Abstract
Traumatic brain injury (TBI)-induced agitation and aggression pose major obstacles to clinicians in the acute hospital and rehabilitation settings. Thus, management of these symptoms is crucial. Antipsychotic drugs (APDs) are a common treatment approach for alleviating these symptoms. However, previous preclinical TBI studies have indicated that daily and chronic administration of these drugs (e.g., haloperidol; HAL) can exacerbate cognitive and motor deficits. Quetiapine (QUE) is an atypical APD that differs from many typical APDs, such as HAL, in its relatively rapid dissociation from the D2 receptor. The goal of this study was to test the hypotheses that intermittent HAL and QUE would not hinder recovery of cognitive and motor function following TBI and that daily QUE would also not impair functional recovery, which would be in contrast to HAL. Seventy anesthetized male rats received either a controlled cortical impact or sham injury and were then randomly assigned to TBI and sham groups receiving HAL (0.5 mg/kg) or QUE (10 mg/kg) intraperitoneally once per day or once every other day and compared to each other and vehicle (VEH) controls. Motor function was assessed by beam balance/walk tests on post-operative days 1-5 and cognitive function was evaluated with a Morris water maze task on days 14-19. No differences were revealed among the sham groups in any task, and hence the data were pooled. No overall differences were detected among the TBI groups, regardless of treatment or administration paradigm [p > 0.05], but all were impaired vs. SHAM controls [p < 0.05]. The SHAM controls also performed significantly better in the cognitive test vs. all TBI groups [p < 0.05]. Moreover, the TBI + continuous HAL group performed worse than the TBI + continuous VEH, TBI + continuous QUE, and TBI + intermittent QUE groups [p < 0.05], which did not differ from one another. Overall, the data suggest that QUE does not exacerbate TBI-induced cognitive and motor deficits, which supports the hypothesis. QUE may prove useful as an alternative APD treatment for management of agitation and aggression after clinical TBI. HAL may also be safe, but only if used sparingly.
Keywords: antipsychotic drugs, behavioral outcome, controlled cortical impact, functional recovery, learning and memory, Morris water maze, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a highly prevalent clinical issue affecting an estimated 1.7 million Americans annually [1-3]. TBI contributes to numerous pathophysiological conditions and adverse neuropsychiatric disturbances [4]. In many cases, extensive rehabilitative care is required. However, disinhibited behavior, including severe agitation and aggression, is common after moderate to severe TBI [5-10]. Such symptoms pose a risk to the health and safety of patients and caregivers, as well as significantly impede rehabilitation [6-11]. Management of agitation and aggression is therefore crucial, and typical and atypical antipsychotic drugs (APDs) are frequently employed to alleviate such issues. Extended use of these APDs, however, presents a number of possible problems, as evidence suggests they exacerbate motor and cognitive deficits and slow the rate of recovery [12-15].
Haloperidol (HAL) is a popular first-generation APD frequently used to manage post-TBI agitation. Preclinical studies using fluid percussion and cortical impact TBI models have demonstrated that chronic administration of HAL impairs motor and cognitive recovery [12-15]. The impairment persists whether the drug is administered before or after behavioral testing, suggesting the deleterious effects are not due simply to behavioral sedation, and endure for up to three months after drug discontinuation [12,15]. Like many of the APDs commonly used to alleviate post-TBI agitation, HAL exerts its effects by acting as a high-affinity D2 receptor antagonist. Quetiapine (QUE), on the other hand, is a second-generation APD with considerably lower affinity for D2 receptors [16,17]. Prior research has demonstrated that neither single nor repeated administrations of the atypical APDs clozapine and olanzapine, both of which have D2 receptor affinities comparable to that of QUE, has a negative impact on cognitive and motor performance after TBI [14,18]. The rationale for evaluating QUE is that it is one of the most widely accepted treatments currently for managing agitation and aggression in the clinic.
When considering the use of antipsychotic medications to manage symptoms that may disrupt rehabilitation, treatment strategies may vary depending on short-term versus long-term needs and goals for patient care. Some evidence suggests that a single administration of HAL after injury does not disrupt cognitive and motor recovery except at high doses, while daily administration for five days exacerbates cognitive and neurobehavioral deficits [12,13,15,19]. A realistic clinical strategy may rely on these medications prior to rehabilitation sessions and thus may not entail daily administration. However, the majority of research on APDs following TBI has focused on a daily drug regimen, the effects of which may differ from a periodic and potentially more clinically relevant administration schedule.
Hence, the present study aimed to evaluate the effects of continuous or intermittent treatment with QUE or HAL on short-term functional recovery after a controlled cortical impact (CCI) injury in adult male rats. The intermittent schedule was intended to simulate a clinically relevant course of drug administration where patients may not necessitate APD treatment every day. Motor function, spatial learning, and memory were assessed during this period to compare behavioral outcomes and how they may be affected by the APDs and the treatment schedule.
Materials and methods
Subjects and pre-surgical procedures
Seventy adult male rats (Harlan Sprague-Dawley, Indianapolis, IN) were paired housed in ventilated polycarbonate rat cages and maintained in a temperature (21 ± 1°C) and light (on 0700-1900 h) controlled environment with food and water available ad libitum. During their week of acclimatization, the rats were pre-trained on the beam-walk task and then randomly assigned to one of the following group conditions: TBI + continuous vehicle (1.0 mL/kg; n=10), TBI + continuous haloperidol (0.5 mg/kg; n=10), TBI + continuous quetiapine (10 mg/kg; n=10), TBI + intermittent haloperidol (0.5 mg/kg; n=10), TBI + intermittent quetiapine (10 mg/kg; n=10), and Sham controls for each condition (n=20). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize suffering.
Surgery
Controlled cortical impact (CCI) was produced as previously described [20-23]. Briefly, surgical anesthesia was induced and maintained with 4% and 2% concentrations of isoflurane, respectively, in 2:1 N2O:O2. After endotracheal intubation the rats (275-300 g) were secured in a stereotaxic frame and ventilated mechanically. Core temperature was maintained at 37 ± 0.5°C with a heating pad. Utilizing aseptic procedures a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy (6-mm in diameter) was made in the right hemisphere with a hand held trephine. The bone flap was removed and the craniectomy was enlarged further to accommodate the impact tip (6 mm, flat), which was centered and lowered through the craniectomy until it touched the dura mater. Once confirmed that the impact tip was touching the dura, the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8 mm tissue deformation at 4 m/sec). Anesthesia was discontinued immediately after the impact and the incision was promptly sutured. The rats were subsequently extubated and assessed for acute neurological outcome. Sham rats underwent all surgical procedures, except the impact.
Acute neurological evaluation
Hind limb reflexive ability was assessed immediately following the cessation of anesthesia by gently squeezing the rats’ paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to prone position on three consecutive trials.
Drug administration
HAL (Sigma) and QUE (Tocris) were prepared daily by dissolving in 1:1 dimethyl sulfoxide (DMSO)/saline, which also served as the vehicle (VEH). The dose of HAL was chosen because it has been reported to be comparable to that used clinically to control psychosis [24] and has been used in several brain injury studies investigating functional outcome [12,13,15,19,25]. The dose of QUE was chosen based on the preclinical literature [26]. Treatments began 24 hr after CCI or sham surgery and were provided intraperitoneally once daily or once every other day (i.e., intermittently) for 19 days. Both HAL and QUE were administered after the daily behavioral assessments to circumvent sedative effects, which would confound the results.
Motor performance: beam-balance and beam-walk
Motor function was assessed using the well-established beam-balance and beam-walk tasks [20-23]. Briefly, performance on the beam-balance is assessed by recording the time that the rats can maintain their balance on an elevated narrow wooden beam (90 cm above floor level, 1.5 cm wide, and 34 cm in length). The beam-walk task, modified from that originally devised by Feeney and colleagues [27], and used extensively in our laboratory [20-23], consists of assessing rats using a negative-reinforcement paradigm to escape a bright light, shining at the start point, and white noise by traversing an elevated narrow beam (90 cm above floor level, 2.5 cm wide, and 100 cm in length) and entering a darkened goal box at the opposite end. Performance on the beam-walk consists of recording time to traverse the beam. The rats were trained prior to TBI or sham injury to perform the tasks without errors (i.e., maintain their balance for 60 sec and traverse the beam in under 5 sec). A baseline performance assessment was taken on the day of surgery. Performance was assessed on post-operative days 1-5 and consisted of three trials (60 sec allotted time per trial) per day on each task. The average daily scores for each subject were used in the statistical analyses.
Cognitive performance: spatial learning
Spatial learning was assessed using a well-established Morris water maze (MWM) task [20-23,28]. Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was positioned in a room with prominent extra-maze cues. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Acquisition of spatial learning began on post-operative day 14 and consisted of providing a block of four daily trials for five consecutive days (14-18) to locate the escape platform when it was submerged 2 cm below the water surface. On day 19 the platform was raised 2 cm above the water surface to evaluate visible platform performance, which is incorporated as a control procedure to determine the contributions of non-spatial factors (e.g., sensory-motor function, motivation, and visual acuity) on cognitive performance. For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a quasi-randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. The rats that failed to locate the escape platform within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials (4-min inter-trial interval). The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
Cognitive performance: memory retention
One day after the final acquisition training (day 19), all rats were given a single probe trial to measure memory retention. Briefly, the platform was removed from the pool and the rats were placed in the pool from the location point most distal to the quadrant where the platform was previously located (i.e., “target quadrant”) and allowed to freely explore the pool for 30 sec. The time spent searching in the target quadrant was recorded and used in the statistical analyses. The data were obtained using ANY-maze video tracking software.
Statistical analyses
All analyses were performed using Statview 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA) on data collected by blinded experimenters. The motor and cognitive analyses were conducted using repeated-measures analysis of variance (ANOVA). The acute neurological data (i.e., hind limb withdrawal reflex and righting reflex) as well as the data for the visible platform, probe trial, and swim speed were analyzed using one-factor ANOVAs. When the overall ANOVA revealed significant effects, the Newman-Keuls post-hoc test, which controls for multiple comparisons and reduces the chance for a type 1 error, was used to determine specific group differences. The results are expressed as the mean ± standard error of the mean (S.E.M.) and were considered significant when p ≤ 0.05.
Results
There were no exclusions and thus the statistical analyses were performed on the data from all 70 rats. No significant differences (p’s > 0.05) were observed in any behavioral measure between the sham controls regardless of housing condition so their data were pooled into one group designated as SHAM.
Acute neurological function
No differences were observed among the TBI groups in hind limb withdrawal reflex after a brief paw pinch [left range = 158.1 ± 4.4 sec to 164.4 ± 5.2 sec, p > 0.05; right range = 153.2 ± 1.8 sec to 160.1 ± 5.2 sec, p > 0.05] or for righting reflex [range 346.2 ± 17.8 sec to 380.1 ± 17.6 sec, p > 0.05] following the termination of anesthesia. The lack of significant differences with these acute neurological indices suggests that all groups experienced equivalent levels of injury and anesthesia.
Motor function: beam-balance
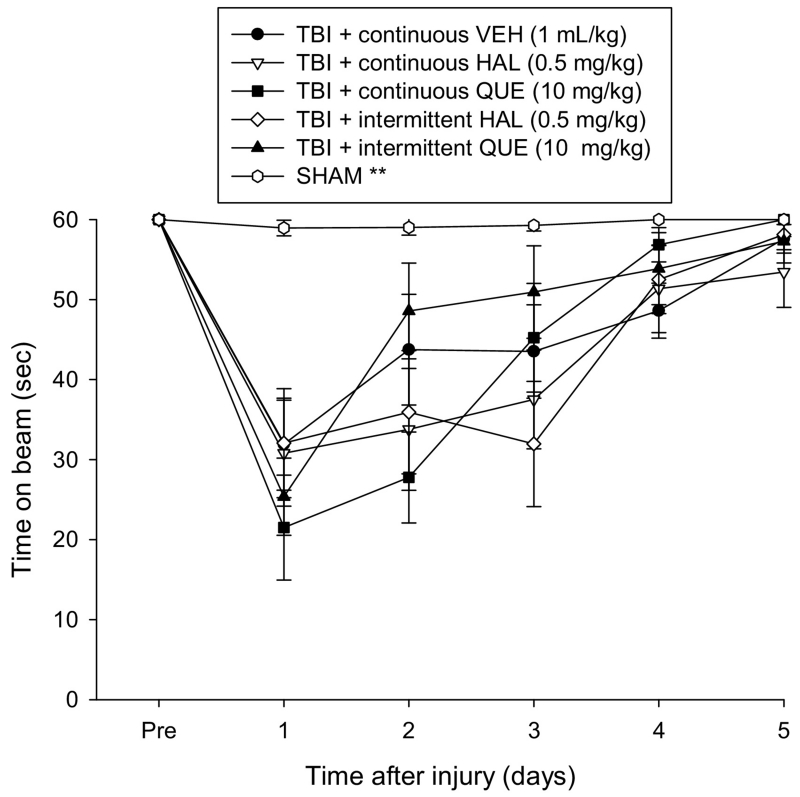
Prior to surgery, each rat was able to balance on the beam for the allotted 60 sec (Fig. 1). After CCI injury there were significant impairments as indicated by the repeated measures ANOVA, which revealed significant Group [F5,64 = 7.810, p < 0.0001] and Day [F5,320 = 52.801, p < 0.0001] differences, as well as a significant Group × Day interaction [F25,320 = 4.614, p < 0.0001]. According to the post-hoc analysis all TBI groups, regardless of treatment or administration paradigm, performed significantly worse than the SHAM controls [p < 0.05] and did not differ from one another overall [p > 0.05].
Fig. 1.
Mean (± S.E.M.) time (sec) balancing on an elevated narrow beam prior to, and after, TBI or sham injury. There were no significant differences among the TBI groups regardless of treatment or continuous or intermittent administration, but they were all significantly impaired relative to the SHAM controls [**p < 0.05].
Motor function: beam-walk
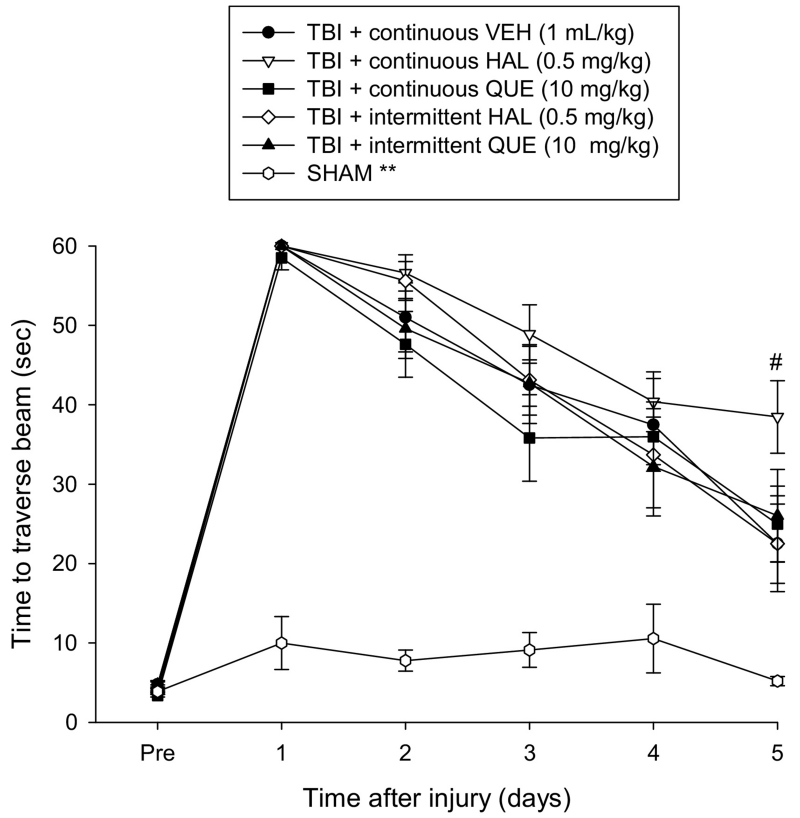
Prior to surgery, each rat consistently traversed the 100 cm beam to the reward box in under 5 sec (Fig. 2). After CCI injury, there was a significant impairment in beam traversal as indicated by the ANOVA, which revealed significant Group [F5,64 = 47.957, p < 0.0001] and Day [F5,320 = 175.483, p < 0.0001] differences, as well as a significant Group × Day interaction [F25,320 = 9.345, p < 0.0001]. According to the post-hoc analysis all TBI groups, regardless of treatment or administration paradigm, performed significantly worse than the SHAM controls [p < 0.05] and did not differ from one another overall [p > 0.05]. However, a single day ANOVA and post-hoc analysis revealed that the TBI + continuous HAL group was significantly impaired on the last day of testing, as indicated by requiring more time to traverse the beam, relative to all other TBI groups [p < 0.05], which did not differ from one another [p > 0.05].
Fig. 2.
Mean (± S.E.M.) time (sec) to traverse an elevated narrow beam after TBI or sham injury. There were no significant overall differences among the TBI groups regardless of treatment or continuous or intermittent administration, but they were all significantly impaired relative to the SHAM controls [**p < 0.05]. However, a single day analysis on the last day of testing (day 5) revealed that the TBI + continuous HAL groups was significantly impaired relative to all other TBI groups [#p < 0.05].
Cognitive function: acquisition of spatial learning, visible platform, and swim speed
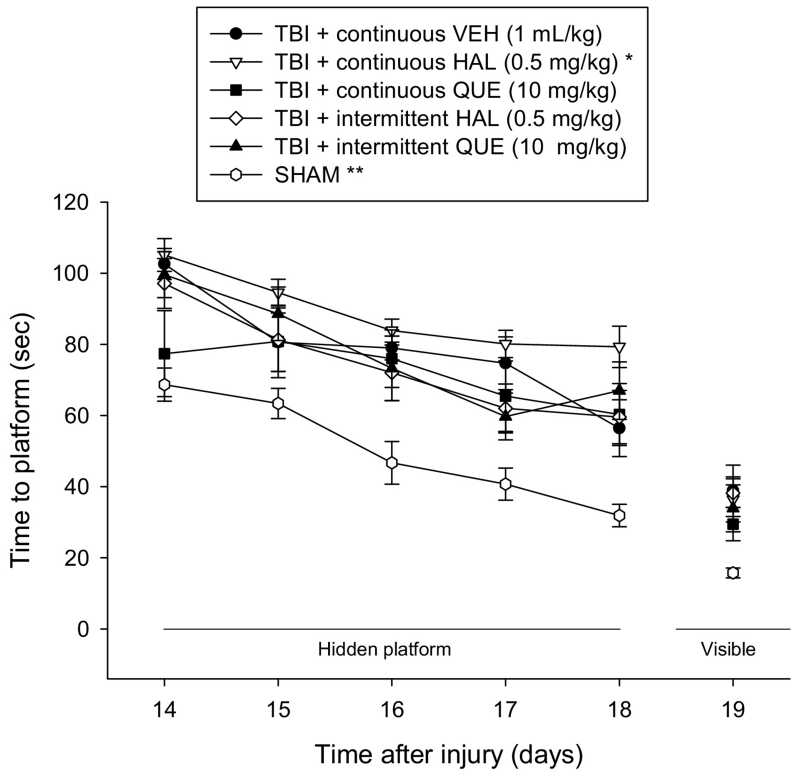
Analysis of spatial learning revealed significant Group [F5,64 = 10.408, p < 0.0001] and Day [F4,256 = 33.919, p < 0.0001] differences. The post-hoc analysis indicated that all TBI groups, regardless of treatment or administration paradigm were significantly impaired relative to the SHAM controls (Fig. 3), as indicated by requiring more time to find the escape platform [p < 0.05]. Additionally, the TBI + continuous HAL group was significantly impaired compared to the TBI + continuous VEH, TBI + continuous QUE, and TBI + intermittent QUE [p < 0.05]. No other comparisons were different [p > 0.05]. The time to locate the visible platform was quicker in the SHAM controls relative to the TBI groups, regardless of treatment or administration paradigm [p < 0.05]. Swim speed did not differ among the groups (range = 26.4 ± 1.6 cm/sec to 29.7 ± 1.4 cm/sec; p > 0.05).
Fig. 3.
Mean (± S.E.M.) time (sec) to locate a hidden and visible platform in the water maze. All TBI groups, regardless of treatment or continuous or intermittent administration, were significantly impaired relative to the SHAM controls [**p < 0.05]. Additionally, the TBI + continuous HAL group was significantly impaired compared to all other TBI groups [p < 0.05]. The time to locate the visible platform was quicker in the SHAM controls relative to the TBI groups, regardless of treatment or administration paradigm [p < 0.05].
Cognitive function: probe trial
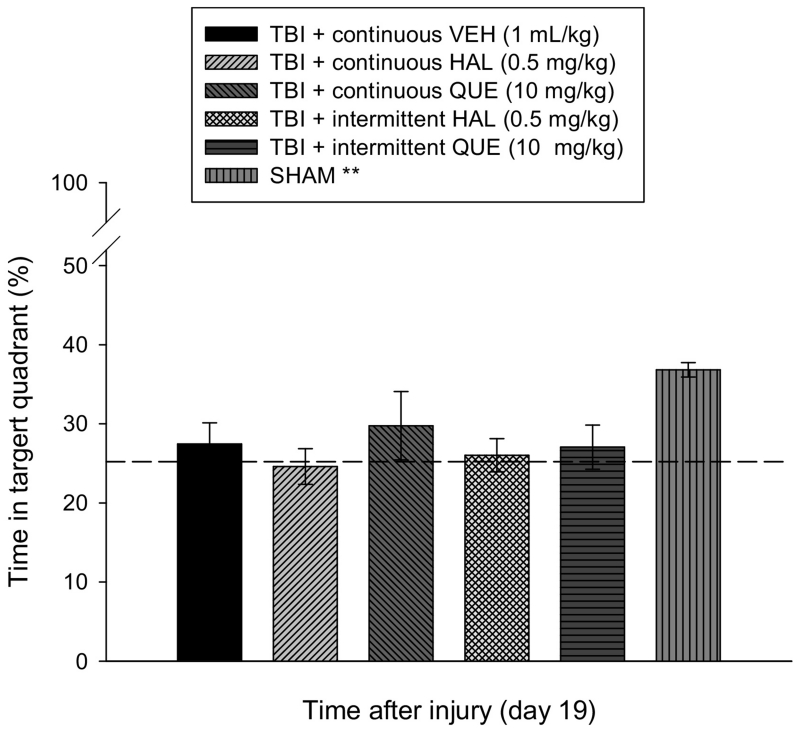
Analysis of the probe data revealed a significant difference among the groups [F5,64 = 3.849, p < 0.0041]. The post-hoc analysis revealed that enhanced memory retention, as demonstrated by a greater percentage of the 30 sec allotted time spent in the target quadrant, was observed only in the SHAM controls (36.8 ± 0.9%) relative to the TBI groups (Fig. 4), which had a range of 24.6 ± 2.3 % to 27.5 ± 2.7 % and did not differ from one another [p > 0.05].
Fig. 4.
Mean (± S.E.M.) percentage of time spent in the target quadrant (i.e., where platform was previously located) following a single probe trial 19 days after cortical impact or sham injury. All TBI groups, regardless of treatment or continuous or intermittent administration, were significantly impaired relative to the SHAM controls [**p < 0.05]. No other comparisons were significant. The dotted line represents performance at the chance level (25%).
Discussion
Previous studies have shown that the typical APD HAL impairs motor and/or cognitive recovery after CCI [12,13,15,19,25], fluid percussion [14], and cortical ablation [27,29] injury. HAL also attenuates the benefits of environmental enrichment, a preclinical model of neurorehabilitation that has been reported to confer significant motor, cognitive, and histological benefits after TBI [30,31]. Moreover, the deleterious effects of HAL persist for up to 3 months after drug withdrawal [15]. In contrast, little is known about the prevalence or duration of any adverse effects of the atypical APD QUE treatment after TBI. However, the findings of several non-TBI studies suggest that it may preserve, and even improve, cognitive function. A prospective study of low-dose daily QUE for the treatment of aggressive symptoms three or more months after injury demonstrated that after six weeks of treatment, patients displayed improvement on measures of cognitive functioning [32]. Among a range of other APDs, QUE produced considerable improvement in global neurocognitive function in a clinical trial of patients with schizophrenia [33]. Rodent studies of both ketamine-induced and stress-induced cognitive impairments have also demonstrated that daily administration of QUE can mitigate performance deficits [34,35]. Additionally, QUE has been shown to effectively reduce agitation and psychotic symptoms after TBI [36]. Taken together, these findings indicate that the mechanisms of QUE that attenuate agitation and aggression are independent of their effect on neurological functions. As such, QUE treatment may be a better alternative than HAL for managing agitated symptoms after TBI. Hence, the goal of this study was to describe the effects of daily and intermittent treatment with the APDs HAL and QUE on neurobehavioral and spatial learning after brain trauma produced by the well-established CCI injury paradigm.
Performance on the beam-balance and beam-walk motor tasks was relatively unaffected by the continuous or intermittent treatments when considering outcomes over the five days of testing. However, when performance was evaluated on the last day of testing, which is a critical endpoint measurement, there was a significant delay in traversing the beam for the TBI group receiving daily HAL, relative to the other TBI conditions. This finding suggests that daily HAL delays beam-walking performance and is in accord with previous CCI studies [12,13,15]. The acquisition of spatial learning was also substantially delayed in the HAL group treated once per day. No such delay was observed in the intermittent HAL group or the QUE-treated groups, regardless of administration paradigm (i.e., daily or intermittent).
These findings demonstrate that QUE, whether administered daily or intermittently after CCI, produces comparable behavioral and cognitive outcomes to those resulting from intermittent HAL treatment. Importantly, the behavioral results of these three treatment paradigms did not differ significantly from those observed in the VEH-treated TBI group. These data indicate that an intermittent treatment schedule with either QUE or HAL inhibits the deleterious cognitive and behavioral outcomes previously observed after daily administration with HAL or risperidone [12,13,15]. An intermittent administration schedule may present an alternative means of treatment with HAL to reduce agitation and aggression in TBI patients without compromising recovery. Additionally, these findings suggest that QUE may present a further treatment option, potentially with a more flexible variety of dosing schedules within a relative margin of safety for patient outcomes.
It is generally acknowledged that HAL exerts deleterious effects of functional outcome after experimental TBI by antagonizing D2 receptors [6,8,12-15,18,19,25]. This theory is supported further by the plethora of data showing that D2 receptor agonists, such as bromocriptine, methylphenidate, and amantadine enhance motor and cognitive performance after TBI [15,37-41]. Moreover, the APD aripiprazole, which is a partial D2 receptor agonist, does not impede recovery, but rather facilitates spatial learning after CCI injury [15]. QUE on the other hand exhibits moderate to high affinities for α1-adrenergic and 5-HT2A receptors and lesser affinity for D2 receptors [16,17]. QUE also exhibits faster dissociation from the D2 receptor versus HAL [42,43], which may be a mediating factor in its actions on behavioral outcomes after TBI and other disorders.
For example, it has been reported that QUE transiently disrupts avoidance behavior in a conditioned avoidance response task because it only transiently blocks D2 receptors [44]. It has also been reported that QUE decreases object recognition deficits in a rat model of malformations of cortical development [26], stress-induced spatial working memory impairment [45], and reverses methamphetamine-induced cognitive deficits [46]. The benefits attributed to QUE could be due, in part to, to increased levels of DA in the frontal cortex. Specifically, Silverstone and colleagues (2012) reported that QUE and the 5-HT1A receptor agonist buspirone significantly increased release of DA compared to controls [47]. However, there was no additive effect of the combined treatments, which lead the authors to suggest that the intrinsic partial 5-HT1A agonist activity of QUE on its own may have led to a ceiling effect. Ichikawa and colleagues (2002) also reported an increase in DA release in the medial prefrontal cortex with QUE relative to saline controls [48]. These positive findings with QUE all have in common the same theme of increased DA neurotransmission that correlates with behavioral and cognitive improvement. It is possible that manipulating the dose of QUE may lead to behavioral improvement after TBI as was seen with the APD aripiprazole, which is also a partial 5-HT1A receptor agonist. Indeed, 5-HT1A receptor agonists have been reported numerous times to confer significant benefits after TBI [for comprehensive review, see 49].
In conclusion, although, QUE did not increase cognitive performance after CCI, it also did not exacerbate TBI-induced cognitive and motor deficits as was seen with daily HAL in the current study and reported in others [12-15,18,19,25]. QUE may prove useful as an alternative APD treatment for management of agitation and aggression after clinical TBI. Furthermore, the data suggests that HAL may also be safe, but only if used sparingly.
Highlights.
-
➢
Intermittent haloperidol does not negatively impact functional outcome after experimental brain trauma
-
➢
Intermittent quetiapine does not negatively impact functional outcome after experimental brain trauma
-
➢
Daily administration of haloperidol negatively impacts functional outcome after experimental brain trauma
-
➢
Daily administration of quetiapine does not negatively impact functional outcome after experimental brain trauma
-
➢
These findings suggest that quetiapine may be a safer alternative to haloperidol for managing TBI-induced agitation
Acknowledgements
This work was supported, in part, by the National Institutes of Health grants NS060005, HD069620, HD069620-S1, NS084967 (AEK), NS094950 (COB), and the University of Pittsburgh Physicians /UPMC Academic Foundation (COB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Faul M, Xu l., Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta (GA): 2010. [Google Scholar]
- [2].Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- [3].Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- [4].Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog. Neurobiol. 2016;142:45–67. doi: 10.1016/j.pneurobio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury-a state-of-the-art review. J. Rehabil. Res. Dev. 2009;46:851–879. doi: 10.1682/jrrd.2008.09.0120. [DOI] [PubMed] [Google Scholar]
- [6].Lombard LA, Zafonte RD. Agitation after traumatic brain injury: considerations and treatment options. Am. J. Phys. Med. Rehabil. 2005;84:797–812. doi: 10.1097/01.phm.0000179438.22235.08. [DOI] [PubMed] [Google Scholar]
- [7].McNett M, Sarver W, Wilczewski P. The prevalence, treatment and outcomes of agitation among patients with brain injury admitted to acute care units. Brain Inj. 2012;26:1155–1162. doi: 10.3109/02699052.2012.667587. [DOI] [PubMed] [Google Scholar]
- [8].Elovic EP, Jasey NN, Eisenberg ME. The use of atypical antipsychotics after traumatic brain injury. J. Head Trauma Rehabil. 2008;23:132–135. doi: 10.1097/01.HTR.0000314532.07530.e5. [DOI] [PubMed] [Google Scholar]
- [9].Rao V, Koliatsos V, Ahmed F, Lyketsosm C, Kortte K. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin. Neurol. 2015;35:64–82. doi: 10.1055/s-0035-1544241. [DOI] [PubMed] [Google Scholar]
- [10].Stanislav SW. Cognitive effects of antipsychotic agents in persons with traumatic brain injury. Brain Inj. 11(1997):335–341. doi: 10.1080/026990597123494. [DOI] [PubMed] [Google Scholar]
- [11].Sandel ME, Mysiw JW. The agitated brain injured patient. Part 1: Definitions, differential diagnosis, and assessment. Arch. Phys. Med. Rehab. 1996;77:617–623. doi: 10.1016/s0003-9993(96)90306-8. [DOI] [PubMed] [Google Scholar]
- [12].Kline AE, Hoffman AN, Cheng JP, Zafonte RD, Massucci JL. Chronic administration of antipsychotics impede behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 2008;448:263–267. doi: 10.1016/j.neulet.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoffman AN, Cheng JP, Zafonte RD, Kline AE. Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 2008;83:602–607. doi: 10.1016/j.lfs.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wilson MS, Gibson CJ, Hamm RJ. Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am. J. Phys. Med. Rehab. 2003;82:871–879. doi: 10.1097/01.PHM.0000091982.33232.CB. [DOI] [PubMed] [Google Scholar]
- [15].Phelps TI, Bondi CO, Ahmed RH, Olugbade YT, Kline AE. Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J. Neurotrauma. 2015;32:590–597. doi: 10.1089/neu.2014.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jibson MD, Tandon R. New atypical antipsychotic medications. J. Psychiatr. Res. 1998;32:215–228. doi: 10.1016/s0022-3956(98)00023-5. [DOI] [PubMed] [Google Scholar]
- [17].Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- [18].Goldstein LB, Bullman S. Differential effects of haloperidol and clozapine on motor recovery after sensorimotor cortex injury in rats. Neurorehab. Neural Repair. 2002;16:321–325. doi: 10.1177/154596830201600402. [DOI] [PubMed] [Google Scholar]
- [19].Kline AE, Massucci JL, Zafonte RD, Dixon CE, DeFeo JR, Rogers EH. Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental brain trauma. Crit. Care Med. 2007;35:919–924. doi: 10.1097/01.CCM.0000256722.88854.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil. Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matter AM, Folweiler KA, Curatolo LM, Kline AE. Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair. 2011;25:558–564. doi: 10.1177/1545968310397206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J. Neurotrauma. 2012;29:2684–2688. doi: 10.1089/neu.2012.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosengarten H, Quartermain D. The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three-and eighteen-month-old rats. Prog. Neuro-psychopharmacol. Bio. Psychiat. 2002;26:1047–1054. doi: 10.1016/s0278-5846(02)00221-x. [DOI] [PubMed] [Google Scholar]
- [25].Folweiler KA, Bondi CO, Ogunsanya EA, LaPorte MJ, Leary JB, Radabaugh HL, Monaco CM, Kline AE. Combining the antipsychotic drug haloperidol and environmental enrichment after traumatic brain injury is a double-edged sword. J. Neurotrauma. 2016 doi: 10.1089/neu.2016.4417. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ma L, Yang F, Zhao R, Li L, Kang X, Xiao L, Jiang W. Quetiapine attenuates cognitive impairment and decreases seizure susceptibility possibly through promoting myelin development in a rat model of malformations of cortical development. Brain Res. 2015;1622:443–451. doi: 10.1016/j.brainres.2015.07.012. [DOI] [PubMed] [Google Scholar]
- [27].Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- [28].Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [29].Hovda DA, Feeney DM. Haloperidol blocks amphetamine induced recovery of binocular depth perception after bilateral visual cortex ablation in cat. Proc. West Pharmacol. Soc. 1985;28:209–211. [PubMed] [Google Scholar]
- [30].Bondi CO, Klitsch KC, Leary JB, Kline AE. Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J. Neurotrauma. 2014;31:873–888. doi: 10.1089/neu.2014.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bondi CO, Semple BD, Noble-Haeusslein LJ, Osier ND, Carlson SW, Dixon CE, Giza CC, Kline AE. Found in translation: Understanding the biology and behavior of experimental traumatic brain injury. Neurosci Biobehav Rev. 2015;58:123–146. doi: 10.1016/j.neubiorev.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim E, Bijlani M. A pilot study of quetiapine treatment of aggression due to traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2006;18:547–549. doi: 10.1176/jnp.2006.18.4.547. [DOI] [PubMed] [Google Scholar]
- [33].Johnsen E, Jørgensen HA, Kroken RA, Løberg EM. Neurocognitive effectiveness of quetiapine, olanzapine, risperidone, and ziprasidone: a pragmatic, randomized trial. European Psychiatry. 2013;28:174–184. doi: 10.1016/j.eurpsy.2011.10.003. [DOI] [PubMed] [Google Scholar]
- [34].Nikiforuk A, Popik P. Effects of quetiapine and sertindole on subchronic ketamine-induced deficits in attentional set-shifting in rats. Psychopharmacology. 2012;220:65–74. doi: 10.1007/s00213-011-2487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang HN, Peng Y, Tan QR, Chen YC, Zhang RG, Qiao YT, Wang HH, Liu L, Kuang F, Wang BR, Zhang ZJ. Quetiapine ameliorates anxiety-like behavior and cognitive impairments in stressed rats: implications for the treatment of posttraumatic stress disorder. Physiol. Res. 2010;59:263–271. doi: 10.33549/physiolres.931756. [DOI] [PubMed] [Google Scholar]
- [36].Daniels JP, Felde A. Quetiapine treatment for mania secondary to brain injury in 2 patients. J. Clin. Psychiatry. 2008;69:497–498. doi: 10.4088/jcp.v69n0324a. [DOI] [PubMed] [Google Scholar]
- [37].Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- [38].Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J. Neurotrauma. 2004;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- [39].Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci. Lett. 2000;280:163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- [40].Leary LB, Bondi CO, LaPorte MJ, Carlson LC, Cheng JP, Kline AE. The therapeutic efficacy of environmental enrichment and methylphenidate alone and in combination after controlled cortical impact injury. J. Neurotrauma. 2016 doi: 10.1089/neu.2016.4438. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dixon CE, Kraus MF, Kline AE, Ma X, Yan HQ, Griffith RG, Wolfson BM, Marion DW. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 1999;14:285–294. [PubMed] [Google Scholar]
- [42].Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J. Psychiatry Neurosci. 2000;25:161–166. [PMC free article] [PubMed] [Google Scholar]
- [43].Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch. Gen. Psychiatry. 2000;57:553–559. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- [44].Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- [45].Amin SN, Gamal SM, Esmail RS, Aziz TM, Rashed LA. Cognitive effects of acute restraint stress in male albino rats and the impact of pretreatment with quetiapine versus ghrelin. J. Integr. Neurosci. 2014;13:669–692. doi: 10.1142/S0219635214500253. [DOI] [PubMed] [Google Scholar]
- [46].He J, Yang Y, Yu Y, Li X, Li XM. The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav. Brain Res. 2006;172:39–45. doi: 10.1016/j.bbr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- [47].Silverstone PH, Lalies MD, Hudson AL. Quetiapine and buspirone both elevate cortical levels of noradrenaline and dopamine in vivo, but do not have synergistic effects. Front. Psychiatry. 2012;14:82. doi: 10.3389/fpsyt.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ichikawa J, Li Z, Dai J, Meltzer HY. Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res. 2002;956:349–357. doi: 10.1016/s0006-8993(02)03570-9. [DOI] [PubMed] [Google Scholar]
- [49].Cheng JP, Leary JB, Sembhi A, Edwards CM, Bondi CO, Kline AE. 5-hydroxytryptamine1A (5-HT1A) receptor agonists: A decade of empirical evidence supports their use as an efficacious therapeutic strategy for brain trauma. Brain Res. 2016;1640:5–14. doi: 10.1016/j.brainres.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]