Abstract
Objective
We tested mediation of birth weight and ADHD symptoms by multiple biologically plausible neurocognitive functions and evaluated familiality of observed indirect effects.
Method
647 youth from 284 multiplex families with ADHD completed the Arithmetic, Digit Span, Vocabulary, and Block Design subtests of the Wechsler Intelligence Scale for Children (WISC). Multiple mediation tested WISC subtests as mediators of birth weight and multi-informant ADHD symptoms. Familiality of indirect effects was estimated via moderated mediation comparing conditional indirect effects across siblings concordant and discordant for ADHD.
Results
Controlling for IQ and demographic factors, Arithmetic uniquely mediated birth weight and ADHD symptoms. Conditional indirect effects through Arithmetic did not differ across ADHD concordant and discordant siblings.
Conclusion
These cross-sectional findings support previous prospective longitudinal research implicating Arithmetic (i.e., fluid reasoning) as a preliminary causal mediator of birth weight and ADHD symptoms, and suggest that this pathway is independent of genetic influences on ADHD.
Keywords: ADHD, birth weight, neurocognitive functioning, multiple mediation, familiality
Meta-analytic and prospective longitudinal evidence similarly suggest that birth weight is reliably associated with individual differences in ADHD (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009; Bhutta, Cleves, Casey, Cradock, & Anand, 2002; Halmøy, Klungsøyr, Skjærven, & Haavik, 2012; Martel, Lucia, Nigg, & Breslau, 2007; Morgan, Loo, & Lee, 2016; Nigg & Breslau, 2007). Moreover, there is replicated evidence that birth weight predicts ADHD symptoms in co-twin control designs, providing quasi-experimental evidence for a causal relation that is independent of environmental and genetic confounds (Groen-Blokhuis, Middeldorp, van Beijsterveldt, & Boomsma, 2011; Pettersson et al., 2015). Thus, birth weight is unlikely to predict ADHD symptoms due to shared variance with other correlates of poor fetal development (e.g., gestational age, teratogen exposure) and is instead a preliminary independent causal factor for the pathogenesis of ADHD symptoms.
If birth weight is a causal predictor of ADHD symptoms, elucidating its mechanisms of influence is necessary to develop early interventions that prevent the onset of symptoms (Sonuga-Barke & Halperin, 2010); surprisingly few studies, however, have examined mediators of this association. To date, predictions of ADHD symptoms from birth weight were statistically mediated by separable neurocognitive functions, including sensorimotor and visuospatial domains in young children (Hatch, Healey, & Halperin, 2014; Martel et al., 2007) and response variability in youth aged 6 to 17 years (Wiggs, Elmore, Nigg, & Nikolas, 2016). To our knowledge, however, only one study evaluated temporally ordered mediators of birth weight and ADHD symptoms, which is necessary to infer causal mediation (Kraemer, Stice, Kazdin, Offord, & Kupfer, 2001). Specifically, baseline scores on the Arithmetic subtest of the Wechsler Intelligence Scale for Children (WISC) at ages 5 to 10 uniquely mediated the association of birth weight with multi-method/informant ADHD symptoms at a 2-year follow-up (ages 7-13), controlling for demographic factors, co-occurring psychopathology, and baseline ADHD symptoms (Morgan et al., 2016). Indirect effects of other WISC subtests (e.g., Digit Span, Vocabulary) were not significant. Although these longitudinal findings implicated fluid reasoning, the primary construct reflected by Arithmetic (Keith, Fine, Taub, Reynolds, & Kranzler, 2006; Weiss, Keith, Zhu, & Chen, 2013), as a potential causal mediator, the results require replication. However, the four extant studies on mediators of birth weight and ADHD have evaluated different domains of neurocognitive functioning, precluding direct comparisons across samples. The current study addresses this important gap directly.
Given the need to prosecute indirect effects from birth weight to ADHD symptoms, the present study evaluated higher-order neurocognitive functions as mediators (i.e., performance on WISC Arithmetic, Digit Span, Vocabulary, and Block Design). Although Arithmetic is sensitive to multiple cognitive domains (e.g., working memory, quantitative reasoning), factor analyses suggest that it primarily assesses fluid reasoning (Keith et al., 2006; Weiss et al., 2013). Additionally, Digit Span measures short-term memory and verbal working memory, Vocabulary primarily assesses verbal comprehension (but may also involve crystallized knowledge), and Block Design reflects perceptual reasoning (Keith et al., 2006; Wechsler, 1991; Weiss et al., 2013). We specifically prioritized Arithmetic, Digit Span, Vocabulary, and Block Design based on their (a) previous evaluation as temporally ordered mediators of birth weight and ADHD symptoms within the context of a prospective longitudinal design (with the exception of Block Design), and (b) biological plausibility as causal mediators. That is, all four proposed WISC subtests, and their primary underlying constructs, are reliably correlated with birth weight (Aarnoudse-Moens et al., 2009; Bhutta et al., 2002; Hutchinson, De Luca, Doyle, Roberts, & Anderson, 2013; Lahat, Van Lieshout, Saigal, Boyle, & Schmidt, 2014; Skranes et al., 2013) and with ADHD (Doyle, Biederman, Seidman, Reske-Nielsen, & Faraone, 2005; Martin, Hamshere, Stergiakouli, O'Donovan, & Thapar, 2015; Nigg, 2006; Tamm & Juranek, 2012; Willcutt et al., 2010; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Moreover, the proposed WISC mediators are associated with neurodevelopmental impairments (e.g., reduced cortical surface area, thickness, volume) that are secondary to low birth weight (Martinussen et al., 2005; Skranes et al., 2013; Walhovd et al., 2012) and central to ADHD etiology (Narr et al., 2009; Shaw et al., 2012). For example, young adults with low birth weight exhibited reduced cortical surface area that correlated with a composite score from Arithmetic and Digit Span specifically in regions where reduced surface area was observed in youth with ADHD (e.g., superior frontal and medial temporal gyri; Shaw et al., 2012; Skranes et al., 2013). Similar results were identified with a composite score from Vocabulary and Block Design (Martinussen et al., 2005). Thus, these four neurocognitive factors are biologically plausible mediators of birth weight and ADHD symptoms.
The present study extends the existing literature on mediators of birth weight and ADHD in several ways. First, this is a relatively large sample (n = 647), consisting of affected and unaffected siblings from multiplex families with ADHD. Although a previous study identified significant indirect effects through Arithmetic, but not Digit Span and Vocabulary, the latter tests may have been underpowered given the modest sample size (n = 222; Morgan et al., 2016). Second, previous studies on neurocognitive mediators of birth weight and ADHD symptoms did not control for IQ. Because g loads substantially across all four hypothesized mediators (.55-.79), and particularly Arithmetic (Keith et al., 2006), control of IQ is necessary to specify indirect effects of birth weight on ADHD symptoms. Third, beyond prosecution of the hypothesized mediators, the present sample affords a unique opportunity to consider familiality of mediating pathways from birth weight to ADHD symptoms. Birth weight, reasoning/executive function (EF) domains (e.g., fluid reasoning, working memory), and ADHD symptoms are substantially heritable. Fetal and maternal genetic factors accounted for 31% and 22% of the variance in birth weight, respectively (Lunde, Melve, Gjessing, Skjærven, & Irgens, 2007), and heritability estimates for ADHD range from .70 to .95 (Hawi et al., 2015; Thapar, Cooper, Eyre, & Langley, 2013). In addition, genome-wide complex trait analysis highlights a strong additive influence of common single nucleotide polymorphisms on IQ, reasoning, and EF (Loo et al., 2012; Robinson et al., 2015), and polygenic ADHD risk scores have predicted IQ and working memory (Martin et al., 2015). Although co-twin control studies suggest that the total effect of birth weight on ADHD symptoms is independent of genetic confounds (Groen-Blokhuis et al., 2011; Pettersson et al., 2015), no study has directly evaluated potential genetic confounding of the corresponding indirect effects (although Wiggs et al. controlled for parental ADHD in indirect effects from birth weight to offspring ADHD symptoms). Unlike tests of the total effect, tests of indirect effects are susceptible to bias from unmeasured confounders of mediator-outcome associations (Loeys, Moerkerke, & Vansteelandt, 2015). Thus, genetic factors common to neurocognitive functioning and ADHD (e.g., Martin et al., 2015) may confound indirect effects from birth weight to ADHD symptoms. Crucially, if ADHD and the hypothesized indirect effects are genetically independent, indirect effects will be similar for ADHD concordant siblings and discordant siblings (Schachar et al., 2005; Tsuang, Faraone, & Lyons, 1993). Thus, moderated mediation analysis comparing conditional indirect effects of birth weight on ADHD symptoms across probands, affected siblings, and unaffected siblings is a critical preliminary test of whether effects are independent of or confounded by genetic influences on ADHD. This distinction has key clinical implications, given that environmentally based pathologies may be more amenable to prevention and intervention compared to genetically driven disorders (Moffitt, Caspi, & Rutter, 2006).
To review, although birth weight may be a causal predictor of ADHD symptoms, the mechanisms underlying this prediction are largely unknown. We examined biologically plausible neurocognitive mediators in a sample of affected and unaffected siblings from multiplex families with ADHD. To expand upon prior mediational investigations of birth weight and ADHD symptoms, the present study had two aims: (a) to simultaneously test WISC Arithmetic, Digit Span, Vocabulary, and Block Design as statistical mediators of birth weight and ADHD symptoms in a multiple mediation framework, using stringent control of IQ and relevant demographic factors; and (b) to investigate familiality of observed indirect effects by comparing conditional indirect effects across siblings concordant and discordant for ADHD.
Method
Participants
Participants were 647 youth aged 5 to 19 years (M = 10.47, SD = 3.53 years; 40.96% female) from 284 families who were assessed within a larger genetic study of multiplex families with ADHD. Complete demographic data and descriptive statistics are presented in Table 1. Families were recruited from a large metropolitan city in California via referrals from local psychiatry, pediatric, and community outlets (see Smalley et al., 2000, for additional details regarding recruitment). At least two siblings from each family met criteria for ADHD, with the oldest ADHD youth designated as the proband; unaffected siblings were also included in the sample. ADHD status required a positive diagnosis on the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997), a semi-structured interview with the parent and youth keyed to Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association, 1994) criteria. For a small number of families, data were not available for a second affected sibling, such that the sample consisted of ADHD probands (n = 284), affected siblings (n = 255), and unaffected siblings (n = 108). Participants were required to be fluent in English and have biological parents available to participate in the study. Exclusion criteria consisted of an IQ < 70 or a diagnosis of schizophrenia, autism spectrum disorder, or a known ADHD-linked genetic condition (e.g., tuberous sclerosis, fragile X syndrome, generalized resistance to thyroid hormone).
Table 1.
Sample Demographics and Descriptive Statistics.
| % of sample | |
|---|---|
| Sex (female) | 40.96 |
| Ethnicity | |
| Caucasian | 71.52 |
| African American/Black | 4.80 |
| Hispanic/Latino | 8.51 |
| Asian | 2.17 |
| Mixed | 10.22 |
| Other | 2.79 |
| ADHD diagnosis | 83.44 |
| ODD diagnosis | 40.19 |
| CD diagnosis | 1.86 |
| M (SD), range | |
| Age, years | 10.47 (3.53), 5-19 |
| SES | 2.63 (0.91), 1-5 |
| Birth weight, ounces | 119.55 (20.41), 26-176 |
| WISC Arithmetic | 10.94 (3.42), 1-19 |
| WISC Digit Span | 10.31 (2.94), 3-19 |
| WISC Vocabulary | 11.44 (3.18), 1-19 |
| WISC Block Design | 11.07 (3.42), 1-19 |
| Full Scale IQ | 108.87 (15.65), 71-152 |
| Total ADHD symptoms | 11.17 (5.00), 0-18 |
Note. ODD = oppositional defiant disorder; CD = conduct disorder; SES = socioeconomic status assessed with the Hollingshead Scale on an ordinal scale from 1 = highest to 5 = lowest; WISC = Wechsler Intelligence Scale for Children.
Procedures
All study procedures were approved by the Institutional Review Board. After receiving verbal and written explanations of study requirements, parents and youth provided written informed consent/assent. The K-SADS-PL interview was administered to parents (95% mothers), and then separately to youth (if ≥ 8 years of age). In addition, mothers reported birth weight data, youth completed a neurocognitive assessment, and rating scales were mailed to teachers. All youth completed the neurocognitive assessment free of stimulant medication for at least 24 hours. Informants were asked to assign ratings according to youth unmedicated behavior, if possible. All assessments were conducted by intensively trained clinical psychologists or master's degree-level research assistants. “Best estimate” diagnoses were determined by senior clinicians after individual review of diagnostic data. Multi-informant ADHD symptom counts were generated across parent and youth K-SADS-PL ratings for each symptom; teacher ratings were used to supplement the interview data to achieve “best estimate” symptom counts using all available data. Interrater reliability among senior clinicians is reflected by a kappa of 1.0 for ADHD diagnoses and a mean weighted kappa of .84 across all diagnoses with > 5% occurrence in the sample. See Smalley et al. (2000) for additional details regarding assessment procedures and reliability.
Measures
Birth weight
Mothers retrospectively reported youth birth weights in pounds and ounces, which were converted to ounces for all analyses (M = 119.55, SD = 20.41, range = 26-176). Maternal recall of offspring birth weight is highly correlated with medical record data into offspring adulthood (e.g., intraclass correlation [ICC] = .99 in Yawn, Suman, & Jacobsen, 1998; also, see Buka, Goldstein, Spartos, & Tsuang, 2004; Jaspers, de Meer, Verhulst, Ormel, & Reijneveld, 2010; O'Sullivan, Pearce, & Parker, 2000; Rice et al., 2007; Walton et al., 2000).
Neurocognitive functioning
Neurocognitive functioning was assessed using the Arithmetic, Digit Span, Vocabulary, and Block Design subtests of the WISC-III (Wechsler, 1991), which demonstrates excellent psychometric properties (Wechsler, 1991). The primary constructs assessed by these tests are fluid reasoning (Arithmetic), short-term memory and verbal working memory (Digit Span), verbal comprehension (Vocabulary), and perceptual reasoning (Block Design; Keith et al., 2006; Wechsler, 1991; Weiss et al., 2013), although they may also tap other domains of functioning. We used scaled scores for each subtest. Full Scale IQ was also derived from the WISC to be used as a covariate in analyses.
ADHD
As described above, ADHD diagnostic status and “best estimate” symptom counts were generated from parent and youth ratings on the K-SADS-PL (Kaufman et al., 1997) and informed by teacher ratings on the Teacher Report Form (TRF; Achenbach & Rescorla, 2001). The TRF is a normed 113-item rating scale yielding eight narrowband syndrome scales, including an Attention Problems Scale with both inattention and hyperactivity/impulsivity items. Both the K-SADS-PL and TRF have been extensively validated and demonstrate excellent psychometric properties (Achenbach & Rescorla, 2001; Kaufman et al., 1997).
Statistical Analysis
Missing data
Approximately 20% of youth were missing data on at least one key study variable (e.g., birth weight). Thus, we used full information maximum likelihood (FIML) estimation to maximize sample size for all analyses described below. FIML optimally remediates missing data when the amount of missingness is up to 50% and data are missing at random or missing completely at random (MCAR; Schlomer, Bauman, & Card, 2010). Evaluation of missing data patterns via Little's MCAR Test (Little, 1988) indicated that data were indeed MCAR in the present sample, χ2(176) = 190.60, p = .21.
Multiple mediation
ICCs indicated substantial between-family variation on the key study variables: birth weight (ICC = .47), Arithmetic (ICC = .33), Digit Span (ICC = .24), Vocabulary (ICC = .38), Block Design (ICC = .28), and ADHD symptoms (ICC = .04). Thus, we used multilevel structural equation modeling (MSEM; Preacher, Zyphur, & Zhang, 2010) in Mplus 7.0 (Muthén & Muthén, 1998-2015). Unlike traditional multilevel modeling approaches to mediation that conflate within- and between-level indirect effects (i.e., produce a single mean slope that combines the within- and between-level coefficients), MSEM separates within- and between-level effects into their orthogonal components and calculates separate coefficients for each level (Preacher et al., 2010). This distinction is nontrivial given that the former approach often produces biased estimates (Preacher et al., 2010). Moreover, in simulation studies, MSEM was superior to conflated and unconflated multilevel modeling-based mediation with respect to bias, power, and efficiency (Preacher, Zhang, & Zyphur, 2011). Thus, to evaluate Arithmetic, Digit Span, Vocabulary, and Block Design as statistical mediators of birth weight and ADHD symptoms, we constructed an MSEM path analysis that simultaneously tested direct effects from birth weight to all mediators and ADHD symptoms, and from all mediators to ADHD symptoms, controlling for age, sex, race-ethnicity, socioeconomic status (SES), and IQ. Given that this analysis stringently controlled for IQ and intercorrelations among the WISC mediators (bivariate correlations ranged from r = .17 to .46, p < .001 for all tests), demographic covariates unrelated to ADHD symptoms were dropped from the analysis to preserve power. Because bootstrapped confidence intervals (CIs) cannot be computed with multilevel data, 95% CIs for the total and specific indirect effects of the mediators were calculated using 20,000 Monte Carlo simulations (Preacher & Selig, 2012; statistical significance is assumed when the interval excludes zero). Monte Carlo CIs for indirect effects are superior to other methods that are compatible with multilevel data (e.g., delta method) with respect to power, Type I error, and robustness to non-normal data (Mackinnon, Lockwood, & Williams, 2004; Preacher & Selig, 2012). Effect sizes were calculated for significant indirect effects using the completely standardized indirect effect (Preacher & Kelley, 2011), which can be interpreted on a scale of .01 = small, .09 = medium, and .25 = large. Tests of observed indirect effects were repeated but with mediator and outcome reversed to improve directional inferences derived from the cross-sectional data.
Moderated mediation
We also evaluated moderation of observed indirect effects by proband, affected sibling, or unaffected sibling status to estimate familiality. Specifically, we calculated conditional indirect effects (i.e., an indirect effect conditioned on a particular value of the moderator) for probands (n = 284), affected siblings (n = 255), and unaffected siblings (n = 108), and calculated post hoc differences between these effects (Hayes, 2015). Moderated mediation calculations were based on addition of two dummy codes for the sibling status variable as well as two dummy codes for a sibling status × birth weight interaction term to the full multiple mediation model described above, such that conditional indirect effects were identically adjusted for covariates and the other WISC mediators.
Results
WISC Subtests as Mediators of Birth Weight and ADHD Symptoms
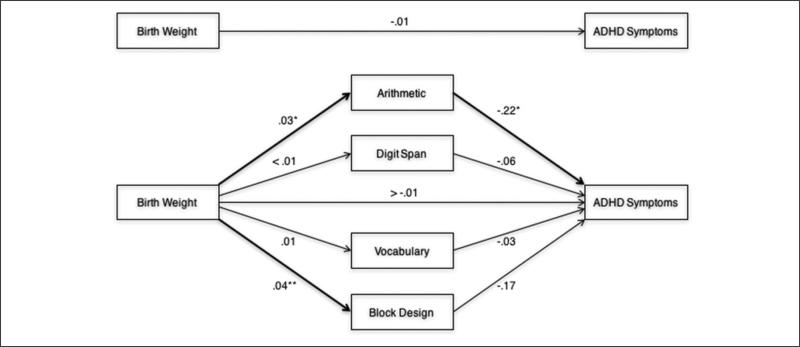
We first evaluated multiple mediation of birth weight and ADHD symptoms by Arithmetic, Digit Span, Vocabulary, and Block Design simultaneously, controlling for age, sex, and IQ (Figure 1). Race-ethnicity and SES were not controlled given they were unrelated to ADHD symptoms (p > .17 for each). At the within level (i.e., individual youth within families), birth weight was positively associated with Arithmetic (B = .03, SE = .01, p = .01) and Block Design (B = .04, SE = .01, p < .001), but unrelated to Digit Span (B < .01, SE = .01, p = .77) and Vocabulary (B = .01, SE = .01, p = .53). In turn, Arithmetic was inversely associated with ADHD symptoms (B = −.22, SE = .10, p = .02), but Digit Span (B = −.06, SE = .10, p = .53), Vocabulary (B = −.03, SE = .14, p = .84), and Block Design were not (B = −.17, SE = .10, p = .12). Neither the total effect of birth weight on ADHD symptoms nor its corresponding direct effect (i.e., controlling for the mediators) was significant (respectively, B = −.01, SE = .02, p = .45; B > −.01, SE = .02, p = .98). However, the within-level total indirect effect of birth weight on ADHD symptoms through the mediators (i.e., the difference between the total effect and direct effect) was significant, such that Arithmetic mediated the association of birth weight with ADHD symptoms (Table 2). Specific indirect effects of Digit Span, Vocabulary, and Block Design were not significant (Table 2). The effect sizes for the total indirect effect and the specific indirect effect of Arithmetic were −.05 and −.03, respectively, indicating small to medium effects. When the observed indirect effect through Arithmetic was tested with mediator and outcome reversed, there was no significant indirect effect of birth weight on Arithmetic through ADHD symptoms (B = −.001, SE = .001, 95% CI = [−.004, .002]). Given that data were cross-sectional, the absence of a reverse indirect effect additionally supports Arithmetic as a mediating pathway. No between-level direct or indirect effects among birth weight, mediators, and ADHD symptoms were observed (results available upon request), suggesting that mediation by Arithmetic was present across individuals (i.e., youth within families), but not across families.
Figure 1.
Multiple mediation of birth weight and ADHD symptoms by Wechsler Intelligence Scale for Children neurocognitive functions, controlling for age, sex, and IQ.
*p < .05. **p < .001.
Table 2.
Indirect Effects of Birth Weight on ADHD Symptoms Through the WISC Subtests.
| 95% Monte Carlo CI |
||||
|---|---|---|---|---|
| Point Est. | SE | Lower | Upper | |
| Total | −.013 | .006 | −.028 | −.002 |
| Arithmetic | −.006 | .003 | −.015 | −.002 |
| Digit Span | >−.001 | .001 | −.003 | .002 |
| Vocabulary | >−.001 | .001 | −.005 | .004 |
| Block Design | −.007 | .005 | −.017 | .002 |
Note. Boldface indicates significant mediation; WISC = Wechsler Intelligence Scale for Children; Point est. = point estimate of the indirect effect; CI = confidence interval.
Moderation of the Indirect Effect Through Arithmetic by Sibling Status
We additionally evaluated moderation of the indirect effect of birth weight on ADHD symptoms through Arithmetic by sibling status to estimate potential genetic influences on this mediating pathway. Conditional indirect effects for each sibling group were significant: probands (B = −.009, SE = .004, p = .03), affected siblings (B = −.011, SE = .005, p = .02), and unaffected siblings (B = −.014, SE = .005, p = .01). Post hoc tests of differences among the conditional indirect effects revealed that the indirect effect through Arithmetic did not differ significantly between probands and affected siblings (B = −.001, SE = .002, p = .39) or between probands and unaffected siblings (B = −.004, SE = .002, p = .08). Thus, the indirect effect through Arithmetic was similar across siblings concordant and discordant for ADHD.
Discussion
We evaluated mediation of birth weight and ADHD symptoms by biologically plausible neurocognitive functions in a sample of youth from multiplex families with ADHD. WISC Arithmetic statistically mediated the association of birth weight with ADHD symptoms, controlling for IQ and relevant demographic factors; Digit Span, Vocabulary, and Block Design were not significant mediators. Additionally, the indirect effect of birth weight on ADHD symptoms through Arithmetic did not differ between siblings concordant and discordant for ADHD. Although cross-sectional, these findings (a) support previous prospective longitudinal research implicating individual differences in Arithmetic as a preliminary causal mediator of birth weight and ADHD symptoms, and (b) provide preliminary evidence that this mediating pathway is independent of genetic influences on ADHD.
The significant indirect effect through Arithmetic and absence of indirect effects through Digit Span and Vocabulary in this study converge with prior prospective longitudinal research (Morgan et al., 2016), including a nearly identical effect size for the indirect effect through Arithmetic; this is especially notable given important differences between these samples. In particular, the previous study investigated WISC mediators at ages 5 to 10 and ADHD at ages 7 to 13 in unrelated youth with and without ADHD as well as controlled for co-occurring internalizing and externalizing symptoms (Morgan et al., 2016). In contrast, the present study extended hypotheses to a larger sample consisting of siblings from a broad age range with high genetic load for ADHD (although age was controlled in all analyses), and controlled for IQ. Contrary to Morgan et al. (2016) as well as the larger literature on birth weight and ADHD, but consistent with other mediational studies of birth weight and ADHD symptoms (e.g., Wiggs et al., 2016), we did not observe a significant total effect of birth weight on ADHD symptoms (i.e., association of birth weight with ADHD symptoms unadjusted for the mediators). This may be attributable to several factors, including higher power to detect indirect effects relative to the total effect (Loeys et al., 2015), or the elevated ADHD symptom severity of this high-risk sample relative to case-control or population-based samples previously used to establish birth weight–ADHD associations. Thus, future studies should investigate whether the association between birth weight and ADHD severity relates to heterogeneity within youth with ADHD.
Whereas Arithmetic loads moderately onto working memory and modestly onto verbal reasoning domains in traditional four-factor WISC models, there is replicated evidence that it loads strongly onto fluid reasoning in superior five-factor models (Keith et al., 2006; Weiss et al., 2013). Thus, although Arithmetic may recruit additional neurocognitive functions, it appears to primarily reflect fluid reasoning. That fluid reasoning statistically mediated the association of birth weight with ADHD symptoms converges with prior evidence of fluid reasoning deficits in youth born with low birth weight (e.g., Lahat et al., 2014), and with evidence for fluid reasoning deficits and hypoactivation in relevant brain regions in youth with ADHD (Tamm & Juranek, 2012). However, given the complexity of the constructs underlying Arithmetic, we await studies that disentangle these separable components to further improve traction on fluid reasoning pathways from birth weight to ADHD. In this regard, follow-up mediation analyses employing other measures of fluid reasoning and the Arithmetic Process Approach, which involves administration of the subtest in multiple formats that decrease working memory, attentional, quantitative, and oral expressive demands, are promising avenues. Although stringent control of IQ in the present study lends critical precision to inferences regarding Arithmetic, distillation of cognitive components underlying Arithmetic that are most critical to birth weight and ADHD will further enhance the clinical utility of the present findings.
Several key limitations should be noted. First, whereas this study was cross-sectional, temporally ordered predictors, mediators, and outcomes are necessary to infer causal mediation (Kraemer et al., 2001). However, that secondary analysis using a reverse mediation framework did not identify a significant indirect effect of birth weight on Arithmetic through ADHD symptoms, coupled with the convergence of these findings with prior prospective longitudinal research, provides key support for the mediated pathway. Second, because the featured WISC subtests may tap other domains of functioning in addition to fluid reasoning, working memory, verbal comprehension, and perceptual reasoning, replication with more specific measures of these constructs is necessary; this is especially true not only for fluid reasoning but also working memory given evidence that Digit Span may reflect only short-term memory and not working memory (e.g., Colom, Abad, Rebollo, & Shih, 2005). Third, birth weight was assessed via maternal recall, which although highly correlated with medical record data (e.g., Yawn et al., 1998), is less accurate. Fourth, despite the relatively large sample (n = 647), which significantly exceeds that required to adequately power product-of-coefficients tests of mediation using resampling methods for path coefficients of even small effect (Fritz & Mackinnon, 2007; Mackinnon et al., 2004), larger samples may be required to test the complex models examined herein (e.g., moderated mediation with multiple mediators). For example, the indirect effect for Block Design was of the same magnitude as that for Arithmetic, but was not significant. Additionally, due to the relatively low number of unaffected siblings (n = 108), the analysis to detect a difference between probands and unaffected siblings on the indirect effect of Arithmetic may have been underpowered. Thus, further evaluation of these hypotheses in large prospective longitudinal samples is warranted. Fifth, although comparison of ADHD concordant and discordant siblings affords insight into potential genetic influences (Schachar et al., 2005; Tsuang et al., 1993) and indeed was a novel feature of this study, follow-up with additional behavioral genetic designs that estimate heritability (e.g., co-twin control) will provide further clarification of whether the mediated effect through Arithmetic is truly independent of genetic influences on ADHD. Finally, whereas we found that fluid reasoning statistically mediated the pathway from birth weight to ADHD symptoms, substantial variance remained unexplained given the small effect observed. That is, additional neurocognitive functions (e.g., response variability) may mediate parallel pathways from birth weight (Wiggs et al., 2016), and even from other risk factors. For example, working memory and response inhibition are potential endophenotypes for ADHD from dopaminergic genes (Kamradt, Nigg, Friderici, & Nikolas, 2016; Loo et al., 2008). Thus, evaluation of diverse biologically plausible causal mediators for ADHD symptoms must be a continued priority.
Consistent with prior prospective longitudinal research, we found that Arithmetic (i.e., fluid reasoning) uniquely mediated the association of individual differences in birth weight with ADHD symptoms. Additionally, these data suggest that the observed indirect effect through Arithmetic was independent of genetic risk for ADHD. If replicated further, fluid reasoning will reflect a single step in a multilevel neurodevelopmental pathway from birth weight to ADHD. It will therefore be important to characterize the proximal mechanisms that mediate the associations of birth weight with fluid reasoning and fluid reasoning with ADHD symptoms. To this end, deficient in utero nourishment preceding birth weight and postnatal complications arising from birth weight (e.g., neonatal malnutrition; De Curtis & Rigo, 2004) are promising mechanisms underlying neurodevelopmental impairments that elicit fluid reasoning deficits and ADHD (Georgieff, 2007; Groen-Blokhuis et al., 2011). Also, deep phenotyping across multiple levels of analysis (e.g., cellular, neural, behavioral) should be prioritized (Bilder, Howe, Howe, & Sabb, 2013). Ultimately, delineation of causal processes underlying ADHD symptoms will highlight precise targets for early interventions to reduce the burden associated with ADHD.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Health grants MH58277 and NS54124.
Biography
Julia E. Morgan, MA, is a doctoral student in clinical psychology at University of California, Los Angeles (UCLA) and is mentored by Drs. Steve Lee and Sandra Loo. Her research aims to elucidate causal mechanisms underlying individual differences in youth ADHD with a focus on prenatal/perinatal and genetic influences on ADHD symptoms.
Steve S. Lee, PhD, is an associate professor of psychology at UCLA. His work uses developmentally sensitive, genetically informative designs to understand the etiology, development, and prediction of ADHD and co-occurring psychopathology dimensions (e.g., conduct problems, substance use disorders).
Sandra K. Loo, PhD, is a professor of psychiatry and biobehavioral sciences at the UCLA Semel Institute for Neuroscience and Human Behavior. Her work focuses on characterizing gene-brain-behavior pathways in ADHD as well as identifying brain-based biological markers of psychopathology and treatment response for additional child psychiatric disorders (e.g., obsessive-compulsive disorder, Tourette's syndrome, autism spectrum disorders).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. doi:10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age: Forms & profiles. Achenbach System of Empirically Based Assessemnt; Burlington, VT: 2001. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. Journal of the American Medical Association. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Howe AG, Howe AS, Sabb FW. Multilevel models from biology to psychology: Mission impossible? Journal of Abnormal Psychology. 2013;122:917–927. doi: 10.1037/a0032263. doi:10.1037/a0032263. [DOI] [PubMed] [Google Scholar]
- Buka SL, Goldstein JM, Spartos E, Tsuang MT. The retrospective measurement of prenatal and perinatal events: Accuracy of maternal recall. Schizophrenia Research. 2004;71:417–426. doi: 10.1016/j.schres.2004.04.004. doi:10.1016/j.schres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Colom R, Abad FJ, Rebollo I, Shih PC. Memory span and general intelligence: A latent-variable approach. Intelligence. 2005;33:623–642. doi:10.1016/j.intell.2005.05.006. [Google Scholar]
- De Curtis M, Rigo J. Extrauterine growth restriction in very-low-birthweight infants. Acta Paediatrica. 2004;93:1563–1568. doi: 10.1080/08035250410022198. doi:10.1080/08035250410022198. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Biederman J, Seidman LJ, Reske-Nielsen JJ, Faraone SV. Neuropsychological functioning in relatives of girls with and without ADHD. Psychological Medicine. 2005;35:1121–1132. doi: 10.1017/s0033291705004496. doi:10.1017/S0033291705004496. [DOI] [PubMed] [Google Scholar]
- Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. doi:10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff MK. Nutrition and the developing brain: Nutrient priorities and measurement. American Journal of Clinical Nutrition. 2007;85:614–620. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- Groen-Blokhuis MM, Middeldorp CM, van Beijsterveldt CEM, Boomsma DI. Evidence for a causal association of low birth weight and attention problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:1247–1254. doi: 10.1016/j.jaac.2011.09.007. doi:10.1016/j.jaac.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Halmøy A, Klungsøyr K, Skjærven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;71:474–481. doi: 10.1016/j.biopsych.2011.11.013. doi:10.1016/j.biopsych.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Hatch B, Healey DM, Halperin JM. Associations between birth weight and attention-deficit/hyperactivity disorder symptom severity: Indirect effects via primary neuro-psychological functions. Journal of Child Psychology and Psychiatry. 2014;55:384–392. doi: 10.1111/jcpp.12168. doi:10.1111/jcpp.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawi Z, Cummins TDR, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA. The molecular genetic architecture of attention deficit hyperactivity disorder. Molecular Psychiatry. 2015;20:289–297. doi: 10.1038/mp.2014.183. doi:10.1038/mp.2014.183. [DOI] [PubMed] [Google Scholar]
- Hayes AF. An index and test of linear moderated mediation. Multivariate Behavioral Research. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. doi:10.1080/00273171.2014.962683. [DOI] [PubMed] [Google Scholar]
- Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131:e1053–e1061. doi: 10.1542/peds.2012-2311. doi:10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- Jaspers M, de Meer G, Verhulst FC, Ormel J, Reijneveld SA. Limited validity of parental recall on pregnancy, birth, and early childhood at child age 10 years. Journal of Clinical Epidemiology. 2010;63:185–191. doi: 10.1016/j.jclinepi.2009.05.003. doi:10.1016/j.jclinepi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Kamradt JM, Nigg JT, Friderici KH, Nikolas MA. Neuropsychological performance measures as intermediate phenotypes for attention-deficit/hyperactivity disorder: A multiple mediation analysis. Development and Psychopathology. 2016 doi: 10.1017/S0954579416000195. Advance online publication. doi:10.1017/S0954579416000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. doi:10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keith TZ, Fine JG, Taub GE, Reynolds MR, Kranzler JH. Higher order, multisample, confirmatory factor analysis of the Wechsler Intelligence Scale for Children–Fourth Edition: What does it measure? School Psychology Review. 2006;35:108–127. [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Lahat A, Van Lieshout RJ, Saigal S, Boyle MH, Schmidt LA. ADHD among young adults born at extremely low birth weight: The role of fluid intelligence in childhood. Frontiers in Psychology. 2014;5:1–7. doi: 10.3389/fpsyg.2014.00446. doi:10.3389/fpsyg.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- Loeys T, Moerkerke B, Vansteelandt S. A cautionary note on the power of the test for the indirect effect in mediation analysis. Frontiers in Psychology. 2015;5:1–8. doi: 10.3389/fpsyg.2014.01549. doi:10.3389/fpsyg.2014.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Rich EC, Ishii J, McGough J, McCracken J, Nelson S, Smalley SL. Cognitive functioning in affected sibling pairs with ADHD: Familial clustering and dopamine genes. Journal of Child Psychology and Psychiatry. 2008;49:950–957. doi: 10.1111/j.1469-7610.2008.01928.x. doi:10.1111/j.1469-7610.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- Loo SK, Shtir C, Doyle AE, Mick E, McGough JJ, McCracken J, Nelson SF. Genome-wide association study of intelligence: Additive effects of novel brain expressed genes. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:432–440. e2. doi: 10.1016/j.jaac.2012.01.006. doi:10.1016/j.jaac.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Lunde A, Melve KK, Gjessing HK, Skjærven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. American Journal of Epidemiology. 2007;165:734–741. doi: 10.1093/aje/kwk107. doi:10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39(1):1–24. doi: 10.1207/s15327906mbr3901_4. doi:10.1207/s15327906mbr3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Lucia VC, Nigg JT, Breslau N. Sex differences in the pathway from low birth weight to inattention/hyperactivity. Journal of Abnormal Child Psychology. 2007;35:87–96. doi: 10.1007/s10802-006-9089-9. doi:10.1007/s10802-006-9089-9. [DOI] [PubMed] [Google Scholar]
- Martin J, Hamshere ML, Stergiakouli E, O'Donovan MC, Thapar A. Neurocognitive abilities in the general population and composite genetic risk scores for attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2015;56:648–656. doi: 10.1111/jcpp.12336. doi:10.1111/jcpp.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen M, Fischl B, Larsson HB, Skranes J, Kulseng S, Vangberg TR, Dale AM. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain. 2005;128:2588–2596. doi: 10.1093/brain/awh610. doi:10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. doi:10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Loo SK, Lee SS. Neurocognitive functioning mediates the prospective association of birth weight with youth ADHD symptoms. Journal of Clinical Child & Adolescent Psychology. 2016 doi: 10.1080/15374416.2016.1183498. Advance online publication. doi:10.1080/15374416.2016.1183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. 7th ed. Author; Los Angeles, CA: 1998-2015. [Google Scholar]
- Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del'Homme M, Levitt JG. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:1014–1022. doi: 10.1097/CHI.0b013e3181b395c0. doi:10.1097/CHI.0b013e3181b395c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. What causes ADHD? The Guilford Press; New York, NY: 2006. [Google Scholar]
- Nigg JT, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:362–369. doi: 10.1097/01.chi.0000246054.76167.44. doi:10.1097/01.chi.0000246054.76167.44. [DOI] [PubMed] [Google Scholar]
- O'Sullivan JJ, Pearce MS, Parker L. Parental recall of birth weight: How accurate is it? Archives of Disease in Childhood. 2000;82:202–203. doi: 10.1136/adc.82.3.202. doi:10.1136/adc.82.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson E, Sjölander A, Almqvist C, Anckarsäter H, D'Onofrio BM, Lichtenstein P, Larsson H. Birth weight as an independent predictor of ADHD symptoms: A within-twin pair analysis. Journal of Child Psychology and Psychiatry. 2015;56:453–459. doi: 10.1111/jcpp.12299. doi:10.1111/jcpp.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Kelley K. Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods. 2011;16:93–115. doi: 10.1037/a0022658. doi:10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Selig JP. Advantages of Monte Carlo confidence intervals for indirect effects. Communication Methods and Measures. 2012;6:77–98. doi:10.1080/19312458.2012.679848. [Google Scholar]
- Preacher KJ, Zhang Z, Zyphur MJ. Alternative methods for assessing mediation in multilevel data: The advantages of multilevel SEM. Structural Equation Modeling. 2011;18:161–182. doi:10.1080/10705511.2011.557329. [Google Scholar]
- Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychological Methods. 2010;15:209–233. doi: 10.1037/a0020141. doi:10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- Rice F, Lewis A, Harold G, van den Bree M, Boivin J, Hay DF, Thapar A. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: The influence of maternal and child characteristics. Early Human Development. 2007;83:497–504. doi: 10.1016/j.earlhumdev.2006.09.015. doi:10.1016/j.earl-humdev.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Kirby A, Ruparel K, Yang J, McGrath L, Anttila V, Hakonarson H. The genetic architecture of pediatric cognitive abilities in the Philadelphia Neurodevelopmental Cohort. Molecular Psychiatry. 2015;20:454–458. doi: 10.1038/mp.2014.65. doi:10.1038/mp.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar RJ, Crosbie J, Barr CL, Ornstein TJ, Kennedy J, Malone M, Pathare T. Inhibition of motor responses in siblings concordant and discordant for attention deficit hyperactivity disorder. American Journal of Psychiatry. 2005;162:1076–1082. doi: 10.1176/appi.ajp.162.6.1076. doi:10.1176/appi.ajp.162.6.1076. [DOI] [PubMed] [Google Scholar]
- Schlomer GL, Bauman S, Card NA. Best practices for missing data management in counseling psychology. Journal of Counseling Psychology. 2010;57(1):1–10. doi: 10.1037/a0018082. doi:10.1037/a0018082. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;72:191–197. doi: 10.1016/j.biopsych.2012.01.031. doi:10.1016/j.bio-psych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J, Løhaugen GCC, Martinussen M, Håberg A, Brubakk AM, Dale AM. Cortical surface area and IQ in very-low-birth-weight (VLBW) young adults. Cortex. 2013;49:2264–2271. doi: 10.1016/j.cortex.2013.06.001. doi:10.1016/j.cortex.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Del'Homme M, Newdelman J, Gordon E, Kim T, McCracken JT. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1135–1143. doi: 10.1097/00004583-200009000-00013. doi:10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyper-activity disorder: Potential targets for early intervention? Journal of Child Psychology and Psychiatry. 2010;51:368–389. doi: 10.1111/j.1469-7610.2009.02195.x. doi:10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Tamm L, Juranek J. Fluid reasoning deficits in children with ADHD: Evidence from fMRI. Brain Research. 2012;1465:48–56. doi: 10.1016/j.brainres.2012.05.021. doi:10.1016/j.brainres.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Cooper M, Eyre O, Langley K. Practitioner review: What have we learnt about the causes of ADHD? Journal of Child Psychology and Psychiatry. 2013;54:3–16. doi: 10.1111/j.1469-7610.2012.02611.x. doi:10.1111/j.1469-7610.2012.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV, Lyons MJ. Identification of the phenotype in psychiatric genetics. European Archives of Psychiatry Clinical Neuroscience. 1993;243:131–142. doi: 10.1007/BF02190719. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Dale AM. Long-term influence of normal variation in neonatal characteristics on human brain development. Proceedings of the National Academy of Sciences. 2012;109:20089–20094. doi: 10.1073/pnas.1208180109. doi:10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KA, Murray LJ, Gallagher AM, Cran GW, Savage MJ, Boreham C. Parental recall of birthweight: A good proxy for recorded birthweight? European Journal of Epidemiology. 2000;16:793–796. doi: 10.1023/a:1007625030509. doi:10.1023/A:1007625030509. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Examiner's manual: Wechsler Intelligence Scale for Children. 3rd ed. Psychological Corporation; New York, NY: 1991. [Google Scholar]
- Weiss LG, Keith TZ, Zhu J, Chen H. WISC-IV and clinical validation of the four- and five-factor interpretative approaches. Journal of Psychoeducational Assessment. 2013;31:114–131. doi:10.1177/0734282913478032. [Google Scholar]
- Wiggs K, Elmore AL, Nigg JT, Nikolas MA. Pre- and perinatal risk for attention-deficit hyperactivity disorder: Does neuropsychological weakness explain the link? Journal of Abnormal Child Psychology. 2016 doi: 10.1007/s10802-016-0142-z. Advance online publication. doi:10.1007/s10802-016-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Betjemann RS, McGrath LM, Chhabildas NA, Olson RK, DeFries JC, Pennington BF. Etiology and neuropsychology of comorbidity between RD and ADHD: The case for multiple-deficit models. Cortex. 2010;46:1345–1361. doi: 10.1016/j.cortex.2010.06.009. doi:10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. doi:10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Suman VJ, Jacobsen SJ. Maternal recall of distant pregnancy events. Journal of Clinical Epidemiology. 1998;51:399–405. doi: 10.1016/s0895-4356(97)00304-1. [DOI] [PubMed] [Google Scholar]