 years and older have at least one limitation in ADLs or IADLs.4 Among Medicare recipients, 14% of those who had no ADL impairment were hospitalized per year, compared to 30%, 38% and 50% of those who had 1–2, 3–4 and 5–6 ADL limitations that required assistance, respectively.2 Medicare beneficiaries who had 3 or more ADL limitations that required assistance had a higher unplanned hospital readmission rate within 30 days compared to those with intact ADLs (18.2% versus 13.5%).1 Older adults with unmet needs for managing ADL tasks were also more likely to experience one or repeated hospitalizations.5,6 With the expanding life expectancy, the share of US population that is aged 65 and older is projected to increase from 13.7% in 2012 to 20.3% in 2030.7 Understanding the relationships among diverse disability patterns and associated hospitalization risk are imperative to improve older patient’s health and reduce costs.
As defined by the International Classification of Functioning, Disability and Health (ICF),8 ADLs entail self-care functions of eating, toileting, bathing or showering, getting in/out of bed or chairs, and walking; IADLs include domestic life functions of telephoning, managing money, preparing meals, doing light housework, shopping for personal items and doing heavy housework. Although counts of ADL and IADL limitations are associated with hospitalization among older adults,2,9–11 such counts do not indicate which specific ADL or IADL activities are limited. This makes it difficult to predict services needed to ameliorate specific ADL impairment leading to hospitalization. For example, limitation in bathing (which might pose a risk of slip and fall-related injury) versus limitation in eating (which might be associated with a risk of aspiration) will have very different implications for sources and types of assistance needed to avert hospitalization and to allow older adults to remain at home.
The HealthyPeople-2020 objectives and the Patient Protection and Affordable Health Care Act of 2010 endorse the need for standard data aggregation approaches to identify disability related disparities.12 Stineman and colleagues developed separate ICF-based activity limitation stage systems for ADLs and IADLs to characterize groups of individuals with homogeneous activity limitation.13–15 Five ADL stages (0-IV) and five IADL stages (0-IV) characterize the severity and types of disability experienced and specify clinically meaningful patterns of increasing difficulty with self-care and domestic management tasks. Stages were labeled by the ICF convention of “performance qualifiers” for activity limitation as follows: no (stage 0), mild (stage I), moderate (stage II), severe (stage III), and complete (stage IV) activity limitation. Stage III was designed as a non-fitting stage to accommodate people with unusual patterns of limitation. Limitation categories and their associated ADL and IADL stages are presented in the Appendix I. In the Longitudinal Study of Aging, these stages have been found to have ordered associations with one-, five-, and ten-year mortality, as well as to nursing home admission.16–18 In the population of older Medicare beneficiaries, these stages have ordered associations with three-year mortality.19 Whether and how activity limitation stage is associated with hospitalization has yet to be examined.
Our current study aimed to determine the extent to which disability stage is independently associated with hospitalization above and beyond its association with sociodemographic and clinical characteristics. We were interested in two outcomes: time to first hospitalization and time to recurrent hospitalizations. As disability stage can change over time,20 we examined a one-year follow-up interval after ascertainment of stage. Our hypothesis was that higher ADL or IADL stages are associated with greater risks of first and subsequent hospitalizations during the year following the ascertainment of stage, with the possible exception of the non-fitting stage III.13,15
Methods
Data Source and Design
We used data from the Medicare Current Beneficiary Survey (MCBS). The MCBS is a rotating panel survey of a nationally representative sample of the Medicare beneficiaries.21 The MCBS beneficiaries provide information of their sociodemographics, health and functioning status, and medical encounters. A respondent is usually interviewed annually for four years. Interviews to collect data on health and physical functioning are conducted during the months of September to December of each year. The MCBS releases annual Cost and Use files that link Medicare claims to the survey. Medicare claims data are available for three consecutive years starting on January 1st of the year following the initial survey.
We included the 2005–2009 entry panels of community-dwelling Medicare beneficiaries aged 65 years and older, due to unavailability of data beyond 2010. The entry cohorts in year 2005 through 2007 were followed up for up to three years, and those entering in 2008 and 2009 were followed for two years and one year, respectively. We excluded survey beneficiaries who enrolled in a managed care program in any one-year follow-up period because of incomplete claims data for these individuals.
This study was approved by the University of Pennsylvania Institutional Review Board.
Outcome
The outcomes of interest were time to first hospitalization and time to recurrent hospitalizations in a year. For the outcome of time to recurrent hospitalizations, the time interval restarts after each hospitalization. In other words, time to first hospitalization referred to the interval from the start of the follow-up (January 1st of the year following the baseline interview) to the first hospital admission, and time to second hospitalization was the interval from the first hospital discharge to the second hospital admission. The same rule applied to time to the following sequential hospitalizations. Due to small numbers, 5th or later hospitalizations for the same subject were excluded (3% of all hospitalizations). Inpatient hospitalizations were identified using Medicare claims data.
Ascertainment of Activity Limitation Stages
Respondents were asked about their ability to perform six ADL activities (eating, toileting, dressing, bathing, moving in/out of bed/chair, and walking) and six IADL activities (using the telephone, managing money, preparing meals, doing light housework, shopping for personal items, and doing heavy housework). Each respondent was classified into an ADL and IADL stage derived from the method described previously.13,15 ADL and IADL stages were ascertained prior to the start of each follow-up year.
Ascertainment of Covariates
Covariates were ascertained during the same surveys in which ADL or IADL stages were measured. The covariates included age (65–74, 75–84, or ≥85 years), gender (male or female), race (non-Hispanic white, non-Hispanic black, Hispanic, or other), education (less than high school, high school, some college, or college and above), dual eligibility for Medicare and Medicaid, living arrangement (living alone, with spouse, with children, or with others), smoking, and comorbidities. We used dual eligibility for Medicare and Medicaid as a poverty measure because the income variable (< $25000 and ≥ $25000) in MCBS is a crude measure and greater than 10% of the whole sample had a missing value in income. Smoking was classified as never, past, or current. Comorbidities were conditions that a doctor ever told the beneficiary that she or he had, including Alzheimer’s disease, amputation, chronic heart disease, arthritis, broken hip, cancer, heart failure, depression, diabetes, chronic obstructive pulmonary disease (COPD), hardening of the arteries, heart rhythm disease, heart valve disease, hypertension, incontinence, mental disorders, retardation, myocardial infarction, osteoporosis, paralysis, Parkinson’s disease, rheumatoid arthritis and stroke. To account for multiple years of data, we controlled for the year of survey entry as a categorical variable (2005, 2006, 2007, 2008, and 2009).
Statistical Analysis
Baseline sample characteristics by activity limitation stage were compared using Chi-Square tests. Proportional hazards models were used to examine the association between activity limitation stage and time to first hospitalization and time to recurrent hospitalizations over the subsequent year. Results were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). Death and loss to follow-up were considered censoring events.
For time to first hospitalization in a year, we used multiple records available for each survey respondent. For example, if a respondent entered the survey in 2005, this individual would have up to 4 annual survey interviews and hospitalization records for up to 3 years after the initial interview, and would contribute up to 3 records in the Cox regression model. To assess the validity of the proportional hazards assumption, we tested the interaction term between stage and time to hospitalization, and examined the stability of hazard ratios over time. Although the interaction was statistically significant due to the large sample size, the HRs remained relatively stable over a year.
For time to recurrent hospitalizations, we used data from the baseline interview and the hospitalizations in the subsequent year. We applied a conditional model,22 in the sense that a respondent is assumed not to be at risk for a subsequent event until a prior event has occurred. We first detected inconsistent effect of stage on hospitalization over first four sequential hospitalizations (1st, 2nd, 3rd, and 4th). We then stratified the sample by the outcome (e.g., strata 1 – time to first hospitalization, strata 2 – time to the second hospitalization, strata 3 – time to the third hospitalization, strata 4 – time to the fourth hospitalization) and conducted a stratified analysis.
Covariates included in the models were decided a priori. Partially adjusted models included sociodemographics (age, gender, race/ethnicity, education, dual eligibility for Medicare and Medicaid, and living arrangement), smoking status, and year of survey entry. Fully adjusted models further included self-reported comorbidities. All models used only complete cases.
All models accounted for the complex survey design of MCBS (such as weight, clustering and stratification) and non-independence of observations within the same individual when applicable. The analysis was conducted in SAS 9.4 (SAS Institute, Cary, NC).
Results
Descriptive Statistics
Table 1 lists baseline characteristics of Medicare beneficiaries by ADL and IADL stage. In total, the MCBS 2005–2009 entry panels included 9,447 beneficiaries who were community-dwelling, aged 65 and older, and not enrolled in a managed care program at baseline. A great majority of the population experienced no functional loss (stage 0) in ADLs (71%; all %’s reported thereafter are weighted) or IADLs (65%). The percentage distribution of beneficiaries at ADL stage I through IV was 15%, 7%, 6%, and 0.9% respectively, and at corresponding IADL stages was 16%, 7%, 9%, and 2% respectively. Higher levels of disability were observed in beneficiaries who were older (85 and older), female, Non-White, with high school education or less, and dually eligible for Medicare and Medicaid. In general, higher stages, especially stage III and IV, were associated with diagnoses of medical comorbidities, most strikingly Alzheimer's disease, depression, incontinence, retardation, Parkinson's disease, paralysis, and stroke.
Table 1.
Weighted sample distribution of baseline covariates by Activity of Daily Living (ADL) Stage among enrollees (≥ 65) of the 2005–2009 entry panels of the Medicare Current Beneficiaries Survey (MCBS).
| Weighted Percent by Activity of Daily Living (ADL) Stage |
||||||
|---|---|---|---|---|---|---|
| Variable | Class | Stage 0 | Stage I | Stage II | Stage III |
Stage IV |
| Total sample weighted percent (raw size) | 71 (N=6433) |
15 (N=1561) |
7 (N=767) |
6 (N=576) |
0.9 (N=105) |
|
| Sociodemographics | ||||||
| Age | 65–74 | 59 | 41 | 33 | 37 | 27 |
| 75–84 | 33 | 41 | 42 | 37 | 45 | |
| 85+ | 8 | 18 | 25 | 26 | 28 | |
| Gender | Male | 46 | 41 | 36 | 35 | 45 |
| Female | 54 | 59 | 64 | 65 | 55 | |
| Race/Ethnicity | White | 86 | 83 | 81 | 80 | 76 |
| Black | 6 | 8 | 9 | 9 | 6 | |
| Hispanic | 5 | 6 | 6 | 7 | 11 | |
| Other | 3 | 4 | 4 | 4 | 7 | |
| Education | Less than High School | 21 | 31 | 37 | 38 | 42 |
| High School | 30 | 30 | 25 | 24 | 31 | |
| Some College | 27 | 24 | 24 | 22 | 20 | |
| College and above | 23 | 15 | 14 | 16 | 7 | |
| Dual Eligibility in Medicare and Medicaid |
10 | 18 | 28 | 27 | 45 | |
| Living Arrangement |
Alone | 31 | 34 | 35 | 34 | 24 |
| Spouse | 58 | 47 | 39 | 43 | 44 | |
| Children | 7 | 12 | 18 | 16 | 23 | |
| Other | 4 | 7 | 8 | 8 | 9 | |
| Self-reported medical comorbidities | ||||||
| Alzheimer’s disease | 1 | 2 | 7 | 7 | 20 | |
| Amputation | 0 | 1 | 1 | 3 | 3 | |
| Broken hip | 2 | 6 | 9 | 9 | 10 | |
| Cancer | 16 | 22 | 21 | 25 | 24 | |
| Chronic heart disease | 8 | 13 | 15 | 12 | 17 | |
| Chronic obstructive pulmonary disease (COPD) |
11 | 21 | 23 | 24 | 23 | |
| Depression | 12 | 23 | 29 | 30 | 37 | |
| Diabetes | Type 1 Diabetes | 1 | 3 | 4 | 4 | 4 |
| Type 2 Diabetes | 13 | 21 | 25 | 22 | 20 | |
| Other Type | 5 | 6 | 5 | 5 | 11 | |
| Heart failure | 4 | 9 | 16 | 16 | 13 | |
| Hardening of the arteries | 6 | 10 | 12 | 13 | 18 | |
| Heart rhythm disease | 14 | 22 | 23 | 29 | 29 | |
| Heart valve disease | 7 | 10 | 11 | 12 | 7 | |
| Hypertension | 57 | 71 | 71 | 69 | 74 | |
| Incontinence | No dialysis or Catheterization |
23 | 38 | 51 | 52 | 64 |
| Dialysis/Catheterization | 0 | 1 | 1 | 4 | 10 | |
| Mental / psychiatric disorders | 1 | 2 | 3 | 3 | 5 | |
| Myocardial infarction | 10 | 19 | 18 | 19 | 24 | |
| Osteoporosis | 14 | 21 | 27 | 24 | 22 | |
| Paralysis | 1 | 3 | 5 | 8 | 22 | |
| Parkinson’s disease | 1 | 2 | 3 | 3 | 9 | |
| Retardation | 0 | 0 | 1 | 1 | 5 | |
| Rheumatoid arthritis | 6 | 12 | 16 | 15 | 13 | |
| Arthritis other than rheumatoid | 39 | 61 | 61 | 63 | 48 | |
| Stroke | 7 | 15 | 21 | 22 | 38 | |
| Smoking | ||||||
| Smoker | Non-Smoke | 45 | 41 | 43 | 48 | 57 |
| Ever-Smoke | 44 | 45 | 50 | 44 | 33 | |
| Current-Smoke | 11 | 14 | 8 | 8 | 10 | |
Note. When a medical condition is classified as yes (present) or no (absent), only the yes category is displayed. The association of ADL stage with each covariate was assessed with the Chi-square test, and all p values < 0.001.
Of the 9,447 beneficiaries included at baseline, 6,424 and 4,516 were included in the second and third year, respectively, with most of the attrition due to loss of 2008–2009 entry panels and a small percentage due to missing covariates (0.4%). Average follow-up time was 0.86 year (standard error 0.28). The percentage of persons hospitalized over three years remained relatively stable: 20.5% of beneficiaries had at least one hospitalization in year one, 21.8% in year two, and 22.5% in year three.
Association of Activity Limitation Stages and Risk of the First Hospitalization
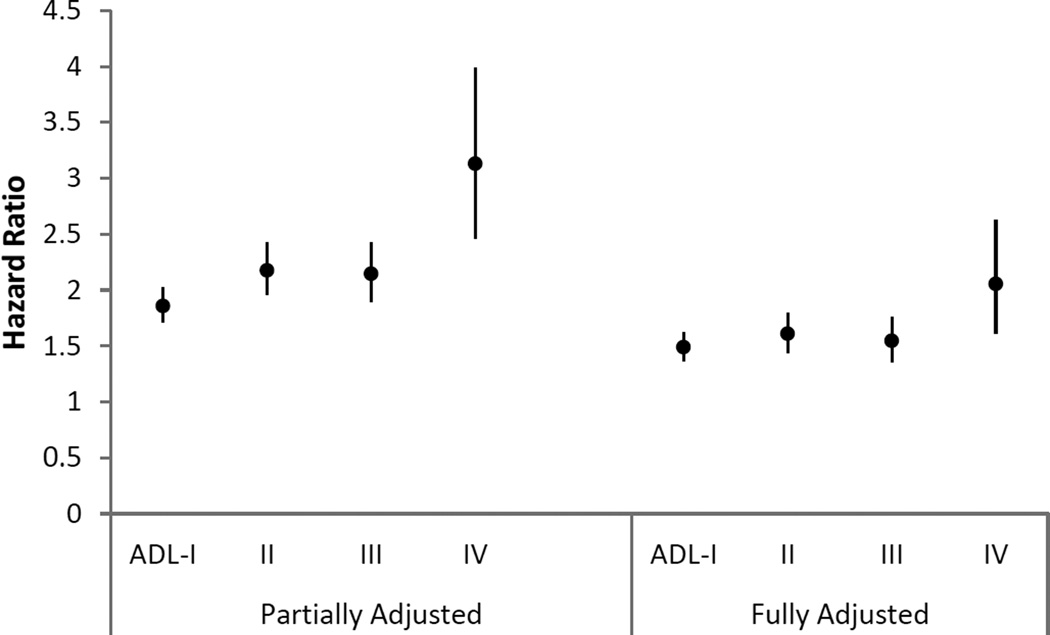
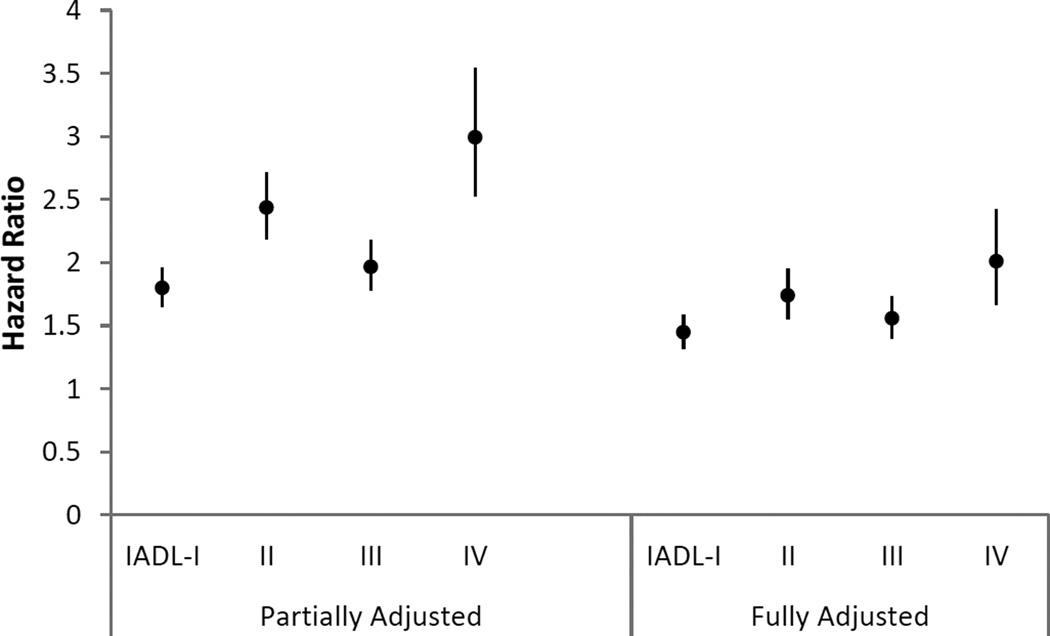
Figures 1 and 2 display the hazard ratios and 95% CIs for the association between activity limitation stages and first hospitalization in the year after stage was measured. In the models that adjusted only for sociodemographic factors, smoking status, and year of survey entry, hazard ratios of first hospitalization increased with higher stages (I to IV) versus stage 0 for both ADL (HRs and 95% CIs at 1.86 (1.71–2.02), 2.18 (1.96–2.42), 2.15 (1.9–2.43), and 3.13 (2.46–3.98)) and IADL (HRs and 95% CIs at 1.80 (1.65–1.96), 2.44 (2.19–2.71), 1.97 (1.78–2.18) and 2.99 (2.53–3.54)), except for stage III, which was non-fitting by design. In the fully-adjusted models that further controlled for self-reported comorbidities, hazard ratios of stages I to IV were attenuated, yet the incremental pattern (except stage III) still held. For instance, the hazard ratios and 95% CIs of the next hospitalization for ADL stages I to IV compared to stage 0 were 1.49 (1.36–1.63), 1.61 (1.44–1.8), 1.54 (1.35–1.76) and 2.06 (1.61–2.63), respectively. A similar pattern was found for IADL stages, with HRs and 95% CIs for stage I to IV at 1.45 (1.32–1.58), 1.74 (1.55–1.95), 1.56 (1.4–1.73) and 2.01 (1.67–2.42). Tests of the proportional hazard assumption for the models showed no violation of the assumption.
Figure 1.
Hazard ratio and 95% confidence intervals for the association of Activity of Daily Living (ADL) stage with first hospitalization in the subsequent year
Figure 2.
Hazard ratio (95% confidence interval) for the association of Instrumental Activity of Daily Living (IADL) stage with first hospitalization in the subsequent year
Note. Partially adjusted models were adjusted for sociodemographics (age, gender, race/ethnicity, education, dual eligibility in Medicare and Medicaid, and living arrangement), smoking status, and year of survey entry. Fully adjusted models were further adjusted for self-reported comorbidities. Comorbidities included Alzheimer’s disease, amputation, broken hip, cancer, chronic heart disease, chronic obstructive pulmonary disease (COPD), depression, diabetes, heart failure, hardening of the arteries, heart rhythm disease, heart valve disease, hypertension, incontinence, mental disorders, myocardial infarction, osteoporosis, paralysis, Parkinson’s disease, retardation, rheumatoid arthritis, arthritis other than rheumatoid, and stroke. All covariates were categorized as in table 1.
Association of Activity Limitation Stage and Risk of Recurrent Hospitalizations
There were in total 2108 first hospitalizations, 625 second hospitalizations, 327 third hospitalizations, and 149 fourth hospitalizations. For the stratified analysis by the sequence of hospitalizations, we found baseline ADL and IADL stage was associated with time to recurrent hospitalizations in the first stratum (1st hospital episode), but not in the second, third or fourth strata (Table 2). This may suggest that baseline activity limitation stage was only associated with time from baseline to first hospitalization. Stage was not associated with time from the first to second, second to third, or third to fourth hospitalization.
Table 2.
Hazard Ratios (95% Confidence Intervals) for the associations of activity limitation stages with recurrent hospitalizations within one year
| 1st hospitalization (N=2108) |
2nd hospitalization (N= 625) |
3rd hospitalization (N = 327) |
4th hospitalization (N = 149) |
|
|---|---|---|---|---|
| ADL Stage (ref: Stage 0) | ||||
| ADL- I | 1.58 (1.46 – 1.71) | 1.10 (0.97 – 1.25) | 0.85 (0.70 – 1.03) | 0.98 (0.73 – 1.31) |
| ADL-II | 1.76 (1.59 – 1.95) | 1.09 (0.94 – 1.27) | 0.90 (0.71 – 1.13) | 0.99 (0.70 – 1.40) |
| ADL-III | 1.69 (1.50 – 1.90) | 1.12 (0.93 – 1.34) | 0.99 (0.76 – 1.30) | 1.29 (0.89 – 1.88) |
| ADL-IV | 2.39 (1.88 – 3.03) | 1.05 (0.75 – 1.49) | 0.63 (0.36 – 1.10) | 1.28 (0.62 – 2.62) |
| IADL stage (ref: Stage 0) | ||||
| IADL- I | 1.56 (1.38 – 1.77) | 1.20 (0.98 – 1.46) | 0.86 (0.62 – 1.19) | 0.84 (0.51 – 1.38) |
| IADL-II | 2.00 (1.72 – 2.33) | 1.37 (1.10 – 1.72) | 1.14 (0.82 – 1.59) | 1.09 (0.67 – 1.77) |
| IADL-III | 1.83 (1.59 – 2.11) | 1.22 (0.98 – 1.52) | 0.80 (0.55 – 1.14) | 1.06 (0.63 – 1.81) |
| IADL-IV | 2.37 (1.83 – 3.07) | 0.93 (0.62 – 1.41) | 0.71 (0.36 – 1.38) | 2.01 (0.85 – 4.78) |
Note. Reference category is stage 0. ADL: Activity of Daily Living; IADL: Instrumental Activity of Daily Living. The models were adjusted for sociodemographics (age, gender, race/ethnicity, education, income, living arrangement), smoking status, self-reported comorbidities, and year of survey entry. Comorbidities included Alzheimer’s disease, amputation, broken hip, cancer, chronic heart disease, chronic obstructive pulmonary disease (COPD), depression, diabetes, heart failure, hardening of the arteries, heart rhythm disease, heart valve disease, hypertension, incontinence, mental disorders, myocardial infarction, osteoporosis, paralysis, Parkinson’s disease, retardation, rheumatoid arthritis, arthritis other than rheumatoid, and stroke. All covariates were categorized as in table 1.
Discussion
Interpretations and Implications of Findings
Using a nationally representative, longitudinal survey of older Americans, this study identified an association between ADL and IADL stages with hospitalization within a year, after accounting for sociodemographics, smoking status, year of survey entry, and self-reported comorbidities. In analyses that were adjusted only for sociodemographics, smoking status and entry cohorts, the associations with stages followed a gradient with the exception of stage III, such that higher stages were associated with higher risk for the next hospitalization compared to stage 0. When the models were further adjusted for medical comorbidities, the strength of the association declined, yet the graded relationship persisted. Our results are consistent with previous research that identified the count of ADL or IADL limitations as a significant predictor of hospitalization among Medicare enrollees.2,10,11 The advantage of the stage systems over other classifications of ADL or IADL limitations lies in the fact that the stage systems convey greater specificity and transparency of disability status. In addition, our study suggests that ADL and IADL stages have similar strength in their association with an early hospitalization in the subsequent year. Thus, either stage system can be conveniently used in clinical and administrative settings for assessment of the relative hazard of the next hospitalization, even when no medical information is available.
In the analysis of the risk of recurrent hospitalizations, baseline activity limitation stages showed an elevated risk for the first hospitalization, but not for subsequent hospitalizations. This might be due to the instability of functional status over time among the elderly, even within a year. It also suggests that the relationship between disability and hospitalization is likely to be bidirectional: hospitalization may trigger transitions in activity limitation stages, and changed activity limitation stage due to a previous hospitalization may affect the risk of a subsequent hospitalization (readmission). Previous research has provided support for both directions. Hospitalization was a risk factor for change in functional status in older adults, especially in functional decline. Decline in ADLs was observed during hospital stay,23 by hospital discharge and within 12 months of hospital discharge.24,25 Post-baseline hospitalizations were found to be the most robust predictor of functional decline among the older Medicare beneficiaries.26 Although our current study is the first to elucidate the association of activity limitation stages with hospitalization, the time-sensitive nature of functional status among older adults, especially with respect to hospitalization, was supported by previous research. On the other hand, activity limitation stage was not associated with repeated hospitalizations in the long run. This may be because unmeasured post-discharge functional impairment may predispose vulnerable elderly to rehospitalization, especially in the early recovery period.27 For instance, recently discharged frail seniors are at risk for falls and injuries that can lead to rehospitalization. In addition, unmet need for assistance to manage ADL limitation was associated with increased risk for hospital readmission in a year among the elderly, demonstrating need for support to fulfill their self-care functions at home as a risk reduction strategy.6 Even though some studies have suggested that pre-hospitalization ADL limitation is a key factor in hospital readmission,1 our study adds to previous findings that prehospitalization activity limitation stage appears to contribute to future hospitalization risk, but the effect is sensitive to time and to the sequence of hospitalizations.
As two major risk factors for hospitalization, morbidity and disability are related to some extent. People with disabilities report much poorer health status than their non-disabled counterparts.14,28 They are more likely to be obese, to smoke, and have heart diseases29 and certain types of cancer.14 As shown in our analysis, the attenuated effect of stages after comorbidity adjustment suggests that the risk of hospitalization can be in part explained by comorbidity. Longitudinal studies reported that the absence of health risks can reduce prevalence of disability and postpone disability onset from 7 to 12 years among the elderly.30 Population-based interventions to diminish adverse health events such as hospitalization should focus on prevention of diseases, disability onset and functional decline. Previous research found that fall prevention interventions contributed to fall reduction and functional improvement and that community-based care after hospital discharge significantly reduced re-hospitalization risk.31 For chronic diseases for which we do not have preventive or mitigating treatments, it is important to plan for disability management. In addition, as revealed in our study, several sociodemographic characteristics were associated with greater activity limitation, including older age, racial or ethnic minority status, lower levels of education, and dual eligibility in Medicare and Medicaid (a poverty measure). Intervention programs should intentionally target these at-risk subgroups. Assessment of hospitalization risk among older adults at different activity limitation stages can inform policy makers of specific at-risk subgroups and facilitate evidence-based monitoring of the health service system to ameliorate adverse outcomes. Furthermore, investigators may explore the effects of the constellation of research that points to ADL/IADL ramifications on the adaptation of health system preventive care.
Strengths and Limitations
Our study used the novel activity limitation stage systems as a data aggregation technique intended to enhance understanding of disability-related disparities in health and guide evaluation of interventions and services. The stage systems showed promising capacity to differentiate degrees of vulnerability to hospitalization among the elderly with different disability profiles. We used a large nationally representative survey of Medicare beneficiaries linked to claims data of health service use, with annual self- or proxy-reported functional status and medical conditions. The timeline to hospital events was accurately assessed with the billing data rather than from subjective recalls. Recognizing the dynamic nature of disability, disability stage was assessed annually prior to the follow-up in a repeated measures design for our primary analysis. The repeated measures design in the primary analysis increased our statistical precision due to almost tripled sample size. We studied two types of related outcomes, which provided a more comprehensive view on the association between disability and hospitalization. Our analysis of time to recurrent hospitalizations revealed a proximal effect of baseline functional status on future hospitalization, which revealed the time-sensitive effect of prehospitalization activity limitation stages on future hospitalizations and highlighted the importance of recency in functional assessment among the elderly.
There are limitations in our study, which also give rise to future opportunities. Our study did not establish a causal relationship between baseline stages and future hospitalizations. Although a strong association of stages with first hospitalization in one year emerged from our analysis, the effect of unknown confounders could not be ruled out. The lack of significant effect of activity limitation stages on higher-order (2nd, 3rd, 4th) hospitalizations needs to be interpreted with caution, because it may partly due to the smaller sample sizes in these strata. With respect to the survey, although the MCBS data contains rich information on the health of elderly beneficiaries, it also has some limitations. We derived disability stages from the MCBS annual assessment of functional status. Functional status of the elderly however is more fluid, and transitions across activity limitation stages within a year, with or without hospitalization, are expected for some elders. It may be desirable for the MCBS or other surveys of senior health to adopt more frequent assessment cycles to accurately reflect changing disability in this population. To prevent functional deterioration and facilitate transitional care, assessment of ADL and IADL stages after hospitalization and major medical events can be instrumental. Furthermore, although self- and proxy-reported ADL and IADL assessments reduce resource utilization by avoiding the need for performance-based testing, recall and non-response biases in self-reported data could cause misclassification of the primary exposure. In addition, like many large-scale surveys, the MCBS is subject to non-response bias 32 and panel attrition. Bias in estimation was greatly reduced by MCBS nonresponse weights, but was not entirely eliminated. With regard to generalizability, our results do not apply to the whole elderly Medicare population because we excluded elderly beneficiaries who participated in the Medicare managed care programs and those who resided in long-term care facilities during the study period. Future studies may investigate these subpopulations.
Conclusion
Activity limitation stage systems are effective clinical tools to predict adverse health outcomes such as hospitalization. Stages with greater transparency are informative for matching resources to rehabilitation and care needs.20 Due to the fluid nature of functional stages, frequent assessment of functional status at the population-level is necessary in predicting future hospital service use. Stages are especially useful to assess hospitalization risk proximal in time to ascertainment of stage. The study has clinical and policy implications. Financially feasible community programs to prevent disability onset and manage acute and chronic conditions among the elderly should be established to forestall early hospitalization and enhance performance of ADLs and IADLs among the elderly. The multifactorial determinants of hospitalization suggest prevention programs should be informed by activity limitation stages, sociodemographics, and health conditions.
Acknowledgments
Disclosures: The research for this manuscript was supported by the grant from the National Institutes of Health (R01AG040105).
We thank Dr. Margaret Margaret G. Stineman MD, MPH for conceptualizing the study, obtaining funding, and providing input in the analysis.
Appendix I
Classification of Activity Limitation Stages for the Medicare Current Beneficiary Survey
| Limitation Stages | ADL Stages | IADL Stages |
|---|---|---|
| 0 = no limitation | Able to eat, toilet, dress, bathe/shower, get in/out of bed /chairs, and walk without difficulty. | Able to use telephone, manage money, prepare meals, do light housework, shop for personal items, and do heavy housework without difficulty. |
| I = mild limitation | Able to eat, toilet, dress, bathe / shower without difficulty. May have some difficulty getting in/out of bed/chairs and/or walking. | Able to use telephone, manage money, prepare meals, and do light housework without difficulty. May have difficulty shopping and/or doing heavy housework. |
| II = Moderate limitation | Able to eat and toilet without difficulty. May have some difficulty dressing, bathing/showering, getting in/out of bed/chairs and/or walking. | Able to use telephone and manage money without difficulty. May have difficulty preparing meals, doing light housework, shopping and/or doing heavy housework. |
| III = Severe limitation | Difficulty with eating and/or toileting, but not with all ADLs. | Has difficulty using telephone and/or managing money, but not all IADLs. |
| IV = Complete limitation | All ADLs are difficult. | All IADLs are difficult. |
Note. ADL: Activity of Daily Living; IADL: Instrumental Activity of Daily Living.
Appendix II
Weighted Sample Distribution of Baseline Covariates by Instrumental Activity of Daily Living (IADL) Stage
| Weighted Percent by Instrumental Activity of Daily Living (IADL) Stage |
||||||
|---|---|---|---|---|---|---|
| Variable | Class | Stage 0 | Stage I | Stage II | Stage III | Stage IV |
| Total sample weighted percent (raw size) |
65 (N=5807) |
16 (N=1646) |
7 (N=725) |
9 (N=1035) |
2 (N=229) |
|
| Sociodemographics | ||||||
| Age | 65–74 | 60 | 44 | 41 | 32 | 23 |
| 75–84 | 32 | 39 | 41 | 43 | 37 | |
| 85+ | 8 | 16 | 19 | 25 | 39 | |
| Gender | Male | 50 | 26 | 29 | 49 | 38 |
| Female | 50 | 74 | 71 | 51 | 62 | |
| Race/Ethnicity | White | 86 | 86 | 77 | 82 | 74 |
| Black | 6 | 6 | 10 | 6 | 15 | |
| Hispanic | 5 | 5 | 6 | 7 | 7 | |
| Other | 3 | 3 | 6 | 4 | 4 | |
| Education | Less than High School | 19 | 28 | 38 | 41 | 45 |
| High School | 29 | 31 | 26 | 26 | 30 | |
| Some College | 27 | 25 | 23 | 20 | 15 | |
| College and above | 24 | 16 | 13 | 13 | 10 | |
| Dual eligibility in Medicare and Medicaid |
8 | 17 | 29 | 27 | 39 | |
| Living Arrangement |
Alone | 30 | 37 | 35 | 32 | 20 |
| Spouse | 59 | 45 | 42 | 46 | 39 | |
| Children | 6 | 12 | 15 | 17 | 29 | |
| Other | 4 | 6 | 8 | 6 | 11 | |
| Self-reported medical comorbidities | ||||||
| Alzheimer’s disease | 1 | 1 | 2 | 9 | 35 | |
| Amputation | 0 | 1 | 1 | 1 | 3 | |
| Broken hip | 1 | 5 | 9 | 8 | 11 | |
| Cancer | 16 | 22 | 24 | 22 | 18 | |
| Chronic heart disease | 7 | 12 | 14 | 13 | 18 | |
| Chronic obstructive pulmonary disease (COPD) |
10 | 20 | 28 | 20 | 17 | |
| Depression | 10 | 24 | 31 | 26 | 37 | |
| Diabetes | Type 1 Diabetes | 1 | 2 | 6 | 3 | 5 |
| Type 2 Diabetes | 13 | 20 | 23 | 19 | 19 | |
| Other Type | 5 | 4 | 6 | 5 | 8 | |
| Heart failure | 3 | 10 | 15 | 12 | 18 | |
| Hardening of the arteries | 6 | 10 | 12 | 12 | 15 | |
| Heart rhythm disease | 14 | 22 | 27 | 23 | 28 | |
| Heart valve disease | 6 | 11 | 13 | 10 | 9 | |
| Hypertension | 56 | 70 | 73 | 70 | 61 | |
| Incontinence | No dialysis/ Catheterization |
21 | 42 | 47 | 40 | 59 |
| Dialysis/Catheterization | 0 | 1 | 2 | 1 | 7 | |
| Mental / psychiatric disorders | 1 | 2 | 3 | 4 | 4 | |
| Myocardial infarction | 10 | 16 | 20 | 19 | 20 | |
| Osteoporosis | 13 | 27 | 27 | 21 | 22 | |
| Paralysis | 1 | 2 | 5 | 5 | 13 | |
| Parkinson’s disease | 0 | 2 | 2 | 3 | 6 | |
| Retardation | 0 | 0 | 0 | 1 | 3 | |
| Rheumatoid arthritis | 5 | 13 | 15 | 12 | 16 | |
| Arthritis other than rheumatoid | 38 | 63 | 63 | 51 | 49 | |
| Stroke | 6 | 12 | 17 | 21 | 39 | |
| Smoking | ||||||
| Smoker | Non-Smoke | 43 | 47 | 47 | 42 | 55 |
| Ever-Smoke | 45 | 43 | 41 | 49 | 39 | |
| Current-Smoke | 12 | 10 | 12 | 10 | 6 | |
Note. When a medical condition is classified as yes (present) or no (absent), only the yes category is displayed. The association of ADL stage with each covariate was assessed with the Chi-square test, and all p values < 0.01.
Footnotes
There are no personal conflicts of interest of any of the authors, and no authors reported disclosures beyond the funding source. The opinions and conclusions of the authors are not necessarily those of the sponsoring agency. We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated. This material has not been previously presented at a meeting.
References
- 1.Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in medicare seniors. JAMA Intern Med. 2015;175(4):559–565. doi: 10.1001/jamainternmed.2014.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan L, Beaver S, Maclehose RF, Jha A, Maciejewski M, Doctor JN. Disability and health care costs in the medicare population. Arch Phys Med Rehabil. 2002;83(9):1196–1201. doi: 10.1053/apmr.2002.34811. [DOI] [PubMed] [Google Scholar]
- 3.Miller EA, Weissert WG. Predicting elderly people's risk for nursing home placement, hospitalization, functional impairment, and mortality: A synthesis. Med Care Res Rev. 2000;57(3):259–297. doi: 10.1177/107755870005700301. [DOI] [PubMed] [Google Scholar]
- 4.Older americans 2012: Key indicators of well-being. Washington, DC: U.S. Government Printing Office; 2012. [Accessed December 3, 2015]. Federal Interagency Forum on Aging-Related Statistics. http://www.agingstats.gov/Main_Site/Data/2012_Documents/Health_Status.aspx. [Google Scholar]
- 5.Xu H, Covinsky KE, Stallard E, Thomas J, 3rd, Sands LP. Insufficient help for activity of daily living disabilities and risk of all-cause hospitalization. J Am Geriatr Soc. 2012;60(5):927–933. doi: 10.1111/j.1532-5415.2012.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depalma G, Xu H, Covinsky KE, et al. Hospital readmission among older adults who return home with unmet need for ADL disability. Gerontologist. 2013;53(3):454–461. doi: 10.1093/geront/gns103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortman JM, Velkoff VA, Hogan H. An aging nation: The older population in the united states, 2014. US Census Bureau. 2014 May 5; 2015. [Google Scholar]
- 8.World Health Organization. International classification of functioning, disability and health: ICF. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 9.Leon-Munoz LM, Lopez-Garcia E, Graciani A, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. Functional status and use of health care services: Longitudinal study on the older adult population in spain. Maturitas. 2007;58(4):377–386. doi: 10.1016/j.maturitas.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36(6):804–817. doi: 10.1097/00005650-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hastings SN, Oddone EZ, Fillenbaum G, Sloane RJ, Schmader KE. Frequency and predictors of adverse health outcomes in older medicare beneficiaries discharged from the emergency department. Med Care. 2008;46(8):771–777. doi: 10.1097/MLR.0b013e3181791a2d. [DOI] [PubMed] [Google Scholar]
- 12.HealthyPeople.gov 2020. [Accessed December/15, 2015];Topics and objectives: Disability and health. http://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health#two. Updated 2015.
- 13.Stineman MG, Streim JE, Pan Q, Kurichi JE, Schussler-Fiorenza Rose SM, Xie D. Activity limitation stages empirically derived for activities of daily living (ADL) and instrumental ADL in the U.S adult community-dwelling medicare population. PM R. 2014;6(11):976–987. doi: 10.1016/j.pmrj.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stineman MG, Xie D, Pan Q, Kurichi JE, Saliba D, Streim J. Activity of daily living staging, chronic health conditions, and perceived lack of home accessibility features for elderly people living in the community. J Am Geriatr Soc. 2011;59(3):454–462. doi: 10.1111/j.1532-5415.2010.03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurichi JE, Streim JE, Bogner HR, Xie D, Kwong PL, Hennessy S. Comparison of predictive value of activity limitation staging systems based on dichotomous versus trichotomous responses in the medicare current beneficiary survey. Disabil Health J. 2016;9(1):64–73. doi: 10.1016/j.dhjo.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stineman MG, Xie D, Pan Q, et al. All-cause 1-, 5-, and 10-year mortality in elderly people according to activities of daily living stage. J Am Geriatr Soc. 2012;60(3):485–492. doi: 10.1111/j.1532-5415.2011.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Xie D, Kurichi JE, Streim J, Zhang G, Stineman MG. Mortality predictive indexes for the community-dwelling elderly US population. J Gen Intern Med. 2012;27(8):901–910. doi: 10.1007/s11606-012-2027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stineman MG, Xie D, Streim JE, et al. Home accessibility, living circumstances, stage of activity limitation, and nursing home use. Arch Phys Med Rehabil. 2012;93(9):1609–1616. doi: 10.1016/j.apmr.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennessy S, Kurichi JE, Pan Q, et al. Disability stage is an independent risk factor for mortality in medicare beneficiaries aged 65 years and older. PM R. 2015;7(12):1215–1225. doi: 10.1016/j.pmrj.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stineman MG, Zhang G, Kurichi JE, et al. Prognosis for functional deterioration and functional improvement in late life among community-dwelling persons. PM R. 2013;5(5):360–371. doi: 10.1016/j.pmrj.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services. [Accessed October/10, 2015];Medicare current beneficiary survey (MCBS) 2015 CMS.gov Web site. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/index.html?redirect=/MCBS. Updated 2015.
- 22.Hosmer DWJ, Lemeshow S. Applied survival analysis: Regression modeling of time to event data. New York: John Wiley; 1999. [Google Scholar]
- 23.Wakefield BJ, Holman JE. Functional trajectories associated with hospitalization in older adults. West J Nurs Res. 2007;29(2):161–177. doi: 10.1177/0193945906293809. discussion 178-82. [DOI] [PubMed] [Google Scholar]
- 24.Mudge AM, O'Rourke P, Denaro CP. Timing and risk factors for functional changes associated with medical hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2010;65(8):866–872. doi: 10.1093/gerona/glq069. [DOI] [PubMed] [Google Scholar]
- 25.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolinsky FD, Bentler SE, Hockenberry J, et al. Long-term declines in ADLs, IADLs, and mobility among older medicare beneficiaries. BMC Geriatr. 2011;11 doi: 10.1186/1471-2318-11-43. 43-2318-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iezzoni LI. Eliminating health and health care disparities among the growing population of people with disabilities. Health Aff. 2011;30(10):1947–1954. doi: 10.1377/hlthaff.2011.0613. [DOI] [PubMed] [Google Scholar]
- 29.Altman BM, Bernstein AB. Disability and health in the united states, 2001–2005. Hyattsville, MD: National Center for Health Statistics; 2008. [Accessed August 10, 2015]. http://stacks.cdc.gov/view/cdc/6983. [Google Scholar]
- 30.Fries JF. Frailty, heart disease, and stroke: The compression of morbidity paradigm. Am J Prev Med. 2005;29(5 Suppl 1):164–168. doi: 10.1016/j.amepre.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: A systematic review and meta-analysis. Lancet. 2008;371(9614):725–735. doi: 10.1016/S0140-6736(08)60342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kautter J, Khatutsky G, Pope GC, Chromy JR, Adler GS. Impact of nonresponse on medicare current beneficiary survey estimates. Health Care Financ Rev. 2006;27(4):71–93. [PMC free article] [PubMed] [Google Scholar]