Abstract
Understanding how tissues and organs acquire and maintain an appropriate size and shape remains one of the most challenging areas in developmental biology. The eye lens represents an excellent system to provide insights into regulatory mechanisms because in addition to its relative simplicity in cellular composition (being made up of only two forms of cells, epithelial and fiber cells), these cells must become organized to generate the precise spheroidal arrangement that delivers normal lens function. Epithelial and fiber cells also represent spatially distinct proliferation and differentiation compartments, respectively, and an ongoing balance between these domains must be tightly regulated so that the lens achieves and maintains appropriate dimensions during growth and ageing. Recent research indicates that reciprocal inductive interactions mediated by Wnt-Frizzled and Notch-Jagged signaling pathways are important for maintaining and organizing these compartments. The Hippo-Yap pathway has also been implicated in maintaining the epithelial progenitor compartment and regulating growth processes. Thus, whilst some molecules and mechanisms have been identified, further work in this important area is needed to provide a clearer understanding of how lens size and shape is regulated.
Keywords: Lens epithelial cells, Lens fiber cells, Lens morphogenesis, Epithelial cell proliferation, Fiber cell differentiation, FGF, Notch-jagged, Wnt-frizzled, Hippo-yap
1. Introduction
Developmental biology has made remarkable advances in recent decades. As a result we now have a much better understanding of how cells differentiate into the different cell types that make up tissues and organs. Advances in cell and molecular biology have contributed significantly to our understanding of how genes are regulated by transcription and signaling networks and how these in turn are influenced by extracellular signals from growth factors and extracellular matrix. However, one area of development that is still poorly understood relates to morphogenesis and how cells come together to form tissues and organs with the required three-dimensional cellular architecture. This is well illustrated in the eye because, in addition to tissues requiring the correct combinations and juxtapositions of their different cellular components, tissues must also acquire appropriate dimensions for the eye to function normally. The lens provides a particularly good example of this because, together with transparency, its precise dimensions are critical for its function of focussing light on the retina. The lens also provides a useful system to study the regulatory mechanisms involved in its morphogenesis, growth and pathology given its relative simplicity in the sense that it is comprised of only two main cell types, epithelial cells and fiber cells. Moreover, these cells are in spatially distinct compartments and their interactions and dynamics can be readily studied. Whilst these interactive processes are still relatively poorly understood, some recent developments have been encouraging, as they have at least identified some starting points for further research into how lens growth and three dimensional architecture might be regulated.
1.1. Formation and maintenance of epithelial and fiber compartments
A critical step in lens development is the formation and segregation of the two distinct forms of lens cell, epithelial and fiber cells. This begins with the formation of a hollow ball of lens vesicle cells that invaginate from the surface ectoderm that overlies the optic vesicle. Cells in the posterior half of the lens vesicle then elongate and differentiate into primary fiber cells whereas cells in the anterior half differentiate into a monolayer of epithelial cells that cover the anterior tips of the fibers. This characteristic lens polarity appears to be determined by the position of the lens vesicle in the optic cup as indicated by classical lens inversion experiments (see Coulombre and Coulombre, 1963). Once the spheroidal lens structure has formed, it grows by epithelial cell proliferation and differentiation of their progeny that shift below the lens equator into secondary fiber cells. Again the Coulombre experiment emphasises the importance of the ocular environment in maintaining lens polarity with epithelial and fiber phenotypes maintained and promoted by aqueous and vitreous environments, respectively. Further studies using lens epithelial explants (reviewed in Lovicu et al., 2011) identified FGFs as the triggers for fiber differentiation and the lens gradient hypothesis was put forward. Essentially this depends on vitreous providing a higher level of FGF stimulation than aqueous (Fig.1). One way this is achieved is by the vitreous being a reservoir of more FGF (and enhancers of FGF signaling) than aqueous; however, other considerations include the presence of recently identified growth factor signaling inhibitors, Sef, Sprouty and Spreds in the epithelium (reviewed in Lovicu et al., 2015). Whilst differential levels of growth factor signaling provide a good explanation for the generation of these two lens compartments, questions remain surrounding how these compartments are maintained and regulated so that the lens retains its polarity and three-dimensional architecture as well as fulfilling its growth requirements.
Fig. 1.
FGF gradient model. Lens cell behavior correlates with anterior - posterior differences in FGF concentration in the ocular media.
An underlying theme that has emerged from recent studies has underscored the importance of interactions between epithelial cells and fiber cells. Although it is well known that one of the key features of differentiating fibers in the intact lens is that they become regularly aligned and polarized towards the overlying epithelial sheet, until recently no mechanism had been identified. Studies in our laboratory indicate that as fibers undergo early stages of elongation, their alignment and orientation depends on the Wnt-Frizzled/Planar Cell Polarity (Wnt-Fz/PCP) signaling pathway (Chen et al., 2008; Sugiyama et al., 2010, 2011). These lens explant studies also show that FGF upregulates Wnt-Fz signaling and this involves translocation of Fz and the centrosome to the leading edge (apical tip) of similarly polarized groups of elongating fiber cells (Dawes et al., 2013). Further studies with FGF-treated explants that retain islands of epithelial cells have shown that elongating fiber cells always become oriented/aligned towards the epithelial islands. The polarization of the fibers is shown by the observation that in virtually every one of these aligned fiber cells the centrosome that defines the apical tip of each cell is juxtaposed to the epithelium (Fig. 2; Dawes et al., 2014). This mimics the alignment and polarized behavior that fibers exhibit in vivo (Sugiyama et al., 2010; see Fig. 3), and clearly shows that the epithelium provides a polarising cue that ensures the elongating fibers align/orient appropriately towards the epithelium. Experiments with Wnt producing cells also indicate that this polarizing influence may be due, at least in part, to an epithelial-derived Wnt (Dawes et al., 2014). The observation that explanted lens cells can migrate along a Wnt concentration gradient also led to the hypothesis that a higher concentration of Wnt ligand at the anterior pole of the lens could provide the polarizing signal that promotes directed migration to the poles to form the lens sutures. During this phase of fiber differentiation the elongating cells develop convex curvature and this appears to be an important factor for maintaining the spheroidal lens shape. For example, in transgenic mice overexpressing the Wnt-Fz signaling inhibitor, Sfrp2, the fibers do not develop convex curvature and their lenses end up having a relatively flat anterior surface (Chen et al., 2008; Sugiyama et al., 2010). Other studies that interfere with the directed migration of elongating fibers towards the poles by blocking other cellular processes, give similar results and support the idea that the shape of the lens, at least in part, depends on the fibers developing convex curvature and forming normal sutures (Grove et al., 2004; Rivera et al., 2009).
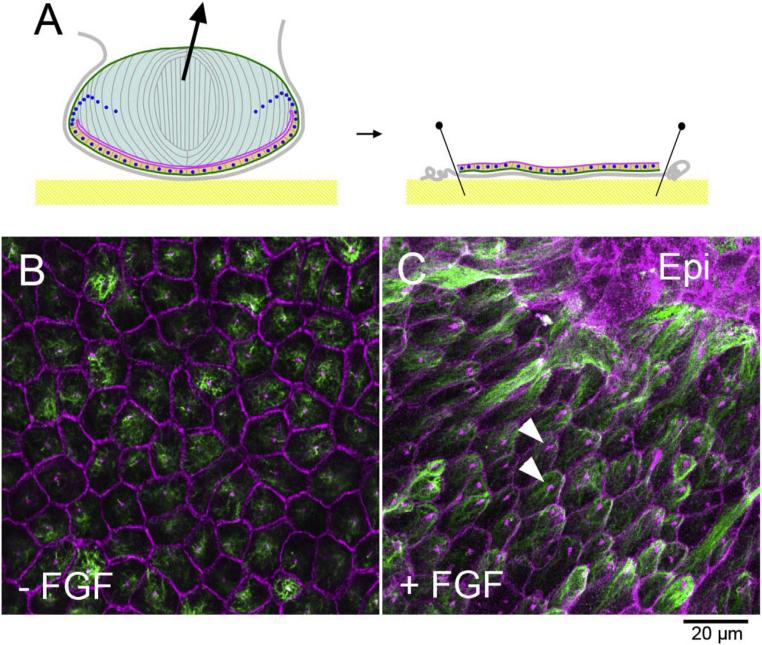
Fig. 2. Lens epithelial explants.
A Lens capsule with attached epithelial cells is pinned onto the bottom of culture dish. B, C Explant immunostained with β-catenin/pericentrin (purple, to show cell boundaries and centrosomes) and acetyl-tubulin (green, to detect cilia and microtubules) antibodies. Without FGF lens epithelial cells maintain cobblestone-like appearance (B). FGF induces groups of cells to elongate into fibers, whereas other groups of cells retain epithelial (epi) phenotype (C). The elongating fibers have hexagonal apical surfaces and cilia/centrosomes (arrowheads) are polarised towards the islands of epithelial cells (C).
Fig. 3.
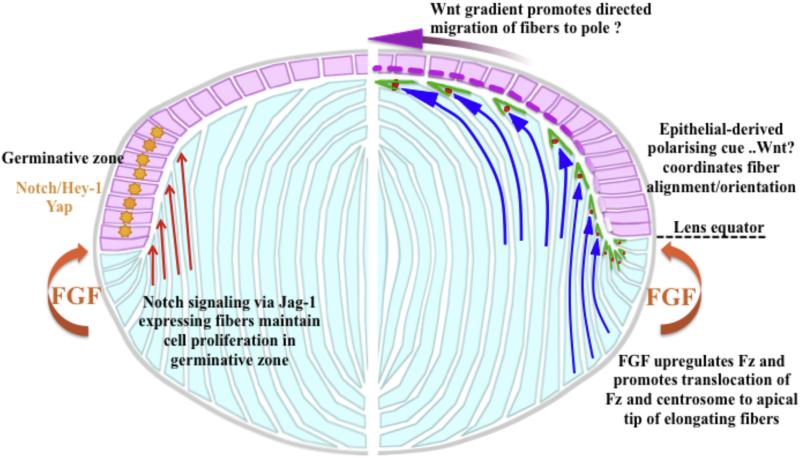
Proposed model for interactions between the epithelial-fiber (pink and light blue, respectively) compartments and key signaling pathways that regulate their dynamics. Right hand side of the diagram (Wnt-Frizzled signaling) shows that when epithelial cells shift below the lens equator, fiber differentiation is initiated in response to FGF in the posterior segment. This includes upregulation of Wnt-Fz signaling components and pathway activation that plays a key role in fiber elongation and differentiation (Dawes et al., 2013). This response also involves translocation of Fz (green) and the centrosome (red spot) to the apical tip of fibers as they elongate, and we propose this acts as the organizing center for cytoskeletal assembly and dynamics. Early in the elongation process the fibers have concave curvature (concave blue arrows) as they orient towards overlying epithelia in response to an epithelial-derived polarizing cue, possibly a Wnt (pink-purple dashed line in epithelium). As elongation progresses, the fibers take on convex curvature (convex blue arrows) as they undergo directed migration towards the pole, possibly in response to a Wnt concentration gradient. Left hand side shows that where the Jag-1 expressing cortical fibers (orange arrows) abut the epithelium determines the germinative zone, with nuclear localization of Notch-Jagged effector, Hey-1 and nuclear localization of Yap (orange star). Diagram modified from original in Dawes et al., 2014.
Whilst these studies have identified an epithelial to fiber inductive interaction, in development we know that inductive interactions between two tissues are usually reciprocal. Indeed, earlier work from several groups showed that early differentiating fibers have an important influence on the overlying epithelial cells of the germinative zone. Studies using both in vivo and in vitro rodent models showed that Notch signaling is required for maintenance of this population of proliferating cells. The first indications came from studies that conditionally deleted the Notch signaling effector, RPB-J, in the lens. This resulted in premature epithelial cell cycle exit and precocious fiber cell differentiation (Jia et al., 2007; Rowan et al., 2008). Similarly, null mutation of Jagged-1 (Jag-1) resulted in loss of anterior epithelial cells, with abnormal proliferation and defective secondary fiber differentiation (Le et al., 2009; Rowan et al., 2008; Saravanamuthu et al., 2009). The activation of the Notch pathway was also shown to be mediated by Jag-1-expressing fiber cells (Rowan et al., 2008; Le et al., 2009; Saravanamuthu et al., 2009, 2012). Consistent with this our explant studies showed that Jag-1 was strongly expressed in elongated fibers that were juxtaposed to the proliferating epithelial cells expressing the Notch effector, Hey1. We also showed that when we applied the Notch signaling inhibitor DAPT, Jag-1 was reduced in the FGF-treated explants and a proliferating population of lens epithelial cells was not maintained (Dawes et al., 2014). Taken together these studies show that, when given the appropriate stimuli and provided the two populations of cells are present and can interact, an intrinsic mechanism is activated that ensures two sharply delineated lens compartments are maintained (summarised in Fig. 3).
1.2. Balancing proliferation and differentiation during growth and ageing
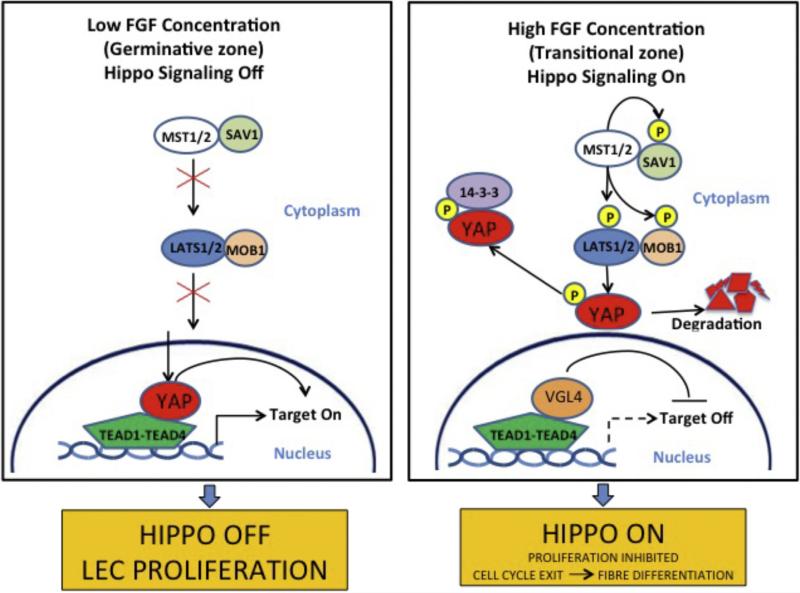
Whilst the Notch signaling studies have provided strong evidence that differentiating fibers play a role in maintaining the proliferative epithelial compartment, the question remains as to how the balance between proliferation and differentiation is regulated? This presents an ongoing challenge given that these processes continually need to be fine-tuned so that new fibers form to enable the lens to achieve the appropriate size for its developmental stage, and at the same time retain an appropriately sized progenitor pool of epithelial cells. Recent analyses of cell populations in post-natal mouse lenses have shown that the rates of cell production and differentiation are not always in equilibrium, thereby indicating the processes of cell proliferation and differentiation may be independently regulated to some degree (Shi et al., 2015). Studies on Hippo-Yap signaling in the lens have provided some useful insights into possible regulatory mechanisms that might influence at least part of this equation. The Hippo pathway has been identified as having an important influence in determining organ size through its ability to regulate Yes Associated Protein (Yap) and in turn negatively regulate tissue growth (Fig. 4; George et al., 2012). A role for the Hippo pathway in the lens has been identified in Merlin/NF2- and Yap-knockout mice. Merlin/NF2 is a negative regulator of Yap, a key effector of the Hippo pathway. In mice with Merlin/NF2 conditionally deleted from the lens, fiber cells did not fully exit the cell cycle and continued to express epithelial cell markers, such as FoxE3 and E-cadherin (Wiley et al., 2010). Later studies with mice that had Yap conditionally deleted from the lens, showed that the lens epithelium was substantially reduced (Song et al., 2014). The reduced lens epithelial progenitor pool resulted from reduced self-renewal and apoptosis. In addition, this study showed that Yap-deficient cells precociously exited the cell cycle and expressed the β-crystallin marker for fiber differentiation. Localization studies showed that normally Yap expression is restricted to the lens epithelium and that it localizes to the nucleus of cells that co-label with BrdU, whereas in the cells of the transitional zone where fiber differentiation is initiated, Yap is excluded from the nucleus and then disappears from the differentiating fibers. This is significant because the dogma is that in its unphosphorylated state, Yap translocates to the nucleus (in the absence of Hippo upstream signaling) where it mediates proliferative activity. In contrast, in the presence of Hippo signaling, phosphorylated Yap exits the nucleus and is sequestered in the cytoplasm. Taken together these results reveal a key role for the Hippo-Yap pathway for maintaining the lens epithelial progenitor pool. Furthermore, evidence is emerging that contact inhibition may regulate Taz, which itself is a regulator of Yap. In the lens a feedback mechanism has been suggested whereby sensitivity to cell density could be an important regulator of proliferative activity in the epithelial progenitor pool (Song et al., 2014). Somewhat related to this is the evidence that changes in tissue tension and/or mechanical cues can also influence Yap/Taz activation and regulate proliferation (Halder et al., 2012; Maller et al., 2013). In the lens context, an intriguing possibility could be that mechanical stimulation provided by accommodative changes in lens shape could also translate into changes that influence Yap/Taz activity and subsequently lens growth processes.
Fig. 4.
Hippo pathway. With low FGF stimulus, the Hippo pathway of off and YAP localizes to the nucleus and promotes proliferation. When high FGF stimulus, Hippo is on and YAP is phosphorylated. It is restricted to the cytoplasm where it is degraded.
There is recent evidence for co-operation between Hippo and Notch pathways. Studies on neural crest development have shown that Notch and Hippo pathways can converge to regulate cell differentiation (Manderfield et al., 2015). Neural crest-specific deletion of Yap and Taz produces neural crest precursors that migrate normally, but fail to produce vascular smooth muscle, with Notch target genes such as Jag-1 failing to activate normally. The study showed that Yap is normally recruited to a tissue-specific Jag-1 enhancer by directly interacting with the Notch intracellular domain (NICD). The Yap-NICD complex is recruited to chromatin by the DNA-binding protein RBP-J. Thus, Hippo-Yap signaling can modulate Notch signaling outputs, and components of the Hippo and Notch pathways physically interact. Our recent studies have also shown that FGF upregulates Hippo signaling components and levels of phosphorylated Yap in lens epithelial explants and this coincides with initiation of cell elongation and fiber differentiation (Lucy Dawes, preliminary results). Thus, an FGF-induced switch from proliferation to differentiation could be mediated by Hippo phosphorylation of Yap, thereby suppressing its Notch-promoting proliferative function. Taken together, appropriate convergence of FGF, Hippo and Notch pathways could be at the heart of a mechanism that achieves the correct balance between proliferation and differentiation that is clearly critical for proper regulation of lens growth.
Achieving a correct proliferation/differentiation balance is an ongoing challenge for the lens because as the eyeball grows the lens must adjust its growth rate accordingly. Thus, a progenitor pool must be maintained, whilst at the same time generate fibers at the appropriate rate to ensure lens growth keeps pace with eyeball growth. In addition to being a fundamental developmental question, this may also have relevance to important clinical issues in light of evidence that lens dimensions, notably its thickness, are abnormal in two major eye diseases, namely myopia (Mutti et al., 2012; Iribarren et al., 2012) and cataract (Klein et al., 1998, 2000). Whilst it is not clear if this is due to disturbed regulation of lens growth processes or other physical factors, one thing that is certain is that changes in lens growth rate must occur throughout life. Most obviously the relatively rapid growth rate characteristic of fetal and early postnatal life, must be moderated thereafter. Suggestions as to how this might be achieved have included changes in cell density and related contact inhibition-activated pathways influencing the proliferation/differentiation nexus (Song et al., 2014). External factors that can influence lens cell proliferation and differentiation age-dependently such as growth factors and oxygen, are also likely have a regulatory role. For example, lens cells become less responsive to the fiber-inducing influence of FGF with increasing age (Lovicu and McAvoy, 1992). Also, whilst it is well known that hypoxia induced HIF-1 suppresses proliferation, it was only capable of inhibiting the proliferation of lens cells later in life (Shui et al., 2008; Shui and Beebe, 2008). Finally, a recent study showed that hypoxic conditions differentially regulate Yap and Taz in cancer cells (Yan et al., 2014). This returns us to the interesting idea that Yap/Taz might function as the ‘gatekeeper’ that regulates the dynamics between proliferation and differentiation. Thus, Yap/Taz might be a cellular hub where diverse pathways, representing both intrinsic and extrinsic influences, converge in order to maintain the ‘Goldilocks Scenario’ where the balance between proliferation and differentiation is kept ‘just right’. As noted earlier, intrinsic factors could include cell density- and tissue tension-induced signaling, whereas extrinsic factors could include local growth factor bioavailability and oxygen concentration.
1.3. Future perspectives
Clearly there are still many challenges to overcome before we fully understand how lenses acquire their appropriate size and shape. Some progress has been made in identifying molecules and mechanisms that regulate proliferation and differentiation and we now have some candidates that may have a role in regulating the dynamics between these two compartments and how this might be regulated by intrinsic and extrinsic factors. However, our knowledge is still fragmentary and extensive experimentation needs to be directed at testing the key hypotheses and adding detail to the existing framework to provide a clearer understanding of mechanisms that regulate lens growth. Similarly, our understanding of how the lens acquires and modulates its precise curvature is rudimentary. So far, some indications that the curvature of the lens fibers contributes to overall lens shape comes from observations that normal curvature is not achieved if the fibers do not themselves develop convex curvature. Further research into the relationship between fiber cell curvature and lens curvature is needed to determine if, and to what degree, the fibers influence lens shape. Also, the capsule that surrounds the lens cells should not be overlooked in this context because there are strong indications that it may play a key role in determining lens shape. Support for this comes from experiments with monkeys in which the lens cells were removed and the capsular bag filled with flexible gel. If the capsule had no function other than to contain the lens cells, intuitively one would expect the capsular bag, as it was filled with gel, to form a sphere somewhat like the effect of blowing up a balloon. However, the studies showed that the lens mostly retained its characteristic shape and curvature differences between anterior and posterior domains as well as its ability to accommodate in the absence of fibers (for example, see Haefliger et al., 1987). Whilst this supports a role for the capsule in maintaining lens shape, it still leaves unaddressed the key question of how such a lens-moulding structure is generated in the first place. Lens shape is likely influenced by regional differences in thickness and composition of capsular components, particularly between anterior, posterior and equatorial regions. Earlier studies on lens development in rats, mice and chicks identified differences in capsule components and their patterns of deposition at different stages and in different regions of the lens (see for example, Johnson and Beebe, 1984; Parmigiani and McAvoy, 1989, 1991; Lovicu and McAvoy, 1993a,b; Kelley et al., 2002). Further studies are needed that focus on how such regional differences in components and dynamics of capsule growth are regulated and how they impact on lens dimensions and its physical properties. Clearly, answering these fundamental questions about how the lens takes shape still offers many challenges, but to those of us working in this area, the lens represents one of the best model systems for investigating how tissues/organs achieve their structural properties that are often critical for their functions.
Information gleaned from these basic studies will also have important implications for future clinical practice. Tissue engineering and regenerative medicine are fast growing areas of research and for the lens understanding the basic mechanisms that control its size and shape will clearly be important for promoting regeneration after cataract surgery. Whilst it has been known for some time that lenses of mice and rabbits (Call et al., 2004; Gwon et al., 1993) have good regenerative capacities, a recent study has shown this also to be the case for humans, at least in pediatric patients (Lin et al., 2016). An important key to success in this human study depended on retaining as much of the epithelial layer as possible, rather than the usual attempts to remove it, during surgery. Whilst it is acknowledged that these epithelial cells are the progenitors for the new fibers in these pediatric patients, the importance of the epithelial-fiber inductive interactions for generating a lens with appropriate structural and functional properties (as described above) must not be overlooked. The next major challenge will be to determine if lens regeneration is a feasible prospect for the vast and growing numbers of elderly cataract patients. With these patients there are other problems to overcome, one of which is that older lens epithelial cells are known to respond much slower to growth factor stimuli than younger cells (for example see Lovicu and McAvoy, 1992). However, our growing knowledge of the key growth factor signaling pathways and their regulation in lens cells, throws up the challenge of finding new ways of enhancing the responsiveness of older cells. Application of stem cell technology may also provide another approach to tackle these age-related problems. In this case, one idea might be to seed the lens with appropriately activated embryonic stem (ES) cells. Studies have already shown that when Pax-6 or Six-3 expression vectors were introduced into human ES cells they expressed a lens cell phenotype (Anchan et al., 2014). Further research along these different lines will be needed to determine the best approaches to the problem. One certainty however, is that novel approaches will be needed if we are to deal with the growing problem of cataract in the elderly as human life span continues to increase.
Acknowledgments
The authors would like to acknowledge the support for this work from the National Institutes of Health (R01 EY0-3177), the National Health and Medical Research Council (NHMRC), the National Foundation for Medical Research and Innovation, the Sydney Medical School Foundation and the Ophthalmic Research Institute of Australia.
References
- Anchan RM, Lachke SA, Gerami-Naini B, Lindsey J, Ng N, Naber C, Nickerson M, Cavallesco R, Rowan S, Eaton JL, Xi Q, Maas RL. Pax6-and Six3-mediated induction of lens cell fate in mouse and human ES cells. PLoS One. 2014;9:e115106. doi: 10.1371/journal.pone.0115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA. Lens regeneration in mice: implications in cataracts. Exp. Eye Res. 2004;78:297–299. doi: 10.1016/j.exer.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev. Biol. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Lens development: fiber elongation and lens orientation. Science. 1963;142:1489–1490. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Tanedo AS, Lovicu FJ, McAvoy JW. Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Invest Ophthalmol. Vis. Sci. 2013;54:1582–1590. doi: 10.1167/iovs.12-11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Lovicu FJ, Harris C, Shelley EJ, McAvoy JW. Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism. Dev. Biol. 2014;385:291–303. doi: 10.1016/j.ydbio.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Day CE, Boerner BP, Johnson RL, Sarvetnick NE. Hippo signaling regulates pancreas development through inactivation of Yap. Mol. Cell Biol. 2012;32:5116–5128. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol. Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwon A, Gruber L, Mantras C, Cunanan C. Lens regeneration in New Zealand albino rabbits after endocapsular cataract extraction. Invest. Ophthalmol. Vis. Sci. 1993;34:2124–2129. [PubMed] [Google Scholar]
- Haefliger E, Parel JM, Fantes F, Norton EW, Anderson DR, Forster RK, Hernandez E, Feuer WJ. Accommodation of an endocapsular silicone lens (Phaco-Ersatz) in the nonhuman primate. Ophthalmology. 1987;94:471–477. doi: 10.1016/s0161-6420(87)33422-0. [DOI] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- Iribarren R, Morgan IG, Chan YH, Lin X, Saw SM. Changes in lens power in Singapore Chinese children during refractive development. Invest Ophthalmol. Vis. Sci. 2012;53:5124–5130. doi: 10.1167/iovs.12-9637. [DOI] [PubMed] [Google Scholar]
- Jia J, Lin M, Zhang L, York JP, Zhang P. The Notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol. Cell Biol. 2007;27:7236–7247. doi: 10.1128/MCB.00780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MC, Beebe DC. Growth, synthesis and regional specialization of the embryonic chicken lens capsule. Exp. Eye Res. 1984;38:579–592. doi: 10.1016/0014-4835(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Kelley PB, Sado Y, Duncan MK. Collagen IV in the developing lens capsule. Matrix Biol. 2002;21:415–423. doi: 10.1016/s0945-053x(02)00014-8. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Moss SE. Correlates of lens thickness: the Beaver Dam Eye Study. Invest Ophthalmol. Vis. Sci. 1998;39:1507–1510. [PubMed] [Google Scholar]
- Klein BE, Klein R, Moss SE. Lens thickness and five-year cumulative incidence of cataracts: the Beaver Dam Eye Study. Ophthalmic Epidemiol. 2000;7:243–248. doi: 10.1076/opep.7.4.243.4176. [DOI] [PubMed] [Google Scholar]
- Le TT, Conley KW, Brown NL. Jagged 1 is necessary for normal mouse lens formation. Dev. Biol. 2009;328:118–126. doi: 10.1016/j.ydbio.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RA, Xu Y, Chung C, Zhang Y, Lin D, Patel S, Wu F, Cai H, Hou J, Wen C, Jafari M, Liu X, Luo L, Zhu J, Qiu A, Hou R, Chen B, Chen J, Granet D, Heichel C, Shang F, Li X, Krawczyk M, Skowronska-Krawczyk D, Wang Y, Shi W, Chen D, Zhong Z, Zhong S, Zhang L, Chen S, Morrison SJ, Maas RL, Zhang K, Liu Y. Lens regeneration using endogenous stem cells with gain of visual function. Nature. 2016;531:323–328. doi: 10.1038/nature17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Age of rats affects response of lens epithelial ex-plants to FGF: an ultrastructural study. Invest Ophthalmol. Vis. Sci. 1992;33:2269–2278. [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Localization of acidic fibroblast growth factor, basic fibroblast growth factor, and heparan sulphate proteoglycan in rat lens: implications for lens polarity and growth patterns. Invest Ophthalmol. Vis. Sci. 1993a;34:3355–3365. [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW, de Iongh RU. Understanding the role of growth factors in embryonic development: insights from the lens. Phil Trans. R. Soc. Lond B. Biol. Sci. 2011;366:1204–1218. doi: 10.1098/rstb.2010.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Shin EH, McAvoy JW. Fibrosis in the lens. Sprouty regulation of TGFb-signaling prevents lens EMT leading to cataract. Exp. Eye Res. 2015 May 20; doi: 10.1016/j.exer.2015.02.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Localisation of aFGF, bFGF and HSPG in rat lens: implications for lens polarity and growth patterns. Invest Ophthalmol. Vis. Sci. 1993b;34:3355–3365. [PubMed] [Google Scholar]
- Maller O, DuFort CC, Weaver VM. YAP forces fibroblasts to feel the tension. Nat. Cell Biol. 2013;15:570–572. doi: 10.1038/ncb2777. [DOI] [PubMed] [Google Scholar]
- Manderfield LJ, Aghajanian H, Engleka KA, Lim LY, Lui F, Jain R, Li L, Olson EN, Epstein JA. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Development. 2015 Aug 7; doi: 10.1242/dev.125807. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Sinnott LT, Jones-Jordan LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. CLEERE Study Group. Corneal and crystalline lens dimensions before and after myopia onset. Optom. Vis. Sci. 2012;89:251–262. doi: 10.1097/OPX.0b013e3182418213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani CM, McAvoy JW. A morphometric analysis of the development of the rat lens capsule. Curr. Eye Res. 1989;8:1271–1277. doi: 10.3109/02713688909013906. [DOI] [PubMed] [Google Scholar]
- Parmigiani CM, McAvoy JW. The roles of laminin and fibronectin in the development of the lens capsule. Curr. Eye Res. 1991;10:501–511. doi: 10.3109/02713689109001758. [DOI] [PubMed] [Google Scholar]
- Rivera C, Yamben IF, Shatadal S, Waldof M, Robinson ML, Griep AE. Cell-autonomous requirements for Dlg-1 for lens epithelial cell structure and fiber cell morphogenesis. Dev. Dyn. 2009;238:2292–2308. doi: 10.1002/dvdy.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Conley KW, Le TT, Donner AL, Maas RL, Brown NL. Notch signaling regulates growth and differentiation in the mammalian lens. Dev. Biol. 2008;321:111–122. doi: 10.1016/j.ydbio.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev. Biol. 2009;332:166–176. doi: 10.1016/j.ydbio.2009.05.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanamuthu SS, Le TT, Gao CY, Cojocaru RI, Pandiyan P, Liu C, Zhang J, Zelenka PS, Brown NL. Conditional ablation of the Notch2 receptor in the ocular lens. Dev. Biol. 2012;362:219–229. doi: 10.1016/j.ydbio.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, De Maria A, Lubura S, Šikić H, Bassnett S. The penny pusher: a cellular model of lens growth. Invest Ophthalmol. Vis. Sci. 2015;56:799–809. doi: 10.1167/iovs.14-16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui YB, Beebe DC. Age-dependent control of lens growth by hypoxia. Invest Ophthalmol. Vis. Sci. 2008;49:1023–1029. doi: 10.1167/iovs.07-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui YB, Arbeit JM, Johnson RS, Beebe DC. HIF-1: an age-dependent regulator of lens cell proliferation. Invest Ophthalmol. Vis. Sci. 2008;49:4961–4970. doi: 10.1167/iovs.08-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Park R, Kim JY, Hughes L, Lu L, Kim S, Johnson RL, Cho SH. Dual function of Yap in the regulation of lens progenitor cells and cellular polarity. Dev. Biol. 2014;386:281–290. doi: 10.1016/j.ydbio.2013.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Stump RJ, Nguyen A, Wen L, Chen Y, Wang Y, et al. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev. Biol. 2010;338:193–201. doi: 10.1016/j.ydbio.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis. 2011;7:191–201. doi: 10.4161/org.7.3.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Dattilo LK, Kang KB, Giovannini M, Beebe DC. The tumor suppressor merlin is required for cell cycle exit, terminal differentiation, and cell polarity in the developing murine lens. Invest Ophthalmol. Vis. Sci. 2010;51:3611–3618. doi: 10.1167/iovs.09-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Cai Q, Xu Y. Hypoxic conditions differentially regulate TAZ and YAP in cancer cells. Arch. Biochem. Biophys. 2014;562:31–36. doi: 10.1016/j.abb.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]