Abstract
Systemic lupus erythematosus (SLE, lupus) is characterized by autoantibody-mediated organ injury. Follicular helper T cells (Tfh) orchestrate physiological germinal center (GC) B cell responses, while in lupus they promote aberrant GC responses with autoreactive memory B cell development and plasma cell-derived autoantibody production. IL-21, a Tfh cell-derived cytokine, provides instructional cues for GC B cell maturation, with disruption of IL-21 signaling representing a potential therapeutic strategy for autoantibody-driven diseases such as SLE. We used blockade of IL-21 to dissect the mechanisms by which this cytokine promotes autoimmunity in murine lupus. Treatment of lupus-prone B6.Sle1.Yaa mice with an anti-IL-21 blocking antibody reduced titers of autoantibodies, delayed progression of glomerulonephritis and diminished renal infiltrating Tfh and T helper 1 (Th1) cells, and improved overall survival. Therapy inhibited excessive accumulation of Tfh cells co-expressing IL-21 and IFN-γ, and suppressed their production of the latter cytokine, albeit while not affecting their frequency. Anti-IL-21 treatment also led to a reduction in GC B cells, CD138hi plasmablasts, IFN-γ-dependent IgG2c production, and autoantibodies, indicating that Tfh-cell derived IL-21 is critical for pathological B cell cues in lupus. Normalization of GC responses were, in part, due to uncoupling of Tfh-B cell interactions, as evidenced by reduced expression of CD40L on Tfh cells and reduced B cell proliferation in treated mice. Our work provides mechanistic insight into the contribution of IL-21 to the pathogenesis of murine lupus, while revealing the importance of T-B cellular cross-talk in mediating autoimmunity, demonstrating that its interruption impacts both cell types leading to disease amelioration.
Introduction
Systemic lupus erythematosus (SLE, lupus) is an inflammatory disorder characterized by the generation of autoantibodies that promote tissue injury. Both adaptive and innate immune cells contribute to the aberrant immune response in SLE, with follicular helper T (Tfh) cells playing a central role given their direct effects in promoting the proliferation and maturation of B cells in germinal centers (GCs) of secondary lymphoid organs (SLOs) (1, 2). While much is known about how Tfh cells function and interact with other immune components during normal immune responses, such as in infection or immunization, less is understood about their function in SLE (3).
In murine models of lupus, GCs expand with concomitant increase of Tfh cells in both follicular and extrafollicular compartments (2, 4, 5). The number of circulating Tfh-like (cTfh) cells likewise increases in human SLE compared to control subjects, with correlation to autoantibody production and disease severity (6, 7). Defining the mechanisms by which Tfh cells promote autoimmunity either through autoreactive B cell responses and/or influencing the function of other immune cells is critical for understanding the molecular and cellular origins of autoimmunity, and ultimately, for developing novel treatment strategies.
IL-21, the signature effector cytokine secreted by Tfh cells in SLOs (1), promotes B cell proliferation, immunoglobulin (Ig) class switching, and plasma cell differentiation (8-11). IL-21R-deficient mice have weakened humoral responses to T-dependent antigens, with reduced GC B cell formation due to diminished induction of the transcription factor B-cell lymphoma 6 protein (Bcl6) necessary for GC B cell proliferation and immunoglobulin (Ig) gene mutation, and defective plasma cell formation and impaired development of memory B cells (8, 10, 11). Accordingly, IL-21 transgenic mice exhibit expansion of plasma cells, hypergammaglobulinemia, and an increased frequency of class switched Igs (12). IL-21 is markedly elevated in autoimmune prone mice, including the lupus-prone BXSB-Yaa strain (12-14). Moreover, lupus severity is diminished in the absence of IL-21 or IL-21R signaling in the lupus-prone strains BXSB-Yaa and MRL/MpJ-Faslpr (MRL/Faslpr) (13-15). Treatment of MRL/Faslpr mice with an IL-21R-Fc fusion protein as an IL-21 sequestering agent lowered levels of circulating autoantibodies and diminished deposition of glomerular immune complexes (16). While a subsequent study of BXSB-Yaa mice treated with an IL-21 blocking agent demonstrated minimal beneficial effect (17), a lack of IL-21R expression on B cells, but not T cells, nonetheless protected BSXB-Yaa mice from disease, thereby demonstrating a B-cell intrinsic requirement for IL-21 signaling to support GC formation and plasma cell differentiation in autoimmunity (14). The protective effect of B-cell restricted IL-21R deficiency was not simply limited to modulation of autoantibody titers, as these animals also had improvement in non-B cell features, such as reduced numbers of splenic myeloid cells which have been associated with lupus severity (18, 19). Together these studies demonstrate that effective targeting of a specific population of immune cells may have ripple effects throughout the immune system that can lead to greater therapeutic impact. Based on these data, we hypothesized that IL-21 signaling from Tfh cells to B cells represents a critical node in mediating the pathologic cellular networks that lead to disease pathology and severity in murine lupus.
To better understand the temporal molecular and cellular responses to IL-21 in autoimmunity, we have used a novel anti-IL-21 monoclonal antibody to interrupt IL-21 signaling in lupus-prone C57BL/6J (B6) Sle1.Yaa mice (E. Wakeland, UTSW). These mice harbor an introgressed Sle1 locus from the NZM2410 strain, and carry a duplication of Tlr7 (TLR7) via the Y-linked autoimmune accelerating (Yaa) locus (20-23). The combination of these genetic components causes spontaneous immunological aberrations, including abnormal GC responses, expansion of splenic T, B, and myeloid cells, and development of autoantibody-mediated, fatal lupus glomerulonephritis (13, 22, 24). Anti-IL-21 treatment improved disease parameters in these animals, including blockade of pathological B cell expansion and organ damage. Although we anticipated that the effects of anti-IL-21 therapy would primarily derive from down-modulation of B cell responses, we found that, despite maintenance of overall splenic Tfh cell frequency, IL-21 blockade significantly inhibited the disease-associated expansion of Tfh cells that produced both IL-21 and IFN-γ, in particular reducing IFN-γ synthesis and concomitant pathogenic IgG2c autoantibody production. These results demonstrate that IL-21 signaling plays an essential role in the pathological cellular crosstalk that drives autoimmunity in murine lupus, including the aberrant expansion of pathogenic IFN-γ-producing Tfh cells. Our findings will help develop a better framework for understanding current lupus therapies and for evaluating and designing new therapeutics for autoimmune diseases.
Materials and Methods
Mice
B6.Sle1.Yaa mice (22) were provided by E. Wakeland (University of Texas Southwestern Medical School), and bred and maintained in specific pathogen-free (SPF) conditions and handled according to protocols approved by Yale Institutional Animal Care and Use Committee.
Generation of anti-mouse IL-21 monoclonal antibody (mAb)
The rat anti-mouse IL-21 hybridoma mAb was generated by immunization of rats with cDNA expressing mouse IL-21. Clone BFJ-4H11-B4 was selected as the hybridoma lead based on its strong neutralization activity on mouse IL-21 on a STAT3 phosphorylation bioassay. To enable evaluation in chronic mouse lupus models, a rat/mouse chimeric mAb 4H11-B4 mIgG2a/k was constructed and generated in HEK293 cells via transient transfection. 4H11-B4 mIgG2a/k exhibits strong neutralization activity on mouse IL-21, with an IC50 of approximately 30 pM.
Quantification of the anti-IL-21 antibody in serum samples
Murine anti-IL-21 serum samples were analyzed with a Meso Scale Discovery (MSD) assay employing biotinylated murine IL-21 (PR-1464132, lot 2130004; 0.05 μg/mL) for capture and goat anti-mouse sulfotag antibody for detection (MSD Cat# R32AC-1; 0.5 μg/mL). Samples were diluted 1:100 in assay buffer (1% MSD Blocker A in Tris-buffered saline with 0.02% Tween-20) then further diluted in assay buffer containing serum for a 1% final matrix concentration. MSD standard curve fitting and data evaluation was performed using XLfit4 software (Version 4.2.1 Build 16). A calibration curve was prepared in serum and was plotted from MSD luminescence units versus theoretical standard concentrations. A four-parameter logistic model was used for curve fitting. The regression equation for the calibration curve was then used to back calculate the measured concentrations. The lower limit of quantitation (LLOQ) was 0.021 μg/ml.
Anti-IL-21 treatment in vivo
Eight-week old male B6.Sle1.Yaa mice were separated randomly into two groups and injected intraperitoneally (i.p.) with anti-IL-21 monoclonal antibody (4H11-B4; AbbVie Pharmaceutical) at 30 mg/kg, two times a week for the first four weeks followed by three times a week for three months. Control mice were injected with PBS in the same manner. Serum samples were collected by retro-orbital bleeding using heparinized capillary tubes, and sera were stored at −80°C.
Preparation of single cell suspensions
Spleens were homogenized by crushing between two frosted glass slides followed by straining through a 40-μm cell strainer. Red blood cells were lysed by hypotonic shock, and washed with DPBS before counting cells. To isolate renal lymphocytes, mice were perfused with DPBS and kidneys were removed, minced, and strained through a 70-μm cell strainer. Cell suspensions were loaded onto Ficoll-Paque, followed by density-gradient centrifugation. Cells were harvested from the opaque layer in the interface and washed twice with DPBS before staining for flow cytometry.
Flow cytometry
Antibodies and dilutions used for staining included CD4 (RM4-5, 1:200), CD44 (IM7, 1:200), ICOS (C398.4A, 1:100), TCRβ (H57-597, 1:100), F4/80 (BM8, 1:400), and T-bet (c4B10, 1:50) all from eBioscience; PSGL-1 (2PH1, 1:1000), B220 (RA3-6B2, 1:400), GL-7 (GL7, 1:400), CD95 (Jo2, 1:400), CD138 (281-2, 1:400), CD11b (M1/70, 1:400), Gr-1 (RB6-8C5, 1:400), CD8 (53-6.7, 1:200), Bcl6 (K112-91, 1:50), and CD40L (MR1, 1:50), all from BD Bioscience; PD-1 (RMP1-30, 1:200), IgD (11-26, 1:400), CXCR5 (L138D7, 1:1000), Ki-67 (16A8, 1:100), and IFN-γ (XMG1.2, 1:100), all from BioLegend; granzyme B (GB11, 1:200, Invitrogen) and IL-21R–FC (1:50, R&D Systems). For intracellular cytokine staining, cells were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) for 2 hours with Golgi Plug (BD Bioscience) for an additional 2 hours. Cells were fixed (BD CytoFix/CytoPerm™) and permeabilized (BD PERM/Wash™) following the manufacturer's protocol. IL-21 was detected by PE-conjugated F(ab')2 (1:400, Jackson ImmunoResearch). For intracellular transcription factor staining, surface molecule-stained cells were fixed and permeabilized using the buffer (eBioscience) without stimulation, and followed by staining for transcription factors. Stained cells were analyzed using a LSRII multilaser flow cytometry (BD) and followed by FlowJo software (Tree Star).
ELISA and ELISPOT analysis
To determine anti-dsDNA titers, ELISA plates were coated with methylated BSA, followed by calf thymus DNA. Diluted sera were added to the plates and allowed to bind overnight. Anti-dsDNA antibodies were detected with goat anti-mouse IgG conjugated with horseradish peroxidase (HRP; Southern Biotechnology). MRL/Faslpr serum samples were used as an internal standard. Plates were developed with TMB Microwell Peroxidase Substrate (SureBlue, KPL) and the optical density read at 450 nm. To determine autoantibody-forming cells (AFC), ELISPOT analysis was carried out similarly. MultiScreen filter plates (EMD Millipore) were coated with either chromatin or DNA. Single cell suspensions of splenocytes and kidney cells were prepared in 10% FBS containing RPMI1640 media at 2 × 106/ml concentration. Cells were plated directly onto coated plates in triplicate and incubated for 24 hours at 37°C. Spots were developed with Vector Blue Substrate Kit (Vector Laboratories) and read by AID EliSpot Reader.
Histology
Kidneys were removed, bisected, formalin-fixed, paraffin-embedded, and hematoxylin and eosin–stained. Kidneys were scored for glomerulonephritis by M.K. in a blinded manner, on a scale of 0 to 6 performed as previously reported (25). For immunofluorescence staining, frozen kidneys stored in −80°C were cut into 7 μm sections, fixed in acetone, and stained with FITC-conjugated anti-mouse IgG2a (R19-15, 1:200, BD Bioscience) and FITC-conjugated anti-mouse C3 antibodies (1:200, MP Biomedicals). Antibody deposits within glomeruli were analyzed using a fluorescence microscope (Olympus BX40).
Statistical analysis
All data were presented as the mean ± SEM. The significance of the difference between two groups were evaluated by the two-tailed Student's t-test. Each dot in a scatter plot corresponds to an individual mouse. Pearson correlation coefficient with two-tailed p value was determined in the analysis of correlations. P values less than 0.05 were considered significant. Data were analyzed with Prism software (version 5.0d for Macintosh; GraphPad Software).
Results
IL-21 blockade inhibits disease progression in B6.Sle1.Yaa mice
Twenty-one 8-week old male B6.Sle1.Yaa mice were injected i.p. with anti-IL-21 mAb (4H11-B4) at 30 mg/kg, two times a week for four weeks followed by three times a week for an additional 12 weeks. Mice were selected for intervention at 8 weeks of age, as animals at this age have developed evidence of aberrant T and B activation, albeit without apparent deep organ injury(22). As a control, 18 age-matched male B6.Sle1.Yaa mice were injected with PBS. Nine mice from each group were sacrificed at 4 months of age (after 8 weeks of treatment), and all surviving animals were sacrificed at age 24 weeks (16 weeks of treatment).
Control B6.Sle1.Yaa mice had enlarged spleens with increased cellularity, compared to animals treated with anti-IL-21 (Fig. 1A, 1B). Anti-IL-21 treated mice had significant reduction in nephritis at ages 4 and 6 months (Fig.1C, 1D, right), by contrast to kidneys from controls that exhibited hypercellular glomeruli with crescent formation, suggestive of necrosis (Fig. 1D, left). As expected, spleen weights correlated with glomerulonephritis scores (Fig. 1E). Serum creatinine concentrations, used as a measure of renal function, were similar between both groups before treatment, but were significantly elevated in control mice at 4 months of age, suggesting compromised kidney function in the untreated animals, whereas they remained low in anti-IL-21-treated mice (Fig. 1F). Serum creatinine levels and kidney histopathology scores were correlated (Fig. 1G). Control B6.Sle1.Yaa mice had significant cumulative mortality beginning approximately at 4 months of age and > 50% mortality at 6 months (22). By contrast, nearly all mice treated with anti-IL-21 survived to age 6 months (Fig. 1H). Moreover, clinical phenotypes in treated animals had an inverse correlation with the sera concentrations of the anti-IL-21 antibody, as evidenced by decreases in spleen weights in treated mice (Fig. 1I). These data demonstrate that treatment of B6.Sle1.Yaa mice with anti-IL-21 decreases the severity of lupus glomerulonephritis and improves survival.
Figure 1.
Treatment of lupus-prone B6.Sle1.Yaa mice with anti-IL-21 inhibits disease progression and promotes survival. (A, B) Spleen weight (A) and total number of splenocytes (B) from control (closed circles) or anti-IL-21 treated (open circles) B6.Sle1.Yaa mice at indicated ages. (C, D) Scores of glomerulonephritis on a scale of 0 to 6 (C) and representative images of H & E stained kidney sections from 4-month old control (D, left) and anti-IL-21-treated animals (D, right). (E) Correlation between glomerulonephritis and spleen weights from all animals sacrificed at ages 4 and 6 months. Each circle represents an individual animal; controls in closed, and anti-IL-21-treated animals in open, circles. (F) Measurement of creatinine using QuantiChrom Creatinine Assay Kit (BioAssay Systems) in sera from all animals at indicated age (months). (G) Correlation between glomerulonephritis and serum creatinine from all animals sacrificed at ages 4 months. (H) Survival of B6.Sle1.Yaa mice treated with anti-IL-21 (n = 12), compared to PBS-treated controls (n = 9), using Mantel-Cox test. Correlations were determined using Pearson's correlation analyses. (I) Correlation between the amount of anti-IL-21 in sera and spleen weights at 6 months of age.
IL-21 blockade inhibits GC maturation and autoantibody production
Due to the necessary role that IL-21 plays in B cell differentiation and GC responses (8-11), we asked if IL-21 blockade impaired B cell proliferation, maturation in GCs, and development into plasmablasts in B6.Sle1.Yaa mice. In control animals, greater than 60% of activated B220+IgDlo splenic B cells expressed intracellular Ki-67 as a marker of proliferation at age 6 months, whereas only 20% of B cells from mice treated with anti-IL-21 were Ki-67+ (Fig. 2A), suggesting IL-21 blockade suppressed their proliferation. Moreover, numbers of TCRβ−B220+CD95hiGL7hi GC B cells were significantly reduced by IL-21 blockade (Fig. 2B). IL-21 blockade also caused significant reduction in formation of TCRβ− B220loCD138hi plasmablasts (Fig. 2C). In concert with anti-IL-21-mediated reduction in GC B cells and plasmablasts in B6.Sle1.Yaa mice, treated animals had a trend toward reduced formation of IgG1 and IgG2c anti-chromatin antibodies as measured by ELISPOT assays, compared to controls (Fig. 2D), as well as a reduction in IgG anti-dsDNA antibody amounts as determined by ELISA (Fig. 2E). Accordingly, glomerular immune complex deposition was reduced in anti-IL-21-treated animals, compared to controls, as determined by immunofluorescence analysis for IgG2c and C3 deposition (Fig. 2F). These data indicate that IL-21 blockade prevents proliferation and expansion of B cells, GC maturation and plasmablast differentiation, as well as autoantibody generation in B6.Sle1.Yaa mice.
Figure 2.
Anti-IL-21 treatment of B6.Sle1.Yaa mice inhibits GC B cell maturation, plasmablast differentiation, and autoantibody production. (A-C) Representative flow cytometry plots of control (left, closed circles) and anti-IL-21-treated (middle, open circles) animals and scatter plot (right) of the percentage of Ki-67+ among IgDloB220hi activated B cells in mice at 6 months (A) and GL7hiCD95hi GC B cells among B220+ B cells at 4 months (B), and the percentage of TCRβ−B220loCD138hi plasmablasts among total splenocytes at 4 months (C). Histogram in gray in (A) is IgDloB220hi naive B cells. (D) Representative ELISPOT analysis (left) and number of splenocytes secreting anti-chromatin antibodies in 4-month old animals (right). (E) IgG anti-dsDNA IgG autoantibodies from 4 month old control and anti-IL-21 treated B6.Sle1.Yaa mice. Each dot indicates an individual animal. Each scatter plot is a compilation of 2-3 independent experiments. (F) Immunofluorescent staining for IgG2c and C3 deposits in the glomeruli of control and anti-IL-21-treated mice at 4 months of age. The significance of the difference between two groups was evaluated by two-tailed Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
IL-21 blockade blocks accumulation of IL-21+ IFN-γ+ Tfh cells in B6.Sle1.Yaa mice
Activated CD4+ T cells expand as B6.Sle1.Yaa mice age, with robust expression of transcripts for Il21, Ifng, and Il4, with expansion of a population bearing Tfh cell surface markers (22). We likewise found that >90% of splenic CD4+ T cells from 4-month old control lupus mice were activated, as evidenced by CD44hi staining, with <10% CD44lo naive, finding similar percentages at age 6 months (Fig. 3A, top). By contrast, mice treated with anti-IL-21 had significant reduction in percentages of activated CD44hi cells (~60%) at age 4 months, with a concomitant increase in their naive counterparts (~40%), with similar results at age 6 months (Fig. 3A, top). In a like manner, anti-IL-21 treated animals had significant reduction in total CD4+ T cell numbers compared to controls at age 4 months, with fewer such cells also found at age 6 months (Fig. 3A, bottom). By contrast to the ameliorative effects of IL-21 blockade on numbers and percentages of activated CD4+ T cells, the frequency and number of CD4+CD44hiPSGL-1loCXCR5hiPD-1hi Tfh cells were relatively unaltered by anti-IL-21 treatment (Fig. 3B, C). These results show that while IL-21 blockade inhibits the overall activation and expansion of CD4+ T cells, the Tfh cell population appears largely unchanged numerically.
Figure 3.
Altered phenotype of splenic Tfh cells in B6.Sle1.Yaa mice is restored by anti-IL-21 treatment. (A) Quantification of percentage (top) and total numbers (bottom) of CD44loPSGL-1hi naive and CD44hi activated CD4+ T cell subsets from 4 month- and 6 month-old B6.Sle1.Yaa mice. (B, C) Strategy for identification of Tfh cells from control (top, closed circles) or anti-IL-21-treated (bottom, open circles) animals by CD44hiPSGL-1lo gating of CD4+ T cells (left) followed by that of CXCR5hiPD-1hi cells (right), with quantification of percentage (left) and total number (right) of Tfh cells from 4 month- and 6 month-old B6.Sle1.Yaa mice. (D, E) Representative flow cytometry plot from control (top) and anti-IL-21-treated (bottom) animals showing intracellular IL-21 and IFN-γ staining in Tfh cells (D) or Th1 cells (E) in black dot plots overlaid on naive CD4+ T cells staining in gray contour plot. The percentages of total IL-21+ cells (red box) or IL-21+ IFN-γ+ double positive cells (blue box) from control (closed circles) vs. anti-IL-21-treated (open circles) B6.Sle1.Yaa mice are shown at indicated ages. (F) The percentages of CD40Lhi Tfh cells from control compared to anti-IL-21-treated animals at 6 months of age. The gate was set based on CD40L expression on naive CD4+ cells. Each dot indicates an individual animal. Scatter plots are compilations of 2-3 independent experiments. The significance of the difference between two groups was evaluated by a two-tailed Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
We next asked if IL-21 blockade affected cytokine production by splenic Tfh cells, determining expression of IL-21 and IFN-γ in CD44hiPSGL-1loPD-1hiCXCR5hi Tfh and CD44hi PSGL-1hiPD-1loCXCR5lo Th1 cells. IL-21 was robustly produced by Tfh cells in control mice, findings similar to those earlier reported for Il21 transcripts in BXSB-Yaa mice (13), with IL-21 protein also produced by Th1 cells (Fig. 3D, E), also consistent with earlier findings of its transcript in BXSB-Yaa Th1 cells (13). IL-21 producers from both populations commonly expressed IFN-γ, with more IL-21+ IFN-γ+ double positive cells in the Th1 population (~40%) than in the Tfh group (~30%) in control animals (Fig. 3D, 3E), while relatively few cells produced only IFN-γ. Treatment with anti-IL-21 led to a significant decrease in cytokine double positive cells among both the Tfh and Th1 subsets at age 4 months, and for Tfh cells, also at age 6 months with a trend toward a decrease in Th1 cells, without significantly impacting the frequency of IL-21 single positive cells (Fig. 3D, 3E). The frequency of Tfh cells expressing CD40L was also significantly reduced in animals treated with IL-21 blockade (55% vs. 45%; control vs. IL-21 blockade, respectively) (Fig. 3F). Hence, IL-21 blockade resulted in the reduction of the expression of IFN-γ by Tfh and Th1 cells in B6.Sle1.Yaa mice, as well as that of CD40L, critical for GC B maturation to plasma cells (9).
To further investigate the phenotype of splenic Tfh cells in B6.Sle1.Yaa mice, we measured the flow cytometric expression of Bcl6 and T-box transcription factor Tbx21 (T-bet), subset-associated transcription factors for Tfh and Th1 cells, respectively. Bcl6 expression by Tfh cells, determined as geometric mean fluorescence index (gMFI), was lower in control animals compared to those treated with anti-IL-21 (Fig. 4A, left and center panels). To compensate for differences in Bcl6 staining among experiments, Bcl6 expression also was normalized to the degree of expression in naive CD4+ T cells (Fig. 4A, right panel). Regardless of whether we normalized its expression, there was significantly less Bcl6 in Tfh cells of control animals compared the treated cohort. Bcl6 expression also was inversely correlated with spleen weights and kidney disease (Fig. 4B, left and right, respectively). T-bet expression in splenic Tfh cells from control animals was also significantly lower than that observed in cells from anti-IL-21-treated animals (Fig. 4C), albeit not different between Th1 cells in either group (Fig. 4D). These results suggest that Tfh cells in B6.Sle1.Yaa mice with advanced disease, as manifested by splenomegaly and glomerulonephritis, do not conform to the canonical Tfh cell phenotype, as evidenced by lower Bcl6 and T-bet expression, and by expression of the inflammatory cytokine IFN-γ. The lack of correlation between T-bet expression and IFN-γ production is surprising and suggests there are T-bet independent mechanisms of IFN-γ production in lupus Tfh cells. For example, in the chronic inflammatory milieu of active lupus characterized by robust cytokine production, chromatin modifications necessary for Ifng expression can be acquired in the absence of T-bet (Craft Lab, unpublished data). While treatment with anti-IL-21 blockade did not affect the generation of Tfh cells, it significantly altered their phenotypic and functional properties, reverting them toward a more classical Tfh cell phenotype with enhanced Bcl6 expression and reduced numbers of IL-21 IFN-γ double positive cells compared to cells in control mice.
Figure 4.
Altered phenotype of splenic Tfh cells in B6.Sle1.Yaa mice is correlated with disease progression. (A) Representative flow cytometry plots from control (top, closed circles) and anti-IL-21-treated (bottom, open circles) animals showing Bcl6 expressions by Tfh cells (black line) overlaid on naive cells (filled gray) and scatter plots of geometric mean fluorescence intensity (gMFI) of Bcl6 in Tfh cells (middle) and normalized Bcl6 gMFI in Tfh cells (right). (B) Correlation between the ratio of Bcl6 gMFI and spleen weights (left), and the ratio of Bcl6 gMFI and kidney disease scores (right). Correlations were determined using Pearson's correlation analyses. (C, D) Representative flow cytometry plots from control (top) and anti-IL-21-treated (bottom) animals showing T-bet expression by Tfh cells (C) and Th1 cells (D) in black overlaid on naive cells (filled gray) and scatter plots of normalized T-bet gMFI in Tfh cells (C) and Th1 cells (D). Each dot indicates an individual animal. Scatter plots are compilations of 2-3 independent experiments. The significance of the difference between two groups was evaluated by two-tailed Student's t-test. n.s. not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Effect of IL-21 blockade on myeloid cells and CD8+ T cells in B6.Sle1.Yaa mice
Cellular abnormalities exhibited by B6.Sle1.Yaa mice are not limited to CD4+ T and B lymphocytes, but are also apparent in the myeloid cell compartment (24), the expansion of which is caused in part by altered expression of IL-6 (26, 27). We assessed expression of myeloid cell markers, including CD11b, F4/80, and Gr-1 among TCRβ− B220− splenocytes using flow cytometry. At 4 months of age, TCRβ− B220− cells constituted approximately 40% of the splenocytes in control animals, increasing to 60% at 6 months of age (Fig. 5A). The expanded TCRβ− B220− cell pool included CD11bhiF4/80hiGr-1med monocytes (Fig. 5B) and CD11bhiF4/80loGr-1hi granulocytes (Fig. 5C). By contrast, TCRβ− B220− cells constituted < 20% of the splenocytes and the proportion remained unchanged at 6 months of age in the anti-IL-21-treated animals. Accordingly, monocyte and granulocyte populations were significantly reduced by anti-IL-21 treatment (Fig. 5B, 5C).
Figure 5.
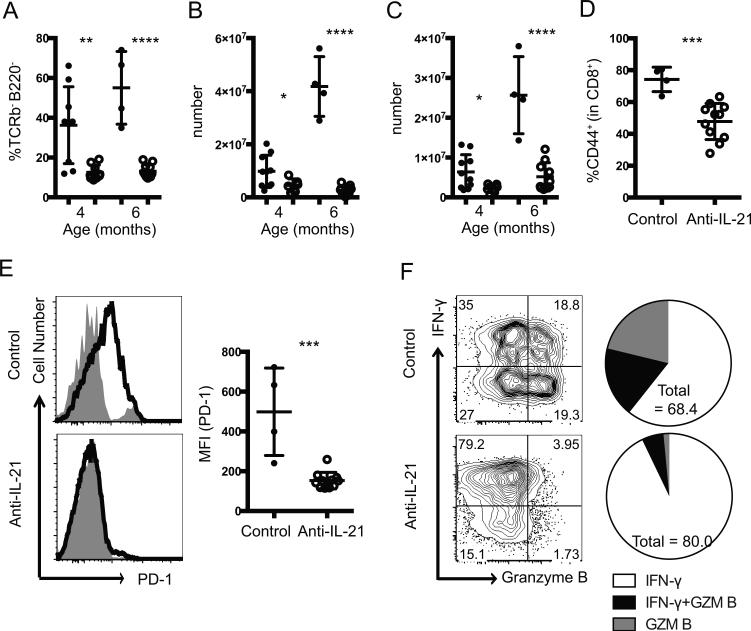
IL-21 blockade prevents abnormal expansion of leukocytes in B6.Sle1.Yaa mice. (A-C) Quantification of the percentage of TCRβ−B220− cells (A) from control (closed circles) or anti-IL-21 treated (open circles) mice, total numbers of monocytes (B), and granulocytes (C) among total splenocytes from control (closed circles) or anti-IL-21 treated (open circles) B6.Sle1.Yaa mice at indicated ages. (D, E) The percentages of CD44hi cells among splenic CD8+ T cells (D) and representative flow cytometry plots from control (top) and anti-IL-21-treated (bottom) animals showing PD-1 expression by CD44hiCD8+ cells in black overlaid on naive CD44loCD8+cells (filled gray) and scatter plots of PD-1 gMFI in CD44hiCD8+ cells at age of 6 months (E). (F) Representative flow cytometry plots showing intracellular staining for IFN-γ and granzyme B among splenic CD8+ T cells from 6 months old B6.Sle1.Yaa animals treated with PBS (top) or anti-IL-21 (bottom) and the fraction of the total CD8+ T cells expressing IFN-γ, granzyme B, or both (right). Each dot indicates an individual animal. The significance of the difference between two groups was evaluated by two-tailed Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
IL-21 signaling is necessary for sustained function of CD8+ T cells during chronic viral infection (28-30) and for expansion of central memory and suppressor CD8+ T cells in autoimmune BXSB-Yaa mice (14). We therefore characterized splenic CD8+ T cells in control and treated B6.Sle1.Yaa mice at age 6 months. CD8+ T cells from control mice comprised >70% of CD44hi cells, while anti-IL-21 treated animals had significant reduction to 40% of CD44hi cells (Fig. 5D). Expression of PD-1 on CD44hiCD8+ T cells, which was correlated with CD8+ T cell activation, was high in control animals, but decreased to that of naïve CD8+ T cells in treated animals (Fig. 5E). CD8+CD44hi T cells from control animals commonly expressed the cytolytic molecule granzyme B (Fig. 5F, top). By contrast, granzyme B-expressing CD8+CD44hi T cells were markedly reduced in anti-IL-21-treated animals (Fig. 5F, bottom), suggesting that CD8+ T cell expansion in murine lupus is dependent upon IL-21, as in viral infection (28-30), with IL-21 blockade reducing the cytolytic potential of this population.
Anti-IL-21 treatment abrogates infiltration of inflammatory CD4+ T cells in the kidney
Infiltration of inflammatory cells into the kidney is a key feature of murine lupus nephritis (31), with the diseased human kidney containing infiltrates of B cells and Tfh cells located in close proximity (32), suggesting local, pathological Tfh-B cell interaction. We therefore characterized the nature of the T cell infiltration in kidneys of B6.Sle1.Yaa mice. At 4 months of age, the total number of kidney-infiltrating leukocytes, as determined by forward and side scatter gating followed by a live cell gate, was significantly increased in control B6.Sle1.Yaa mice compared to animals treated with anti-IL-21 (Fig. 6A). Infiltrating CD4+ T cells mostly bore a CD44hiPSGL-1hi Th1 phenotype, while CD44hiPSGL-1lo Tfh cells were less commonly observed (Fig. 6B). Kidney infiltrating Th1 cells produced IL-21, with IL-21 IFN-γ double positive cells also increased compared to those observed in the spleen (60% vs. 40%, Fig. 6C; compare to Fig. 3E). CD44hiPSGL-1lo Tfh cells in the kidney likewise contained IL-21 IFN-γ double producers (Fig. 6D). Although the total numbers of renal Th1 and Tfh cells were reduced by anti-IL-21 therapy, their cytokine phenotypes did not change (Fig. 6C, D), nor did the proliferative capacity of the Th1 pool (Fig. 6E). These data indicate that infiltrating renal CD4+ T cells exhibit a Th1 phenotype in B6.Sle1.Yaa mice, with fewer Tfh-like CD44hiPSGL-1lo cells, with both populations reduced numerically by anti-IL-21 therapy.
Figure 6.
Infiltration of inflammatory CD4+ T cells into the kidney is abrogated by treatment with anti-IL-21 in B6.Sle1.Yaa animals. (A, B) Quantification of total number of leukocytes (A), from control (closed circles) or anti-IL-21 treated (open circles), and CD4+ T cell subsets (B) isolated from kidneys from 4 months old B6.Sle1.Yaa mice treated with PBS (closed circle) or anti-IL-21 (open circle). (C, D) Scatter plot of the percentages of IL-21+ IFN-γ+ cells among Th1 cells (C) and PSGL-1lo cells (D) isolated from kidney from control or anti-IL-21 treated B6.Sle1.Yaa mice at 4 months of age. (E) Representative flow cytometry plots from control (top) and anti-IL-21-treated (bottom) animals showing Ki-67 expression in Th1 cells (black line) overlaid on naive cells (filled gray) and the percentage of Ki67+ cells in Th1 cells (right) at 6 months of age. Each dot indicates an individual mouse. Each scatter plot is compiled from 2 independent experiments. The significance of the difference between two groups was evaluated by two-tailed Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Effect of IL-21 blockade in B6.Sle1.Yaa mice with advanced disease
We next assessed the effect of anti-IL-21 blockade in older B6.Sle1.Yaa mice, initiating therapy in twelve, 3-month old mice. Six animals were treated with either 30 mg/kg anti-IL-21 or PBS two times a week for four weeks, with all animals sacrificed at age 4 months; one PBS-treated animal died during treatment. While anti-IL-21 therapy did not affect spleen weights and total number of splenocytes (Fig. 7A, B, respectively), or the overall frequency and numbers of splenic Tfh (not shown) or GC B cells (Fig. 7C), CD138hi plasmablasts were dramatically diminished in animals treated with IL-21 blockade (Fig. 7D). Likewise, potentially pathogenic IgG2c plasmablasts from anti-IL-21-treated animals were significantly reduced compared to those from control animals (10% versus 30%, respectively), with both groups having similar numbers of IgG1 plasmablasts (Fig. 7E, F). Numbers of such cells producing anti-dsDNA antibodies were also significantly reduced by anti-IL-21 treatment (Fig. 7G), suggesting that IL-21 signaling is required for the expansion and maintenance of autoantibody-secreting plasmablasts even in advanced disease. Yet, the frequency of B cells with CD38hiGL7lo memory phenotype was significantly increased by treatment (Fig. 7I), suggesting IL-21 signaling is dispensable in memory B cell formation in murine lupus as it appears to be in normal physiological contexts (10, 11). At this later stage of disease, one month of anti-IL-21 blockade had no significant effect on renal disease, as serum creatinine concentrations had increased from 0.13 ± 0.03 mg/dl mg/dl (mean ± SD) at age 2 months (Fig. 1F) to 0.17 ± 0.03 and 0.18 ± 0.07 mg/dl (mean ± SD) at ages 3 and 4 months (Fig. 7H).
Figure 7.
Reduced autoantibody response by a brief treatment with IL-21 blockade in B6.Sle1.Yaa mice with advanced disease. (A, B) Spleen weight (A) and total number of splenocytes (B) from control (closed circles) or anti-IL-21 treated (open circles) B6.Sle1.Yaa mice at age of 4 months. (C) Scatter plots of the percentage of GL7hiCD95hi GC B cells among B220hiIgDlo B cells. (D) Scatter plot of the percentage of TCRβ−B220loCD138hi plasmablasts among total splenocytes. (E, F) Representative flow cytometry plots from control (top) and anti-IL-21-treated (bottom) animals showing intracellular IgG1 and IgG2a staining among TCRβ−B220loCD138hi plasmablasts (E) and scatter plots of the percentage of intracellular IgG1+ (left) or IgG2a+ (right) cells among TCRβ−B220loCD138hi plasmablasts in control vs. anti-IL-21-treated B6.Sle1.Yaa mice at age of 4 month (F). (G) Number of splenocytes secreting anti-dsDNA antibodies in 4-month old animals. (H) Measurement of creatinine in sera from control (closed circles) or anti-IL-21 treated (open circles) B6.Sle1.Yaa mice at indicated ages (months). (I) Scatter plots of the percentage of CD38hiGL7lo memory B cells among B220hiIgDlo B cells. Each dot indicates an individual mouse. The significance of the difference between two groups was evaluated by two-tailed Student's t-test. n.s. not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
Lupus-prone B6.Sle1.Yaa mice spontaneously develop expansion of T and B cells, with an abnormal GC output leading to pathological autoantibody-mediated glomerulonephritis (22). We show that treatment of these animals, before onset of pathological renal disease but after that of immunological abnormalities with T and B cell activation(22), with a novel anti-IL-21 antibody reduced B cell proliferation, GC responses, CD138+ plasma cell formation, and autoantibody secretion. These findings were in parallel with reduction in renal immune-complex deposits and cellular infiltrates, resulting in amelioration of histological kidney disease and improvement in survival. Although B cells with a CD38+GL7lo memory phenotype were relatively unaffected by IL-21 blockade, this finding is consistent with previous experiments demonstrating unaltered formation of memory B cells with in the absence of IL-21 signaling (10, 11). In aggregate, our results support earlier studies in other lupus models using anti-IL-21 blockade, or interruption in IL-21 signaling using genetic approaches, that this pathway is essential to the pathogenesis of lupus, most notably through driving aberrant B cell responses (13-17, 33).
IL-21 blockade was also effective when applied to an older cohort with advanced renal disease as evidenced by elevated blood creatinine levels. Treatment of older animals suppressed production of CD138+ plasma cells that secrete IgG2c pathogenic autoantibodies, although this cohort was too small to effectively evaluate changes in renal infiltrates or histological renal disease. Nonetheless, creatinine concentrations were not altered compared to control mice, despite significant reduction in pathological B cell responses, suggesting that renal inflammation and/or damage had progressed to a point independent of immune-complex triggering. It is also possible these later changes were independent of autoantibody-triggered inflammation altogether; however, this conclusion is at odds with our findings that earlier intervention with anti-IL-21 dramatically reduced renal inflammatory changes in conjunction with reduction in abnormal B cell responses and glomerular antibody and complement deposition. It is possible that longer-term treatment of older animals is needed to achieve a significant therapeutic effect.
Pathogenic autoantibodies play a fundamental role in pathogenesis of human lupus (34) and the production of such autoantibodies requires T cell-dependent GC B cell responses (3). SLE patients have elevated blood concentrations of IL-21 compared to healthy controls (35, 36) in association with increased numbers of circulating IL-21+ Tfh-like cells and disease activity (36). Evidence from both human and murine lupus studies unequivocally indicates that IL-21 plays a critical role in the pathogenesis of lupus and consequently, is an attractive therapeutic target in human SLE. A fully humanized anti-IL-21 receptor antibody (ART-107) has been used safely in a small number of healthy subjects, albeit with bioavailability concern (37). IL-21 blockade has also been evaluated in a phase I clinical trial for SLE, albeit with early termination (ClinicalTrials.gov Identifier: NCT01689025) (38). Our work provides pre-clinical rationale for these approaches.
IL-21 blockade also inhibited aberrant development of Th1 and Tfh cells. B6.Sle1.Yaa mice spontaneously develop and accumulate Tfh cells that promote aberrant GC responses with concomitant production of pathogenic IgG2c autoantibodies (22, 24). We found that Tfh cells in diseased B6.Sle1.Yaa mice robustly produced IL-21 and IFN-γ, perhaps surprisingly in parallel with downregulation of Bcl6 and T-bet expression, with expression of the former transcription inversely correlated with renal disease and spleen weight, indicative of an atypical Tfh cell phenotype in diseased B6.Sle1.Yaa mice. IL-21+ Tfh cells that coproduce IFN-γ with pathogenic drive have also been reported in other murine lupus models (22, 39, 40), and in our hands, in circulating Tfh-like cells in patients with SLE (our unpublished data). Tfh cells with a similar phenotype are found following viral challenge in mice (41, 42) and humans (43), albeit they are short-lived. By contrast, they may persist in chronic type I immune challenge of mice (44) and humans (43, 45). IFN-γ production by Tfh cells promotes protective immunity via generation of high affinity isotype-specific antibodies appropriate to the invading pathogen (46). By contrast, its effects in chronic infection or autoimmunity may be detrimental. Tfh-derived IFN-γ promotes Ig isotype switching by autoreactive B cells to inflammatory IgG2a (or IgG2c in C57Bl6 background) (47) and IgG3, with Fc receptor activation and complement fixation, potentially contributing to disease severity (48, 49). Moreover, Th1-derived IFN-γ, and presumably that derived from Tfh cells, contributes locally to macrophage activation, and renal inflammation and tissue damage (50, 51), including in human lupus nephritis (52).
Anti-IL-21 treatment suppressed CD4 T cell activation, leading to a decrease in the number and frequency of activated CD44hi CD4+ T cells. While Tfh cell numbers were unchanged by treatment, they nonetheless underwent phenotypic change. Tfh cells in treated animals produced significantly less IFN-γ, albeit with no change in IL-21 production, and with increased Bcl6 expression. These changes revealed reversion of the lupus Tfh cell phenotype in treated animals from pathogenic potential to one more conventional. Tfh cells in treated animals also had significant reduction in CD40L expression. Its expression on GC Tfh cells is regulated by antigen-dependent ICOS triggering via GC B-cell ICOSL (53). Thus, IL-21 blockade interrupts bidirectional collaboration between Tfh cells and GC B cells, affecting both cell populations. We speculate that the effects of IL-21 blockade on B cells are direct, given previous results (14), while the effects on Tfh cell cytokine production are indirectly mediated via B cells, as shown in our recent collaborative studies(54). Thus, our observation of pleiotropic immunological effects of IL-21 blockade suggests that IL-21 plays a critical role in maintaining the pathologic cellular interactions that drive persistent autoimmunity in lupus. Along these lines, anti-IL-21 associated downregulation of IFN-γ expression by Tfh cells, may be of particular benefit in controlling the pathogenic inflammation characteristic of lupus.
IFN-γ+ Th1 cells were significantly diminished in treated mice, with a 10-fold decrease in Th1 cell infiltrates in the kidney. We also found that blockade of IL-21 signaling reduced potentially cytolytic CD8+ T cells, which require IL-21 for their survival and function in chronic inflammatory states (28-30), as well as IL-6-dependent myeloid cells (24), with the latter result consistent with the role of CD4+ T cell-secreted IL-21 in promoting myeloid cell migration to survival niches and activation in experimental type I diabetes mellitus (55).
In summary, we report that blocking IL-21 signaling with anti-IL-21 in lupus-prone B6.Sle1.Yaa mice ameliorated disease with improved survival, via down-modulation of pathological B cell responses but also by regulation of pathogenic Tfh and Th1 cell function. These results add to the rationale for anti-IL-21 therapeutic intervention in SLE, and offer insight into the mechanism by which IL-21 promotes bidirectional collaboration between Tfh and GC B cells and disease progression in lupus.
Acknowledgements
The authors thank Stephen Clarke, David Duignan and Christine Grinnell (AbbVie), and members of the Craft lab, for helpful discussions and review of the manuscript, and Martha Mayo and Donna McCarthy (AbbVie) for their help with in vitro and in vivo assays.
This work was supported in part by grants provided by AbbVie, the Alliance for Lupus Research and the NIH (R37AR040072, R01AR068994 and P30AR053495) (to JC). Abhinav Seth was supported by a Scientist Development Award from the American College of Rheumatology Research Foundation, by NIH T32 AR07107, and a grant from the Robert E. Leet and Clara Guthrie Patterson Trust.
Footnotes
The authors have no financial conflicts of interest to disclose regarding this work.
References
- 1.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nature reviews. Rheumatology. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. The Journal of experimental medicine. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 4.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 5.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of experimental medicine. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, Goodnow CC, Vinuesa CG, Cook MC. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 7.Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, Borba EF, Goncalves CR, Costa PR, Kallas EG, Bonfa E, Craft J. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis & rheumatology. 2015;67:988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. Journal of immunology. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 10.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of experimental medicine. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. The Journal of experimental medicine. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. Journal of immunology. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 13.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPhee CG, Bubier JA, Sproule TJ, Park G, Steinbuck MP, Schott WH, Christianson GJ, Morse HC, 3rd, Roopenian DC. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. Journal of immunology. 2013;191:4581–4588. doi: 10.4049/jimmunol.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y, Keegan S, Senices M, Stedman N, Ryan M, Bloom L, Medley Q, Collins M, Nickerson-Nutter C, Craft J, Young D, Dunussi-Joannopoulos K. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJFas(lpr/lpr)/J mice. Journal of immunology. 2012;188:1656–1667. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. Journal of immunology. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 17.Bubier JA, Bennett SM, Sproule TJ, Lyons BL, Olland S, Young DA, Roopenian DC. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Annals of the New York Academy of Sciences. 2007;1110:590–601. doi: 10.1196/annals.1423.063. [DOI] [PubMed] [Google Scholar]
- 18.Becker AM, Dao KH, Han BK, Kornu R, Lakhanpal S, Mobley AB, Li QZ, Lian Y, Wu T, Reimold AM, Olsen NJ, Karp DR, Chowdhury FZ, Farrar JD, Satterthwaite AB, Mohan C, Lipsky PE, Wakeland EK, Davis LS. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PloS one. 2013;8:e67003. doi: 10.1371/journal.pone.0067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. The Journal of experimental medicine. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 21.Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis and rheumatism. 1979;22:1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, Casco J, Li QZ, Connolly JE, Wakeland EK. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. European journal of immunology. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Science translational medicine. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang A, Fairhurst AM, Tus K, Subramanian S, Liu Y, Lin F, Igarashi P, Zhou XJ, Batteux F, Wong D, Wakeland EK, Mohan C. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. Journal of immunology. 2009;182:4448–4458. doi: 10.4049/jimmunol.0801920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113:4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 29.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGaha TL, Madaio MP. Lupus Nephritis: Animal Modeling of a Complex Disease Syndrome Pathology. Drug discovery today. Disease models. 2014;11:13–18. doi: 10.1016/j.ddmod.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, Carlesso G, Herbst R, Utset TO, Labno C, Peng Y, Jiang Y, Giger ML, Clark MR. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Science translational medicine. 2014;6:230ra246. doi: 10.1126/scitranslmed.3008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Yu G, Chan B, Pearson JT, Rathanaswami P, Delaney J, Ching Lim A, Babcook J, Hsu H, Gavin MA. Interleukin-21 receptor blockade inhibits secondary humoral responses and halts the progression of preestablished disease in the (NZB x NZW)F1 systemic lupus erythematosus model. Arthritis & rheumatology. 2015;67:2723–2731. doi: 10.1002/art.39233. [DOI] [PubMed] [Google Scholar]
- 34.Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 35.Lan Y, Luo B, Wang JL, Jiang YW, Wei YS. The association of interleukin-21 polymorphisms with interleukin-21 serum levels and risk of systemic lupus erythematosus. Gene. 2014;538:94–98. doi: 10.1016/j.gene.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Zhao P, Ma L, Shan Y, Jiang Z, Wang J, Jiang Y. Increased interleukin 21 and follicular helper T-like cells and reduced interleukin 10+ B cells in patients with new-onset systemic lupus erythematosus. The Journal of rheumatology. 2014;41:1781–1792. doi: 10.3899/jrheum.131025. [DOI] [PubMed] [Google Scholar]
- 37.Hua F, Comer GM, Stockert L, Jin B, Nowak J, Pleasic-Williams S, Wunderlich D, Cheng J, Beebe JS. Anti-IL21 receptor monoclonal antibody (ATR-107): Safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. Journal of clinical pharmacology. 2014;54:14–22. doi: 10.1002/jcph.158. [DOI] [PubMed] [Google Scholar]
- 38.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nature reviews. Drug discovery. 2014;13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 39.Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, Walters G, Vinuesa CG. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Coquery CM, Loo WM, Wade NS, Bederman AG, Tung KS, Lewis JE, Hess H, Erickson LD. BAFF regulates follicular helper t cells and affects their accumulation and interferon-gamma production in autoimmunity. Arthritis & rheumatology. 2015;67:773–784. doi: 10.1002/art.38950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, Kaech SM. Differential Expression of Ly6C and T-bet Distinguish Effector and Memory Th1 CD4+ Cell Properties during Viral Infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, International AVIPCPI, Poignard P, Crotty S. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, Kallies A, Hansen DS. Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell reports. 2016;14:68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Cubas R, van Grevenynghe J, Wills S, Kardava L, Santich BH, Buckner CM, Muir R, Tardif V, Nichols C, Procopio F, He Z, Metcalf T, Ghneim K, Locci M, Ancuta P, Routy JP, Trautmann L, Li Y, McDermott AB, Koup RA, Petrovas C, Migueles SA, Connors M, Tomaras GD, Moir S, Crotty S, Haddad EK. Reversible Reprogramming of Circulating Memory T Follicular Helper Cell Function during Chronic HIV Infection. Journal of immunology. 2015;195:5625–5636. doi: 10.4049/jimmunol.1501524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Goldschmidt T, Salter H. Possible allelic structure of IgG2a and IgG2c in mice. Molecular immunology. 2012;50:169–171. doi: 10.1016/j.molimm.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer seminars in immunopathology. 2006;28:175–184. doi: 10.1007/s00281-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 49.Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-gamma and systemic autoimmunity. Discovery medicine. 2013;16:123–131. [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. Journal of immunology. 1998;161:494–503. [PubMed] [Google Scholar]
- 51.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T, Fukuda K, Kanai H, Nakashima H, Otsuka T, Hirakata H. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis and rheumatism. 2001;44:2097–2106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 53.Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, Luo D, Qi H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517:214–218. doi: 10.1038/nature13803. [DOI] [PubMed] [Google Scholar]
- 54.Shulman Z, Gitlin AD, Weinstein JS, Lainez B, Esplugues E, Flavell RA, Craft JE, Nussenzweig MC. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Belle TL, Nierkens S, Arens R, von Herrath MG. Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity. 2012;36:1060–1072. doi: 10.1016/j.immuni.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]