Abstract
Endothelial cells express a diverse array of ion channels including members of the strong inward rectifier family composed of KIR2 subunits. These two-membrane-spanning-domain channels are modulated by their lipid environment, and exist in macromolecular signaling complexes with receptors, protein kinases and other ion channels. Inward rectifier K+ channels (KIR) currents display a region of negative slope conductance at membrane potentials positive to the K+ equilibrium potential that allows outward current through the channels to be activated by membrane hyperpolarization, permitting KIR to amplify hyperpolarization induced by other K+ channels and ion transporters. Increases in extracellular K+ concentration activate KIR allowing them to sense extracellular K+ concentration and transduce this change into membrane hyperpolarization. These properties position KIR to participate in the mechanism of action of hyperpolarizing vasodilators and contribute to cell-cell conduction of hyperpolarization along the wall of microvessels. Expression of KIR in capillaries in electrically active tissues may allow KIR to sense extracellular K+, contributing to functional hyperemia. Understanding the regulation of expression and function of microvascular endothelial KIR will improve our understanding of the control of blood flow in the microcirculation in health and disease and may provide new targets for development of therapeutics in the future.
Keywords: Potassium channels, KIR2.1, KCNJ2, endothelial cells, microcirculation, arterioles, hyperpolarization, vasodilation, functional hyperemia
Introduction
Ion channels in the membranes of endothelial cells contribute to all aspects of the function of these cells [57]. Calcium influx and release through members of these membrane proteins determines intracellular Ca2+ concentration. This second messenger importantly regulates a number of endothelial cell processes related to the regulation of vascular function including endothelial cell production of autacoids such as NO, prostaglandins and epoxides of arachidonic acid (EETs) [31], as well as the activity of Ca2+-dependent ion channels [31]. Intracellular Ca2+ also regulates endothelial cell barrier function [39,64], gene expression [82,93], and proliferation [83,87]. Cell volume regulation also depends on the activity of plasma membrane ion channels [52]. Importantly, ion channels determine and regulate endothelial cell membrane potential. Modulation of this separation of charge, through changes in ion channel activity, functions as an important signal for endothelial cell-endothelial cell communication and endothelial cell-smooth muscle cell communication, because these cells are electrically coupled by gap junctions [23]. Membrane potential also affects the electrochemical gradient for movement of all ions across the plasma membrane, potentially modulating Ca2+ influx through endothelial cell Ca2+ channels [6,46,47,51,87], although this topic remains controversial [16,26,77,80,107]. Thus, the physiology and pathophysiology of endothelial cells depends heavily on the expression and function of ion channels.
As in all cells [50], K+ channels play a central role in setting and modulating membrane potential of endothelial cells. At the resting membrane potential of microvascular endothelial cells in intact pressurized vessels (~30–40 mV [32,102,103,116]), with physiological ion gradients (3–5 mM K+ in the extracellular fluid, ~140 mM K+ in the cytosol), the electrochemical gradient for diffusion of K+ across endothelial cell membranes is outward. Thus, K+ will flow out of cells when K+ channels open, producing membrane hyperpolarization. Closure of open K+ channels will have the opposite effect, membrane depolarization.
Endothelial cells may express four or more classes of K+ channels [57]. The expression and function of strong inward rectifier K+ (KIR2.X) channels will be the focus of this review. Because ion channel expression and function change dramatically during cell culture [7,8,14,87,99], emphasis will be placed on data originating from intact microvessels and freshly isolated endothelial cells. Earlier literature may be accessed from prior reviews [87–89].
Structure of KIR channels
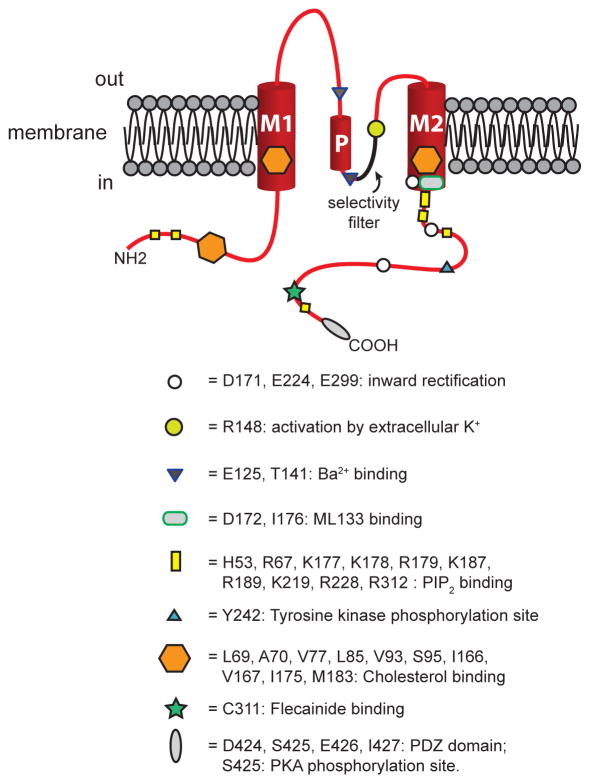
Inward rectifier K+ channels in the KIR2.1 – 2.4 family represent the products of four genes (loci: KCNJ2, KCNJ12, KCNJ4, KCNJ14, respectively) in the 15 member family of KIR channels [49]. These channels consist of a tetramer of pore-forming α-subunits [49,86]. Each α-subunit has two membrane spanning domains (M1 and M2, Figure 1) with intracellular carboxy and amino termini [49,86]. A P-loop links the membrane spanning domains (Figure 1). The P-loop and M2 form the ion-conducting pore [49,86]. Conserved amino acids in the P-loop (T142-I143-G144-Y145-G146-F147-R148 in KIR2.1) comprise the channels’ K+ ion selectivity filter [49,86,108] (Figure 1). Voltage-dependent block of outward K+ currents by intracellular polyamines [34,37,73] and Mg2+ [78,112] causes the distinguishing KIR channel current inward rectification (Figure 2) [75]. Magnesium ions and polyamines interact with basic residues in M2 (D172 in KIR2.1, D173 in KIR2.2) and in the carboxy terminus (E224, E299 and D255 in KIR2.1; E225, E300 and D256 in KIR2.2) to produce voltage-dependent block of outward current flow at membrane potentials positive to the K+ equilibrium potential [75,108] (Figures 1 and 2). Based on studies of the crystal structure of chicken KIR2.2 [108], K+ ions also will interact with these same residues, explaining why inward rectification shifts to more positive membrane potentials as extracellular K+ concentration is elevated.
Figure 1.
Structure of KIR2 channels. Shown is a schematic representation of one KIR2 channel subunit positioned in the lipid bilayer of a cell membrane as shown. These channels have two membrane spanning helical domains, denoted M1 and M2 connected by a P-loop that contains a helical domain (P in the drawing). The channel’s pore is formed by the P-loop and M2, with the selectivity filter sequence highlighted in the drawing. Cytosolic amino (NH2) and carboxy (COOH) termini are also shown. Approximate locations of sites where regulatory molecules interact with the channel protein are shown as indicated. See text for references and more information.
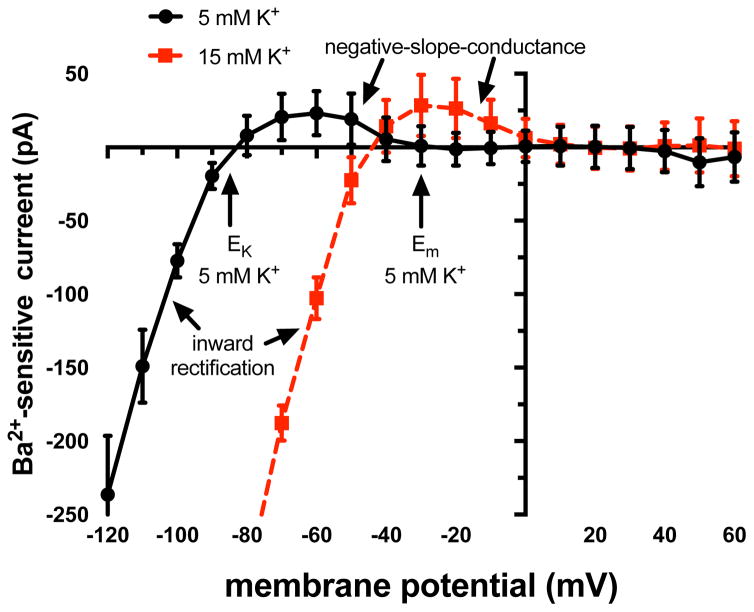
Figure 2.
KIR currents in arteriolar endothelial cells. Shown are mean ± SE (n = 5) current-voltage (I–V) relationships for Ba2+ -sensitive currents recorded from enzymatically isolated endothelial cell tubes (see [55] for more information). At membrane potentials (Ems) negative to the K+ equilibrium potential (EK), inward currents are carried by the channels (inward rectification). At membrane potentials positive to EK, outward currents are carried by the channels, however, the outward currents diminish as the membrane becomes more positive producing a region of negative slope conductance, where hyperpolarization increases outward current flow through the channels. At normal resting Em (5 mM K+ outside and 140 mM K+ inside, ~−30 mV), little current flows through these channels due to block by intracellular polyamines and Mg2+. However, membrane hyperpolarization will recruit current through the channels and amplify the initial hyperpolarization. Also shown is the shift in the I–V relationship with an increase in extracellular K+ (due to the shift in EK). Note that at the resting level of Em (−30 mV) with 15 mM K+ outside, there is now outward current through KIR channels that will tend to hyperpolarize the membrane toward the new EK. Data from [55] and graph modified from the same publication, with permission.
Negative-slope-conductance and the KIR channel current-voltage relationship
Although KIR channels are named for the inward rectification of current, it is the small outward “hump” in the current-voltage relationship (i.e., the region of negative slope conductance [29,98], Figure 2) that is present between the K+ equilibrium potential and resting membrane potential (~−40 to −30 mV [32,102,103,116]) of endothelial cells that is important for the physiological function of KIR channels. Membrane hyperpolarization from the resting potential will increase outward KIR channel current, amplifying the original hyperpolarization [12,55,58,72,104,106] (Figure 2). Microvascular endothelial cells have high membrane resistance at resting membrane potential (2–100 GΩ; [63,106,114]). Thus, activation of only a few KIR channels can effectively modulate endothelial cell membrane potential; in mouse mesenteric arteries, there are on the order of only 144 functional KIR channels per endothelial cell that significantly contribute to regulation of vessel function [106]. In KIR channels containing KIR2.2 subunits, steady-state membrane hyperpolarization decreases the channel’s open-state probability in a time-dependent fashion, a feature unique to this KIR2 family member [49].
Membrane lipids modulate KIR channels
The lipid environment around KIR channels strongly regulates their function. Phosphatidylinositol 4,5-bisphosphate (PIP2) activates KIR2 channels by interacting with basic amino acids in M2 (K177, K178, R179 for KIR2.1) and in the cytoplasmic tails of the channels (H53, R67, K187, K188, R189, K219, R228, and R312 for KIR2.1) [49] (Figure 1). This interaction opens the potential for KIR channel modulation by phospholipase-mediated hydrolysis of PIP2 and its synthesis via kinases [49]. It is possible that activation of Gq/11-coupled receptors and the subsequent activation of phospholipase Cβ-mediated PIP2 hydrolysis could inhibit KIR2.2 and 2.3, because they have a relatively low PIP2 affinity [28]. In contrast, KIR2.1 affinity for PIP2 binding is sufficiently high that phospholipase-induced PIP2 hydrolysis may not remove activator PIP2 from these channels [28]. Angiotensin II, vasopressin and GTPγS in the patch pipette inhibit KIR currents in porcine cerebral capillary endothelial cells, in vitro [54]. These data suggest that microvascular endothelial cell KIR channels may be physiologically modulated by activation of G-protein coupled receptors. However, it is not known if these effects were due to stimulation of PIP2 hydrolysis.
Membrane cholesterol also modulates KIR channel function. Increases in membrane cholesterol inhibit, while decreases in membrane cholesterol stimulate currents through KIR2 channels in macrovascular endothelial cells [33,36,95–97] and in heterologous expression systems [94]. The hinge region of M1 (L85, V93, S95 in KIR2.1) and M2 (I166, V167, I175, M183 in KIR2.1) and the region between M1 and the cytosolic domains (L69, A70, V77 in KIR2.1) are the sites where cholesterol interacts with the channels [96] (Figure 1).
KIR channels reside in signaling complexes
KIR2 channels reside in cholesterol-rich lipid rafts [94]. Loss of cholesterol results in channel movement out of these microdomains [109]. The caveolar protein, Caveolin-1, also inhibits KIR2 channel function [45]. Inward rectifier K+ channels exist in membrane signaling microdomains in cardiac myocytes [67,117], where they interact with receptors, other ion channels, protein kinases, etc. [9]. Heterologously expressed KIR2.1 channels interact with AKAP79 that targets these channels to protein complexes, which include PKA, calcineurin and other signaling proteins [22]. The carboxy terminus of KIR2.1 contains the PDZ domain recognition sequence, (E424-S425-E426-I427; Figure 1), that interacts with postsynaptic density protein (PSD) 95 [84]. This adaptor protein, which is present in endothelial cells [110], interacts with AKAPS, nitric oxide synthase and other signaling proteins [11,110]. Thus, KIR2.1 channels are likely located in signaling complexes in endothelial cells, providing significant potential for modulation of channel function, although this has not been explored in microvascular endothelial cells.
Protein kinases modulate KIR channels
Studies in other systems suggest that there is considerable potential for KIR channel regulation by kinases. There is a protein kinase A (PKA) consensus sequence in the C-terminus of KIR2.1 (S425 in the PDZ domain in KIR2.1; Figure 1) [119]. However, the effects of phosphorylation of this residue on KIR channel function is far from clear. Phosphorylation of S425 by PKA inhibits inward KIR currents at potentials more negative than the K+ equilibrium potential [113,118], but increases outward currents at more positive potentials [113] when the channels are expressed in COS cells. Activation of PKA inhibits native KIR channels in the heart [113]. However, in contrast, inward KIR2.1 channels expressed in Xenopus oocytes are activated by PKA [35], whereas native KIR2.1 channels in cultured pulmonary endothelial cells are unaffected by activation of PKA [60]. Thus, how KIR channels are modulated by PKA depends on the particular cell type in which the channels are expressed, and, most likely, also on the composition and configuration of the signaling complexes in which they are located. Regulation of microvascular endothelial cell KIR channels by PKA has not been studied.
There is also a tyrosine kinase consensus sequence in KIR2.1 at Y242 [118]. However, as with PKA, how phosphorylation of this site affects KIR2.1 channel activity remains unclear with evidence for both decreased [118] and increased [123] activity reported. In addition, the tyrosine kinase Src inhibits currents through channels containing KIR2.2 independent from protein kinase C (PKC), but does not inhibit homomeric KIR2.1 channels [125]. In contrast, PKC mediates α1A-adrenergic receptor-induced inhibition of KIR2.3 channels [125]. Also, Gβγ-subunits inhibit KIR2.3-containing channels [17].
Channels containing KIR2.2 or 2.3 are inhibited by PKC [28,126], with no effect on KIR2.1 channels in mammalian expression systems [28,48], or in cultured endothelial cells [60]. However, activation of β3-adrenoreceptors activates KIR2.1 channels through a mechanism involving PKC in Xenopus oocytes [100]. In vascular smooth muscle cells that express KIR2.1 and KIR2.2, hypoosmotic-induced cell swelling inhibits KIR channels through a mechanism involving PKC [120].
There is also evidence for modulation of the expression and function of KIR channels by other protein kinases. Calmodulin-dependent protein kinase II increases protein expression and activates currents through KIR2.1 channels in cultured macrovascular endothelial cells [92]. Surface expression of KIR2.1 is decreased via AMP kinase-dependent phosphorylation of the ubiquitin ligase, Nedd4-2 [3].
Other modulators of KIR channels
Intracellular acidosis inhibits KIR channels through effects on residues in the N-terminal domain of the channel [49]. In taste buds, acid-induced closure of KIR2.1 contributes to acid-induced depolarization that is involved with the transduction of sour taste [121]. Whether pH modulates the function of endothelial KIR channels has not been studied.
Fluid shear stress activates KIR2.1 channels in macrovascular endothelial cells [53,68,90] and in heterologous expression systems through a mechanism that may involve a tyrosine kinase [53]. Whether a similar mechanism functions in microvascular endothelial cells remains to be established, but would be predicted to produce endothelial cell hyperpolarization and vasodilation. Shear stress also modulates transcription of KIR channels in cultured human coronary endothelial cells, with KIR2.2, 2.3 and 2.4 being upregulated by increased shear stress [61]. It is not known if altered shear stress produces similar changes in native microvascular endothelial cells. The impact of sheer stress-induced changes in endothelial KIR channel expression on microvascular endothelial cell function also has not been established. However, such altered KIR channel isoform expression would be predicted to modify the modulation of KIR channel function by protein kinases, for example, potentially depressing or augmenting KIR channel-mediated changes in endothelial cell membrane potential.
Increases in nitric oxide result in S-nitrosylation of a cysteine residue in the amino terminus of KIR2.1 (C76) and an increase in KIR channel activity in the heart and in heterologously expressed channels [40]. This may account for the increase in KIR channel activity induced by NO in vascular smooth muscle KIR channels [101].
KIR channel pharmacology
Extracellular Ba2+ potently blocks KIR channels in a voltage-dependent fashion [43]: at physiological membrane potentials (−30 to −40 mV) the Kd = 19–30 μM for KIR2.1 [2,69]; for KIR2.2, the Kd = 9 μM [69], and for KIR2.3, the Kd = 70 μM [69]. Barium ions also block KATP channels (IC50 = 100 μM [10]). Barium block of KIR2.1 channels involves interactions with two residues, one in the outer vestibule of the channel (E125) and one just before the selectivity filter (T141) (Figure 1) [2]. In addition to Ba2+, extracellular Cs+ ions block KIR channels [49], as they do all K+ channels.
Currents through KIR2 channels are inhibited by ML133 (IC50 = 1.9 μM for KIR 2.1; 2.3 μM for KIR2.2 and 4 μM for KIR2.3) [115]. This compound blocks KIR channel currents in endothelial cells from rat middle cerebral arteries [63], and in rat mesenteric artery endothelial cells [106]. Residues in M2 (D172 and I176 in KIR2.1) are involved in the mechanism of action of ML133 [115]. This compound also blocks KATP channels composed of KIR6.2 subunits (IC50 = 7.7 μM) [115], channels that are likely expressed in some microvascular endothelial cells [38]. Inward rectifier K+ channels are also non-specifically blocked by a number of drugs including: antihistamines such as diphenhydramine and mepyramine; the antimalarial, choloroquin; and the class Ia antiarrhythmic, quinidine [49].
Potassium ions are an important physiological agonist of KIR channels [72], increasing KIR channel conductance proportional to the square root of the extracellular K+ concentration [44,66,72,74,76,98]. Extracellular K+ interacts with a residue near the K+ selectivity filter (R148 in KIR2.1) [49]. Furthermore, increases in extracellular K+ concentration, by moving the K+ equilibrium potential to more positive potentials, will move the negative slope conductance region to more depolarized potentials (Figure 2). This results from interactions of K+ ions with the negatively charged residues that bind Mg2+ and polyamines and which are responsible for inward rectification [108]. The K+-induced shift in the current-voltage relationship will recruit outward current through the channels, also promoting membrane hyperpolarization (Figure 2). Thus, as with vascular smooth muscle KIR channels, endothelial cell KIR channels can serve as sensitive sensors of extracellular K+ concentration, transducing small changes (e.g., 3–5 mM to 8–15 mM K+) into membrane hyperpolarization [72].
The antiarrhythmic drug, flecainide activates cardiac KIR2.1 channels by interacting with a cysteine residue in the carboxy terminus of the channel (C311 in KIR2.1; Figure 1), a residue that is not found in KIR2.2 or 2.3 [13]. This activation results from decreased affinity of the channels for polyamines, like spermine, through an allosteric mechanism [13]. Other drugs, such as the antiarrhythmic, propafenone and the β-adrenergic receptor antagonist, timolol may activate KIR2.1 channels through a similar mechanism [41].
Expression of KIR channels in microvascular endothelial cells
Early studies of cultured cells suggested that microvascular endothelial cells might not express KIR channels [1,87]. However, more recent investigation of freshly isolated microvascular endothelial cells have clearly shown Ba2+-sensitive KIR channel currents [18,21,54,55,63,70,72,114] (Figure 2). Barium also blocks endothelial cell KIR currents in rat superior mesenteric arteries, in situ [15].
Capillary endothelial cells reportedly express mRNA for KIR2.1, 2.2 and possibly 2.3 [69,81]. Rat cremaster muscle arteriolar endothelial cells express protein for KIR2.1, but not KIR2.2 (Figure 3). Similar results were observed in mouse arteriolar endothelial cells [57]. Inward rectifier K+ currents are blocked by the selective KIR2 inhibitor, ML 133 [115], in rat middle cerebral artery [63] and mouse 3rd-order mesenteric artery [106] endothelial cells. Knockout of endothelial cell expression of KIR2.1 suppresses Ba2+-sensitive KIR currents in mouse 3rd-order mesenteric artery [106] and brain capillary [70] endothelial cells. Taken together, these data indicate that KIR2.1 is an essential subunit of the native endothelial KIR channels expressed in rats and mice. The presence and functions of other KIR2 channel subunits in microvascular endothelial cells remains to be established.
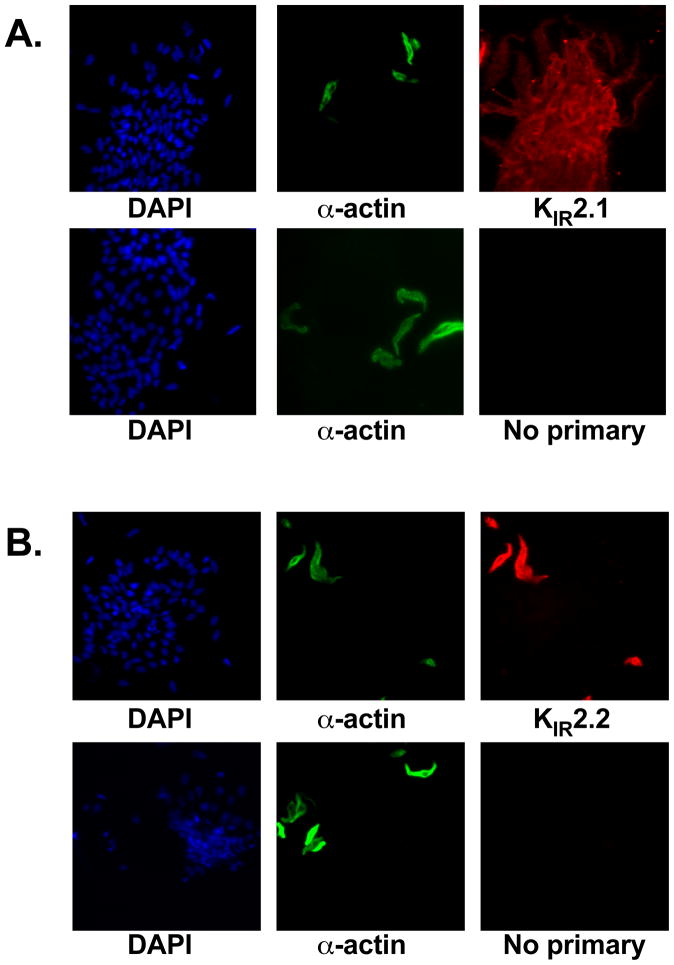
Figure 3.
Expression of KIR2.1 in arteriolar endothelial cells – Shown are fluorescent micrographs of enzymatically isolated rat cremaster arteriolar smooth muscle and endothelial cells that were fixed in 4% paraformaldehyde, permeabilized with Triton X-100, and labeled with DAPI (all cell nuclei – Left panels), FITC-labeled primary antibody for α-smooth muscle actin to positively identify smooth muscle cells (1:1000 – Sigma, Middle Panels), and primary antibodies for (A) KIR2.1 (1:200 - Alomone) and (B) KIR2.2 (1:400 – Alomone) (Right Panels). The secondary antibodies for the KIR channels were Texas-red-labeled donkey anti-rabbit (Jackson Immunoresearch). Bottom row of panels in A and B shows results in the absence of primary antibodies. Panel A shows that endothelial cells display KIR2.1 immunoreactivity, consistent with expression of KIR2.1, while staining of smooth muscle cells in the same preparation was weak. Panel B shows that KIR2.2 immunoreactivity is not present in endothelial cells, but is robustly expressed in smooth muscle cells as a positive control. Data are representative of 3 experiments. Similar results were obtained for mouse arteriolar endothelial cells [55].
Functions of endothelial KIR channels
Vascular smooth muscle KIR channels amplify hyperpolarization induced by the activation of other K+ channels, and transduce small increases in extracellular K+ into cell hyperpolarization [59,62,72,85,104,122]. However, the function of endothelial KIR channels is not as clear, based solely on studies utilizing rat and mouse mesenteric arteries where functional KIR channels are confined to the endothelium [18,25,27,42,104,106,107]. Barium attenuates endothelium-dependent hyperpolarization and conducted vasodilation in rat mesenteric arteries in normotensive rats [42]. In addition, Ba2+ inhibits K+-induced hyperpolarization and vasodilation in these arteries [25,42]. Studies in mouse 3rd-order mesenteric arteries demonstrate that endothelial cell KIR channels amplify the effects of drugs that act via endothelial cell hyperpolarization [106]. Endothelial KIR channels can also mediate K+-induced vasodilation in these vessels [106]. Importantly, Sonkusare et al. [106] showed that endothelial cell-selective knockout of KIR2.1 abolished these effects, demonstrating the crucial role for KIR2.1 in murine resistance artery endothelial cells as end-stage boosters of membrane hyperpolarization. These studies are consistent with a significant role for endothelial cell KIR channels in the vasoreactivity of small mesenteric arteries.
In contrast, other studies in rat mesenteric arteries do not support a major role for endothelial cell KIR channels. Takano, et al. [107] found that Ba2+ had no effect on conducted vasodilation induced by agents that hyperpolarized either endothelial cells or smooth muscle cells. Barium also had no effect on conducted dilation or K+-induced vasodilation in mesenteric arteries, in contrast to cerebral and coronary arteries that show robust expression of smooth muscle KIR channels [104]. Thus, there is evidence both for and against a significant role for endothelial cell KIR channels.
The level of agonist-induced activation of the smooth muscle could be one possible explanation that might reconcile the opposing views of the role-played by endothelial KIR channels. Increased agonist-induced activation of smooth muscle has been shown to inhibit Ba2+-sensitive K+-induced dilation of rat mesenteric arteries [25]. However, Goto et al. [42] (who observed a role for endothelial KIR channels), Takano et al. [107] and Smith et al. [104] (who both found no role for endothelial KIR channels) used similar concentrations of phenylephrine (~1μM) to pre-constrict their rat mesenteric arteries, and yet observed disparate results. Thus, there does not appear to be a simple reason that can reconcile these opposing views.
Nonetheless, the disparate findings outlined in the preceding paragraphs do suggest that endothelial cell KIR channel function may be modulated, allowing fine-tuning of vascular function to maintain homeostasis, as well as dysregulation of these channels in disease states. Consistent with this latter proposition, endothelium-dependent hyperpolarization and conducted vasodilation are depressed in mesenteric arteries from spontaneously hypertensive rats, and Ba2+ is without effect in these arteries suggesting that hypertension decreases endothelial cell KIR channel function [42]. The mechanism responsible for the hypertension-induced KIR channel dysfunction has not been determined.
Ischemia [91] and stress [71] also depress KIR channel function in cerebral vascular smooth muscle cells. The stress-induced suppression of KIR channel function results from glucocorticoid-mediated decreases in KIR2.1 channel transcript levels and reduced expression of functional KIR channels in the smooth muscle cells [71]. Diabetes alters retinal pericyte KIR channel function through increased polyamine synthesis and increased inward rectification [79]. Mutations in KCNJ2 result in a multi-system disorder (Andersen-Tawil syndrome) that includes cardiac arrhythmias, periodic paralysis and dysmorphogenesis related to decreased KIR channel function [111]. These data support the hypothesis that KIR channels are targets for modulation during disease states, and that mutations that lead to altered KIR channel function produce significant pathologies. The impact of diseases and mutations on microvascular endothelial cell KIR channel function remains to be established.
In arterioles, endothelial cell KIR channels also may be positioned to sense changes in extracellular K+ concentration in the restricted space between the endothelium and overlying smooth muscle cells, as has been hypothesized for smooth muscle KIR channels [30] (Figure 4). As noted above, in mesenteric arteries, endothelial KIR channels have been shown to mediate dilation induced by elevated extracellular K+ [25,42]. Thus, local increases in K+ produced by the activity of other endothelial cell or smooth muscle cell K+ channels also may activate endothelial cell KIR channels (Figure 4). This provides another mechanism (in addition to activation by hyperpolarization), to amplify the activity of other K+ channels, in either the endothelium or smooth muscle layers, and could contribute to the regulation of endothelial cell membrane potential and other microvascular functions.
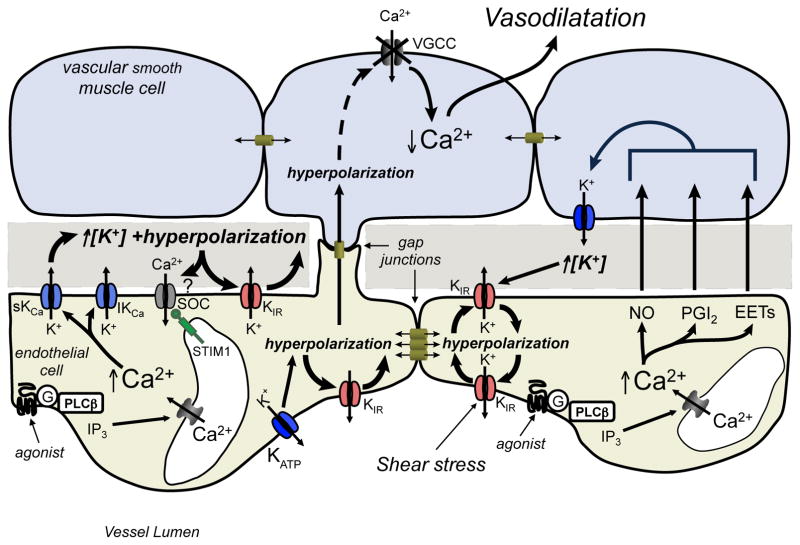
Figure 4.
Endothelial KIR channels: Amplifiers and sensors of extracellular K+. Hyperpolarization of endothelial cells, due to Ca2+-dependent activation of endothelial cell IKCa and SKCa, or by activation of other endothelial cell K+ channels such as ATP-dependent K+ channels (KATP) can activate endothelial cell KIR channels, amplifying the initial hyperpolarization in a positive feed-back manner. Conduction of this hyperpolarization to adjacent endothelial cells that are electrically coupled by gap junctions, can also recruit KIR channels amplifying the hyperpolarization and promoting conduction of this electrical signal. Membrane hyperpolarization may promote Ca2+ entry into the endothelial cells through store-operated channels, although this is controversial. The resulting endothelial cell hyperpolarization can then be conducted, through myoendothelial gap junctions, to overlying smooth muscle cells, deactivating smooth muscle voltage-gated Ca2+ channels, reducing intracellular Ca2+ and promoting vasodilatation. Endothelial cell K+ channels also can be recruited by elevation of extracellular K+ concentration in their microenvironment by adjacent endothelial cell or smooth muscle cell K+ channels. This mechanism may allow endothelium-derived vasodilators, such as NO, prostacyclin (PGI2) or epoxides of arachidonic acid (EETs), which act, in part, by activating smooth muscle K+ channels, to utilize endothelial cell KIR channels in their mechanism of action. See text for more information. Figure redrawn and adapted from [56], with permission.
Capillary endothelial cell KIR channels
Cells in excitable tissues like brain, heart and skeletal muscle rely on the opening of K+ channels and efflux of K+ ions to repolarize the cells during each action potential. During periods of increased activity, this results in accumulation of K+ in the interstitium that can routinely be on the order of 8–10 mM, more than sufficient to activate KIR channels [72]. Expression of KIR channels in capillary endothelial cells opens the exciting possibility that capillaries may be ideally positioned to sense these increases in extracellular K+, transducing this signal into hyperpolarization that could be transmitted, via gap junctions, to upstream arterioles to elicit vasodilation and increases in capillary blood flow to match the increased activity of the tissue (functional hyperemia) [72].
This hypothesis has recently been tested in mouse cerebral microcirculation. Using a novel ex vivo system composed of pressurized cerebral parenchymal arterioles with attached capillaries, Dabertrand and colleagues [21] have shown that microapplication of 10 mM K+ onto the capillaries results in vasodilation of the upstream arterioles that can be blocked by Ba2+ and which is absent in vessels isolated from endothelial cell KIR2.1−/− mice. Similarly, Longden et al, [70] demonstrated that application of 10 mM K+ to brain capillaries, in vivo, results in upstream arteriolar dilation and increased red blood cell flux through the capillaries. Whether similar mechanisms operate in heart and skeletal muscle remains to be established. Coronary capillary endothelial cells display robust KIR channel currents and express mRNA for KIR channels [69,114] suggesting a potential role in the heart. In skeletal muscle, KIR channels have been implicated in both the rapid-onset of vasodilation and steady-state increases in blood flow associated with muscle contraction [4,19,20]. However, the location of the KIR channels (endothelium, smooth muscle, skeletal muscle fibers, etc.) that mediate skeletal muscle functional hyperemia is not known. While, conduction of signals from capillaries to arterioles has long been proposed [5,24,65,105,124], and conduction of electrical activity from capillaries to arterioles has been observed [5], Dabertrand and colleagues’ study is the first to define the ion channel that mediates such a response and to exclude other cell types for initiation of signal transmission from capillaries to arterioles.
Conclusions
While KIR channel expression in endothelial cells has been known for some time, the physiological function of these ion channels is just beginning to be unraveled. Current evidence suggests that these K+ channels serve to boost hyperpolarization induced by opening of other K+ channels or transporters (such as the Na+/K+ ATPase), and to transduce changes in extracellular K+ concentration into endothelial cell membrane hyperpolarization. The hyperpolarization booster function of KIR channels will be particularly effective for small hyperpolarizations due to opening of only few K+ channels, for example. Large hyperpolarizations, that drive the membrane potential close to the K+ equilibrium potential on their own, will be little affected by the recruitment of current through KIR channels, because of the shape of the KIR channel current-voltage relationship (Figure 2).
These functions position endothelial cell KIR channels (along with their smooth muscle counterparts) to be involved in the mechanism of action of all hyperpolarizing vasodilators, with modulation of KIR channel function providing an additional mechanism to fine tune the reactivity of arterioles in the microcirculation. Endothelial KIR channels also may contribute to conduction of hyperpolarization along the wall of microvessels, participating in the coordination of local blood flow regulation. The expression of KIR channels in capillary endothelial cells positions these ion channels to play a major role in sensing extracellular K+ and contributing to functional hyperemia in electrically active tissues such as the brain, heart and skeletal muscle.
How the expression and function of native microvascular endothelial cell KIR channels is modulated during cell signaling processes in health and disease remains to be established. However, based on studies in other systems, it seems likely that microvascular endothelial cell KIR channels also may be modulated in diseases like hypertension, diabetes, and stress. It also is likely that endothelial KIR channels reside in macromolecular signaling complexes. However, knowledge of the protein partners with which these channels interact is simply lacking. At a more fundamental level, our knowledge and understanding of the expression and function of the full complement of ion channels in microvascular endothelial cells, smooth muscle cells and pericytes remains in its infancy. Molecular approaches need to be applied to define the complete ion channel transcriptome and proteome in native, not cultured microvascular cells, in multiple vascular beds. For example, we know much about the function of endothelial KIR channels from the study of mesenteric arteries. However, how these findings apply to other vascular beds, is simply not known. Functional assays of ion channel function using patch-clamp approaches need to be applied so that the actual currents, in the cells of interest, which are responsible for a physiological response, are identified and characterized to verify what is often inferred from the application of pharmacology to complex systems. This will mean developing new, or adapting old techniques to isolate microvascular cells from the particular vessels of interest, including capillaries. The use of cell specific and hopefully, conditional knockouts of KIR channels should help delineate the function of KIR channels in specific microvascular cells. However, given that these ion channels are likely part of much larger signaling complexes, such knockouts have the potential to disrupt more than just the function of a single ion channel. Thus, multiple approaches, including careful pharmacology, must be applied, rather than relying on any single strategy. Understanding the control of expression and function of microvascular endothelial cell KIR channels will improve our understanding of local blood flow control in health and disease, and may, in the future, provide new targets for the development of therapeutics directed at these important ion channels.
Acknowledgments
Special thanks are offered to Dr. Erika Boerman and Erica Lange, M.S. for performing the experiments shown in Figure 3. Dr. Jackson was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award numbers RO1 HL32469 and P01 HL070687. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts:
None
References
- 1.Adams DJ, Hill MA. Potassium channels and membrane potential in the modulation of intracellular calcium in vascular endothelial cells. J Cardiovasc Electrophysiol. 2004;15:598–610. doi: 10.1046/j.1540-8167.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- 2.Alagem N, Dvir M, Reuveny E. Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. Journal of Physiology-London. 2001;534:381–393. doi: 10.1111/j.1469-7793.2001.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alesutan I, Munoz C, Sopjani M, Dermaku-Sopjani M, Michael D, Fraser S, Kemp BE, Seebohm G, Foller M, Lang F. Inhibition of Kir2.1 (KCNJ2) by the AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;408:505–510. doi: 10.1016/j.bbrc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach JM, McGahren ED, Duling BR. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am J Physiol. 1998;275:H1489–1496. doi: 10.1152/ajpheart.1998.275.4.H1489. [DOI] [PubMed] [Google Scholar]
- 6.Behringer EJ, Segal SS. Membrane potential governs calcium influx into microvascular endothelium: integral role for muscarinic receptor activation. J Physiol. 2015;593:4531–4548. doi: 10.1113/JP271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergdahl A, Gomez MF, Wihlborg A-K, Erlinge D, Eyjolfson A, Xu S-Z, Beech DJ, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005;288:C872–880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- 8.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev. 2014;66:513–569. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonev AD, Nelson MT. ATP-sensitive potassium channels in smooth muscle cells from guinea pig urinary bladder. Am J Physiol. 1993;264:C1190–1200. doi: 10.1152/ajpcell.1993.264.5.C1190. [DOI] [PubMed] [Google Scholar]
- 11.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 12.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caballero R, Dolz-Gaiton P, Gomez R, Amoros I, Barana A, Gonzalez de la Fuente M, Osuna L, Duarte J, Lopez-Izquierdo A, Moraleda I, Galvez E, Sanchez-Chapula JA, Tamargo J, Delpon E. Flecainide increases Kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification. Proc Natl Acad Sci USA. 2010;107:15631–15636. doi: 10.1073/pnas.1004021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol. 2003;285:C959–967. doi: 10.1152/ajpcell.00511.2002. [DOI] [PubMed] [Google Scholar]
- 15.Climent B, Zsiros E, Stankevicius E, de la Villa P, Panyi G, Simonsen U, Garcia-Sacristan A, Rivera L. Intact rat superior mesenteric artery endothelium is an electrical syncytium and expresses strong inward rectifier K+ conductance. Biochem Biophys Res Commun. 2011;410:501–507. doi: 10.1016/j.bbrc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Cohen KD, Jackson WF. Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells. Microcirculation. 2005;12:169–182. doi: 10.1080/10739680590904973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen NA, Sha Q, Makhina EN, Lopatin AN, Linder ME, Snyder SH, Nichols CG. Inhibition of an inward rectifier potassium channel (Kir2.3) by G-protein betagamma subunits. J Biol Chem. 1996;271:32301–32305. doi: 10.1074/jbc.271.50.32301. [DOI] [PubMed] [Google Scholar]
- 18.Crane GJ, Walker SD, Dora KA, Garland CJ. Evidence for a differential cellular distribution of inward rectifier K channels in the rat isolated mesenteric artery. J Vasc Res. 2003;40:159–168. doi: 10.1159/000070713. [DOI] [PubMed] [Google Scholar]
- 19.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2013;305:H29–40. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steady-state exercise hyperemia in humans. Am J Physiol Heart Circ Physiol. 2014;307:H782–791. doi: 10.1152/ajpheart.00212.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabertrand F, Brayden JE, Nelson MT. Novel Intact Ex Vivo Preparation of Pressurized Intracerebral Arterioles and Capillaries Reveals Conducted Upstream Vasodilation and Endothelium-dependent Vasodilation Amplification by Inward Rectifier Potassium Channels. Journal of General Physiology. 2015;146:4a–4a. [Google Scholar]
- 22.Dart C, Leyland ML. Targeting of an A kinase-anchoring protein, AKAP79, to an inwardly rectifying potassium channel, Kir2.1. J Biol Chem. 2001;276:20499–20505. doi: 10.1074/jbc.M101425200. [DOI] [PubMed] [Google Scholar]
- 23.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich HH, Tyml K. Capillary as a Communicating Medium in the Microvasculature. Microvascular Research. 1992;43:87–99. doi: 10.1016/0026-2862(92)90008-d. [DOI] [PubMed] [Google Scholar]
- 25.Dora KA, Garland CJ. Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol. 2001;280:H2424–2429. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- 26.Dora KA, Garland CJ. Linking hyperpolarization to endothelial cell calcium events in arterioles. Microcirculation. 2013;20:248–256. doi: 10.1111/micc.12041. [DOI] [PubMed] [Google Scholar]
- 27.Doughty JM, Boyle JP, Langton PD. Blockade of chloride channels reveals relaxations of rat small mesenteric arteries to raised potassium. Br J Pharmacol. 2001;132:293–301. doi: 10.1038/sj.bjp.0703769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 29.Edwards FR, Hirst GD. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 31.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 32.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 33.Epshtein Y, Chopra AP, Rosenhouse-Dantsker A, Kowalsky GB, Logothetis DE, Levitan I. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc Natl Acad Sci USA. 2009;106:8055–8060. doi: 10.1073/pnas.0809847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakler B, Brandle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- 35.Fakler B, Brandle U, Glowatzki E, Zenner HP, Ruppersberg JP. Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron. 1994;13:1413–1420. doi: 10.1016/0896-6273(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 36.Fang Y, Mohler ER, 3rd, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circ Res. 2006;98:1064–1071. doi: 10.1161/01.RES.0000218776.87842.43. [DOI] [PubMed] [Google Scholar]
- 37.Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- 38.Foster MN, Coetzee WA. KATP Channels in the Cardiovascular System. Physiol Rev. 2016;96:177–252. doi: 10.1152/physrev.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goddard LM, Iruela-Arispe ML. Cellular and molecular regulation of vascular permeability. Thromb Haemost. 2013;109:407–415. doi: 10.1160/TH12-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez R, Caballero R, Barana A, Amoros I, Calvo E, Lopez JA, Klein H, Vaquero M, Osuna L, Atienza F, Almendral J, Pinto A, Tamargo J, Delpon E. Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ Res. 2009;105:383–392. doi: 10.1161/CIRCRESAHA.109.197558. [DOI] [PubMed] [Google Scholar]
- 41.Gomez R, Caballero R, Barana A, Amoros I, De Palm SH, Matamoros M, Nunez M, Perez-Hernandez M, Iriepa I, Tamargo J, Delpon E. Structural basis of drugs that increase cardiac inward rectifier Kir2.1 currents. Cardiovasc Res. 2014;104:337–346. doi: 10.1093/cvr/cvu203. [DOI] [PubMed] [Google Scholar]
- 42.Goto K, Rummery NM, Grayson TH, Hill CE. Attenuation of conducted vasodilatation in rat mesenteric arteries during hypertension: role of inwardly rectifying potassium channels. The Journal of Physiology. 2004;561:215–231. doi: 10.1113/jphysiol.2004.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- 45.Han H, Rosenhouse-Dantsker A, Gnanasambandam R, Epshtein Y, Chen Z, Sachs F, Minshall RD, Levitan I. Silencing of Kir2 channels by caveolin-1: cross-talk with cholesterol. J Physiol. 2014;592:4025–4038. doi: 10.1113/jphysiol.2014.273177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He P, Curry FE. Depolarization modulates endothelial cell calcium influx and microvessel permeability. AmJPhysiolHeart CircPhysiol. 1991;261:H1246–H1254. doi: 10.1152/ajpheart.1991.261.4.H1246. [DOI] [PubMed] [Google Scholar]
- 47.He P, Curry FE. Endothelial cell hyperpolarization increases [Ca2+]i and venular microvessel permeability. Journal of Applied Physiology. 1994;76:2288–2297. doi: 10.1152/jappl.1994.76.6.2288. [DOI] [PubMed] [Google Scholar]
- 48.Henry P, Pearson WL, Nichols CG. Protein kinase C inhibition of cloned inward rectifier (HRK1/KIR2.3) K+ channels expressed in Xenopus oocytes. J Physiol. 1996;495(Pt 3):681–688. doi: 10.1113/jphysiol.1996.sp021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 50.Hille B. Ionic channels of excitable membranes. 3. Sunderland, Mass: Sinauer; 2001. [Google Scholar]
- 51.Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 53.Hoger JH, Ilyin VI, Forsyth S, Hoger A. Shear stress regulates the endothelial Kir2.1 ion channel. Proc Natl Acad Sci USA. 2002;99:7780–7785. doi: 10.1073/pnas.102184999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoyer J, Popp R, Meyer J, Galla HJ, Gogelein H. Angiotensin II, vasopressin and GTP[gamma-S] inhibit inward-rectifying K+ channels in porcine cerebral capillary endothelial cells. J Membr Biol. 1991;123:55–62. doi: 10.1007/BF01993963. [DOI] [PubMed] [Google Scholar]
- 55.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson WF. Silent Inward Rectifier K+ Channels in Hypercholesterolemia. Circ Res. 2006;98:982–984. doi: 10.1161/01.RES.0000222140.93190.7f. [DOI] [PubMed] [Google Scholar]
- 57.Jackson WF. Endothelial cell ion channel expression and function in arterioles and resistance arteries. In: Levitan I, Dopico AM, editors. Vascular Ion Channels in Physiology and Disease. 1. Switzerland: Springer International Publishing; 2016. p. 431. [Google Scholar]
- 58.Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291:H1319–1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- 59.Jantzi MC, Brett SE, Jackson WF, Corteling RL, Vigmond EJ, Welsh DG. Inward Rectifying Potassium Channels Facilitate Cell-to-Cell Communication in Hamster Retractor Muscle Feed Arteries. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- 60.Kamouchi M, Van Den Bremt K, Eggermont J, Droogmans G, Nilius B. Modulation of inwardly rectifying potassium channels in cultured bovine pulmonary artery endothelial cells. J Physiol. 1997;504(Pt 3):545–556. doi: 10.1111/j.1469-7793.1997.545bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kefaloyianni E, Coetzee WA. Transcriptional remodeling of ion channel subunits by flow adaptation in human coronary artery endothelial cells. J Vasc Res. 2011;48:357–367. doi: 10.1159/000323475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 1996;492(Pt 2):419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kochukov MY, Balasubramanian A, Abramowitz J, Birnbaumer L, Marrelli SP. Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 65.Krogh A. The anatomy and physiology of capillaries. New Haven: Yale University Press; 1922. Rev. and enl. ed. edn. [Google Scholar]
- 66.Kubo Y, Baldwin TJ, Nung Jan Y, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- 67.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, 3rd, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J Biol Chem. 2004;279:22331–22346. doi: 10.1074/jbc.M400285200. [DOI] [PubMed] [Google Scholar]
- 68.Lieu DK, Pappone PA, Barakat AI. Differential membrane potential and ion current responses to different types of shear stress in vascular endothelial cells. Am J Physiol Cell Physiol. 2004;286:C1367–1375. doi: 10.1152/ajpcell.00243.2003. [DOI] [PubMed] [Google Scholar]
- 69.Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Longden TA, Dabertrand F, Gonzales AL, Koide M, Nelson MT. Potassium Sensing by Capillary KIR Channels Regulates Cerebral Blood Flow. Journal of General Physiology. 2015;146:10a–10a. [Google Scholar]
- 71.Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proceedings of the National Academy of Sciences. 2014;111:7462–7467. doi: 10.1073/pnas.1401811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Longden TA, Nelson MT. Vascular inward rectifier k(+) channels as external k(+) sensors in the control of cerebral blood flow. Microcirculation. 2015;22:183–196. doi: 10.1111/micc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopatin AN, Makhina EN, Nichols CG. Potassium Channel Block by Cytoplasmic Polyamines as the Mechanism of Intrinsic Rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 74.Lopatin AN, Nichols CG. [K+] dependence of open-channel conductance in cloned inward rectifier potassium channels (IRK1, Kir2.1) Biophys J. 1996;71:682–694. doi: 10.1016/S0006-3495(96)79268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 76.Makhina EN, Kelly AJ, Lopatin AN, Mercer RW, Nichols CG. Cloning and expression of a novel human brain inward rectifier potassium channel. J Biol Chem. 1994;269:20468–20474. [PubMed] [Google Scholar]
- 77.Marrelli SP, Eckmann MS, Hunte MS. Role of Endothelial Intermediate Conductance KCa Channels in Cerebral EDHF-Mediated Dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- 78.Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- 79.Matsushita K, Puro DG. Topographical heterogeneity of KIR currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol (Lond) 2006;573:483–495. doi: 10.1113/jphysiol.2006.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McSherry IN, Spitaler MM, Takano H, Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium. 2005;38:23–33. doi: 10.1016/j.ceca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Millar ID, Wang S, Brown PD, Barrand MA, Hladky SB. Kv1 and Kir2 potassium channels are expressed in rat brain endothelial cells. Pflugers Arch. 2008;456:379–391. doi: 10.1007/s00424-007-0377-1. [DOI] [PubMed] [Google Scholar]
- 82.Minami T. Calcineurin-NFAT activation and DSCR-1 auto-inhibitory loop: how is homoeostasis regulated? J Biochem. 2014;155:217–226. doi: 10.1093/jb/mvu006. [DOI] [PubMed] [Google Scholar]
- 83.Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent patents on anti-cancer drug discovery. 2006;1:105–119. doi: 10.2174/157489206775246502. [DOI] [PubMed] [Google Scholar]
- 84.Nehring RB, Wischmeyer E, Doring F, Veh RW, Sheng M, Karschin A. Neuronal inwardly rectifying K(+) channels differentially couple to PDZ proteins of the PSD-95/SAP90 family. J Neurosci. 2000;20:156–162. doi: 10.1523/JNEUROSCI.20-01-00156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 86.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 87.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 88.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium. 2003;10:5–15. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 89.Nilius B, Viana F, Droogmans G. Ion channels in vascular endothelium. Annu Rev Physiol. 1997;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- 90.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 91.Povlsen GK, Longden TA, Bonev AD, Hill-Eubanks DC, Nelson MT. Uncoupling of neurovascular communication after transient global cerebral ischemia is caused by impaired parenchymal smooth muscle Kir channel function. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16638350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qu L, Yu L, Wang Y, Jin X, Zhang Q, Lu P, Yu X, Zhong W, Zheng X, Cui N, Jiang C, Zhu D. Inward Rectifier K+ Currents Are Regulated by CaMKII in Endothelial Cells of Primarily Cultured Bovine Pulmonary Arteries. PLoS One. 2015;10:e0145508. doi: 10.1371/journal.pone.0145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quinlan KL, Naik SM, Cannon G, Armstrong CA, Bunnett NW, Ansel JC, Caughman SW. Substance P activates coincident NF-AT- and NF-kappa B-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol. 1999;163:5656–5665. [PubMed] [Google Scholar]
- 94.Romanenko VG, Fang Y, Byfield F, Travis AJ, Vandenberg CA, Rothblat GH, Levitan I. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys J. 2004;87:3850–3861. doi: 10.1529/biophysj.104.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol. Biophys J. 2002;83:3211–3222. doi: 10.1016/S0006-3495(02)75323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenhouse-Dantsker A, Noskov S, Durdagi S, Logothetis DE, Levitan I. Identification of novel cholesterol-binding regions in Kir2 channels. J Biol Chem. 2013;288:31154–31164. doi: 10.1074/jbc.M113.496117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosenhouse-Dantsker A, Noskov S, Logothetis DE, Levitan I. Cholesterol sensitivity of KIR2.1 depends on functional inter-links between the N and C termini. Channels. 2013;7:303–312. doi: 10.4161/chan.25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakmann B, Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol. 2009;297:H1–7. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- 100.Scherer D, Kiesecker C, Kulzer M, Gunth M, Scholz EP, Kathofer S, Thomas D, Maurer M, Kreuzer J, Bauer A, Katus HA, Karle CA, Zitron E. Activation of inwardly rectifying Kir2.x potassium channels by beta 3-adrenoceptors is mediated via different signaling pathways with a predominant role of PKC for Kir2.1 and of PKA for Kir2.2. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:311–322. doi: 10.1007/s00210-007-0167-5. [DOI] [PubMed] [Google Scholar]
- 101.Schubert R, Krien U, Wulfsen I, Schiemann D, Lehmann G, Ulfig N, Veh RW, Schwarz JR, Gago H. Nitric oxide donor sodium nitroprusside dilates rat small arteries by activation of inward rectifier potassium channels. Hypertension. 2004;43:891–896. doi: 10.1161/01.HYP.0000121882.42731.6b. [DOI] [PubMed] [Google Scholar]
- 102.Segal SS, Beny JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol. 1992;263:H1–7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- 103.Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 104.Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586:1147–1160. doi: 10.1113/jphysiol.2007.145474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song H, Tyml K. Evidence for sensing and integration of biological signals by the capillary network. Am J Physiol. 1993;265:H1235–1242. doi: 10.1152/ajpheart.1993.265.4.H1235. [DOI] [PubMed] [Google Scholar]
- 106.Sonkusare SK, Dalsgaard T, Bonev AD, Nelson MT. Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J Physiol. 2016 doi: 10.1113/JP271652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takano H, Dora KA, Spitaler MM, Garland CJ. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J Physiol. 2004;556:887–903. doi: 10.1113/jphysiol.2003.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science (New York, NY) 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tikku S, Epshtein Y, Collins H, Travis AJ, Rothblat GH, Levitan I. Relationship between Kir2.1/Kir2.3 activity and their distributions between cholesterol-rich and cholesterol-poor membrane domains. Am J Physiol Cell Physiol. 2007;293:C440–450. doi: 10.1152/ajpcell.00492.2006. [DOI] [PubMed] [Google Scholar]
- 110.Tran QK, VerMeer M, Burgard MA, Hassan AB, Giles J. Hetero-oligomeric Complex between the G Protein-coupled Estrogen Receptor 1 and the Plasma Membrane Ca2+-ATPase 4b. J Biol Chem. 2015;290:13293–13307. doi: 10.1074/jbc.M114.628743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tristani-Firouzi M, Etheridge SP. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch. 2010;460:289–294. doi: 10.1007/s00424-010-0820-6. [DOI] [PubMed] [Google Scholar]
- 112.Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci USA. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vega AL, Tester DJ, Ackerman MJ, Makielski JC. Protein kinase A-dependent biophysical phenotype for V227F-KCNJ2 mutation in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2009;2:540–547. doi: 10.1161/CIRCEP.109.872309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Beckerath N, Dittrich M, Klieber HG, Daut J. Inwardly rectifying K+ channels in freshly dissociated coronary endothelial cells from guinea-pig heart. J Physiol. 1996;491(Pt 2):357–365. doi: 10.1113/jphysiol.1996.sp021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang HR, Wu M, Yu H, Long S, Stevens A, Engers DW, Sackin H, Daniels JS, Dawson ES, Hopkins CR, Lindsley CW, Li M, McManus OB. Selective inhibition of the K(ir)2 family of inward rectifier potassium channels by a small molecule probe: the discovery, SAR, and pharmacological characterization of ML133. ACS Chem Biol. 2011;6:845–856. doi: 10.1021/cb200146a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol. 1998;274:H178–186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 117.Willis BC, Ponce-Balbuena D, Jalife J. Protein assemblies of sodium and inward rectifier potassium channels control cardiac excitability and arrhythmogenesis. Am J Physiol Heart Circ Physiol. 2015;308:H1463–1473. doi: 10.1152/ajpheart.00176.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wischmeyer E, Doring F, Karschin A. Acute suppression of inwardly rectifying Kir2.1 channels by direct tyrosine kinase phosphorylation. J Biol Chem. 1998;273:34063–34068. doi: 10.1074/jbc.273.51.34063. [DOI] [PubMed] [Google Scholar]
- 119.Wischmeyer E, Karschin A. Receptor stimulation causes slow inhibition of IRK1 inwardly rectifying K+ channels by direct protein kinase A-mediated phosphorylation. Proc Natl Acad Sci USA. 1996;93:5819–5823. doi: 10.1073/pnas.93.12.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu BN, Luykenaar KD, Brayden JE, Giles WR, Corteling RL, Wiehler WB, Welsh DG. Hyposmotic challenge inhibits inward rectifying K+ channels in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H1085–1094. doi: 10.1152/ajpheart.00926.2006. [DOI] [PubMed] [Google Scholar]
- 121.Ye W, Chang RB, Bushman JD, Tu YH, Mulhall EM, Wilson CE, Cooper AJ, Chick WS, Hill-Eubanks DC, Nelson MT, Kinnamon SC, Liman ER. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc Natl Acad Sci USA. 2016;113:E229–238. doi: 10.1073/pnas.1514282112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 123.Zhang DY, Wu W, Deng XL, Lau CP, Li GR. Genistein and tyrphostin AG556 inhibit inwardly-rectifying Kir2.1 channels expressed in HEK 293 cells via protein tyrosine kinase inhibition. Biochim Biophys Acta. 2011;1808:1993–1999. doi: 10.1016/j.bbamem.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 124.Zhang T, Wu DM, Xu GZ, Puro DG. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol. 2011;589:2383–2399. doi: 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zitron E, Gunth M, Scherer D, Kiesecker C, Kulzer M, Bloehs R, Scholz EP, Thomas D, Weidenhammer C, Kathofer S, Bauer A, Katus HA, Karle CA. Kir2.x inward rectifier potassium channels are differentially regulated by adrenergic alpha1A receptors. J Mol Cell Cardiol. 2008;44:84–94. doi: 10.1016/j.yjmcc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 126.Zitron E, Kiesecker C, Luck S, Kathofer S, Thomas D, Kreye VA, Kiehn J, Katus HA, Schoels W, Karle CA. Human cardiac inwardly rectifying current IKir2.2 is upregulated by activation of protein kinase A. Cardiovasc Res. 2004;63:520–527. doi: 10.1016/j.cardiores.2004.02.015. [DOI] [PubMed] [Google Scholar]