Abstract
Although HIV services are expanding, few have reached the scale necessary to support universal viral suppression of individuals living with HIV. The purpose of this systematic review was to summarize the qualitative evidence evaluating public health HIV interventions to enhance linkage to care, antiretroviral drug (ARV) adherence, and retention in care. We searched 19 databases without language restrictions. The review collated data from three separate qualitative evidence reviews addressing each of the three outcomes along the care continuum. 21,738 citations were identified and 24 studies were included in the evidence review. Among low and middle-income countries in Africa, men living with HIV had decreased engagement in interventions compared to women and this lack of engagement among men also influenced the willingness of their partners to engage in services. Four structural issues (poverty, unstable housing, food insecurity, lack of transportation) mediated the feasibility and acceptability of public health HIV interventions. Individuals living with HIV identified unmet mental health needs that interfered with their ability to access HIV services. Persistent social and cultural factors contribute to disparities in HIV outcomes across the continuum of care, shaping the context of service delivery among important subpopulations.
Keywords: HIV, Public health, Intervention, Qualitative, Systematic review
Highlights
-
•
Among low and middle-income countries in Africa, men living with HIV had decreased engagement in HIV interventions compared to women.
-
•
Several structural issues mediated the feasibility and acceptability of HIV interventions.
-
•
Persistent social and cultural factors contribute to disparities in HIV outcomes across the continuum of care.
Qualitative research such as in-depth interviews can be useful to better understand social factors that influence the success or failure of public health interventions. This study combined data from three separate systematic reviews of qualitative evidence. The purpose was to better understand the social context of interventions focused on improving HIV services. Men living with HIV were less likely to receive HIV services like routine HIV testing compared to women. A wide range of non-health issues, such as access to poverty and unstable housing, influenced the feasibility of HIV interventions.
1. Introduction
Although HIV services are expanding across the world, few have reached the scale necessary to support universal viral suppression of individuals living with HIV. Striking disparities in HIV service delivery have been prominent since the earliest days of the epidemic (Cleary, 2010, Farmer, 1992). For example, poor people living with HIV have faced challenges receiving services at multiple points along the HIV continuum of care (Boyer et al., 2009, Marcellin et al., 2008, Souteyrand et al., 2008). Public health interventions may facilitate more equitable health service delivery of both HIV and non-HIV related prevention and care. Widespread and equitable HIV services will be essential for achieving the UNAIDS goal of 90% of individuals living with HIV virally suppressed by 2020 (UNAIDS, 2014).
Evidence from the last three decades has underlined the importance of improving linkage to care, adherence, and retention. Linkage to care is confirmation of HIV infection or first HIV-specific clinical visit (WHO, 2013). Antiretroviral (ARV) adherence is the extent to which taking ARVs corresponds with agreed recommendations from a provider (World Health Organization (WHO), 2014). Retention in HIV care is the continued engagement in health services, from enrollment in care to discharge or death (Stricker et al., 2014). Previous systematic reviews have examined facilitators and barriers at each of these three separate steps within the HIV continuum of care (Tso et al., 2016, Ma et al., 2016, Hall et al., n.d). Although each of these steps is important, comprehensive interventions that address multiple steps are necessary. The current challenge is to integrate strategies at each of these points into sustainable systems for improving HIV service delivery (Piot et al., 2015). Few reviews have holistically examined facilitators and barriers that are relevant across multiple steps within the HIV continuum of care.
Qualitative systematic reviews play an important role in evaluating public health HIV interventions because of three reasons. First, qualitative systematic reviews examine the social context that facilitates or stymies service delivery. This local social context is not captured in quantitative reviews of HIV interventions (Govindasamy et al., 2012, Govindasamy et al., 2014, Barnighausen et al., 2011, Mills et al., 2006, Ortego et al., 2011, Okeke et al., 2014). Second, qualitative systematic reviews can help identify structural issues to consider when tailoring evidence-based interventions for specific vulnerable groups. Third, review findings from qualitative systematic reviews bring greater rigor and generalizability compared to review findings from individual qualitative research studies. The CERQual (Confidence in the Evidence from Reviews of Qualitative Research) approach, analogous to the GRADE (Grading of Recommendations Assessment Development and Evaluation) approach for quantitative systematic reviews, allows researchers to transparently assess confidence in qualitative systematic review findings (Lewin et al., 2015). Recognizing the importance of qualitative research to evaluate public health HIV interventions, we undertook a meta-synthesis and systematic review of qualitative evaluations of interventions to enhance HIV linkage to care, adherence, and retention.
2. Methods
2.1. Search Strategy
We searched the literature in order to identify all relevant English language studies regardless of publication status. The search was undertaken as a set of three search strategies developed by an information specialist focusing on the following three key aspects of the HIV care continuum: linkage to care (Tso et al., 2016), adherence (Ma et al., 2016), and retention in care (Hall et al., n.d.) (Additional Data 1). The search was implemented according to each of the three subsearches on linkage to care (21 February 2015), adherence (17 February 2015), and retention (17 February 2015). Each subsearch was conducted in the following electronic journal and dissertation/thesis databases: CENTRAL (Cochrane Central Register of Controlled Trials), EMBASE, LILACS, PsycINFO, PubMed (MEDLINE), Web of Science/Web of Social Science, CINAHL, British Nursing Index and Archive, Social Science Citation Index, AMED (Allied and Complementary Medicine Database), DAI (Dissertation Abstracts International), EPPI-Centre (Evidence for Policy and Practice Information and Coordinating Centre), ESRC (Economic and Social Research Council), Global Health (EBSCO), Anthrosource, and JSTOR. Conference proceedings including the Conferences on Retroviruses and Opportunistic Infections (CROI), International AIDS Conference (IAC), and alternating years of International AIDS Society (IAS) clinical meetings were searched during the last two years. Reference lists of included manuscripts were also searched for possible relevant titles.
2.2. Study Selection
Each title was evaluated by a single individual and then all abstracts were evaluated by two independent individuals. Differences of opinion at the abstract level were brought to a third individual to consider and make a final decision. Each full text article was reviewed by two independent reviewers using the following standardized inclusion criteria: (1) focused on evaluating an public health intervention that improves HIV linkage to care, ARV adherence, or retention in care; (2) clearly identified as a qualitative study defined by both qualitative research methodologies (e.g., in-depth interviews, focus groups) and qualitative data analysis (e.g., framework analysis, thematic analysis); (3) presented original research data.
2.3. Methodological Rigor Assessment
The methodological rigor of each qualitative research study was investigated using a set of seven items adapted from the CASP (Critical Appraisal Skills Program) tool (Critical Appraisal Skills Programme, 2006), including the following: (1) qualitative research; (2) study context; (3) researcher reflexivity; (4) sampling methodology; (5) data collection; (6) analysis; (7) sufficient evidence to support claims. This assessment has been used in other qualitative evidence reviews (Munro et al., 2007, Carlsen et al., 2007). Methodological rigor was rated as having minor, moderate, or major concerns based on evaluation of two independent reviewers. Differences of opinion on the methodological rigor were brought to a third independent individual to consider and make a final decision. Methodological rigor was not used to screen studies for exclusion.
2.4. Data Extraction
Data were extracted using a standardized data extraction tool that included the following elements: (1) primary source data (e.g., quotes from individuals living with HIV); (2) secondary source data (e.g., interpretation from authors); (3) characteristics of the studies such as location of trial (country, city), type of intervention, key findings, study size, mean age of participants, analytical methodology, study limitations and implications, and inclusions of subpopulation or key populations. The following potential subgroup analyses were specified a priori: men, key populations (sex workers, people who inject drugs, transgender individuals, men who have sex with men, prisoners), pregnant women and breastfeeding women, children, adolescents, and individuals initiating antiretroviral therapy (ART) with a CD4 count greater than 500 cells/mm3. All data was extracted by two people and differences were resolved by discussing with a third individual. More details about the methodology can be found in the original systematic review papers (Tso et al., 2016, Ma et al., 2016, Hall et al., n.d).
2.5. Qualitative Synthesis
We used a framework thematic synthesis approach based on the theory of conceptual saturation (Booth et al., 2012, Thomas and Harden, 2008). Thematic synthesis is one of the approaches recommended by the Cochrane Qualitative Review Methods Group (Noyes, 2011). The thematic synthesis had three steps. First, the two independent individuals who undertook the respective searches identified cross-cutting themes that emerged independent of intervention type. Then, these 26 initial cross-cutting themes were compared across the three types of interventions to identify themes that were present in interventions at more than one point in the care continuum (e.g., adherence and linkage to care). Finally, once tentative cross-cutting themes were identified, primary (i.e., participant quotes), secondary (i.e., author interpretation), and tertiary (e.g., study team interpretations) data were explored with respect to each theme.
Qualitative review findings were assessed based on the CERQual approach (GRADE-CERQUAL, 2015). This is analogous to the GRADE tool for quantitative systematic reviews, providing a transparent method for evaluating confidence in the review finding based on standardized criteria (GRADE-CERQUAL, 2015). The CERQual approach evaluates specific review findings according to the following four components: (1) methodological rigor of the individual studies contributing to the review finding; (2) adequacy of the data contributing to a review finding; (3) coherence of the review finding; and (4) relevance of the review question. Methodological rigor was evaluated using the adapted CASP tool as described above. Adequacy of the data was evaluated by the number of contributing studies, the richness of individual study data, geographic regions included, and World Bank classification of country where the individual study was undertaken. Coherence was assessed by examining how the review finding formed a clear pattern and accounted for variations as appropriate. Relevance examined the extent to which the review finding was applicable in terms of population, setting, and phenomena of interest. Each of these four components was assessed and determined to have minor, moderate, or major concerns. Each of these four components was assessed to achieve an overall confidence level for each review finding as high, moderate, low, or very low. A draft version of the review was presented at the World Health Organization's ARV Operational Guideline Development Group for feedback. Subgroup analysis was not attempted because of insufficient data.
2.6. Reporting
Each of the three individual systematic reviews that contributed to this meta review were formally registered in PROSPERO (CRD42015017252, CRD42015017248, CRD42015017328). We included the ENTREQ statement as supplemental material (Tong et al., 2012).
3. Results
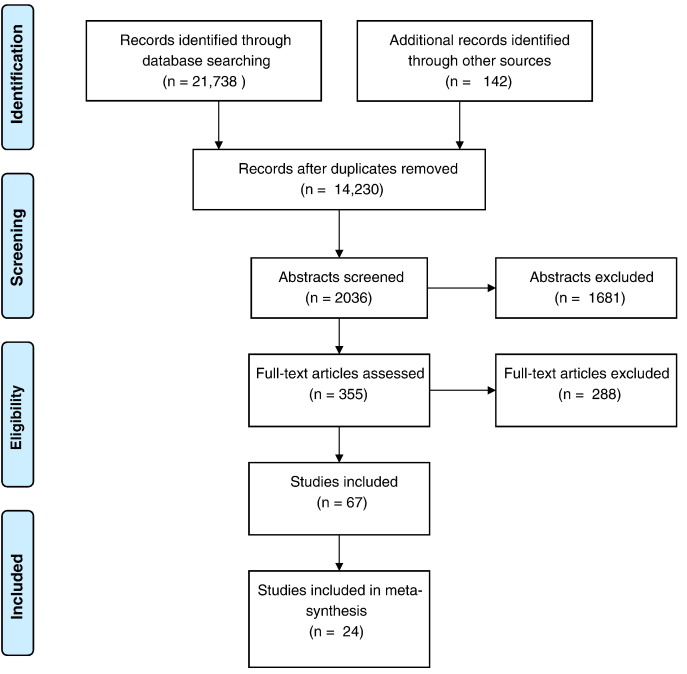
A total of 22,046 potentially relevant citations were identified and duplicates were removed (Fig. 1, Table 1). A total of 12,456 titles were examined, followed by abstracts and full texts. A total of 67 manuscripts were examined at the level of full text. A total of 24 studies were identified. Six studies were from low-income countries (Bezabhe et al., 2014, Busza et al., 2014a, Busza et al., 2014b, MacPherson, 2013, Nsigaye et al., 2009, Shroufi et al., 2013), ten studies were from middle-income countries (Agbonyitor, 2009, Ferguson et al., 2014, Jama and Tshotsho, 2013, Kroeger et al., 2011, Lazarus et al., 2012, Miller et al., 2010, Naik, 2013, Sarna et al., 2014, Smillie et al., 2014, Naik et al., 2013), and eight from high-income countries (Cameron et al., 2009, Garland et al., 2011, Johnson et al., 2003, Magidson et al., 2014, Montoya et al., 2014, Nunn et al., 2010, Prentice et al., 2011, Weiss et al., 2006). We identified seven cross-cutting themes (Table 2).
Fig. 1.
PRISMA flow chart of included studies.
Cross-cutting themes.
Table 1.
Overview of studies included in the qualitative evidence synthesis.
| First author | Year | Country | Intervention type | HIV care continuum step(s) | Method | Population |
|---|---|---|---|---|---|---|
| Bezabhe et al., 2014 | 2013 | Ethiopia | Task shifting | Adherence, retention | SSI, FGD | 2 hospitals in Amhara region |
| Busza et al., 2014a | 2011 | Tanzania | Home-based care | Retention | IDI | Adolescents in rural and urban areas |
| Busza et al., 2014b | 2012 | Zimbabwe | Decentralized | Linkage, adherence, retention | SSI | Children with HIV at 1 clinic |
| MacPherson, 2013 | 2011 | Malawi | Community-based HIVST | Linkage, Retention | SSI | PLHIV adults at 2 clinics |
| Nsigaye et al., 2009 | 2006 | Tanzania | Enhanced referrals | Linkage | IDI, FGD | Rural district |
| Shroufi et al., 2013 | 2011 | Zimbabwe | Peer support for women | Linkage, adherence, retention | IDI | 2 urban districts |
| Agbonyitor, 2009 | 2006 | Nigeria | Home-base care | Linkage, adherence, retention | FGD, IDI | Rural and semi-rural sites |
| Ferguson et al., 2014 | 2010 | Kenya | HIV/pregnancy service intervention | Linkage, retention | SSI | District-level prenatal clinic |
| Jama and Tshotsho, 2013 | 2011 | South Africa | Task shifting | Linkage | FGD | 3 clinics |
| Kroeger et al., 2011 | 2005 | Botswana | Home-based services | Linkage | FGD | Adults in 2 districts |
| Lazarus et al., 2012 | 2009 | India | Peer-led CBO, empowerment | Linkage, adherence | FGD, IDI, EFN | 1 southern state |
| Miller et al., 2010 | 2010 | South Africa | Decentralized | Retention | SSI | 2 treatment sites |
| Naik, 2013 | 2012 | South Africa | Home-based services | Linkage | IDI | Rural adults |
| Sarna et al., 2014 | 2010 | India | Community-based pilot | Linkage | SSI | Adults at 8 clinics |
| Smillie et al., 2014 | 2012 | Kenya | Text messaging | Retention | SSI | Adults at 1 clinic |
| Naik et al., 2013 | 2012 | South Africa | Home-based services | Linkage | IDI | Rural adults |
| Cameron et al., 2009 | 2008 | UK | Housing support | Linkage | SSI | Homeless or marginally housed |
| Garland et al., 2011 | 2008 | US | Enhanced referrals | Linkage | SSI | Adults at 3 sites |
| Johnson et al., 2003 | 2002 | US | Case management | Linkage, adherence, retention | IDI | Youth at 5 sites |
| Magidson et al., 2014 | 2013 | US | Integrated mental health | Adherence | IDI | Adults at 1 urban clinic |
| Montoya et al., 2014 | 2011 | US | mHealth | Retention | FGD | Adults at urban clinic |
| Nunn et al., 2010 | 2007 | US | Enhanced referrals | Linkage, retention | SSI | Adults released from prison |
| Prentice et al., 2011 | 2007 | Canada | Community-based services | Linkage, retention | SSI | Aboriginal youth |
| Weiss et al., 2006 | 2003 | US | Case management | Adherence, retention | SSI | Adults at 4 sites |
SSI: semi-structured interviews; FGD: focus group discussions; IDI: in-depth interviews; EFN: ethnographic field notes.
Table 2.
Summary of qualitative review findings.
| Review finding | Contributing studies | Confidence in the evidencea | Explanation of confidence in the evidence assessment |
|---|---|---|---|
| Participant gender. Among low and middle-income countries in Africa, the HIV-infected individual's gender influenced the uptake of the intervention. Men living with HIV had decreased engagement in HIV care compared to women and also influenced the willingness of their partners to engage HIV services. | MacPherson, 2013, Shroufi et al., 2013, Kroeger et al., 2011, Naik et al., 2013 | High | 4 studies with minor to moderate methodological limitations. Thick data from four African countries, two middle-income countries and low low-income countries. High coherence. |
| Clinical navigation and service orientation. Some people living with HIV (PLHIV) reported that once they reached the hospital, they were disoriented and encountered challenges accessing clinical care or ARVs. Several HIV interventions that improved clinical navigation were feasible and acceptable to PLHIV. | Busza et al., 2014b, Agbonyitor, 2009, Lazarus et al., 2012, Miller et al., 2010, Smillie et al., 2014, Cameron et al., 2009, Johnson et al., 2003, Prentice et al., 2011 | Moderate | 8 studies with minor to significant methodological limitations. Thick data from 8 countries, half in Africa. High coherence. |
| Mental health service integration. PLHIV identified unmet mental health needs that interfered with their ability to embrace interventions. Integration of HIV and mental health services improved intervention uptake and engagement in HIV services across the continuum of care. | Shroufi et al., 2013, Jama and Tshotsho, 2013, Lazarus et al., 2012, Smillie et al., 2014, Cameron et al., 2009, Johnson et al., 2003, Magidson et al., 2014, Montoya et al., 2014, Nunn et al., 2010, Prentice et al., 2011, Weiss et al., 2006 | Moderate | 11 studies with minor methodological limitations. Reasonably thick data from 7 countries, although high-income countries accounted for 7/11 studies. Interventions in middle-income countries were less intensive. High coherence. |
| Participant socioeconomic status. Individual socioeconomic status mediated intervention effectiveness. People with HIV living in poverty faced greater challenges engaging in interventions. Some HIV-infected individuals did not disclose their serostatus to family and friends, further limiting their access to resources. People living with HIV noted that several projects focused on alleviating poverty were feasible and acceptable in the context of larger HIV interventions. | Bezabhe et al., 2014, Busza et al., 2014a, Busza et al., 2014b, Agbonyitor, 2009, Ferguson et al., 2014, Lazarus et al., 2012, Miller et al., 2010, Sarna et al., 2014, Smillie et al., 2014, Naik et al., 2013, Garland et al., 2011 | High | 11 studies with minor methodological limitations. Thick data from 8 countries, predominately low and middle-income countries in Africa. High coherence. |
| Transportation assistance. Lack of regular transport to clinic appointments and to pick up ARVs was difficult for parents living with HIV and those caring for children living with HIV. Transportation assistance to the clinic and to obtain ARVs increased the feasibility and acceptability of HIV interventions. | Bezabhe et al., 2014, Busza et al., 2014b, Nsigaye et al., 2009, Agbonyitor, 2009, Jama and Tshotsho, 2013, Kroeger et al., 2011, Lazarus et al., 2012, Miller et al., 2010, Sarna et al., 2014, Naik et al., 2013, Johnson et al., 2003, Magidson et al., 2014, Nunn et al., 2010 | High | 13 studies with minor to moderate methodological limitations. Thick data from 9 countries, mostly in low and middle-income countries in Africa. High coherence. |
| Housing for homeless and marginally housed individuals. Among individuals from high-income countries, housing support delivered as part of the intervention improved linkage and retention in care. Housing support in this subset of PLHIV provided a more stable environment, enhancing the overall intervention. | Lazarus et al., 2012, Cameron et al., 2009, Johnson et al., 2003, Nunn et al., 2010, Prentice et al., 2011, Anaya et al., 2015 | Moderate | 6 studies with minor methodological limitations. Relatively thick data from three high-income countries in Europe and North America. High coherence. |
| Food insecurity. Lack of food limited the extent to which PLHIV engaged in interventions and were retained in care. Some PLHIV were unable to have sufficient food to accompany their ARVs, influencing their ARV adherence. Food assistance was a critical determinant of several interventions across the HIV continuum of care. | Bezabhe et al., 2014, Busza et al., 2014a, Agbonyitor, 2009, Lazarus et al., 2012, Cameron et al., 2009, Johnson et al., 2003, Nunn et al., 2010, Prentice et al., 2011) | Moderate | 8 studies with minor to significant methodological limitations. Thick data from seven countries, including three studies from low and middle—income countries in Africa. High coherence. |
The CERQual confidence refers to the overall confidence in the review finding based on assessing components related to relevance, adequacy, coherence, and methodological rigor as described in detail in the methods section.
3.1. Participant Gender (High Quality of Evidence) (MacPherson, 2013, Shroufi et al., 2013, Kroeger et al., 2011, Naik et al., 2013)
Two low-income country studies and two middle-income country studies from Africa suggested that the individual's gender influenced the uptake of HIV interventions. Men living with HIV had decreased engagement in HIV care compared to women and also influenced the willingness of their partners to engage HIV services. Three studies found that men were comparatively disinterested in care engagement compared to women (MacPherson, 2013, Shroufi et al., 2013, Kroeger et al., 2011). This disinterest was attributed to gender power norms (MacPherson, 2013, Shroufi et al., 2013), lack of clinic responsiveness to men's perceived needs (MacPherson, 2013), and hesitancy to take women's advice (Shroufi et al., 2013). Two studies found sex-specific delays in intervention uptake (MacPherson, 2013, Shroufi et al., 2013), with particular reluctance from men to seek care until signs of HIV affected sexual function or capacity to work (MacPherson, 2013). Three studies found female participants had concerns about how engaging in HIV services could elicit anger, distrust, or violence from their husbands or male partners (MacPherson, 2013, Shroufi et al., 2013, Kroeger et al., 2011). One mother mentoring intervention promoted partner disclosure which helped women cope with gender differences (Shroufi et al., 2013).
3.2. Participant Socioeconomic Status (High Quality of Evidence) (Bezabhe et al., 2014, Busza et al., 2014a, Busza et al., 2014b, Agbonyitor, 2009, Ferguson et al., 2014, Lazarus et al., 2012, Miller et al., 2010, Sarna et al., 2014, Smillie et al., 2014, Naik et al., 2013, Garland et al., 2011)
Eleven studies from eight countries noted that participant socioeconomic status influenced public health HIV interventions. Most of these studies were from low- and middle-income countries in Africa. Individual socioeconomic status mediated intervention effectiveness. People with HIV living in poverty faced greater challenges engaging in interventions. Some individuals did not disclose their HIV status to family and friends, further limiting their access to resources. Individuals with HIV noted that several projects focused on alleviating poverty were feasible and acceptable in the context of larger HIV interventions. Unemployed people with HIV living in poverty faced greater challenges participating in interventions (Agbonyitor, 2009). Employed people with HIV living in poverty dropped out of care because of competing work demands and the financial cost of missed work time (Busza et al., 2014b, Lazarus et al., 2012, Miller et al., 2010, Sarna et al., 2014, Smillie et al., 2014, Naik et al., 2013). Three studies (Ferguson et al., 2014, Naik et al., 2013, Garland et al., 2011) discussed lack of finances as a barrier to linkage interventions. Six studies (Bezabhe et al., 2014, Busza et al., 2014a, Busza et al., 2014b, Agbonyitor, 2009, Miller et al., 2010, Smillie et al., 2014) noted that people living in poverty faced greater challenges with retention interventions. One study showed how migration to rural areas with better employment prospects disrupted clinic attendance and ultimately adherence (Bezabhe et al., 2014). Individuals living with HIV perceived that entering into care would necessitate all of the financial obligations that comprehensive care entailed and would be a great burden (Garland et al., 2011). Individuals living with HIV noted that the following components focused on alleviating poverty were feasible and acceptable in the context of larger HIV interventions: providing clinical care without cost to the HIV-infected individual (Agbonyitor, 2009, Miller et al., 2010, Smillie et al., 2014), providing ARVs without cost to the HIV-infected individual (Miller et al., 2010, Smillie et al., 2014), providing non-ARV medicines without cost to the HIV-infected individual (Bezabhe et al., 2014), small grants to support groups (Agbonyitor, 2009), paying school fees (Agbonyitor, 2009), and directly giving money to HIV-infected individuals (Agbonyitor, 2009).
3.3. Housing for Homeless and Marginally Housed Individuals (Moderate Quality of Evidence) (Lazarus et al., 2012, Cameron et al., 2009, Johnson et al., 2003, Nunn et al., 2010, Prentice et al., 2011, Anaya et al., 2015)
Six studies from three countries in Europe and North America evaluated housing for the homeless or marginally housed individuals. Housing support delivered as part of the intervention facilitated linkage and retention in care. Housing support in this subset of individuals living with HIV provided a more stable environment, enhancing the overall intervention. Five linkage studies reported on the importance of securing housing and maintaining stable housing for individuals living with HIV (Lazarus et al., 2012, Cameron et al., 2009, Johnson et al., 2003, Nunn et al., 2010, Prentice et al., 2011). One study mentioned how housing support integrated within an HIV intervention created a new local environment that made it easier for them to stay away from alcohol and drugs (Prentice et al., 2011). The achievement of housing support was instrumental to helping patients feel stable to proceed onto linkage of care medical services (Cameron et al., 2009, Anaya et al., 2015). Individuals living with HIV who were homeless and participated in an integrated intervention reported that after they had stable housing, their patient-physician relationship improved and they were more engaged in care (Cameron et al., 2009). Acceptable housing interventions for individuals living with HIV included the following: temporary accommodation for HIV-infected homeless and marginally housed individuals (Cameron et al., 2009), housing for homeless youth (Johnson et al., 2003), housing for sex workers with adherence problems (Lazarus et al., 2012), and shelter-based HIV interventions (Anaya et al., 2015).
3.4. Food Insecurity (Moderate Quality of Evidence) (Bezabhe et al., 2014, Busza et al., 2014a, Agbonyitor, 2009, Lazarus et al., 2012, Cameron et al., 2009, Johnson et al., 2003, Nunn et al., 2010, Prentice et al., 2011)
Eight studies from seven countries focused on food insecurity within public health HIV interventions. Lack of food limited the extent to which individuals living with HIV engaged in interventions and were retained in care. Some individuals living with HIV were unable to have sufficient food to accompany their ARVs, influencing their ARV adherence. Food assistance was a critical determinant of several interventions across the HIV continuum of care. One study found that widows living with HIV were not able to find enough food for themselves and their children (Agbonyitor, 2009). Even among farmers and food sellers living with HIV, they were unable to purchase meat and have a balanced diet (Agbonyitor, 2009). The lack of food prevented individuals from taking their ARV and sometimes affected retention in care (Bezabhe et al., 2014). One study mentioned how free meals within a health center were an incentive to link and be retained in care (Prentice et al., 2011). HIV-infected individuals noted that the following interventions to improve food insecurity were acceptable and feasible: referral to meal assistance (Cameron et al., 2009), reimbursing HIV-infected participants for food purchased for themselves and their children (Agbonyitor, 2009), donated food for HIV-infected individuals (Bezabhe et al., 2014), free meals and snacks (Lazarus et al., 2012, Prentice et al., 2011), dietician advice (Prentice et al., 2011), and donated fertilizer for crops (Bezabhe et al., 2014).
3.5. Transportation Assistance (High Quality of Evidence) (Bezabhe et al., 2014, Busza et al., 2014b, Nsigaye et al., 2009, Agbonyitor, 2009, Jama and Tshotsho, 2013, Kroeger et al., 2011, Lazarus et al., 2012, Miller et al., 2010, Sarna et al., 2014, Naik et al., 2013, Johnson et al., 2003, Magidson et al., 2014, Nunn et al., 2010)
A total of 13 studies, most within low and middle-income countries in Africa, provided evidence on the relationship between transportation and public health HIV interventions. Lack of regular transport to clinic appointments and to pick up ARVs were difficult for parents living with HIV (Busza et al., 2014b, Lazarus et al., 2012, Miller et al., 2010, Naik et al., 2013) and those caring for children with HIV (Busza et al., 2014b). Transportation assistance to the clinic and to obtain ARVs increased the feasibility and acceptability of HIV interventions. Long distances between home and the clinic exacerbated transportation problems (Jama and Tshotsho, 2013, Lazarus et al., 2012). Transportation assistance was particularly useful among poor HIV-infected individuals (Busza et al., 2014b, Agbonyitor, 2009) and those who lived in more remote locations (Agbonyitor, 2009). Transportation assistance included referrals (Cameron et al., 2009, Nunn et al., 2010), money for travel (Bezabhe et al., 2014, Agbonyitor, 2009, Magidson et al., 2014), vouchers (Kroeger et al., 2011), escorts to the clinic (Kroeger et al., 2011), transferring to a proximate clinic (Miller et al., 2010), and home-based services (Agbonyitor, 2009, Kroeger et al., 2011) were all feasible and acceptable to individuals living with HIV.
3.6. Clinical Navigation and Service Orientation (Moderate Quality of Evidence) (Busza et al., 2014b, Agbonyitor, 2009, Lazarus et al., 2012, Miller et al., 2010, Smillie et al., 2014, Cameron et al., 2009, Johnson et al., 2003, Prentice et al., 2011)
Eight studies from eight countries, half in Africa, examined clinical navigation and service orientation. Some individuals living with HIV reported that once they reached the HIV clinic, they were disoriented and encountered challenges accessing clinical care or ARVs. Several public health HIV interventions that aimed to improve clinical navigation were feasible and acceptable to individuals living with HIV. There was confusion about where to be evaluated within medical centers that were often large and intimidating (Agbonyitor, 2009, Lazarus et al., 2012). Long delays in seeing a doctor contributed to a sense of futility among HIV-infected individuals (Busza et al., 2014b, Agbonyitor, 2009, Lazarus et al., 2012). Delays in obtaining ARVs from the pharmacy and stockouts were also common (Busza et al., 2014b, Agbonyitor, 2009, Lazarus et al., 2012, Miller et al., 2010). One retention study found that some HIV-infected individuals referred to missing paperwork (“clinic cards, transfer papers, and proof of travel”) as the reason for not returning for follow-up (Miller et al., 2010). HIV-infected individuals noted that the following interventions to improve clinical navigation were feasible and acceptable: SMS text messaging between HIV-infected individuals and nurses (Smillie et al., 2014), peer support (Lazarus et al., 2012), non-peer support (Agbonyitor, 2009, Cameron et al., 2009), and a one-stop model for care provision (Johnson et al., 2003, Prentice et al., 2011). The one-stop model integrated several types of services within a single site to improve adherence.
3.7. Mental Health Service Integration (Moderate Quality of Evidence) (Shroufi et al., 2013, Jama and Tshotsho, 2013, Lazarus et al., 2012, Smillie et al., 2014, Cameron et al., 2009, Johnson et al., 2003, Magidson et al., 2014, Montoya et al., 2014, Nunn et al., 2010, Prentice et al., 2011, Weiss et al., 2006)
Eleven studies, with seven from high-income countries, explored mental health service integration interventions. Individuals living with HIV identified unmet mental health needs that interfered with their ability to embrace public health interventions. Integration of HIV and mental health services facilitated intervention uptake and engagement in HIV services across the continuum of care. Many HIV-infected individuals were confronted with HIV-associated stigma, anxiety, depression, and fear (Smillie et al., 2014, Johnson et al., 2003, Montoya et al., 2014, Prentice et al., 2011). Four studies addressed how access and treatment for mental health services enhanced linkage to care (Jama and Tshotsho, 2013, Lazarus et al., 2012, Johnson et al., 2003, Nunn et al., 2010). One study used a mental health intervention to improve ARV adherence among HIV-infected individuals (Magidson et al., 2014). Addressing these behavioral and mental health issues increased confidence in adhering to ARVs and retention in care. Integration of mental health and HIV services included: referral to mental health services (Nunn et al., 2010, Weiss et al., 2006), counseling (Shroufi et al., 2013, Nunn et al., 2010, Weiss et al., 2006), co-located services (Johnson et al., 2003), and a group-based adherence intervention for PLHIV with depression (Magidson et al., 2014).
4. Discussion
This systematic review identified cross-cutting themes that impact public health HIV interventions at more than one point in the HIV care continuum. Given the remarkable heterogeneity of interventions evaluated in the qualitative studies, the emergence of coherent cross-cutting themes underlines persistent social and cultural factors of importance. Qualitative research may improve our understanding of intervention benefits and harms, effects on equity/human rights (Tromp et al., 2014), and evaluate intervention feasibility and acceptability (Tso et al., 2016). Our qualitative systematic review expands the literature on public health HIV interventions by incorporating research studies across the HIV care continuum, capturing the lived experience of people with HIV, and formally assessing confidence in the review findings.
We found that participant gender had a strong influence on the uptake of public health HIV interventions in low and middle-income African countries. Among men living with HIV, interventions and clinic systems were perceived not responsive to their needs, contributing to poor engagement. This review finding contrasts research from the United States (Hall et al., 2013) which found minimal sex differences. We speculate that this may be related to different gender power dynamics in African and non-African contexts, although further research is needed. We found that some men believe that engaging in HIV interventions interfered with their masculine identity (Zeglin, 2015, Ganle, 2015, Gibbs et al., 2015). Poor engagement of men living with HIV in interventions may partly explain the increased mortality that has been observed among men versus women living with HIV in Africa and other regions (Castilho et al., 2014, Druyts et al., 2013). A quantitative review of interventions promoting retention of HIV-infected men identified only three studies (Fox and Rosen, 2015), underscoring the need for further research in this field.
Our data revealed four structural issues (poverty, unstable housing, food insecurity, lack of transportation) that mediated the feasibility and acceptability of public health HIV interventions. This is consistent with quantitative reviews of HIV interventions (Govindasamy et al., 2012, Govindasamy et al., 2014, Barnighausen et al., 2011, Mills et al., 2006, Ortego et al., 2011, Okeke et al., 2014). Individuals living with HIV are often members of marginalized groups that face greater barriers to stable income/employment, housing, food, and transportation (Farmer, 1992). These structural issues are often inter-related. For example, people living with HIV who are poor are at greater risk for unstable housing and food insecurity, both of which can influence ARV adherence. Given the overlapping nature of these issues, better understanding these relationships and how they mutually influence HIV service delivery is important. This finding highlights the need for integration of HIV interventions with social services generally and social protection programs specifically (Piot et al., 2015).
Our review also identified the potential need for integration of mental health services alongside public health HIV interventions. High burden of mental health co-morbidities has been noted among individuals living with HIV (Breuer et al., 2011, Catalan et al., 2011), although less is known about the burden of mental illness within low and middle-income contexts that have less developed mental health diagnostic and therapeutic systems (Breuer et al., 2011). Qualitative evidence showed that relatively simple integration such as referrals and counseling can help direct individuals living with HIV into mental health services and are acceptable. Referrals and related methods of low intensity intervention identified in our review may help individuals living with HIV to adjust to local environments that are often stressful. The integration of mental health and HIV outcomes could be useful for improving both mental health and HIV-related outcomes (Magidson et al., 2014).
This review has several limitations. First, we only focused on qualitative research studies and did not include quantitative evaluation studies. Quantitative studies are necessary to establish efficacy and effectiveness. At the same time, qualitative evaluation provides valuable policy-relevant data on potential harms, implications for equity and human rights, and feasibility/acceptability. Second, this meta-review used data collected from three systematic reviews focused on linkage, adherence, and retention, respectively. At the same time, each of these search algorithms, protocols, and analysis plans were standardized in order to facilitate comparisons across reviews. Third, our search algorithms identified fewer qualitative evaluation studies focused on pregnant women, adolescents, and children. This is consistent with the results of quantitative reviews (Govindasamy et al., 2012, Govindasamy et al., 2014, Barnighausen et al., 2011, Mills et al., 2006, Ortego et al., 2011, Okeke et al., 2014) and likely reflects fewer interventions targeting these important subsets of people living with HIV. Fourth, many of the studies we identified were from Africa. The extent to which this influences review findings depends on the scope of the review finding. For example, our finding about men's engagement in HIV services was limited to Africa. However, the review finding about food insecurity was identified in a range of studies from around the world. Finally, our search only covers data through February 2015 and there is growing momentum to examine public health HIV interventions. Given how rapidly this field is expanding, we anticipate that further evidence will become available.
Our data have implications for HIV policy and research. From a policy perspective, this systematic review identified several strategies for tailoring interventions to enhance HIV service delivery at multiple points within the continuum of care. From a research perspective, this qualitative evidence review demonstrates the value of robust qualitative research studies alongside quantitative evaluations. Qualitative evaluations provide detailed observations and perspectives that complement and extend quantitative evaluations. Implementation research on how best to integrate HIV services within larger medical and social systems is increasingly important as stand-alone HIV services are removed and HIV becomes operationally and clinically a chronic disease. Given the high burden of HIV among young people around the world, more implementation research among children and adolescents would be desirable given the additional challenges in these age groups (UNAIDS, 2014).
Persistent social and structural factors contribute to disparities in HIV outcomes across the continuum of care, shaping the context of HIV service delivery among important subpopulations. Although altering the delivery of health services for vulnerable groups living with HIV in low and middle-income countries is no small task, these types of approaches will likely be necessary in order to achieve UNAIDS 90-90-90 targets (UNAIDS, 2014) and ensure an equitable HIV response.
Competing Interests
No competing interests reported.
Financial Disclosure
This project was supported by the World Health Organization and the US NIH (NIAID 1R01AI114310-01, 1D43TW009532-02). The funder had no role in the study design, data collection, data analysis, interpretation, and writing of the report.
Authors' Contributions
JT, BH, and LT conceived of this idea and organized the analysis. The analysis was undertaken by LT, BH, QM, JB, HL, GM, ZR, and KS. JT and ML developed the search strategy. RB and MD advised on analysis. All authors contributed to writing the paper and approved the final manuscript for submission.
Acknowledgements
This research was commissioned by the World Health Organization HIV Department. Additional support was obtained from the US National Institutes of Health (NIAID 1R01AI114310-02, FIC 1D43TW009532-03). We would like to thank the members of the World Health Organization's HIV Operational Guideline Development Group for helpful feedback on this research. We would also like to thank Fengyu Hu, Weiping Cai, Bin Yang, Wei Ma, Kathryn Muessig, and Chongyi Wei for helpful comments.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.01.036.
Contributor Information
Joseph D. Tucker, Email: jdtucker@med.unc.edu.
Lai Sze Tso, Email: ltso@umich.edu.
Brian Hall, Email: brianhall@umac.mo.
Qingyan Ma, Email: qingyan_ma@med.unc.edu.
Rachel Beanland, Email: beanlandr@who.int.
John Best, Email: John.Best@ucsf.edu.
Mellanye Lackey, Email: mellanye.lackey@utah.edu.
Gifty Marley, Email: giftatluck@yahoo.com.
Zachary C. Rich, Email: zackrich@gmail.com.
Ka-lon Sou, Email: soukalon19920126@gmail.com.
Meg Doherty, Email: dohertym@who.int.
Appendix A. Additional Data 1 Search Strategies Used in PubMed for Each of the Three Searches
A.1. Linkage to Care Intervention Subsection
(Care OR treatment) AND (link OR linkage OR linked OR linking OR continuum OR continuity OR cascade OR diagnosis OR retain OR retained OR retention OR barriers OR engage OR engagement OR engaging OR engaged OR entry OR access OR initiation OR initiate OR initiated OR enroll OR enrolled OR enrollment OR enrolls)) OR “continuity of patient care”[tw] OR “client continuity”[tiab] OR “treatment refusal”[tw] OR “western blot”[tiab] OR “western blotting”[tw] OR (appointment AND (doctor OR physician OR clinic OR antenatal OR counseling)) OR cd4 count[tiab] OR cd4 counts[tiab] OR cd4 lymphocyte count[tw] OR cd4 lymphocyte counts[tiab] OR pre-art[tiab] OR pre-treatment[tiab] OR pretreatment[tiab] OR (art OR AND (pre OR eligibility OR eligible OR screening OR screened OR screen)) AND ((HIV[tw] OR “human immunodeficiency virus”[tiab] OR AIDS[tw] OR “acquired immunodeficiency syndrome”[tw] OR serostatus[tw] OR PLHIV[tiab] OR people living with HIV[tiab] OR persons living with HIV[tiab] OR PLWHA[tiab] OR persons living with HIV/AIDS[tiab] OR people living with HIV/AIDS[tiab] OR people living with AIDS[tiab] OR persons living with AIDS[tiab]) OR ((viral OR virally OR HIV) AND (suppress OR suppression OR suppressed))).
A.2. ARV Adherence Subsection
(Adherence[tw] OR adhere[tiab] OR nonadherence[tiab] OR “non adherence”[tiab] OR compliance[tw] ORcomply[tiab] OR noncompliance[tiab] OR “non compliance”[tiab] OR Retain[tiab] OR retention[tw] ORretained[tiab] OR Uptake[tiab]) AND (ARV[tiab] OR ARVs[tiab] OR ART[tiab] OR HAART[tiab] OR antiretroviral[tiab] OR antiretrovirals[tiab] OR “anti-retroviral”[tw] OR “anti-retrovirals”[tiab] OR stavudine[tw] OR D4T[tiab] OR zidovudine[tw] OR AZT[tiab] OR azidothymidine[tw]) AND ((HIV[tw] OR “human immunodeficiency virus”[tiab] OR AIDS[tw] OR “acquired immunodeficiency syndrome”[tw] OR serostatus[tw] OR PLHIV[tiab] OR people living with HIV[tiab] OR persons living with HIV[tiab] OR PLWHA[tiab] OR persons living with HIV/AIDS[tiab] OR people living with HIV/AIDS[tiab] OR people living with AIDS[tiab] OR persons living with AIDS[tiab]) OR ((viral OR virally OR HIV) AND (suppress OR suppression OR suppressed))).
A.3. Retention Interventions Subsection
(((Care OR treatment) AND (continuity OR retain OR retained OR retention OR engage OR engagement OR engaging OR engaged OR access)) OR “continuity of patient care”[tw] OR “client continuity”[tiab] OR “treatment refusal”[tw] OR “loss to follow up”[tiab] OR LTFU[tiab] OR attrition[tiab] OR patient dropout[tiab] OR patient dropouts[tw]) AND ((HIV[tw] OR “human immunodeficiency virus”[tiab] OR AIDS[tw] OR “acquired immunodeficiency syndrome”[tw] OR serostatus[tw] OR PLHIV[tiab] OR people living with HIV[tiab] OR persons living with HIV[tiab] OR PLWHA[tiab] OR persons living with HIV/AIDS[tiab] OR people living with HIV/AIDS[tiab] OR people living with AIDS[tiab] OR persons living with AIDS[tiab]) OR ((viral OR virally OR HIV) AND (suppress OR suppression OR suppressed))).
A.4. Qualitative Term (Used in Each of the Above Subsections)
(((“Semi-structured”[TIAB] OR semistructured[TIAB] OR unstructured[TIAB] OR informal[TIAB] OR “in-depth”[TIAB] OR indepth[TIAB] OR “face-to-face”[TIAB] OR structured[TIAB] OR guide[TIAB] OR guides[TIAB]) AND (interview*[TIAB] OR discussion*[TIAB] OR questionnaire*[TIAB])) OR (“focus group”[TIAB] OR “focus groups”[TW] OR qualitative[TIAB] OR ethnograph*[TIAB] OR fieldwork[TIAB] OR “field work”[TIAB] OR “key informant”[TIAB] OR participant observation[tiab] OR participant observations[tiab] OR anthropology[tw] OR anthropological[tiab] OR narrative[tiab] OR voice[tiab] OR story telling[tiab] OR storytelling[tiab] OR stories[tiab] OR grounded theory[tw])) OR “interviews as topic”[Mesh] OR narration[Mesh] OR qualitative research[Mesh] OR “personal narratives as topic”[Mesh] OR “anecdotes as topic”[Mesh].
Appendix B. Supplementary Data
Supplementary material
References
- Agbonyitor M. Home-based care for people living with HIV/AIDS in Plateau State, Nigeria: findings from a qualitative study. Glob. Public Health. 2009;4(3):303–312. doi: 10.1080/17441690902783165. (PubMed PMID: WOS:000207894200007) [DOI] [PubMed] [Google Scholar]
- Anaya H.D., Butler J.N., Knapp H., Chan K., Conners E.E., Rumanes S.F. Implementing an HIV rapid testing-linkage-to-care project among homeless individuals in Los Angeles County: a collaborative effort between federal, county, and City Government. Am. J. Public Health. 2015;105(1):85–90. doi: 10.2105/AJPH.2014.302213. (PubMed PMID: 25393202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnighausen T., Chaiyachati K., Chimbindi N., Peoples A., Haberer J., Newell M.L. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect. Dis. 2011;11(12):942–951. doi: 10.1016/S1473-3099(11)70181-5. (PubMed PMID: 22030332; PubMed Central PMCID: PMC4250825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezabhe W.M., Chalmers L., Bereznicki L.R., Peterson G.M., Bimirew M.A., Kassie D.M. Barriers and facilitators of adherence to antiretroviral drug therapy and retention in care among adult HIV-positive patients: a qualitative study from Ethiopia. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097353. PubMed PMID: 24828585; PubMed Central PMCID: PMCPmc4020856. (Epub 2014/05/16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A., Papaioannou D., Sutton A. 2012. Systematic Approaches to a Successful Literature Review: Sage Publications. [Google Scholar]
- Boyer S., Marcellin F., Ongolo-Zogo P., Abega S.C., Nantchouang R., Spire B. Financial barriers to HIV treatment in Yaounde, Cameroon: first results of a national cross-sectional survey. Bull. World Health Organ. 2009;87(4):279–287. doi: 10.2471/BLT.07.049643. (PubMed PMID: 19551236; PubMed Central PMCID: PMCPMC2672585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer E., Myer L., Struthers H., Joska J.A. HIV/AIDS and mental health research in sub-Saharan Africa: a systematic review. Afr. J. AIDS Res. 2011;10(2):101–122. doi: 10.2989/16085906.2011.593373. (PubMed PMID: 25859733) [DOI] [PubMed] [Google Scholar]
- Busza J., Besana G.V.R., Mapunda P., Oliveras E. Meeting the needs of adolescents living with HIV through home based care: lessons learned from Tanzania. Child Youth Serv. Rev. 2014;45:137–142. (PubMed PMID: WOS:000342549400016) [Google Scholar]
- Busza J., Dauya E., Bandason T., Mujuru H., Ferrand R.A. “I don't want financial support but verbal support.” How do caregivers manage children's access to and retention in HIV care in urban Zimbabwe? J. Int. AIDS Soc. 2014;17 doi: 10.7448/IAS.17.1.18839. (PubMed PMID: WOS:000335924400001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A., Lloyd L., Turner W., Macdonald G. Working across boundaries to improve health outcomes: a case study of a housing support and outreach service for homeless people living with HIV. Health Soc. Care Community. 2009;17(4):388–395. doi: 10.1111/j.1365-2524.2008.00837.x. 10.1111/j.1365-2524.2008.00837.x Epub 2009/02/04. (PubMed PMID: 19187420) [DOI] [PubMed] [Google Scholar]
- Carlsen B., Glenton C., Pope C. Thou shalt versus thou shalt not: a meta-synthesis of GPs' attitudes to clinical practice guidelines. Br. J. Gen. Pract. 2007;57(545):971–978. doi: 10.3399/096016407782604820. (PubMed Central PMCID: PMC18252073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho J.L., Melekhin V.V., Sterling T.R. Sex differences in HIV outcomes in the highly active antiretroviral therapy era: a systematic review. AIDS Res. Hum. Retroviruses. 2014;30(5):446–456. doi: 10.1089/aid.2013.0208. (PubMed PMID: 24401107; PubMed Central PMCID: PMCPMC4010172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan J., Harding R., Sibley E., Clucas C., Croome N., Sherr L. HIV infection and mental health: suicidal behaviour–systematic review. Psychol. Health Med. 2011;16(5):588–611. doi: 10.1080/13548506.2011.582125. (PubMed PMID: 21745024) [DOI] [PubMed] [Google Scholar]
- Cleary S. Equity and efficiency in scaling up access to HIV-related interventions in resource-limited settings. Curr. Opin. HIV AIDS. 2010;5(3):210–214. doi: 10.1097/COH.0b013e3283384a6f. (PubMed PMID: 20539076) [DOI] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme Qualitative Appraisal Checklist for Qualitative Research 2006 (cited 2015 January 25). Available from: http://www.casp-uk.net/wp-content/uploads/2011/11/CASP_Qualitative_Appraisal_Checklist_14oct10.pdf.
- Druyts E., Dybul M., Kanters S., Nachega J., Birungi J., Ford N. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS (London, England) 2013;27(3):417–425. doi: 10.1097/QAD.0b013e328359b89b. (PubMed PMID: 22948271) [DOI] [PubMed] [Google Scholar]
- Farmer P. xiv. University of California Press; Berkeley: 1992. AIDS and Accusation: Haiti and the Geography of Blame. (338 p.p) [Google Scholar]
- Ferguson L., Grant A.D., Lewis J., Kielmann K., Watson-Jones D., Vusha S. Linking women who test HIV-positive in pregnancy-related services to HIV care and treatment services in Kenya: a mixed methods prospective cohort study. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0089764. (PubMed PMID: 24646492; PubMed Central PMCID: PMCPmc3960101. Epub 2014/03/22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M., Rosen S. WHO ARV Operational Guideline Meeting Geneva; Switzerland: 2015. Systematic Review of Interventions to Facilitate Linkage to Care to Support Development of the WHO 2015 Consolidated Guidelines for the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. [Google Scholar]
- Ganle J.K. Hegemonic masculinity, HIV/AIDS risk perception, and sexual behavior change among young people in Ghana. Qual. Health Res. 2015 doi: 10.1177/1049732315573204. (PubMed PMID: 25721715) [DOI] [PubMed] [Google Scholar]
- Garland P.M., Valverde E.E., Fagan J., Beer L., Sanders C., Hillman D. HIV counseling, testing and referral experiences of persons diagnosed with HIV who have never entered HIV medical care. AIDS Educ. Prev. 2011;23(3 Suppl):117–127. doi: 10.1521/aeap.2011.23.3_supp.117. (PubMed PMID: 21689042) [DOI] [PubMed] [Google Scholar]
- Gibbs A., Jewkes R., Sikweyiya Y., Willan S. Reconstructing masculinity? A qualitative evaluation of the stepping stones and creating futures interventions in urban informal settlements in South Africa. Cult. Health Sex. 2015;17(2):208–222. doi: 10.1080/13691058.2014.966150. (PubMed PMID: 25335905) [DOI] [PubMed] [Google Scholar]
- Govindasamy D., Ford N., Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS (London, England) 2012;26(16):2059–2067. doi: 10.1097/QAD.0b013e3283578b9b. (PubMed PMID: 22781227) [DOI] [PubMed] [Google Scholar]
- Govindasamy D., Meghij J., Kebede Negussi E., Clare Baggaley R., Ford N., Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings–a systematic review. J. Int. AIDS Soc. 2014;17:19032. doi: 10.7448/IAS.17.1.19032. (PubMed PMID: 25095831; PubMed Central PMCID: PMC4122816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADE-CERQUAL What is CERQual Approach? 2015. http://cerqual.org/whatisthecerqualapproach/ Available from:
- Hall H.I., Frazier E.L., Rhodes P., Holtgrave D.R., Furlow-Parmley C., Tang T. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern. Med. 2013;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. (PubMed PMID: 23780395) [DOI] [PubMed] [Google Scholar]
- Hall BJ, Sou K, Beanland R, Lackey M, Tso LS, Ma Q, et al. Barriers and facilitators to interventions improving retention in HIV care: a qualitative evidence meta-synthesis. AIDS Behav. (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Jama N.M., Tshotsho N. Strategies for follow-up care of non-compliant HIV-positive pregnant women. African Journal for Physical, Health Education, Recreation and Dance. 2013;19(Suppl. 1):14–28. (PubMed PMID: 20143127169. Publication Type: Journal Article. Language: English. Subject Subsets: Tropical Diseases) [Google Scholar]
- Johnson R.L., Botwinick G., Sell R.L., Martinez J., Siciliano C., Friedman L.B. The utilization of treatment and case management services by HIV-infected youth. J. Adolesc. Health. 2003;33(2 Suppl):31–38. doi: 10.1016/s1054-139x(03)00158-7. (PubMed PMID: 12888285) [DOI] [PubMed] [Google Scholar]
- Kroeger K., Taylor A., Marlow H., Fleming D.T., Beyleveld V., Alwano M.G. Perceptions of door-to-door HIV counselling and testing in Botswana. Sahara J-Journal of Social Aspects of HIV-AIDS. 2011;8(4):171–178. doi: 10.1080/17290376.2011.9725001. (PubMed PMID: WOS:000305254600002) [DOI] [PubMed] [Google Scholar]
- Lazarus L., Reza-Paul S., Pasha A., Jairam S., Hafeez Ur Rahman S., O'Neil J. Exploring the Role of Community-Based Peer Support in Improving Access to Care and Antiretroviral Treatment for Sex Workers in Mysore, India. Journal of HIV/AIDS & Social Services. 2012;11(2):152–168. (PubMed PMID: 2011557850. Language: English. Entry Date: 20120706. Revision Date: 20140509. Publication Type: journal article) [Google Scholar]
- Lewin S., Glenton C., Munthe-Kaas H., Carlsen B., Colvin C.J., Gulmezoglu M. Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual) PLoS Med. 2015;12(10) doi: 10.1371/journal.pmed.1001895. (PubMed PMID: 26506244; PubMed Central PMCID: PMCPMC4624425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Tso L.S., Rich Z.C., Hall B.J., Beanland R., Li H. Barriers and facilitators of interventions for improving antiretroviral therapy adherence: a systematic review of global qualitative evidence. J. Int. AIDS Soc. 2016;19(1):21166. doi: 10.7448/IAS.19.1.21166. (PubMed PMID: 27756450; PubMed Central PMCID: PMCPMC5069281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson P. The University of Liverpool (United Kingdom); Ann Arbor: 2013. Improving Linkage Into HIV Care Among Adults in Blantyre, Malawi. (Ph.D.) [Google Scholar]
- Magidson J.F., Seitz-Brown C.J., Safren S.A., Daughters S.B. Implementing behavioral activation and life-steps for depression and HIV medication adherence in a community health center. Cogn. Behav. Pract. 2014;21(4):386–403. doi: 10.1016/j.cbpra.2013.10.002. (PubMed PMID: 25419102; PubMed Central PMCID: PMC4238929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellin F., Boyer S., Protopopescu C., Dia A., Ongolo-Zogo P., Koulla-Shiro S. Determinants of unplanned antiretroviral treatment interruptions among people living with HIV in Yaounde, Cameroon (EVAL survey, ANRS 12-116) Trop. Med. Int. Health. 2008;13(12):1470–1478. doi: 10.1111/j.1365-3156.2008.02170.x. (PubMed PMID: 19000156) [DOI] [PubMed] [Google Scholar]
- Miller C.M., Ketlhapile M., Rybasack-Smith H., Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop. Med. Int. Health. 2010;15:48–54. doi: 10.1111/j.1365-3156.2010.02514.x. (PubMed PMID: WOS:000277205500004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.J., Nachega J.B., Bangsberg D.R., Singh S., Rachlis B., Wu P. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11) doi: 10.1371/journal.pmed.0030438. (PubMed PMID: 17121449; PubMed Central PMCID: PMC1637123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J.L., Georges S., Poquette A., Depp C.A., Atkinson J.H., Moore D.J. Refining a personalized mHealth intervention to promote medication adherence among HIV + methamphetamine users. AIDS Care. 2014;26(12):1477–1481. doi: 10.1080/09540121.2014.924213. (PubMed PMID: 24911433; PubMed Central PMCID: PMC4188758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Lewin S., Smith H., Engel M., Fretheim A., Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7) doi: 10.1371/journal.pmed.0040238. (PubMed Central PMCID: PMC17676945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik R. Boston University; Ann Arbor: 2013. Linkage to Care Following Home-Based HIV Counseling and Testing: A Mixed Methods Study in Rural South Africa [D.P.H.] [Google Scholar]
- Naik R., Doherty T., Jackson D., Zembe W., Feeley F. International AIDS Society; Kuala Lumpur, Malaysia: 2013. Client Experiences and Perspectives on the Linkage to Care Following Home-Based HIV Counseling and Testing: A Qualitative Study in Rural South Africa. [Google Scholar]
- Noyes J. S. L. Chapter 6: supplemental guidance on selecting a method of qualitative evidence synthesis, and integrating qualitative evidence with Cochrane intervention reviews. In: Noyes J., Booth A., Hannes K., Harden A., Harris J., Lewin S., editors. Supplementary Guidance for Inclusion of Qualitative Research in Cochrane Systematic Reviews of Interventions Version 1: Cochrane Collaboration Qualitative Methods Group. 2011. [Google Scholar]
- Nsigaye R., Wringe A., Roura M., Kalluvya S., Urassa M., Busza J. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J. Int. AIDS Soc. 2009;12(31) doi: 10.1186/1758-2652-12-31. (PubMed PMID: 19906291; PubMed Central PMCID: PMCPmc2788344. Epub 2009/11/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn A., Cornwall A., Fu J., Bazerman L., Loewenthal H., Beckwith C. Linking HIV-positive jail inmates to treatment, care, and social services after release: results from a qualitative assessment of the COMPASS program. J. Urban Health. 2010;87(6):954–968. doi: 10.1007/s11524-010-9496-7. (PubMed PMID: 21046470; PubMed Central PMCID: PMC3005089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke N.L., Ostermann J., Thielman N.M. Enhancing linkage and retention in HIV care: a review of interventions for highly resourced and resource-poor settings. Curr. HIV/AIDS Rep. 2014;11(4):376–392. doi: 10.1007/s11904-014-0233-9. (PubMed PMID: 25323298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego C., Huedo-Medina T.B., Llorca J., Sevilla L., Santos P., Rodriguez E. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–1396. doi: 10.1007/s10461-011-9942-x. (PubMed PMID: 21468660) [DOI] [PubMed] [Google Scholar]
- Piot P., Abdool Karim S.S., Hecht R., Legido-Quigley H., Buse K., Stover J. Defeating AIDS-advancing global health. Lancet. 2015 doi: 10.1016/S0140-6736(15)60658-4. (PubMed PMID: 26117719) [DOI] [PubMed] [Google Scholar]
- Prentice T., Mill J., Archibald C.P., Sommerfeldt S., Worthington C., Jackson R. Aboriginal youth experiences of accessing HIV care and treatment. Journal of HIV/AIDS & Social Services. 2011;10(4):395–413. (PubMed PMID: 2011363803. Language: English. Entry Date: 20120106. Revision Date: 20140509. Publication Type: journal article) [Google Scholar]
- Sarna A., Sebastian M., Bachani D., Sogarwal R., Battala M. Pretreatment loss-to-follow-up after HIV diagnosis from 27 counseling and testing centers across India: findings from a cohort study. Journal of the International Association of Providers of AIDS Care. 2014;13(3):223–231. doi: 10.1177/1545109712469686. (PubMed PMID: 23418205) [DOI] [PubMed] [Google Scholar]
- Shroufi A., Mafara E., Saint-Sauveur J.F., Taziwa F., Vinoles M.C. Mother to mother (M2M) peer support for women in prevention of mother to child transmission (PMTCT) programmes: a qualitative study. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0064717. (PubMed PMID: 23755137; PubMed Central PMCID: PMC3673995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie K., Van Borek N., van der Kop M.L., Lukhwaro A., Li N., Karanja S. Mobile health for early retention in HIV care: a qualitative study in Kenya (WelTel Retain) Ajar-African Journal of Aids Research. 2014;13(4):331–338. doi: 10.2989/16085906.2014.961939. (PubMed PMID: WOS:000347305800003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souteyrand Y.P., Collard V., Moatti J.P., Grubb I., Guerma T. Free care at the point of service delivery: a key component for reaching universal access to HIV/AIDS treatment in developing countries. AIDS (London, England) 2008;22(Suppl. 1):S161–S168. doi: 10.1097/01.aids.0000327637.59672.02. (PubMed PMID: 18664948) [DOI] [PubMed] [Google Scholar]
- Stricker S.M., Fox K.A., Baggaley R., Negussie E., de Pee S., Grede N. Retention in care and adherence to ART are critical elements of HIV care interventions. AIDS Behav. 2014;18(Suppl. 5):S465–S475. doi: 10.1007/s10461-013-0598-6. 10.1007/s10461-013-0598-6 Epub 2013/12/03. (PubMed PMID: 24292251) [DOI] [PubMed] [Google Scholar]
- Thomas J., Harden A. Methods for the thematic synthesis of qualitative research in systemic reviews. BMC Med. Res. Methodol. 2008;8:45. doi: 10.1186/1471-2288-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A., Flemming K., McInnes E., Oliver S., Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med. Res. Methodol. 2012;12:181. doi: 10.1186/1471-2288-12-181. (PubMed PMID: 23185978; PubMed Central PMCID: PMC3552766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp N., Michels C., Mikkelsen E., Hontelez J., Baltussen R. Equity in utilization of antiretroviral therapy for HIV-infected people in South Africa: a systematic review. Int. J. Equity Health. 2014;13(60) doi: 10.1186/s12939-014-0060-z. (PubMed PMID: 25078612; PubMed Central PMCID: PMCPMC4448289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso L.S., Best J., Beanland R., Doherty M., Lackey M., Ma Q. Facilitators and barriers in HIV linkage to care interventions: a qualitative evidence review. AIDS (London, England) 2016 doi: 10.1097/QAD.0000000000001101. (PubMed PMID: 27058350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS . UNAIDS; Geneva: 2014. Fast Track - Ending the AIDS Epidemic by 2030. [Google Scholar]
- Weiss L., French T., Waters M., Netherland J., Agins B., Finkelstein R. Adherence to HAART: perspectives from clients in treatment support programs. Psychol. Health Med. 2006;11(2):155–170. doi: 10.1080/13548500500159430. (PubMed PMID: 17129905) [DOI] [PubMed] [Google Scholar]
- WHO . Chapter 6. Clinical guidelines across the continuum of care: Linking people diagnosed with HIV infection to HIV care and treatment. In: Po H.I.V., editor. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. 2013. (Consolidated ARV Guidelines). (Geneva 2013, June) [Google Scholar]
- World Health Organization (WHO) 2014. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. [PubMed] [Google Scholar]
- Zeglin R.J. Assessing the role of masculinity in the transmission of HIV: a systematic review to inform HIV risk reduction counseling interventions for men who have sex with men. Arch. Sex. Behav. 2015 doi: 10.1007/s10508-015-0501-9. (PubMed PMID: 25917411) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material