Abstract
Many pathogens, such as the agents of West Nile encephalitis and plague, are maintained in nature by animal reservoirs and transmitted to humans by arthropod vectors. Efforts to reduce disease incidence usually rely on vector control or immunization of humans. Lyme disease, for which no human vaccine is currently available, is a commonly reported vector-borne disease in North America and Europe. In a recently developed, ecological approach to disease prevention, we intervened in the natural cycle of the Lyme disease agent (Borrelia burgdorferi) by immunizing wild white-footed mice (Peromyscus leucopus), a reservoir host species, with either a recombinant antigen of the pathogen, outer surface protein A, or a negative control antigen in a repeated field experiment with paired experimental and control grids stratified by site. Outer surface protein A vaccination significantly reduced the prevalence of B. burgdorferi in nymphal blacklegged ticks (Ixodes scapularis) collected at the sites the following year in both experiments. The magnitude of the vaccine's effect at a given site correlated with the tick infection prevalence found on the control grid, which in turn correlated with mouse density. These data, as well as differences in the population structures of B. burgdorferi in sympatric ticks and mice, indicated that nonmouse hosts contributed more to infecting ticks than previously expected. Thus, where nonmouse hosts play a large role in infection dynamics, vaccination should be directed at additional species.
Keywords: Borrelia, vector, Ixodes, Peromyscus, tick
Of 175 microbial species considered emerging pathogens for humans, 75% are zoonotic, that is, maintained in nature by animal reservoirs (1). Several of these human pathogens are transmitted by arthropod vectors (2, 3). For most vector-borne pathogens, no human vaccines exist; the main approach for reducing the public's risk for disease transmission is vector control. Vaccines directed at animal reservoirs provide additional opportunities to intervene in natural cycles and reduce disease risk by the criterion of number of infected vectors (4). Successful vaccination would decrease the availability of infected hosts to vectors, resulting in a lower proportion of vectors that are infected, and thus, a lower probability of pathogen transmission to humans.
We tested this hypothesis by using Lyme disease (LD), a zoonotic bacterial disease of the northern hemisphere whose etiologic agents are maintained by several vertebrate host species (5). In the eastern United States humans acquire LD when an infected Ixodes scapularis tick attaches and transmits the spirochete Borrelia burgdorferi. The recent surge in the incidence of LD, in the face of the commercial withdrawal of the human vaccine and a general reluctance to apply pesticides, prompts consideration of new approaches to reducing disease risk (6, 7).
Because nymphal I. scapularis cause the majority of LD cases (8), lower disease incidence could be achieved by limiting the overall nymphal abundance, by lowering nymphal infection prevalence (i.e., the proportion of nymphs infected), or both (4). With the second aim in mind, we vaccinated wild populations of the white-footed mouse (Peromyscus leucopus), which is considered to be the main reservoir host species for B. burgdorferi in eastern North America (9, 10). The strategy was to convert the phenotypes of reservoir host populations from competent to noncompetent for B. burgdorferi transmission to I. scapularis larvae, thereby reducing the proportion of larvae that molt into infectious nymphs.
We immunized mice with recombinant B. burgdorferi outer surface protein A (OspA) (11, 12), which is the basis of a currently available vaccine for dogs (13). B. burgdorferi commonly expresses OspA in tick, but not mouse hosts, and consequently, OspA-vaccinated mice, even infected ones, develop antibodies that kill B. burgdorferi in the tick while it feeds (12, 14, 15). A study of our field site revealed that >90% of white-footed mice were infected during the larval host-seeking period and confirmed that these hosts rarely had antibodies to OspA (16).
Although the main end-point of the study was infection prevalence among nymphal ticks, the vaccine was targeted at an abundant vertebrate host species, from whom some proportion of nymphs would have obtained blood meals as larvae in the preceding year. Larval I. scapularis are not host-specific and have been found to feed on many vertebrate species (17). On the basis of previous studies (18, 19), we anticipated that the impact of vaccination on reducing nymphal infection prevalence would correlate positively with the proportional role that mice play in infecting larvae. In other words, a determinant of vaccine effect would be the degree to which alternative hosts infect feeding larvae. The extent to which this happened was a second end-point of the study and was inferred from vaccination results, as well as by comparing the population structure of B. burgdorferi among tick and mouse populations.
Materials and Methods
Field Site. The study grids were located in a 1,400-hectare private mixed hardwood forest in southern Connecticut, where LD is common (7) (Fig. 4, which is published as supporting information on the PNAS web site). From 1998 to 2003, periodic sampling at our field site found nymphal infection prevalence to vary from 23% to 50% at various locations and an average density at the peak nymphal host-seeking period (beginning of June) to range from 0.16 to 0.55 nymphs per m2 as determined by dragcloth sampling (J.I.T., unpublished data). Deer density estimates have ranged from 25 to 120 deer per km2 (20). For each experiment, we chose three sites that were large enough to comprise two grids, one experimental and one control, that had similar landscape features and other ecological factors, that which were also separated by at least 100 m, based on the estimated home range size for P. leucopus (21). Traps were placed 12 m apart and in arrays of 9 × 9 (1998) or 12 × 12 (2001).
Rodent Trapping and Processing. The study protocol (no. 07596) was approved by the Yale University Institutional Animal Care and Utilization Committee. Aluminum Sherman live traps were baited with peanut butter (1998) or crimped oats (2001). Traps were set between 1500 and 1800 hours and checked between 0800 and 0900 hours the next day. Mice captured for the first time each trapping period (i) were anesthetized with ketamine (150 mg/kg), (ii) received a uniquely numbered metal ear tag upon initial capture, (iii) were examined for sex, mass, and number of attached larval and nymphal ticks, (iv) bled of ≤150 μl from the retroorbital sinus, and (v) vaccinated with a vaccine or control immunogen. Upon recovery from anesthesia, mice were rehydrated and released at the point of capture. The blood clot and serum were stored at –20°C. Mice recaptured within the same trapping period were only weighed and examined for ticks; mice recaptured during subsequent trapping periods were processed as described. We trapped mice from early June (1998 and 2001) to late August (1998) or early September (2001). At each site mice were trapped for 2–4 nights per trapping period, with a total of four trapping periods per site and ≈3 weeks between consecutive trapping periods.
Immunizations. In 1998, mice were inoculated s.c. with either nonlipidated recombinant fusion protein of OspA from strain N40 and recombinant GST (22) on vaccine grids or GST alone on control grids. Both immunogens (provided by E. Frikig, Yale University, New Haven, CT) were emulsified with Freund's complete adjuvant for initial immunizations and Freund's incomplete adjuvant for up to two boosters at 3- to 6-week intervals. In a laboratory experiment, this protocol reduced transmission efficiency by 48%, 92%, and 99%, respectively, for one, two, and three doses of OspA, in comparison to GST (15). In 2001, mice were inoculated s.c. no more than twice with either lipidated OspA (provided by R. Huebner, Aventis-Pasteur, Paris) from strain B31 or GST, both delivered with monophosphoryl lipid A and synthetic trehalose dicorynomycolate (Ribi Adjuvant System, Corixa, Seattle); immunogen doses were 10 μg for the first immunization and 5 μg for the second. Previous studies demonstrated cross-protection with an OspA antigen derived from one strain against a variety of other strains found in nature (23, 24).
Tick Collections. In 1999 and 2002, we sampled grids for host-seeking nymphal I. scapularis ticks by drag sampling (25) from late May to August with the aims to collect ticks in proportion to their relative abundance and distribution on grids and to maximize sample sizes for analysis of infection with B. burgdorferi. Ticks were stored either live in environmental chambers at 95% relative humidity until they were frozen and stored at –70°C (1999) or directly immersed in 70% ethyl alcohol (2002).
Antibody Assay. Enzyme immunosorbent assays (EIAs) were performed with recombinant OspA lipoprotein from strain B31 (provided by S. Bergström, Umeå University, Umeå, Sweden) as described (16). Sera from pathogen-free, laboratory-reared P. leucopus (Peromyscus Stock Center, University of South Carolina, Columbia) served as negative controls. Samples with optical density (OD) readings at wavelength 450 nm >3 SD above the mean of the negative controls were considered positive. The OD value for each serum sample was normalized, as an antibody index, by calculating its ratio to an OD value 2 SD below the mean of the negative controls.
DNA Extraction and PCR. In the 1998–1999 study, total DNA was extracted from pulverized ticks by using a DNeasy Tissue kit (Qiagen, Valencia, CA) as described (26, 27) and from 50–100 μl of mouse blood by using Bio101 FastPrep kit (Qbiogene). In 2002, ticks were quartered, and DNA was extracted from both ticks and mouse blood by using a DNeasy Tissue kit (27, 28). DNA extracts from 1999 were screened by PCR with Borrelia genus-specific flaB gene primers as described (29). During the course of the study, a new Borrelia species, provisionally classified as Borrelia miyamotoi (28), was discovered in I. scapularis (30). Remaining samples from flaB PCR-positive ticks were then reanalyzed for the presence of the new species by using p66 gene primers that discriminate between B. burgdorferi and B. miyamotoi (28, 31). In 4 of 81 samples from control grids and 5 of 82 samples from vaccine grids, B. miyamotoi instead of B. burgdorferi was detected. Accordingly, nymphal infection prevalence for all sites and grids were multiplied by a factor of 0.95 (154/163) to adjust for the presence of B. miyamotoi. Tick extracts from 2002 and blood extracts from 2001 were subjected to quantitative multiplex real time (RT) PCR with differential probes for the 16S rDNAs of B. burgdorferi and B. miyamotoi. Forward and reverse primers at 900 nM were, respectively, 5′-GCTGTAAACGATGCACACTTGGT and 5′-GGCGGCACACTTAACACGTTAG. The corresponding dye-labeled probes at 200 nM were 6FAM-TTCGGTACTAACTTTTAGTTAA and VIC-CGGTACTAACCTTTCGATTA modified with a minor groove binding (MGB) protein (Applied Biosystems). The PCR conditions were 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 63°C for 60 s. Samples that were positive for the presence of B. burgdorferi were then subjected to a second multiplex RT-PCR that identified B. burgdorferi genotypes on the basis of the rrs-rrlA intergenic spacer sequence (27). MGB-modified probes 6FAM-AGCCTAATAAAACTATTTAATATCAATAATTT and VIC-AGCTCTTTAATATTAAAAATTCAATACAC specifically identified genotype groups I and II (32, 33). Genotype group III was determined by subtracting from the first RT-PCR numbers the summed numbers for genotype groups I and II from the second PCR. Forward and reverse primers were 5′-AAAACCAGTGTTATTTATGTAAGTTTAAGAGAAGTTT and 5′-CCCCCAAAATGACCTAAAATTAAGTCTA, and annealing was at 62°C. The identities of genotype groups were confirmed for random samples by direct sequencing of the products on a CEQ 8000 capillary sequencer (Beckman Coulter) as described (27). Of 71 samples identified as group III by the above criterion, 68 (96%) were confirmed to contain a group III genotype alone.
Statistical Analyses. We estimated mouse densities from capture-mark-recapture data by using capture (34) and the Mh model, which assumes heterogeneity in capture probabilities and uses a jackknife procedure to estimate the population size for a trapping period. A grid's density of mice was estimated as the average of four trapping period estimates. For continuous variables we used standard parametric statistics, giving the mean ± 95% confidence interval (CI). For proportions we used two methods: (i) exact as well as asymptotic Cochran–Mantel–Haenszel (C–M–H) odds ratio (OR) procedures, which allow for comparison of replicated 2 × 2 tables where replicates are stratified by levels of a covariate, and (ii) a linear regression approach, where continuous and categorical independent variables were entered into the model to predict the arcsine-transformed proportional data. Before analyzing combined data from both experiments, we ascertained that there was no significant variation in the response variable due to a study effect. Statistical analyses were conducted with spss version 11 (SPSS, Chicago) and statxact version 6 (Cytel, San Diego).
Results
Study Design. We conducted separate controlled field experiments in 1998–1999 and 2001–2002, trapping over a total area of 18 hectare of forest in Connecticut. Although sites, grid size, and vaccine formulation varied between the two experiments, each manipulation consisted of three replicates of paired experimental (OspA) and control (GST) trapping grids stratified by site. After mice were captured, sampled, and immunized with OspA or GST during the summers of 1998 and 2001, we compared the prevalence of B. burgdorferi infection in nymphal ticks collected from different treatment grids the following summers. In the 2001–2002 study we also (i) compared population structures of B. burgdorferi in mice and ticks at vaccine and control grids and (ii) determined the prevalence in ticks of B. miyamotoi as another measure of comparability of treatment grids.
A combined 928 mice were captured and immunized on experimental and control grids for both studies. Trapping success was 11.6% of 7,128 trap nights (1998) and 22.9% of 10,080 trap nights (2001). Average mouse densities per ha were 20 ± 9 (1998) and 34 ± 8 (2001). Mark-recapture data showed that 79 ± 4% (1998) and 73 ± 1% (2001) of the mouse population were captured and vaccinated at least once; 68 ± 16% (1998) and 57 ± 7% (2001) were captured and vaccinated at least twice by the last trapping period. Adverse effects of OspA vaccination on mice were not detected. The average proportion of mice captured in a previous trapping period on vaccine and control grids, respectively, was 65 ± 11% and 68 ± 8% (1998) and 65 ± 3% and 63 ± 7% (2001), indicating no effect of the vaccine on survival across both experiments during the study. There was also no difference in the mean weight gain (g/day) between mice at vaccine (0.062 ± 0.014; n = 177) and control grids (0.060 ± 0.015; n = 144) between receipt of first vaccination and next capture at a mean of 28 d later in 2001. Recapture data also suggest that very few mice (0.2% in 1998 and 0.6% in 2001) used multiple grids.
Antibody Responses and Infections of Immunized Mice. Treatments at the vaccine grids manipulated the reservoir competence of the white-footed mice, as defined by the presence of serum antibodies to OspA by EIA (Fig. 1). Mean EIA values for sera of captured mice rose with each trapping period on vaccine grids, but substantially less or not at all on control grids. By the last trapping periods, 71 ± 9% (1998) and 66 ± 17% (2001) of captured mice on vaccine grids had seroconverted to OspA, respectively. For both studies overall, by the peak larval host-seeking period, there was a seroconversion rate of 69 ± 9%, and a capture rate of 79 ± 10%. Thus, an estimated 55 ± 13% of all mice on vaccine grids were immunized.
Fig. 1.
Anti-OspA antibody responses in recombinant OspA- and GST-immunized Peromyscus leucopus populations at a Connecticut field site in 1998 (A) and 2000 (B). The histograms with 95% confidence interval error bars indicate the antibody index by OspA enzyme immunoassay (left y axis) for each group of mice by treatment, site, and trapping period. The dots indicate the percentages of the serum samples in each group that exceeded the mean of negative control sera in the EIA by 3 standard deviations (SD; right y axis). The numbers of P. leucopus sampled each trapping period are given at the top of each graph.
To assess the vaccine's effectiveness in preventing new infections of mice after immunization in 2001, we assayed mice trapped during periods 2–4 and carried out PCR analysis for the presence of B. burgdorferi in the blood, an indication of a newly acquired infection (35) (Table 1). Vaccination with OspA was associated with a proportional reduction of 42 ± 15% in the prevalence of B. burgdorferi in the blood of mice on the grids in comparison to GST immunization. The reduction was greatest at site 5.
Table 1. B. burgdorferi-specific PCR assay of blood of mice captured during trapping periods 2–4 in 2001.
| No. of P. leucopus mice
|
||||||
|---|---|---|---|---|---|---|
| Site 4
|
Site 5
|
Site 6
|
||||
| Grid vaccine | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. |
| GST | 36 | 167 | 19 | 109 | 14 | 104 |
| OspA | 28 | 236 | 9 | 131 | 14 | 154 |
| OR* (Cl) | 0.55 (0.31-0.97) | 0.39 (0.15-0.96) | 0.68 (0.31-1.48) | |||
| C-M-H† (Cl) | 0.54 (0.36-0.79) P = 0.002 | |||||
Pos., positive; Neg., negative.
OR for nymphal infection prevalence at OspA vaccination grid compared to GST grid.
C-M-H stratified χ2 test (95% confidence limits) and two-sided P value.
Nymphal Infection Prevalence. At control grids the average prevalence of B. burgdorferi in nymphal ticks (number of infected ticks/number of tested ticks) was 32.5 ± 10.0% (1999) and 38.6 ± 12.0% (2002). These levels of nymphal infection prevalence were similar to what had previously been observed in Connecticut (36) and New York (10, 37). Vaccination with OspA was associated with a significant reduction in nymphal infection prevalence on experimental grids in each of the two experiments (Table 2). Without adjustment for the migration of uncaptured infectious mice and alternative hosts, which would tend to increase nymphal infections on vaccinated grids, the mean reductions of the odds of infection on vaccinated grids were 19% (1999) and 25% (2002). The area-to-perimeter ratio was 33% greater for 2001–2002 grids than that of 1998–1999 grids; thus, the greater vaccine effect noted in the second study may be partly the consequence of fewer immigrant hosts.
Table 2. Nymphal tick infection in vaccine field studies in 1998–1999 and 2001–2002.
| No. of I. scapularis nymphs infected with B. burgdorferi assayed by PCR
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 sites
|
2002 sites
|
|||||||||||
| Vaccine for P. leucopus
|
1
|
2
|
3
|
4
|
5
|
6
|
||||||
| Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | |
| GST | 90 | 315 | 57 | 89 | 127 | 224 | 57 | 56 | 93 | 221 | 94 | 168 |
| OspA | 87 | 288 | 49 | 121 | 96 | 241 | 41 | 71 | 76 | 178 | 67 | 193 |
| OR (95% CI) | 1.06 (0.76-1.48) | 0.63 (0.39-1.04) | 0.70 (0.5-0.98) | 0.57 (0.32-0.99) | 1.01 (0.69-1.48) | 0.62 (0.42-0.92) | ||||||
| C—M—H OR | 0.81 (0.65-0.99) P = 0.04 | 0.75 (0.59-0.95) P = 0.02 | ||||||||||
Pos., positive; Neg., negative.
In contrast to the findings for B. burgdorferi, the prevalence of nymphs infected with B. miyamotoi was comparable between vaccinated and control grids in 2002. The prevalence of this other Borrelia species was 7 of 113, 25 of 314, and 7 of 262 for GST grids 4–6, and 5 of 112, 9 of 54, and 13 of 260 for OspA grids 4–6 (C-M-H OR = 1.31, 0.737–2.335; P = 0.4). Against the prediction of lower B. burgdorferi burdens per tick from observations of partially immunized laboratory mice (14, 15), there was no significant reduction in the number of spirochetes per infected tick: 9,260 ± 1,772 (n = 184) for OspA grids and 10,252 ± 1,688 (n = 244) for GST grids in 2002. The effect of the vaccine was to reduce the absolute number of infected ticks rather than to lower the spirochete burden in individual infected ticks.
Although there was an overall lower prevalence of B. burgdorferi in nymphs at OspA grids in comparison to GST grids, the magnitude of the response varied among sites (Table 2). The odds of infection were reduced on average by 32% at combined sites 2 and 3 (1999) and 40% at combined sites 4 and 6 (2001), but there was no or little difference in B. burgdorferi prevalence between treatments at sites 1 (1999) and 5 (2002). The apparent lack of a vaccine effect at two of six sites was not associated with lower antibody responses to OspA immunization among mice at these sites. In 2001, the mean increases in OspA EIA optical density values per mouse per day for OspA grids between first and second captures were 0.042 ± 0.008 (n = 87), 0.041 ± 0.009 (n = 46), and 0.039 ± 0.008 (n = 44) for sites 4, 5, and 6, respectively.
Population Structure of B. burgdorferi in Mice and Ticks. To investigate the differences among sites in vaccine effect, we examined the genotypes of B. burgdorferi strains at a polymorphic locus between their 16S and 23S ribosomal RNA genes (27, 32). The rationale was the findings of different population structures among vertebrate taxa for LD Borrelia species in Europe (38) and for B. burgdorferi in New York (39). In other words, the strain structure of B. burgdorferi is an indicator of the relative contributions of mice and other reservoir hosts to infecting larvae (27). Reservoir host composition would be an important determinant of the magnitude of a vaccine's effect when the immunization was directed only at one species.
Indeed, there was a difference between I. scapularis and P. leucopus, which were collected or captured at control sites in 2001–2002, in the distributions of genotype groups I–III among infected vectors and hosts (Table 3). In particular, there was a relative paucity of group III strains in mice in comparison to ticks, suggesting the contribution of alternative hosts that transmit relatively more group III genotypes to larvae than do mice. The ratio of groups I:II:III strains in ticks and mice were similar between years. In ticks the average ratio was 1.1:0.6:1.0, whereas in mice, it was 3.0:2.0:1.0. Because there was no effect of vaccination on overall nymphal infection prevalence at site 5, we specifically compared the prevalence of group III strains at site 5's control grid with group III prevalence at the combined control grids of sites 4 and 6. We found a higher proportion of group III strains in ticks at the control grid of site 5 (34% of 105) than at combined sites 4 and 6 (23% of 171; χ2 = 3.8, P = 0.05). These data indicate that nontargeted hosts, perhaps chipmunks and shrews (10), infected proportionally more larvae at site 5 than at other sites, thus accounting in part for the lack of a vaccination effect at that site.
Table 3. Distribution of B. burgdorferi genotype groups in mice and nymphal ticks at combined sites.
| Genotype group by sequence
|
|||
|---|---|---|---|
| Species/study | I | II | III |
| P. leucopus mice | |||
| 1998-1999 | 11 | 8 | 4 |
| 2001-2002 | 10 | 6 | 3 |
| I. scapularis ticks | |||
| 1998-1999 | 26 | 11 | 25 |
| 2001-2002 | 57 | 37 | 51 |
| C—M—H* | |||
| Species effect | χ2 7.00 df 2 p = 0.03 | ||
| Study effect | χ2 1.04 df 2 p = 0.61 | ||
C—M—H exact test with 10,000 Monte Carlo samples (statxact version 6, Cytel).
An association between population structure and apparent vaccine effect was also observed among nymphs collected in 2002. Although there was no discernible effect of vaccination on the overall prevalence of B. burgdorferi in nymphs at site 5, there was a significant difference by genotype group (Fig. 2). In comparison to group II and III strains, group I strains, which are associated with more severe infections of laboratory mice and humans (33), and which was the most common group in mice at our field site, were significantly lower in prevalence at OspA grids at all sites in 2002. This was evidence that OspA immunization at all sites, including site 5, reduced the prevalence of B. burgdorferi infection of those nymphs that fed as larvae on white-footed mice, the target species for vaccination.
Fig. 2.
Distribution of group I (A), II (B), and III (C) B. burgdorferi genotype groups among questing nymphs collected on GST control (open bars) and OspA vaccine (shaded bars) grids in 2002. Numbers of nymphs assayed per grid are listed within bars. OR and 95% CI given for the odds of a nymph being infected with a given genotype on a vaccinated versus a control grid were determined by an exact Cochran–Mantel–Haenzsel procedure for stratified tables. Some nymphs were infected with two B. burgdorferi genotype groups; thus, the sum of the prevalence of genotype groups was greater than the overall nymphal infection prevalence for some groups.
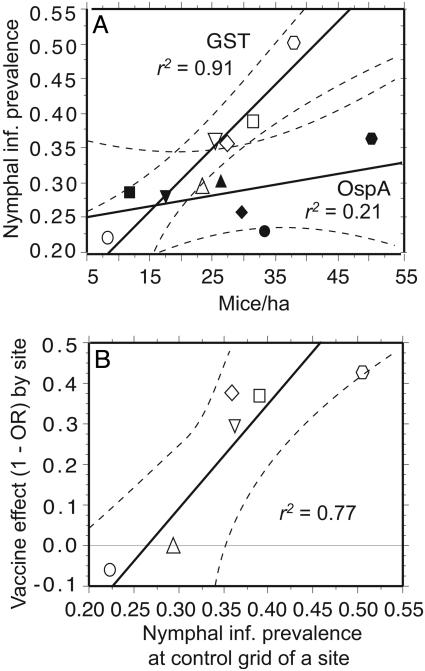
Interaction Effect of Vaccine and Mouse Density. Another gauge of the contributions of different reservoir hosts to infecting larvae was the relationship between mouse density and nymphal infection prevalence (Fig. 3A). On control grids, mouse density and nymphal infection prevalence were positively correlated, supporting the view that mice are important reservoir hosts, but also indicating a strong role of heterogeneity in host community composition among sites. In contrast, OspA immunization reduced the correlation between mouse density and nymphal infection prevalence. A linear regression with nymphal infection prevalence as the dependent variable and mouse density and treatment as independent variables showed a significant interaction effect [r2 = 0.82, F(8, 1) = 12.1; P = 0.008; Fig. 3A], thus providing evidence for both the white-footed mouse's role in determining B. burgdorferi abundance and the vaccine's effect. The differences between sites in the distributions of B. burgdorferi genotypes, notably, the inverse relationship between the proportion of genotype group III and mouse density, was evidence that the densities of alternative host reservoirs did not parallel mouse densities by site.
Fig. 3.
The relationship between mouse density, nymphal infection prevalence, and magnitude of vaccine effect. (A) The relationship between mouse density (mice/ha, hectare) and nymphal infection (inf.) prevalence. Dashed lines represent 95% CI of the slope for the solid regression lines. The coefficient of determination (r2) is given for each treatment. Open and filled symbols represent GST-immunized (control) and OspA-immunized grids, respectively. Grids from the same site are represented by the same symbol: site 1, circle; site 2, square; site 3, inverted triangle; site 4, hexagon; site 5, triangle; site 6, diamond. (B) Vaccine effect (1 – OR) as a function of the control grid nymphal infection prevalence (Table 1). The OR represents the odds of a nymph being infected on a vaccinated grid versus on the control grid at the same site. Symbols for sites are as indicated in A.
Although the overall results confirm the choice of targeting mice for vaccination, the data also show that alternative hosts play an important role in pathogen maintenance, because where mouse densities were relatively lower, nymphal infection prevalences were still >20%. Furthermore, the vaccine's impact at a site increased with mouse density of the grid as well as the nymphal infection prevalence in the matched control grid (Fig. 3B), another indication that, where mice were more dense, they played a proportionately larger role than alternative hosts in infecting larvae.
Discussion
Here we demonstrate that vaccination of wildlife reservoirs can reduce vector infection prevalence, thus lowering the risk of pathogen transmission to humans. In two large-scale experiments, we vaccinated nearly 1,000 white-footed mice, a species considered the most important reservoir of B. burgdorferi in the northeastern United States (9, 10). The number of replicates was restricted to achieve a grid size that not only captured ecological dynamics and heterogeneity on a realistic scale but also provided for sufficient trapping periods for repeated vaccinations, all within a season's transmission period. Although the power of each experiment to detect an effect of vaccination was limited, the results between the two experiments were consistent, despite differences between vaccine formulation, grid size, and grid location, and a combined analysis confirmed trends found in each more limited experiment.
Over both field trials, 69 ± 9% of the recaptured mouse population, or an estimated 55 ± 13% of all mice in each experimental group, had seroconverted to OspA by the peak of the larval host-seeking period. In the succeeding years, there was an overall proportional reduction of 16 ± 12% in the nymphal infection prevalence at vaccine grids in comparison to control grids (Table 2). The size of the reduction in nymphal infection prevalence varied significantly with mouse density, nymphal infection prevalence, and B. burgdorferi population structure, evidence that host composition (i.e., mice vs. nonmouse) strongly influenced local infection dynamics at the field location.
Nonmouse hosts, such as deer, squirrels, and raccoons, are reportedly less competent than white-footed mice as reservoirs for B. burgdorferi transmission to vector ticks (10). These and other observations in both North America (18, 40, 41) and Europe (38, 42, 43) have led to a hypothesis about the importance of diverting larval ticks away from their major reservoir host species to achieve a lower nymphal infection prevalence. Our results are consistent with that hypothesis: there were direct relationships between mouse density and nymphal infection prevalence and between the population structure of B. burgdorferi, a proxy for evaluating reservoir host contributions (39), and nymphal infection prevalence. Extrapolation to the y intercept beyond the data range of Fig. 3 provides an estimate that, under our experiment's conditions, nymphal infection would be reduced to ≈13% in the absence of mice but presence of alternative hosts for larval ticks.
On the other hand, the results of this controlled experimental intervention show that the contributions of alternative hosts to B. burgdorferi maintenance cannot be ignored. The relative contributions of mice and alternative hosts to infecting larvae can be estimated if, for this purpose, we assume linearity between the proportion of mice that were vaccinated one year and nymphal infection prevalence the next (15). If 55 ± 13% vaccination coverage by the last trapping period produced an overall reduction of 16 ± 12% in infections of nymphs, then with 100% vaccination coverage under similar circumstances, nymphal infection prevalence would be predicted to be reduced by 27 ± 21%. This value also provides an estimate of the proportion of infected larvae that can be attributed to mice alone, over the entire range of mouse densities found at all sites. If we only consider site 4, which had the highest mouse density and greatest vaccine effect (Fig. 3), we obtain a maximum estimate for mouse contributions at our field site. The predicted proportion of infected larvae attributable to transmission from mice increases, but only to 55%. Although we recognize that disease transmission dynamics may in fact be nonlinear, especially at the extremes of reservoir host density, the results lead us to conclude that alternative hosts probably have a greater role in infecting larvae than most previous studies would predict. Accordingly, for reducing disease risk for humans, we encourage increased study of and emphasis on nonmouse hosts, including identification of other critical species for infection maintenance.
The present study's protocol of syringe delivery of the vaccine would be impractical in a broader application, but oral delivery of OspA, which was efficacious in protecting laboratory mice against B. burgdorferi (22, 44), could be implemented and administered to white-footed mice and, as indicated, to other reservoir species in areas at high-risk for LD. The immunological response need not be life-long; it need only be efficacious during the larval host-seeking period, which is ≈2 months in the northeastern United States. The successful application of oral recombinant rabies vaccine in food bait in enzootic areas in the United States and Europe demonstrates the feasibility as well as public and regulatory acceptance of field vaccination against zoonoses (45, 46).
For LD and other vector-borne diseases, reduction in the risk of transmission has relied mainly on vector control measures, such as pesticide application and habitat modification. When commercial human vaccines have been available for vector-borne zoonoses (e.g., LD and Rocky Mountain Spotted Fever), they often have not been sufficiently profitable or efficacious to remain on the market, and even a successful vaccine targeted to humans would not reduce the exposure risk for nonimmune individuals. Our study shows that vaccination of a wild vertebrate reservoir population is feasible and capable of reducing the prevalence of a vector-borne zoonotic pathogen. Field vaccination of reservoirs then provides an additional control measure for inclusion in a risk-reduction program, which might include a different mix of strategies, contingent upon the particular ecological dynamics of the pathogen and various social and political constraints on implementation. We further propose that this approach may also be an effective component in an integrated management program for other emerging and reemerging vector-borne zoonoses, such as plague through vaccination of sylvatic or urban rodents, and West Nile encephalitis through vaccination of reservoir bird species.
Supplementary Material
Acknowledgments
We thank L. Beati, S. Bell, J. Derdák, S. Falzone, D. Franz, J. Kenney, M. Levin, N. Madhav, H. Mattaous, J. Miller, H. Miriam, C. Sidor, L. Sheibani, G. Tafesse, E. Wilk, and A. Yeung for field and laboratory contributions; C. Luke for serological assays; E. Fikrig and S. Bergström for their unrestricted gifts of recombinant OspA; R. Huebner and Aventis Pasteur, which is a licensee for some of the OspA patents, for their unrestricted gifts of recombinant OspA; R. Ostfeld for advice; J. Bergelson, M. Leibold, and C. Pfister for comments on earlier versions of the manuscript; G. Dwyer and S. Frank for advice and review of the manuscript; and two anonymous reviewers for helpful critiques. We thank the South Central Connecticut Regional Water Authority for use of its property. This research was supported by National Institutes of Health Grant AI37248 (to A.G.B. and D.F.); U.S. Department of Agriculture/Agriculture Research Service Grant 58-0790-2-072 (to D.F.); the Harold G. and Leila Y. Mathers Charitable Foundation (D.F.); a National Science Foundation Doctoral Dissertation Improvement Grant, National Institutes of Health Grant T32AI07404, and Department of Energy Graduate Assistance in Areas of National Need and American Association of University Women Dissertation Writing Fellowships (to J.I.T.); Environmental Protection Agency Center for Integrating Statistics and the Environment grant (to J.T.W.); Centers for Disease Control and Prevention Grant 919558 (to J.B.); and an Ellison Medical Foundation Senior Scholar in Global Infectious Diseases grant (to A.G.B.).
Author contributions: J.I.T., J.T.W., D.F., and A.G.B. designed research; J.I.T., J.B., M.G.L., D.F., and A.G.B. performed research; J.B. contributed new reagents/analytic tools; J.I.T., J.T.W., J.B., M.G.L., D.F., and A.G.B. analyzed data; and J.I.T. and A.G.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LD, Lyme disease; OspA, outer surface protein A; EIA, enzyme immunosorbent assay; CI, confidence interval, C–M–H, Cochran–Mantel–Haenszel; OR, odds ratio.
References
- 1.Taylor, L. H., Latham, S. M. & Woolhouse, M. E. J. (2001) Philos. Trans. R. Soc. London Ser. B 356, 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolhouse, M. E. J., Taylor, L. H. & Haydon, D. T. (2001) Science 292, 1109–1112. [DOI] [PubMed] [Google Scholar]
- 3.Dobson, A. & Foufopoulos, F. (2001) Philos. Trans. R. Soc. London Ser. B 356, 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mather, T. N., Nickolson, M. C., Donnelly, E. F. & Matyas, B. T. (1996) Am. J. Epidemiol. 144, 1066–1069. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G. & Fish, D. (1993) Science 260, 1610–1616. [DOI] [PubMed] [Google Scholar]
- 6.Steere, A. C., Coburn, J. & Glickstein, L. (2004) J. Clin. Invest. 113, 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (2004) Morbid Mortal Wkly. Rep. 53, 365–369. [Google Scholar]
- 8.Fish, D. (1995) Am. J. Med. 98, S2–S9. [Google Scholar]
- 9.Donahue, J. G., Piesman, J. & Spielman, A. (1987) Am. J. Trop. Med. Hyg. 36, 94–98. [DOI] [PubMed] [Google Scholar]
- 10.LoGiudice, K., Ostfeld, R. S., Schmidt, K. A. & Keesing, F. (2003) Proc. Natl. Acad. Sci. USA 100, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe, T. R., Mayer, L. W. & Barbour, A. G. (1985) Science 227, 645–646. [DOI] [PubMed] [Google Scholar]
- 12.Fikrig, E., Telford, S. R., III, Barthold, S. W., Kantor, F. S., Spielman, A. & Flavell, R. A. (1992) Proc. Natl. Acad. Sci. USA 89, 5418–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma, J., Hine, P. M., Clough, E. R., Fish, D., Coughlin, R. T., Beltz, G. A. & Shew, M. G. (1996) Vaccine 14, 1366–1374. [DOI] [PubMed] [Google Scholar]
- 14.de Silva, A. M., Fish, D., Burkot, T. R., Zhang, Y. & Fikrig, E. (1997) Infect. Immun. 65, 3146–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsao, J., Barbour, A. G., Luke, C. J., Fikrig, E. & Fish, D. (2001) Vect. Born. Zoon. Dis. 1, 65–74. [DOI] [PubMed] [Google Scholar]
- 16.Bunikis, J., Tsao, J., Luke, C. J., Luna, M. G., Fish, D. & Barbour, A. G. (2004) J. Infect. Dis. 189, 1515–1523. [DOI] [PubMed] [Google Scholar]
- 17.Keirans, J. E., Hutcheson, H. J., Durden, L. A. & Klompen, J. S. H. (1996) J. Med. Entomol. 33, 297–318. [DOI] [PubMed] [Google Scholar]
- 18.Van Buskirk, J. & Ostfeld, R. S. (1995) Ecol. Appl. 5, 1133–1140. [Google Scholar]
- 19.Talleklint, L. & Jaenson, T. G. T. (1996) J. Med. Entomol. 33, 805–811. [DOI] [PubMed] [Google Scholar]
- 20.Ramakrishnan, U. (2001) Front. Plant Sci. 53, 7. [Google Scholar]
- 21.Wolff, J. O. (1985) Can. J. Zool. 63, 2657–2662. [Google Scholar]
- 22.Fikrig, E., Barthold, S. W., Kantor, F. S. & Flavell, R. A. (1991) J. Infect. Dis. 164, 1224–1227. [DOI] [PubMed] [Google Scholar]
- 23.Telford, S. R., 3rd, Fikrig, E., Barthold, S. W., Brunet, L. R., Spielman, A. & Flavell, R. A. (1995) J. Exp. Med. 178, 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fikrig, E., Telford, S. R., III, Wallich, R., Chen, M. C., Lobet, Y., Matuschka, F. R., Kimsey, R. B., Kantor, F. S., Barthold, S. W., et al. (1995) J. Exp. Med. 18, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falco, R. C. & Fish, D. (1992) Exp. Appl. Acarol. 14, 165–173. [DOI] [PubMed] [Google Scholar]
- 26.Beati, L. & Keirans, J. (2001) J. Parasit. 87, 32–38. [DOI] [PubMed] [Google Scholar]
- 27.Bunikis, J., Garpmo, U., Tsao, J., Berglund, J., Fish, D. & Barbour, A. G. (2004) Microbiology, 150, 1741–1755. [DOI] [PubMed] [Google Scholar]
- 28.Bunikis, J., Tsao, J., Garpmo, U., Berglund, J., Fish, D. & Barbour, A. G. (2004) Emerg. Infect. Dis., 10, 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbour, A. G., Maupin, G. O., Teltow, G. J., Carter, C. J. & Piesman, J. (1996) J. Infect. Dis. 173, 403–409. [DOI] [PubMed] [Google Scholar]
- 30.Scoles, G. A., Papero, M., Beati, L. & Fish, D. (2001) Vect. Born. Zoon. Dis. 1, 21–34. [DOI] [PubMed] [Google Scholar]
- 31.Bunikis, J., Luke, C. J., Bunikiene, E., Bergstrom, S. & Barbour, A. G. (1998) J. Bacteriol. 180, 1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liveris, D., Wormser, G. P., Nowakowski, J., Nadelman, R., Bittker, S., Cooper, D., Varde, S., Moy, F. H., Forseter, G., Pavia, C. S. & Schwartz, I. (1996) J. Clin. Microbiol. 34, 1306–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wormser, G. P., Liveris, D., Nowakowski, J., Nadelman, R. B., Cavaliere, L. F., McKenna, D., Holmgren, D. & Schwartz, I. (1999) J. Infect. Dis. 180, 720–725. [DOI] [PubMed] [Google Scholar]
- 34.Otis, D. L., Burnham, K. P., White, G. C. & Anderson, D. R. (1978) Wildl. Monogr. 6262, 7–135. [Google Scholar]
- 35.Dolan, M. C., Piesman, J., Schneider, B. S., Schriefer, M., Brandt, K. & Zeidner, N. S. (2004) Infect Immun. 72, 5262–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stafford, K. C. I., Cartter, M. L., Magnarelli, L. A., Ertel, S. & Mshar, P. A. (1998) J. Clin. Microbiol. 36, 1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels, T. J., Boccia, T. M., Varde, S., Marcus, J., Le, J., Bucher, D. J., Falco, R. C. & Schwartz, I. (1998) Appl. Environ. Microbiol. 64, 4663–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanincova, K., Schafer, S. M., Etti, S., Sewell, H. S., Taragelova, V., Ziak, D., Labuda, M. & Kurtenbach, K. (2003) Parasitology 126, 11–20. [DOI] [PubMed] [Google Scholar]
- 39.Brisson, D. & Dykhuizen, D. E. (2004) Genetics 168, 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apperson, C. S., Levine, J. F., Evans, T. L., Braswell, A. & Heller, J. (1993) Exp. Appl. Acarol. 17, 719–731. [DOI] [PubMed] [Google Scholar]
- 41.Casher, L., Lane, R., Barrett, R. & Eisen, L. (2002) Exp. Appl. Acarol. 26, 127–143. [DOI] [PubMed] [Google Scholar]
- 42.Matuschka, F. R., Fischer, P., Heiler, M., Richter, D. & Spielman, A. (1992) J. Infect. Dis. 165, 479–483. [DOI] [PubMed] [Google Scholar]
- 43.Hanincova, K., Taragelova, V., Koci, J., Schafer, S. M., Hails, R., Ullmann, A. J., Piesman, J., Labuda, M. & Kurtenbach, K. (2003) Appl. Environ. Microbiol. 69, 2825–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luke, C. J., Huebner, R. C., Kasmiersky, V. & Barbour, A. G. (1997) Vaccine 15, 739–746. [DOI] [PubMed] [Google Scholar]
- 45.Brochier, B., Kieny, M. P., Costy, F., Coppens, P., Bauduin, B., Lecocq, J. P., Lnaguet, B., Chappuis, G., Desmettre, P., Afiademanyo, K., et al. (1991) Nature 354, 520–522. [DOI] [PubMed] [Google Scholar]
- 46.Krebs, J. W., Wheeling, J. T. & Childs, J. E. (2003) J. Am. Vet. Med. Assoc. 223, 1736–1748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.