Abstract
Migration toward pathology is the first critical step in stem cell engagement during regeneration. Neural stem cells (NSCs) migrate through the parenchyma along nonstereotypical routes in a precise directed manner across great distances to injury sites in the CNS, where they might engage niches harboring local transiently expressed reparative signals. The molecular mechanisms for NSC mobilization have not been identified. Because NSCs seem to home similarly to pathologic sites derived from disparate etiologies, we hypothesized that the inflammatory response itself, a characteristic common to all, guides the behavior of potentially reparative cells. As proof of concept, we show that human NSCs migrate in vivo (including from the contralateral hemisphere) toward an infarcted area (a representative CNS injury), where local astrocytes and endothelium up-regulate the inflammatory chemoattractant stromal cell-derived factor 1α (SDF-1α). NSCs express CXC chemokine receptor 4 (CXCR4), the cognate receptor for SDF-1α. Exposure of SDF-1α to quiescent NSCs enhances proliferation, promotes chain migration and transmigration, and activates intracellular molecular pathways mediating engagement. CXCR4 blockade abrogates their pathology-directed chain migration, a developmentally relevant mode of tangential migration that, if recapitulated, could explain homing along nonstereotypical paths. Our data implicate SDF-1α/CXCR4, representative of the inflammatory milieu characterizing many pathologies, as a pathway that activates NSC molecular programs during injury and suggest that inflammation may be viewed not simply as playing an adverse role but also as providing stimuli that recruit cells with a regenerative homeostasis-promoting capacity. CXCR4 expression within germinal zones suggests that NSC homing after injury and migration during development may invoke similar mechanisms.
Keywords: human stem cells, homing, chain migration, stroke, hypoxia–ischemia
Neural stem cells (NSCs), both mouse and human, have a capacity for precise migration to even widespread and distant areas of pathology in a number of experimental models of CNS disease, including at ages where extensive migration has conventionally been deemed to be limited (1–6). It is both intriguing and perplexing to recognize, however, that NSCs seem to home similarly to pathologic sites derived from disparate etiologies. Because stem cell engagement with a degenerating environment (and niches harboring transiently recapitulated local reparative signals) (5, 7) is the first critical step in regeneration, realizing the therapeutic promise of the NSC depends in part on understanding the mechanisms underlying its mobilization during injury. For such precise homing over long distances and nonstereotypical routes to occur, we judged that signals must be generated at the site of pathology. One of the few characteristics that these otherwise disparate pathologies had in common was an inflammatory signature. We hypothesized that the inflammatory response itself might guide the behavior of potentially reparative cells. Because we have shown that NSCs preferentially home to the site of experimental stroke (3, 4), even if transplanted far from the infarct, and because this model provides a clearly defined pathological region with precise initiation, as proof of concept we used this paradigm to investigate whether products of inflammation, e.g., chemokines, might be good candidates for injury-initiated signals to which human NSCs (hNSCs) respond.
Materials and Methods
NSC Isolation and Culture. We used several NSC lines (n = 5), including bFGF-dependent human and mouse NSCs, isolated and maintained as described (8–11) (see Supporting Text, which is published as supporting information on the PNAS web site).
Migration Assays. The number of cells crossing a fibronectin-coated 8-μm pore membrane when confronted with varying concentrations of stromal cell-derived factor 1α (SDF-1α) in a Boyden chamber was interpreted to represent their migratory capacity; chain migration was observed by direct microscopy (both detailed in Supporting Text).
Induction of Hypoxic–Ischemic (HI) Cerebral Injury. As detailed in Supporting Text, HI was induced by the Vannucci method (4). For organotypic explants, adult C57BL6 mice were subjected to left middle cerebral artery occlusion as described (12) and the brains harvested after 5 days for migration assays under confocal microscopy (see Supporting Text); slice cultures from the right nonischemic hemisphere served as controls for the left ischemic ones.
hNSC Transplantation. hNSCs, prelabeled ex vivo with nondiffusible fluorescent cell tracker (CM-DiI, Cell Tracker, Molecular Probes) were transplanted 3 days after induction of HI into the infarcted hemisphere of each mouse brain or into the contralateral hemisphere, as described (4), and the brains processed 2, 7, and 10 weeks later under confocal microscopy, as detailed in Supporting Text.
Statistical Analysis. Statistical analysis was performed by using the paired Student t test. For migratory analysis, a test for repeated-measures ANOVA with Bonferroni correction for multiple comparisons was used. P < 0.05 was considered significant.
Results
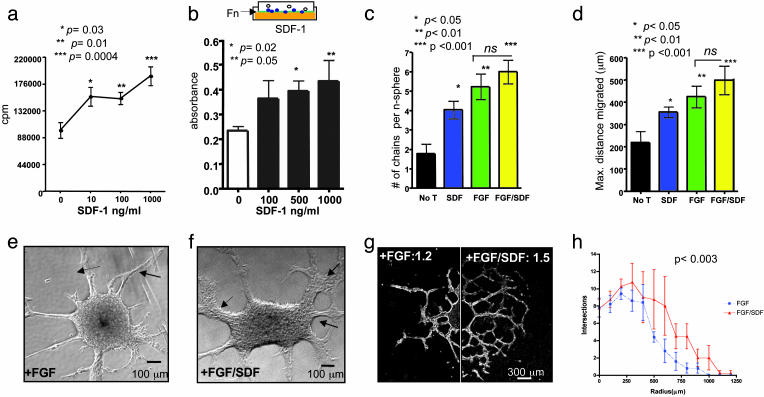
Proliferation of self-renewing multipotent hNSCs in bFGF (6, 8, 9) is enhanced after incubation with increasing doses of recombinant human SDF-1α (hSDF-1α) (Fig. 1a). Furthermore, hNSC transmigration through a fibronectin-coated Boyden chamber membrane (Fig. 1b) to a chemotactic gradient of hSDF-1α is dose-dependent. During development and in some CNS regions, NSCs normally undergo a unique form of tangential migration called chain migration (13, 14), in which neural cells slide and glide along each other, using each other for guidance [e.g., Fig. 6 a and b (arrows), which is published as supporting information on the PNAS web site]. The use of such a mechanism by migrating NSCs in response to injury might explain how pathological regions can be targeted and reached by NSCs even when they are not positioned along stereotypical migratory paths. To investigate a potential role for SDF-1α in promoting and/or directing chain migration, bFGF-perpetuated hNSC neurospheres (n = 125) were seeded onto a fibronectin substrate. In the absence of factors such as bFGF or leukemia-inhibiting factor, hNSCs formed few migratory chains after 6 days in culture (Fig. 6c). However, the addition of SDF-1α induced a significant increase in the number and density of chains of cells emanating from the neurospheres (Fig. 6 d and e). SDF-1α alone was sufficient to increase migratory parameters significantly (Fig. 1 c and d). The combination of SDF-1α and bFGF had minimal additive effect in distance and number of chains per neurosphere (P > 0.05, Fig. 1 c and d). However, this combination did increase the thickness of the migratory chains when compared with bFGF alone (Fig. 1 e and f). Furthermore, it enhanced the complexity of their branching, generating a fractal-like pattern of chain migration. We quantified branching complexity by measuring the fractal dimension (Df) (15, 16) of migrating neurospheres. The combination of SDF-1α and bFGF induced an increase in Df (Fig. 1g). With a second measure of branching complexity, the Sholl method (17), we similarly found that SDF-1α and bFGF were additive in increasing the complexity of radial chains emerging from neurospheres (Fig. 1h). Interestingly, a fractal pattern of bifurcation (15) was generated, a pattern resembling that in the rodent rostral migratory stream, the route by which neural progenitors migrate in chains to the olfactory bulb during normal development and neuronal turnover in adulthood (18) (Fig. 1g).
Fig. 1.
SDF-1α enhances proliferation and induces two forms of migration of hNSCs. (a) Proliferation assay showing significantly increased thymidine incorporation by hNSCs after increasing SDF-1α dosage (cultured in triplicate): 10 ng/ml, P = 0.03; 100 ng/ml, P = 0.01; 1,000 ng/ml, P = 0.0004 compared with control (by Student's t test.) (b Upper) Boyden chamber assay used for assaying migration. hNSCs are allowed to migrate into a fibronectin-coated membrane (green), immersed in medium (orange) containing SDF-1α at different concentrations. The number of cells that cross the membrane and stain with crystal violet (blue) reflects their migratory capacity. (b Lower) Quantification of hNSC migration as measured by optical density (absorbance) of crystal violet after 48 h. There is a significant increase in migration in response to SDF-1α: 500 ng/ml, P < 0.02; 1 μg/ml, P < 0.05, compared with control (by Student's t test). (c) Number of chains migrating from neurospheres. SDF-1α alone significantly increases this number compared with no treatment (No T); P < 0.05 ANOVA. (d) Mean maximal distance migrated per chain/neurosphere: SDF-1α alone had a significant effect compared with untreated (P < 0.05 ANOVA), as did FGF (P < 0.01) and FGF plus SDF-1α (P < 0.001). There was no significant difference between FGF and FGF plus SDF-1α.(e and f) Phase-contrast photomicrograph of neurosphere incubated in FGF (20 ng/ml) alone (e) compared with neurosphere in FGF (20 ng/ml) plus SDF-1α (100 ng/ml) (f), where there appears to be an increase in the number of migrating cells and the complexity, branching, and thickness of migratory chains (arrows in f). (g) Composite dark-field image of neurosphere in bFGF alone compared with one treated with bFGF plus SDF-1α. Such images were used to quantify the Df of chain migration. For bFGF-treated hNSCs, Df = 1.2; for bFGF plus SDF-1α, Df = 1.5 (Df values range from 1 to 2; values approaching 2 denote increasing complexity). The Df for hNSCs exposed to no additives is negligible. (h) Quantification of branching complexity comparing bFGF-exposed with bFGF plus SDF-1α-exposed hNSCs showing a significant increase in branching numbers (P < 0.003). (Branching for hNSCs exposed to no additives is negligible.)
SDF-1α is the only chemokine that has but one cognate receptor, namely CXC chemokine receptor 4 (CXCR4) (19). We identified the constitutive expression of cxcr4 mRNA in hNSCs by RNA protection assay (Fig. 2a, lanes 2 and 3) in two unrelated hNSC lines, which have displayed injury-directed migration in vivo (8, 9). Next, we demonstrated by FACS the expression of CXCR4 protein in the intracellular compartment (Fig. 2b) and on the surface (Fig. 2c) of undifferentiated hNSCs (8) (again assaying multiple unrelated hNSC lines for validation). Confocal microscopy of individual hNSCs confirmed in cultured hNSCs the typical pattern of CXCR4 (20) (Fig. 6 f and g). These findings were confirmed in human neurospheres double-labeled with a different clone of anti-CXCR4 and vimentin (Fig. 2d). Reinforcing the notion that developmental mechanisms might be recapitulated during injury-directed NSC migration, we determined that CXCR4 expression is, in fact, also detected in situ in nestin-expressing cells within the fetal mouse ventricular zone (VZ), among nestin-expressing NSCs isolated from the fetal murine VZ, and maintained in vitro, and in NSCs isolated and cultured from the adult subventricular zone (Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 2.
hNSCs express CXCR4, which activates intracellular molecular pathways upon SDF-1α stimulation. (a) Ribonuclease protection assay showing expression of CXCR4 mRNA in two hNSC lines. Lane 1, yeast RNA (control); lane 2, hNSC clone H6 (6, 8, 9); lane 3, hNSC clone CC-2599 (43). L32, housekeeping gene as control for equal loading. (b) FACS analysis of permeabilized cells revealing intracellular CXCR4 in the two above-mentioned hNSC lines. Black dotted histogram represents results from the isotype control antibody; the red histogram represents staining with a monoclonal antibody against human CXCR4. (c) Membrane expression of CXCR4 in three hNSC lines, H6, CC-2599, and HFB2050, compared with purified CD4+ lymphocytes serving as a positive control. (d) Confocal microscopic image of hNSCs dual-immunostained as neurospheres with antibodies to CXCR4 and vimentin. (e) Activation by SDF-1α of hNSC intracellular signaling pathways. hNSCs were exposed to SDF-1α (100 ng/ml) or to FGF plus EGF (20 ng/ml) followed by Western analysis for phosphorylation of p38MAPK, p90Rsk, c-Jun, and extracellular response kinase at the indicated time points. The phosphorylated form (p-) is shown (Upper) for each molecule. (f) The rapid kinetics of paxilin phosphorylation (p-Paxilin) with an increase after 2 and 5 min and a reduction thereafter.
Exposure of hNSCs to SDF-1α and the consequent induction of CXCR4-mediated signaling triggered a series of intracellular processes associated with fundamental aspects of survival, proliferation, and, importantly, migration [a process that itself involves multiple cooperative pathways and signals (11, 14, 18, 21)]. These steps, dissected in Fig. 2 e and f, include rapid and sustained phosphorylation of p38MAPK kinase [implicated in regulating cytokine-induced cell migration (22–24) (Fig. 2e)]; phosphorylation of the ribosomal S6 kinase (p90RSK) [implicated in phosphorylation of cytoskeletal molecules involved in neurite outgrowth (25, 26) (Fig. 2e)]; phosphorylation of c-Jun [a kinase involved in migratory responses (27) (Fig. 2e)]; rapid activation of extracellular response kinase (involved in proliferative responses), and rapid phosphorylation of paxilin [a scaffold molecule critical for migration (28) (Fig. 2f)].
Having established in vitro that SDF-1α activates molecular pathways responsible for hNSC migration, we next ascertained whether there was in vivo relevance by using a prototypical pathological condition, HI injury. After induction of unilateral HI in mouse brains (4), hNSCs were implanted at a distance from the lesion (in periventricular areas, including on the contralateral side) (Fig. 3 a–c). Although SDF-1α was expressed by meningeal cells on both the ischemic and normal side (29) (Fig. 3 d and e, m), there was a profound up-regulation within the injured parenchyma itself, which was populated by hNSCs that had migrated there from their distant implantation site (Fig. 3 b and e) [and often differentiated into neurons (Fig. 3 f and g)]. hNSCs homed to areas of increased SDF-1α expression in the injured cortex (Fig. 3h), where, often displaying a classic migratory profile (Fig. 3i), they intertwined intimately with SDF-1α-expressing cells (Fig. 8a, which is published as supporting information on the PNAS web site). We found a positive correlation between the expression of SDF-1α per area and the number of hNSCs present in the ischemic brain, the only source of which had to have been via migration (r = +0.6079, P < 0.0001) (Fig. 3 j and k). One of the sources of this increased SDF-1α expression within the injured regions was the large number of reactive astrocytes (coexpressing SDF-1α and glial fibrillary acidic protein) (Fig. 3 m–o). On the uninjured side, astrocytes (in a normal proportion) were nonreactive and did not express SDF-1α (Fig. 3l). Up-regulation of SDF-1α production within astrocytes was plausibly due to the inflammatory environment that characterizes ischemic injury, because treatment of astrocytes with IL-1β up-regulates their SDF-1α production (30). An additional source of SDF-1α may have been blood vessels (31) within the infarct, a region where endothelial cells also up-regulated SDF-1α expression (Fig. 8b). To confirm the role of SDF-1α in NSC migration, we began to dissect the process in vitro by culturing NSCs adjacent to organotypic cortical explants (n = 30) from ischemic (5 days postinfarct) and uninjured murine hemispheres. NSCs isolated from an adult murine subventricular zone and expressing emx2 (32), sox2 (33), and nestin (Fig. 7), when cocultured as neurospheres with ischemic cortical explants in coculture medium, elaborated extensive robust networks of processes containing migrating chains of nestin+ NSCs directed specifically toward and into the explant, compared with nonischemic explants (Fig. 4 a, c–e) [or to no explant in the presence of normal NSCs conditions where extensions were nondirected and multiarrayed (Figs. 6a and 7 t and u)]. The polarization and number of directed migratory chains were significantly increased when confronted with ischemic explants (80% of neurospheres) relative to nonischemic explants (only 12% of neurospheres) (Fig. 4 c and d). These chain-migrating nestin+ NSCs expressed CXCR4 as they made contact with the ischemic explant (Fig. 4b). Addition of a monoclonal blocking antibody against CXCR4 (34, 35) reduced to 14% the proportion of hNSCs migrating toward the ischemic explant (Fig. 4e) and reduced the number and thickness of bridges of migrating nestin+ chains compared with ischemic explants incubated with an isotype control (Fig. 4f).
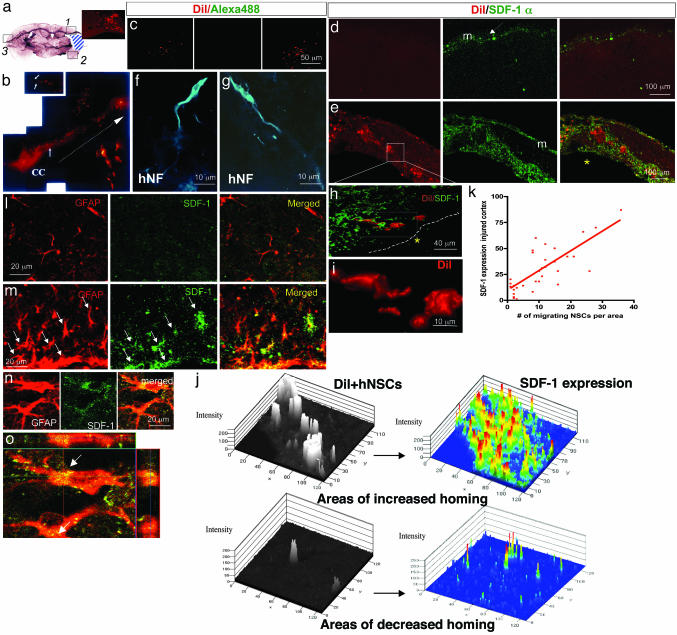
Fig. 3.
hNSCs migrate to areas of SDF-1α up-regulation after stroke. (a) Mouse brain (coronal view) subjected to unilateral stroke. The infarct, largely a necrotic cavity, is delineated by the blue area. Three areas per slide (boxes 1–3) were analyzed. Boxes 1 and 2 include the penumbra; box 3 is from the contralateral uninjured cortex. The hNSCs depicted here were prelabeled ex vivo with chloromethylbenzamido derivate of 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbacyanine perchlorate (DiI) before transplantation. (Inset) Red DiI+ hNSCs have homed to area 1. (b) DiI+ hNSCs (arrows), magnified (Inset), are seen migrating across the corpus callosum (CC) from the contralateral intact hemisphere (site of implantation) to the infarcted hemisphere. (c) DiI fluorescence is specific to hNSCs in ischemic brain (Left) and not seen at the Alexa Fluor 488 wavelength (Center) used for revealing SDF-1α immunoreactivity; merged (Right). (d) Normal contralateral side (box 3) showing absence of DiI+ hNSCs (Left) and only expected meningeal (m) staining of SDF-1α (green) (Center); merge (Right). (e) DiI+ hNSCs that have robustly homed to the penumbra (bottom), some of which have responded to this neurogenic niche by differentiating into neurons (identified by an anti-human-specific NF antibody, an independent marker for hNSC-derived cells) (f and g). SDF-1α immunoreactivity (green) is robust throughout the penumbra. Boxed area in e is viewed at higher power as a merged image in h where a confocal microscopic 3D reconstruction shows the hNSCs (red) intertwined intimately with the abundant SDF-1α-expressing cells (green). The boxed area in h is shown at higher power in i via the red channel capturing a residual classic elongated migratory profile of some DiI+ hNSCs. (j) High-resolution quantitative 2.5-dimensional imaging of hNSCs homing to SDF-1α-enriched areas. Topographical view of multiple areas (n = 30) measured by confocal profile intensity. hNSC values are in gray scale; maximal SDF-1α expression is represented in color. hNSCs colocalize with (home to) areas of high (Upper) but not low (Lower) SDF-1α.(k) Correlation of SDF-1α expression in injured cortex and number of migrating hNSCs (r = 0.679; P < 0.0001). (l upper) Contralateral normal parenchyma showing normal astrocytes (GFAP; red) with no SDF-1α (green) coimmunostaining. (l lower) Infarcted side showing both an increase in GFAP-immunoreactive cells (red) with thick processes (suggestive of reactive astrocytes) and an increase in SDF-1α+ (green) coimmunostaining. Merged images (yellow) suggest that the reactive astrocytes are coexpressing SDF-1α. Colocalization of GFAP (red) and SDF-1α (green) is confirmed by optical dissection and orthogonal reconstruction of the confocal image (o), showing intracellular localization of SDF-1α in two representative reactive astrocytes (arrows).
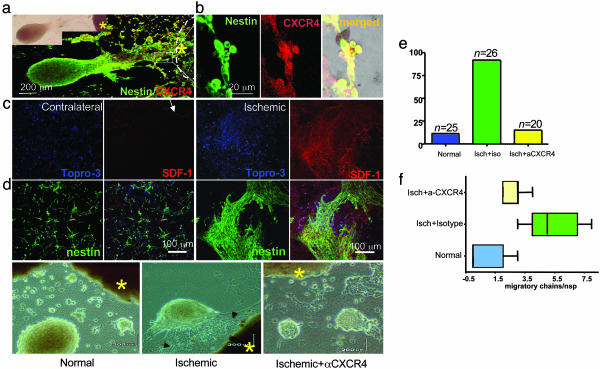
Fig. 4.
Migration in vitro of NSCs from subventricular zone toward explants from ischemic brain is mediated by SDF-1α–CXCR4 interaction. (a) Mouse NSCs (nestin+) that migrate chain-like toward ischemic brain explants express CXCR4. The phase image is shown under fluorescence microscopy below it, dual immunostained for nestin (green) and CXCR4 (red). (b) The area demarcated by the box in a, near the ischemic explant (asterisk), the border of which is indicated by the dotted line, is magnified where the nestin+ NSCs (green) are noted to coexpress CXCR4 (red); dual-immunoreactive cells seen as yellow in merged image. (c Left) Minimal chain migration of nestin+ cells to the contralateral noninjured explant correlating with an absence of SDF-1α in the explant, preserved only in meninges (arrow). (c Right) Robust migration of nestin+ NSCs toward injured explant with an increase in polarization and number of chains (see Figs. 6 and 7). (d) Neurospheres confronted with explants (asterisk) from normal control hemispheres show no migration (Left), neurospheres confronted at the same distance with explants from an ischemic hemisphere (asterisk) elaborate processes containing chains of migrating NSCs directed toward the explants (arrows) (Center); these behaviors are abrogated in neurospheres confronted with an ischemic explant (asterisk) but treated with a purified blocking antibody to CXCR4 (10 μg per explant) (Right). (e) Quantification of the percent of neurospheres with directed migration toward the explants. (f) Quantification of the formation of migratory chains toward the following explants: control, ischemic, or ischemic treated with anti-CXCR4 antibody. The latter condition reduces the number of migratory chains (P < 0.001).
Discussion
The expression of CXCR4 on hNSCs and an appropriate functional response when exposed to its only ligand, SDF-1α, not only help establish their role in injury-mediated homing but likely epitomize a paradigm wherein the many products of inflammation may collaborate to guide somatic stem cell behavior during perturbations of an organ. The SDF-1α–CXCR4 interaction during injury likely recapitulates a developmental role for this dyad in mammalian cerebrogenesis, as suggested by the CXCR4 expression of both nestin-expressing cells within the fetal ventricular zone (VZ) in situ and NSCs cultured from the embryonic VZ and adult subventricular zone (Fig. 7) (36, 37). CXCR4 presumably serves as a guidance molecule that is highly conserved evolutionarily among mammalian NSCs, including in humans.
Our data reinforce the pivotal role played by the SDF-1α/CXCR4 pathway and likely other inflammation-associated mechanisms [including, for example, microglia (F.-J.M. and E.Y.S., unpublished work)] in the migration of NSCs toward regions of brain injury and degeneration. Although known to be critical for hematopoietic homing to the bone marrow (35, 38), the role that such a nonneural mechanism may play in the CNS and that CNS-derived repair cells may be guided by nonneural cues have been underappreciated. Indeed, in a broader sense, inflammation may be viewed as playing more than its commonly recognized adverse role in the CNS and other organs (1, 10, 39) and as also providing stimuli that call in homeostasis-promoting cells. Astrocytes and endothelial cells up-regulate SDF-1α after injury (30, 31) and may work as catalysts and then as signposts for mobilizing and directing quiescent NSCs via ligand–receptor interactions. NSCs migrate to sources of SDF-1α in vivo and, in response to this chemokine, self-organize into directed migrating chains of increasing complexity. The dynamic of SDF-1α's interaction with CXCR4 appears to be at least one requirement for NSC migration toward injury (40).
Our data provide not only a working model for one mechanism underlying innate regenerative programs but also a unifying explanation as to why NSCs appear to behave so similarly when confronted with pathologies of disparate etiologies. The inflammatory response that accompanies many CNS disease processes, regardless of the underlying cause, appears to direct NSCs (including those of human derivation). Intriguingly, prior findings from our group suggest that once NSCs enter the injury site, they begin to produce yet-to-be-identified antiinflammatory molecules (4, 6, 41) [corroborated by others (42)]. The analogy, therefore, is similar to a firefighter who is called to a blaze by the flames but then douses them once he arrives. A better understanding of this complex dynamic may permit us to devise more effective repair strategies by orchestrating the judicious use of antiinflammatory agents that neutralize those aspects of inflammation that are inimical to progenitor well being (1, 10, 39) while enhancing and not negating those aspects that facilitate repair.
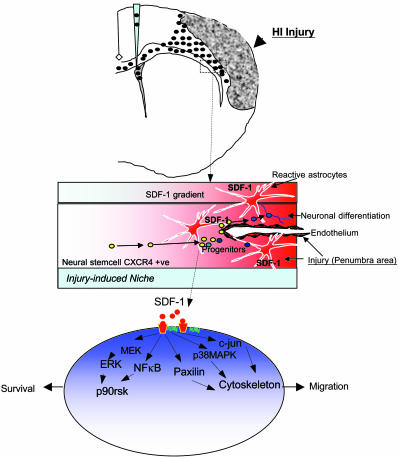
The following scenario might be envisioned (Fig. 5). As a result of injury, surviving or invading glial cells (including activated astrocytes and microglia), the first wave of responders to injury, may produce chemoattractants such as SDF-1α that may direct the migration of NSCs toward the ischemic core and penumbra. Compromised vasculature, too, contributes to the abundance of such chemokines within the infarcted parenchyma. NSCs of exogenous or endogenous origin, by virtue of their expression of chemokine receptors such as CXCR4, respond to SDF-1α and similar chemoattractants that trigger the activation and phosphorylation of scaffold and adapter molecules within the NSCs and direct their migration in a chain-like fashion (even along nonstereotypical migratory routes) toward the sources of the chemokine, allowing them to home to the injured area and to inhabit injury-induced stem cell niches (Fig. 5) harboring local transiently expressed potentially reparative signals. Such a strategy for prompting and directing regenerative processes may be shared by many stem cell systems.
Fig. 5.
Model of inflammation-directed homing of NSCs toward pathology (as modeled by SDF-1α–CXCR4 interaction). As a result of injury, surviving or invading glia, microglia, and endothelial cells, the first responders, may produce chemoattractants (e.g., SDF-1α) that direct NSCs toward the ischemic core or penumbra (shaded area). NSCs of exogenous or endogenous origin (arrows), by virtue of their expression of chemokine receptors (e.g., CXCR4), respond to the chemokines that trigger the activation and phosphorylation of scaffold and adapter molecules within the NSCs and direct NSC migration in a chain-like fashion toward the source of the chemokines, allowing the NSCs to home to the pathology, to produce antiinflammatory/antiscarring molecules, and to engage local transiently expressed injury-induced signals.
Supplementary Material
Acknowledgments
We thank M. Comabella and K. Mahklouf for help with the ribonuclease protection assay, M. Marconi for help with human stem cell cultures, S. Lizarraga for organotypical culture techniques, and B. Zhu and G. Martino for helpful discussions. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (to S.J.K.); the National Institute of Neurological Disorders and Stroke, the March of Dimes, Project ALS, Children's Neurobiological Solutions, and GMP Companies, Inc. (to E.Y.S.); the National Eye Institute (to R.L.S.); and the Stem Cell Research Center of the Korean Ministry for Science and Technology (to K.I.P.).
Author contributions: J.I., K.R., K.I.P., F.-J.M., M.N., Y.D.T., D.F., J.L., R.L.S., E.Y.S., and S.J.K. designed research; J.I., K.R., K.I.P., F.-J.M., M.N., Y.D.T., D.F., and J.L. performed research; J.I., K.I.P., F.-J.M., M.N., D.F., and J.L. contributed new reagents/analytic tools; J.I., K.R., K.I.P., F.-J.M., M.N., Y.D.T., D.F., J.L., R.L.S., C.A.W., E.Y.S., and S.J.K. analyzed data; and J.I., K.R., M.N., E.Y.S., and S.J.K. wrote the paper.
Abbreviations: NSC, neural stem cell; hNSC, human NSC; SDF-1α, stromal cell-derived factor 1α; CXCR4, CXC chemokine receptor 4; HI, hypoxic–ischemic; Df, fractal dimension; DiI, 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbacyanine perchlorate.
Note Added in Proof. While this paper was under review, Kelly et al. (45) reported that hNSCs migrating to stroke lesions express CXCR4.
References
- 1.Imitola, J., Snyder, E. Y. & Khoury, S. J. (2003) Physiol. Genomics 14, 171–197. [DOI] [PubMed] [Google Scholar]
- 2.Imitola, J., Park, K. I., Teng, Y. D., Nisim, S., Lachyankar, M., Ourednik, J., Mueller, F. J., Yiou, R., Atala, A., Sidman, R. L., et al. (2004) Philos. Trans. R. Soc. London B 359, 823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park, K. I., Ourednik, J., Ourednik, V., Taylor, R. M., Aboody, K. S., Auguste, K. I., Lachyankar, M. B., Redmond, D. E. & Snyder, E. Y. (2002) Gene Ther. 9, 613–624. [DOI] [PubMed] [Google Scholar]
- 4.Park, K. I., Teng, Y. D. & Snyder, E. Y. (2002) Nat. Biotechnol. 20, 1111–1117. [DOI] [PubMed] [Google Scholar]
- 5.Snyder, E. Y., Yoon, C., Flax, J. D. & Macklis, J. D. (1997) Proc. Natl. Acad. Sci. USA 94, 11663–11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aboody, K. S., Brown, A., Rainov, N. G., Bower, K. A., Liu, S., Yang, W., Small, J. E., Herrlinger, U., Ourednik, V., Black, P. M., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaBarge, M. A. & Blau, H. M. (2002) Cell 111, 589–601. [DOI] [PubMed] [Google Scholar]
- 8.Flax, J. D., Aurora, S., Yang, C., Simonin, C., Wills, A. M., Billinghurst, L. L., Jendoubi, M., Sidman, R. L., Wolfe, J. H., Kim, S. U. & Snyder, E. Y. (1998) Nat. Biotechnol. 16, 1033–1039. [DOI] [PubMed] [Google Scholar]
- 9.Ourednik, V., Ourednik, J., Flax, J. D., Zawada, W. M., Hutt, C., Yang, C., Park, K. I., Kim, S. U., Sidman, R. L., Freed, C. R. & Snyder, E. Y. (2001) Science 293, 1820–1824. [DOI] [PubMed] [Google Scholar]
- 10.Imitola, J., Comabella, M., Chandraker, A. K., Dangond, F., Sayegh, M. H., Snyder, E. Y. & Khoury, S. J. (2004) Am. J. Pathol. 164, 1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheen, V. L., Ganesh, V. S., Topcu, M., Sebire, G., Bodell, A., Hill, R. S., Grant, P. E., Shugart, Y. Y., Imitola, J., Khoury, S. J., Guerrini, R. & Walsh, C. A. (2004) Nat. Genet. 36, 69–76. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel, D., Huang, Z., Maron, R., Koldzic, D. N., Hancock, W. W., Moskowitz, M. A. & Weiner, H. L. (2003) J. Immunol. 171, 6549–6555. [DOI] [PubMed] [Google Scholar]
- 13.Lois, C., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (1996) Science 271, 978–981. [DOI] [PubMed] [Google Scholar]
- 14.Jacques, T. S., Relvas, J. B., Nishimura, S., Pytela, R., Edwards, G. M., Streuli, C. H. & ffrench-Constant, C. (1998) Development (Cambridge, U.K.) 125, 3167–3177. [DOI] [PubMed] [Google Scholar]
- 15.Haskell, J. P., Ritchie, M. E. & Olff, H. (2002) Nature 418, 527–530. [DOI] [PubMed] [Google Scholar]
- 16.Herman, P., Kocsis, L. & Eke, A. (2001) J. Cereb. Blood Flow Metab. 21, 741–753. [DOI] [PubMed] [Google Scholar]
- 17.Sholl, D. A. (1955) J. Anat. 89, 33–46. [PMC free article] [PubMed] [Google Scholar]
- 18.Conover, J. C., Doetsch, F., Garcia-Verdugo, J. M., Gale, N. W., Yancopoulos, G. D. & Alvarez-Buylla, A. (2000) Nat. Neurosci. 3, 1091–1097. [DOI] [PubMed] [Google Scholar]
- 19.Lu, M., Grove, E. A. & Miller, R. J. (2002) Proc. Natl. Acad. Sci. USA 99, 7090–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bezzi, P., Domercq, M., Brambilla, L., Galli, R., Schols, D., De Clercq, E., Vescovi, A., Bagetta, G., Kollias, G., Meldolesi, J. & Volterra, A. (2001) Nat. Neurosci. 4, 702–710. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson, J. G., Allen, K. M., Fox, J. W., Lamperti, E. D., Berkovic, S., Scheffer, I., Cooper, E. C., Dobyns, W. B., Minnerath, S. R., Ross, M. E. & Walsh, C. A. (1998) Cell 92, 63–72. [DOI] [PubMed] [Google Scholar]
- 22.Sharma, G. D., He, J. & Bazan, H. E. (2003) J. Biol. Chem. 278, 21989–21997. [DOI] [PubMed] [Google Scholar]
- 23.Dechert, M. A., Holder, J. M. & Gerthoffer, W. T. (2001) Am. J. Physiol. 281, C123–C132. [DOI] [PubMed] [Google Scholar]
- 24.Cara, D. C., Kaur, J., Forster, M., McCafferty, D. M. & Kubes, P. (2001) J. Immunol. 167, 6552–6558. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki, Y., Rahman, M. & Mitsuya, H. (2001) J. Immunol. 167, 3064–3073. [DOI] [PubMed] [Google Scholar]
- 26.Wong, E. V., Schaefer, A. W., Landreth, G. & Lemmon, V. (1996) J. Biol. Chem. 271, 18217–18223. [DOI] [PubMed] [Google Scholar]
- 27.Li, G., Gustafson-Brown, C., Hanks, S. K., Nason, K., Arbeit, J. M., Pogliano, K., Wisdom, R. M. & Johnson, R. S. (2003) Dev. Cell 4, 865–877. [DOI] [PubMed] [Google Scholar]
- 28.Huang, C., Rajfur, Z., Borchers, C., Schaller, M. D. & Jacobson, K. (2003) Nature 424, 219–223. [DOI] [PubMed] [Google Scholar]
- 29.Zhu, Y., Yu, T., Zhang, X. C., Nagasawa, T., Wu, J. Y. & Rao, Y. (2002) Nat. Neurosci. 5, 719–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou, Y., Larsen, P. H., Hao, C. & Yong, V. W. (2002) J. Biol. Chem. 277, 49481–49487. [DOI] [PubMed] [Google Scholar]
- 31.Rubin, J. B., Kung, A. L., Klein, R. S., Chan, J. A., Sun, Y., Schmidt, K., Kieran, M. W., Luster, A. D. & Segal, R. A. (2003) Proc. Natl. Acad. Sci. USA 100, 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli, R., Fiocco, R., De Filippis, L., Muzio, L., Gritti, A., Mercurio, S., Broccoli, V., Pellegrini, M., Mallamaci, A. & Vescovi, A. L. (2002) Development (Cambridge, U.K.) 129, 1633–1644. [DOI] [PubMed] [Google Scholar]
- 33.Graham, V., Khudyakov, J., Ellis, P. & Pevny, L. (2003) Neuron 39, 749–765. [DOI] [PubMed] [Google Scholar]
- 34.Avecilla, S. T., Hattori, K., Heissig, B., Tejada, R., Liao, F., Shido, K., Jin, D. K., Dias, S., Zhang, F., Hartman, T. E., et al. (2004) Nat. Med. 10, 64–71. [DOI] [PubMed] [Google Scholar]
- 35.Ponomaryov, T., Peled, A., Petit, I., Taichman, R. S., Habler, L., Sandbank, J., Arenzana-Seisdedos, F., Magerus, A., Caruz, A., Fujii, N., et al. (2000) J. Clin. Invest. 106, 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng, H., Huang, Y., Rose, J., Erichsen, D., Herek, S., Fujii, N., Tamamura, H. & Zheng, J. (2004) J. Neurosci. Res. 76, 35–50. [DOI] [PubMed] [Google Scholar]
- 37.Tran, P. B., Ren, D., Veldhouse, T. J. & Miller, R. J. (2004) J. Neurosci. Res. 76, 20–34. [DOI] [PubMed] [Google Scholar]
- 38.Peled, A., Petit, I., Kollet, O., Magid, M., Ponomaryov, T., Byk, T., Nagler, A., Ben-Hur, H., Many, A., Shultz, L., et al. (1999) Science 283, 845–848. [DOI] [PubMed] [Google Scholar]
- 39.Monje, M. L., Toda, H. & Palmer, T. D. (2003) Science 302, 1760–1765. [DOI] [PubMed] [Google Scholar]
- 40.Sun, L., Lee, J. & Fine, H. A. (2004) J. Clin. Invest. 113, 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teng, Y. D., Lavik, E. B., Qu, X., Park, K. I., Ourednik, J., Zurakowski, D., Langer, R. & Snyder, E. Y. (2002) Proc. Natl. Acad. Sci. USA 99, 3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pluchino, S., Quattrini, A., Brambilla, E., Gritti, A., Salani, G., Dina, G., Galli, R., Del Carro, U., Amadio, S., Bergami, A., et al. (2003) Nature 422, 688–694. [DOI] [PubMed] [Google Scholar]
- 43.Svendsen, C. N., Caldwell, M. A., Shen, J., ter Borg, M. G., Rosser, A. E., Tyers, P., Karmiol, S. & Dunnett, S. B. (1997) Exp. Neurol. 148, 135–146. [DOI] [PubMed] [Google Scholar]
- 44.Lapidot, T. (2001) Ann. N.Y. Acad. Sci. 938, 83–95. [DOI] [PubMed] [Google Scholar]
- 45.Kelly, S., Bliss, T. M., Shah, A. K., Sun, G. H., Ma, M., Foo, W. C., Masel, J., Yenari, M. A., Weissman, I. L., Uchida, N., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 11839–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.