Abstract
Environmental exposure early in development plays a role in susceptibility to disease in later life. Here, we demonstrate that prolonged febrile seizures induced by exposure of rat pups to a hyperthermic environment enhance seizure susceptibility not only in these hyperthermia-treated rats but also in their future offspring, even if the offspring never experience febrile seizures. This transgenerational transmission was intensity-dependent and was mainly from mothers to their offspring. The transmission was associated with DNA methylation. Thus, our study supports a “Lamarckian”-like mechanism of pathogenesis and the crucial role of epigenetic factors in neurological conditions.
Abbreviations: DMR, differentially-methylated region; DNMT, DNA methyltransferases; FS, febrile seizures; KA, kainic acid; LTP, long-term potentiation; MeDIP-seq, massively parallel sequencing; MES, maximal electro-shock
Keywords: Febrile seizures, Transgeneration, Seizure susceptibility, DNA methylation
Highlights
-
•
FS induced enhanced seizure susceptibility was transgenerational transmitted.
-
•
The transgenerational transmission was FS-intensity dependent.
-
•
The transgenerational transmission was mainly from mothers to their offspring.
-
•
The transmission was associated with DNA methylation.
Environmental experience during developmental period plays a critical role. Whether the enhanced seizure susceptibility induced by early life FS transmitted to their unaffected offspring and the underlie characteristic of this transgenerational transmission Here, we used a well-established FS models showed that prolonged febrile seizures enhance seizure susceptibility not only in these hyperthermia-treated rats but also in their future non-FS offspring. This transgenerational transmission was intensity-dependent and was mainly from mothers to their offspring, and was associated with DNA methylation.
1. Introduction
Febrile seizures (FS) are the most common type of seizure in childhood (Shinnar, 1998, Sillanpaa et al., 1998). Retrospective clinical studies have demonstrated a significant relationship between a history of prolonged FS during early childhood and mesial temporal sclerosis, which is responsible for intractable temporal lobe epilepsy (Cendes et al., 1993). However, it is difficult in clinical studies to separate the effect of the FS itself (e.g., seizure type, number, and duration) from the effect of preexisting brain pathology (e.g. genetic or acquired factors) and the influence of treatment.
Well-established models have been developed, in which FS are evoked by exposing rat pups to a hyperthermic environment that raises the body temperature to a level comparable with human fever (Baram et al., 1997, Holtzman et al., 1981, Chen et al., 1999, Dube et al., 2000). Using these models, studies have shown that 100% of FS rats show enhanced susceptibility to kainic acid (KA)-induced seizures (Dube et al., 2000, Dube et al., 2006, Dube et al., 2010). Recently, our study also demonstrated that infantile FS mice showed decreased generalized seizures threshold induced by maximal electro-shock (MES) (Siedlecki & Zielenkiewicz, 2006). However, whether the enhancement of susceptibility is widely existed and maintained for long time and how this phenomenon occurs are still unknown.
It has been demonstrated that exposing young animals to an enriched environment enhances the magnitude of LTP induction in the CA1 hippocampus of themselves as well as their future offspring, even if the offspring are not exposed to enriched environment. Furthermore, it is demonstrated that behavioral and emotional disorders acquired from early-life adverse environmental experiences can be transmitted to their unaffected offspring (Sterba et al., 2007, Kim et al., 2009, Arai et al., 2009). These findings suggest that the acquired enhancement of neuroplasticity can be transmitted to offspring. As neuroplasticity plays an important role in the development of a chronic seizure state (Bough et al., 2004, Schwartzkroin, 2001). Thus a key question is whether the enhanced seizure susceptibility induced by early life FS transmitted to their unaffected offspring and the underlie characteristic of this transgenerational transmission.
Previously studies have shown that early-life environmental exposure led to epigenetic changes which even transgenerational transmission to their unaffected offspring (Jirtle and Skinner, 2007, Skinner et al., 2010, Bohacek et al., 2013). FS was also a disease induced by early-life hyperthermia environmental exposure. Here we investigated whether DNA methylation, a main composition of epigenetic modification, participated in the enhanced seizure susceptibility induced by serious FSs in affected adult rats and their offspring.
Therefore, using a modified model of FS, we investigated the effect of acquired FS in immature rats on the adult seizure susceptibility in their future offspring. Furthermore, the crucial characteristics and a role of epigenetic modulation in the transgenerational transmission were studied.
2. Materials and Methods
2.1. Animals
Sprague-Dawley rats (Grade II, Certificate No. 22-9601018; Experimental Animal Center, Zhejiang University, China) were maintained in individual cages with a 12-h light-dark cycle (lights on from 08:00 to 20:00). Water and food were given ad libitum. Parturition was checked daily, and the day of birth was defined as postnatal day 0 (P0). When weaned (on P21), rats were housed 5 per cage according to sex. Experiments were carried out each day between 10:00 and 17:00. All experiments were carried out in accordance with the Ethical Guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Generation of Experimental FS
Experimental FS were induced in rat pups on P9-P11 using a method adapted from a previously study (Schuchmann et al., 2006, Feng et al., 2016, Chen et al., 2016). After weighing, the body temperature was raised in a chamber at 42–46 °C. Core temperatures were measured at baseline (33.5–34.8 °C) and seizure onset (39.5–42 °C) by a rectal probe (Temperature Control machine, Bowdoinham, ME). Rats were moved to a cool surface for 2 min once a seizure was evoked. The latency to the first onset of a hyperthermia-induced seizure showed little variation (16.4 ± 0.3 min). Pups that had no seizure within 30 min were excluded from further experiments. The behavioral seizures induced by hyperthermia had previously been correlated with EEG seizures (Fig. 1c) and were stereotyped, consisting of sudden movement arrest followed by facial automatisms (chewing), forelimb clonus, and tonic flexion of the body, often associated with a loss of postural control. For 10 FS rats, the total time in the chamber was ~ 56 min, and the time outside the chamber was 18 min (9 times, to decrease the body temperature on a cool surface). The temperature of the chamber (42–46 °C) was maintained for ~ 90 min for 10 FS and ~ 30 min for 4 FS rats. The skin of all pups was intensively examined during the FS, and 15 min, 6 h, 1 day, 2 days, and 3 days later to ensure there were no burns. There were no burns in our experiments.
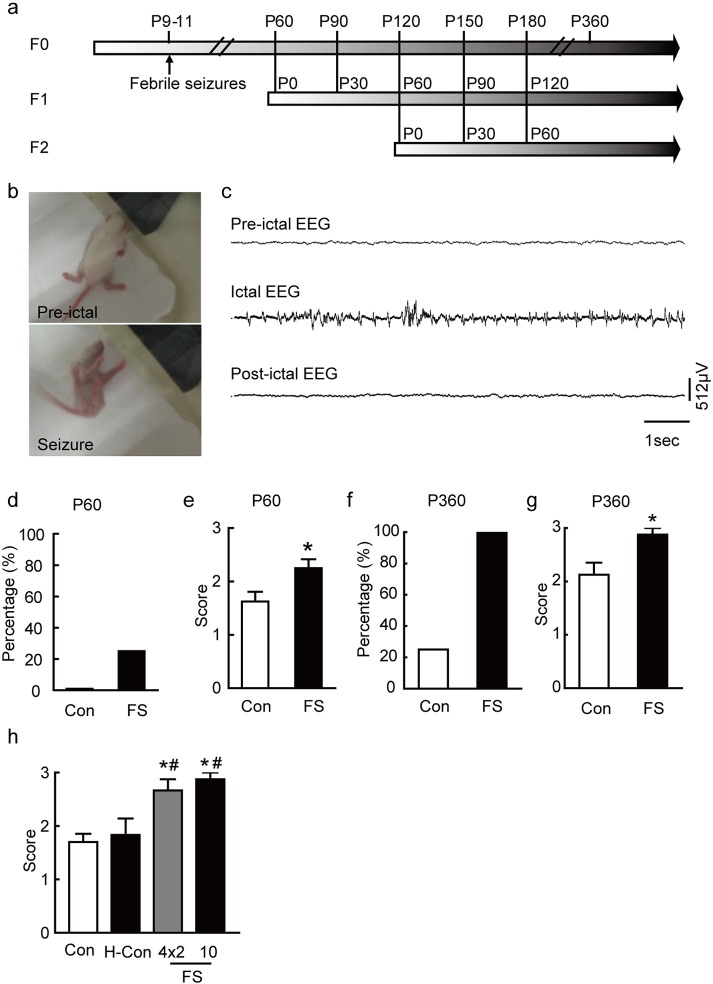
Fig. 1.
Induction of febrile seizures and long-lasting effects of prolonged hyperthermic seizures. (a) Timeline of the experiment. (b) A typical febrile seizure occurred when the core temperature reached threshold. Left, rat before seizure onset; right, behavioral seizure after core temperature reached threshold. (c) Upper trace, EEG before the onset of seizures, showing non-rhythmic waves; middle trace, the onset of a behavioral seizure, with rhythmic discharges of increasing amplitude in the amygdala; lower trace, post-ictal state after behavioral seizures stopped. (d) Percentage of hindlimb seizures and (e) seizure scores induced by MES (40 mA, 0.2 s, 50 Hz) in P90 rats (n = 9 each for the FS and control groups). (f) Percentage of hindlimb seizures and (g) seizure scores induced by MES (65 mA, 0.2 s, 50 Hz) in P360 rats (n = 8 for the FS group, n = 7 for the control group). (h) FS rats (both 10 FSs and 4*2 FSs) showed enhanced susceptibility to MES-(65 mA, 0.2 s, 50 Hz) induced seizures (n = 6 for each group), whereas hyperthermic control group, in which rat pups were exposed to hyperthermia but seizures were suppressed with pentobarbital, and control group showed similar seizures scores to MES. Seizure susceptibility was tested in 360 days old rats. CON: control group; HT: hyperthermic control group; FS:FS group. *P < 0.05 compared with control group; #P < 0.05 compared with hyperthermic control group. Error bars indicate SEM.
To determine whether intensity influenced the transmission of seizure susceptibility (according to the number of hyperthermic seizures), rats were divided into two groups: one group experienced two episodes of FSs: 4 seizures within 30 min on P9 and P11; the other had 10 seizures within 90 min on P9.
After hyperthermia, animals were weighed and moved to a cool surface until the core temperature returned to the normal range for age, and then returned to the home cage. Control rats were littermates of the experimental group. They were separated from the cages for the same duration, and their core temperature was maintained within the normal range for age.
For the hyperthermia controls (H), animals were subjected to 10 episodes of hyperthermia along with the FS 10 group, but seizures were prevented by pretreatment of pentobarbital (25 mg/kg, i.p.).
The body weight of each pup was measured every day until they reached 60 days of age.
2.3. Offspring Generation
At ~ P60, the F0 generation rats that had received different treatments during infancy were allowed to mate. F1 generation rats born to F0 mothers and F2 generation rats born to F1 mothers at 90–150 days of age were used. Male rats were moved out of the cage when the females became pregnant. FS females were mated with non-FS males and vice versa to reveal which parent contributes to the phenomenon of transgenerational transmission.
2.4. Maximal Electroshock (MES)-Induced Seizures
Electroshock was delivered using a Rodent Shocker (Hugo Sachs Elektronik, March-Hugstetten, Germany) with ear electrodes moistened with saline.
In the F0 generation, when rats reached ~ 60 and 360 days old, they were treated with MES (40 mA, 0.2 s, 50 Hz; and 65 mA, 0.2 s, 50 Hz, respectively). In the F1 and F2 generations, when the rats reached 90 days old they were treated with MES (40 mA, 0.2 s, 50 Hz). Three features of the MES-induced convulsions were analyzed as measures of seizure severity: (1) the motor convulsion pattern, (2) the latency and duration of tonic forelimb extension, and (3) the latency and duration of tonic hindlimb extension. The convulsion patterns were assigned scores based on the extent of the spread of tonic extension (Feng et al., 2016): 0, absence of forelimb extension; 1, complete forelimb extension without hindlimb extension; 2, complete forelimb extension with partial hindlimb extension; and 3, complete fore- and hindlimb extension (with hindlimbs fully extended parallel to the tail).
The investigator was blinded to the group allocation during experiments. All data were collected and analyzed in a blinded manner.
2.5. Intrahippocampal KA-Induced Seizures
For intrahippocampal KA injection, the tip of the cannula was located in the CA3 area of the hippocampus (AP − 5.3 mm, L − 5 mm, V − 6 mm). For EEG recording, electrodes were implanted in the right amygdala. The surgery has been described elsewhere (Wu et al., 2008a, Wu et al., 2008b). In brief, when offspring reached ~ 90 days old, under sodium pentobarbital anesthesia (35 mg/kg, i.p.; Abbott, North Chicago, IL, USA), rats were fixed in a stereotaxic apparatus (Narishige, SR-5, Tokyo, Japan), and recording electrodes (each 0.2 mm in diameter) were implanted into the right basolateral amygdala (AP − 2.4 mm, L − 4.8 mm, V − 8.8 mm). The electrodes were bipolar Teflon-coated twisted stainless-steel wires (tip distance 0.5–1.0 mm; A.M. Systems. Inc., USA) and insulated except at the tip (0.5 mm). The electrodes were connected to a miniature receptacle. The cannula and miniature receptacle were attached to the skull with dental cement. All coordinates were measured in mm from bregma.
For intrahippocampal KA-induced seizures, 10 days after surgery, KA (0.5 μg/0.5 μL in normal saline) was injected unilaterally over a period of 5 min through the guide cannula in freely-moving rats (Bragin et al., 2005). Electrical activity was recorded via a digital amplifier (NuAmps, Neuroscan System, USA) for 6–8 h after KA injection. Seizure severity was classified according to Racine (1972)): (1) facial movement; (2) head nodding; (3) unilateral forelimb clonus; (4) bilateral forelimb clonus (BFC) and rearing; and (5) BFC with rearing and falling.
The investigator was blinded to the group allocation during experiments. All data were collected and analyzed in a blinded manner.
2.6. RT-PCR
RNA was extracted from adult rats with different treatments. Total RNA was isolated from the hippocampus using Trizol (Invitrogen). First-strand cDNA synthesis was carried out using 2 μg total RNA with reverse transcriptase. SYBR Green was used to monitor the amplification of template with primers on a real-time thermal cycler. The main procedures were according to a previous study (Lu et al., 2010).
The following PCR primers were used for semi-quantitative RT-PCR analysis:
Dnmt1, 5′-GGGTCTCGTTCAGAGCTG and 5′-GCAGGAATTCATGCAGTAAG; Dnmt3a, 5′-CAGCGTCACACAGAAGCATATCC and 5′-GGTCCTCACTTTGCTGAACTTGG;
Dnmt3b, 5′-GAATTTGAGCAGCCCAGGTTG and 5′-TGAAGAAGAGCCTTCCTGTGCC (Feng et al., 2016);
β-Actin, 5′-GTGGGCCGCTCTAGGCACCAA and 5′-CTCTTTGATGTCACGCACGATTTC.
2.7. Western Blotting
Protein extracts were made from P60 adult rats with different treatments. They were separated by SDS-PAGE on a 7.5% resolving gel with a stacking gel and transferred onto nitrocellulose membrane. Blots were placed in 5% non-fat milk for 1 h at room temperature, and then incubated with primary antibody (diluted in TBS/0.05% Tween) overnight at 4 °C. Then the blots were washed and probed with the respective horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. The following primary antibodies were used: anti-DNMT-1 (1:1000; Cell Signaling; D63A6), anti-DNMT-3A (1:1000; Abcam; ab113430), anti-DNMT-3B (1:1000; Abcam; ab79822), and anti-β-actin (1:1000; Abcam; ab1801).
Blots were visualized with the Odyssey infrared imaging system (LI-COR Biosciences) and analyzed with Odyssey software. The relative optical density was obtained by comparing the measured values with the mean value from the control group.
2.8. DNMT Inhibitor Treatment
Zebularine (Sigma) was dissolved in 10% DMSO and diluted to 2 mg/ml in sterile saline. 5-aza-deoxycytidine (Sigma) was dissolved in 0.8% acetate and diluted to 1 mg/ml in sterile saline. Rats at P9 were administered 0.1 ml DNMT inhibitor (i.p.) immediately after FS and then daily for the following 5 days.
2.9. Histology
At the end of each experiment, the electrode and cannula placements were histologically verified. Coronal brain slices were cut (12 μm) and stained with toluidine blue. Only animals with electrodes and cannula correctly implanted in the targets were included in the statistical analysis.
2.10. MeDIP and Sequencing Library Preparation
For MeDIP, genomic DNA was sonicated to ~ 200–800 bp with a Bioruptorsonicator (Diagenode). The sonicated DNA (800 ng) was end-repaired, A-tailed, and ligated to single-end adapters following the standard Illumina genomic DNA protocol. After agarose size-selection to remove unligated adapters, the adapter-ligated DNA was used for immunoprecipitation using a mouse monoclonal anti-5-methylcytosine antibody (Diagenode). For this, DNA was heat-denatured at 94 °C for 10 min, rapidly cooled on ice, and immunoprecipitated with 1 μL primary antibody overnight at 4 °C with rocking agitation in 400 μL immunoprecipitation buffer (0.5% BSA in PBS). To recover the immunoprecipitated DNA fragments, 200 μL of magnetic beads was added and incubated for an additional 2 h at 4 °C with agitation. After immunoprecipitation, five washes were performed with ice-cold immunoprecipitation buffer. A nonspecific mouse IgG immunoprecipitation was performed in parallel with the methyl DNA immunoprecipitation as a negative control. Washed beads were re-suspended in TE buffer with 0.25% SDS and 0.25 mg/mL proteinase K for 2 h at 65 °C and then allowed to cool to room temperature. MeDIP and supernatant DNA were purified on QiagenMinElute columns and eluted in 16 μL EB (Qiagen). Fourteen cycles of PCR were performed on 5 μL of the immunoprecipitated DNA using the single-end Illumina PCR primers. The resulting reactions were purified with QiagenMinElute columns, after which a final size selection (300–1000 bp) was performed by electrophoresis in 2% agarose. Libraries were quality-controlled by an Agilent 2100 Bioanalyzer. An aliquot of each library was diluted to 5 ng/μL in EB and 1 μL was used in real-time PCR reactions to confirm the enrichment for methylated regions. The library was denatured with 0.1 M NaOH to generate single-stranded DNA, loaded onto channels of the flow cell at 8 pM, and amplified in situ using a TruSeq Rapid SR Cluster Kit (#GD-402-4001, Illumina). Sequencing was carried out by running 100 cycles on an Illumina HiSeq 2000 according to the manufacturer's instructions. After the sequencing platform generated the sequencing images, the stages of image analysis and base-calling were performed using Off-Line Basecaller software (OLB V1.8). After passing the Solexa CHASTITY quality filter, the clean reads were aligned to the rat genome (UCSC RN5) using BOWTIE software (V2.1.0). MeDIP peaks and DMRs were identified by MACS V2.
2.11. Differentially-expressed Genes, Gene Ontology (GO), and Pathway Analysis
RNA quantity and quality were measured by NanoDrop ND-1000. RNA integrity was assessed by standard denaturing agarose gel electrophoresis. The rat 4x44K Gene Expression Array was manufactured by Agilent. Updated content provided full coverage of rat genes and transcripts. Sample labeling and array hybridization were performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technology). Briefly, total RNA from each sample was linearly amplified and labeled with Cy3-UTP. The labeled cRNAs were purified by an RNeasy Mini Kit (Qiagen). The concentration and specific activity of the labeled cRNAs (pmol Cy3/μgcRNA) were measured by NanoDrop ND-1000. One microgram of each labeled cRNA was fragmented by adding 11 μL of 10 × Blocking Agent and 2.2 μL of 25 × Fragmentation Buffer, then the mixture was maintained at 60 °C for 30 min. Finally, 55 μL of 2 × GE hybridization buffer was added to dilute the labeled cRNA. One hundred microliters of hybridization solution was dispensed into the gasket slide and assembled to the gene expression microarray slide. The slides were incubated for 17 h at 65 °C in an Agilent hybridization oven. The hybridized arrays were washed, fixed, and scanned using an Agilent DNA microarray scanner (part number G2505C). Agilent Feature Extraction software (version 11.0.1.1) was used to analyze acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v11.5 software package (Agilent Technologies). After quantile normalization of the raw data, genes that at least 4 out of 20 samples had flags in Detected (“All Targets Value”) were chosen for further data analysis. Differentially-expressed genes with statistically significant differences between two groups were identified by Volcano Plot filtering. Hierarchical clustering was performed using Agilent GeneSpring GX software (version 11.5). GO analysis and pathway analysis were performed using the standard enrichment computation method.
2.12. Statistics
Data are expressed as mean ± SEM. The two-tailed unpaired t-test was used for two-group comparison, and one-way ANOVA (analysis of variance) with Tukey's post-hoc test was used for multiple comparisons. P < 0.05 was considered statistically significant.
3. Results
3.1. Long-lasting Effect of Hyperthermic Seizures in the F0 Generation
Fig. 1a showed the experimental schedule. FS were induced on P9-11. Seizures occurred after exposing rat pups to a hyperthermic environment (Fig. 1b and c). The temperature threshold for the generation of an initial FS was 41.8 ± 0.1 °C and the latency was 16.4 ± 0.3 min. Even 10 episodes of FS (10 FS) did not result in dehydration: the weight before seizures averaged 25.5 ± 0.5 g, and immediately afterwards, 24.7 ± 0.5 g, a weight change of 3.2 ± 0.3% (n = 8).
To further test whether the enhanced seizure susceptibility lasted for a long time, seizures were induced by maximal electroshock (MES) in rats up to 360 days old (Fig. 1a). In P60 rats stimulated at 40 mA, 10 FS rats showed a higher percentage of fore- and hind-limb extension, and higher seizure scores (Fig. 1d and e). Because both male and female FS-rats showed higher scores (data not shown), here we combined these two genders together if not special indicated. Furthermore, in P360 rats stimulated with 65 mA (because old rats were not as excitable as young rats, 65 mA rather than 40 mA was chosen), FS rats showed a higher percentage of complete fore- and hind-limb extension, and higher seizure scores (Fig. 1f and g). However, the hyperthermic control group, in which rat pups were exposed to hyperthermia but seizures were pre-suppressed with pentobarbital, showed similar seizure scores to control rats under MES stimulation (Fig. 1h). Thus, FS-induced enhancement of seizures susceptibility can be maintained for at least 12 months without spontaneous seizures.
3.2. Effect of Hyperthermic Seizures is Transmitted Transgenerationally
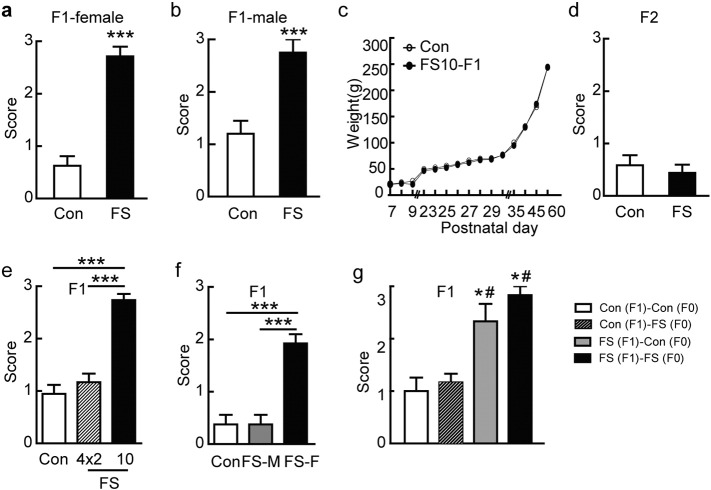
Because FS-induced enhanced seizure susceptibility lasted long enough for rats to bear offspring, whether it was transmitted transgenerationally were tested. Nine-day-old male and female rats were exposed to a hyperthermic environment to evoke FS, or to a conventional environment as controls. They were allowed to mate when they were older than 60 days old age (Fig. 1a). Offspring were raised in a conventional environment for ~ 90 days and then the susceptibility to MES seizures was compared with rats whose parents had not experienced FS. The 90-day-old offspring of FS rats displayed a higher susceptibility to MES-induced seizures compared with offspring of controls. As was shown in Fig. 2a and b, 40 mA current stimulation induced hindlimb extension in 73.3% of the offspring of FS rats and induced higher seizure scores in both genders of the offspring of FS rats. The difference of susceptibility to seizures was not due to a difference in weight, because there was no difference of body weight between the offspring of FS and control rats in the development curve (Fig. 2c).
Fig. 2.
Effect of hyperthermic seizures was transmitted transgenerationally. (a) and (b) MES induced higher scores in female (n = 8 for the FS group and n = 15 for the control group) and male offspring, respectively (n = 7 for the FS group and n = 8 for the control group). (c) The developmental weight curve after FS. The FS group and control group did not show obvious differences. (d) Susceptibility to MES in F2 offspring of control and FS groups (n = 16 for the FS group, n = 12 for the control group). (e) Susceptibility to MES in 90-day-old offspring whose parents experienced 10 seizures or 4 × 2 seizures was compared with age-matched offspring of control rats (n = 15 for the 10 FS group, n = 18 for the 4 × 2 FS group, and n = 23 for the control group). (f) MES-induced seizure scores were assessed in the 90-day-old offspring of 10-FS female and non-FS male rats (n = 13) or in the offspring of non-FS female and 10-FS male rats (n = 8). MES-induced seizure scores in the offspring of non-FS parents are also shown for comparison (n = 13). (g) Maternal care did not affect the transgenerational transmission of seizure susceptibility. Offspring of FS rats, either raised by FS or non-FS rats (n = 6 for each group), displayed higher susceptibility to MES-induced seizures compared with control rats (n = 6); offspring of non-FS rats, reared by FSs foster mothers (n = 6) displayed normal seizure susceptibility compared with control rats. C(F1)-C(F0): offspring of control rats reared by control mothers; C(F1)-FS(F0): offspring of control rats reared by FS mothers; FS(F1)-C(F0): offspring of FS rats reared by control mothers; FS(F1)-FS(F0): offspring of FS rats reared by FS mothers; Error bars indicated SEM. ***P < 0.001, *P < 0.05 compared with control group reared by control mothers; #P < 0.05 compared with control group reared by FS mothers.
3.3. Transgenerational Transmission is Limited to F1 Generation
To determine whether the transmission is limited to the second generation (F1 generation), third generation rats (F2 generation) were bred from the offspring of FS-F1 rats (Fig. 1a). Similar to F1 generation, these rats were raised in a conventional environment so that they also never experienced FS. The results showed that the susceptibility to MES-induced seizures was not statistically different from the offspring of non-FS rats (Fig. 2d), suggesting that the effect of FS was lost in the F2 generation. The 90-day-old offspring of F0 rats that had experienced 10 FS displayed greater susceptibility to MES-induced seizures than the offspring of both control F0 rats and F0 rats that experienced 4 × 2 FS (Fig. 2e). However, although rats that had experienced 4 × 2 FS showed higher scores than control F0 rats (Fig. 1h), their 90-day-old offspring did not show a significant difference in MES-induced seizures compared with the control group (Fig. 2e).
3.4. Transgenerational Transmission of Enhanced Susceptibility was Through the Mother Before Birth
To reveal which parent contributes to the transgenerational transmission, 10 FS females were mated with non-FS males and vice versa. The results showed that only when female rats experienced FS did their offspring display higher MES scores (Fig. 2f). Thus, these results showed that the transmission is through the mother.
We next determined whether the transgenerational transmission occurred before or after birth. Pups born to FS mothers were placed with non-FS foster mothers until they were weaned. Alternatively, pups born to non-FS mothers were placed with FS foster mothers until they were weaned. The results showed that F1 rats whose biological mothers had experienced FS, raised by either FS or non-FS mothers, displayed higher susceptibility to MES than control rats (Fig. 2g). In contrast, rats born to non-FS mothers reared by FS foster mothers did not differ in seizure susceptibility from control rats (Fig. 2g). These results showed that transgenerational effect of FS occurs before birth.
3.5. Effect of Hyperthermic Seizures was Transmitted Transgenerationally in Kainate-induced Seizures
We then investigated whether the transgenerational effect existed for other forms of seizures. Ninety-day-old offspring were tested by intrahippocampal kainate (KA) treatment. The percentage of generalized seizures (stages 4–5) and the average seizure stage were higher in the F1 generation of the FS group than in control rats (Fig. 3a and b). Representative EEG traces of baseline and seizure bursts in amygdala and CA3 after KA injection are shown in Fig. 3d. Furthermore, the seizure stage induced by KA in the F2 generation was not statistically different from that in the offspring of non-FS rats (Fig. 3c). Thus, FS resulted in enhanced susceptibility to KA-induced seizures in F1 rats, which is consistent with MES model of seizures.
Fig. 3.
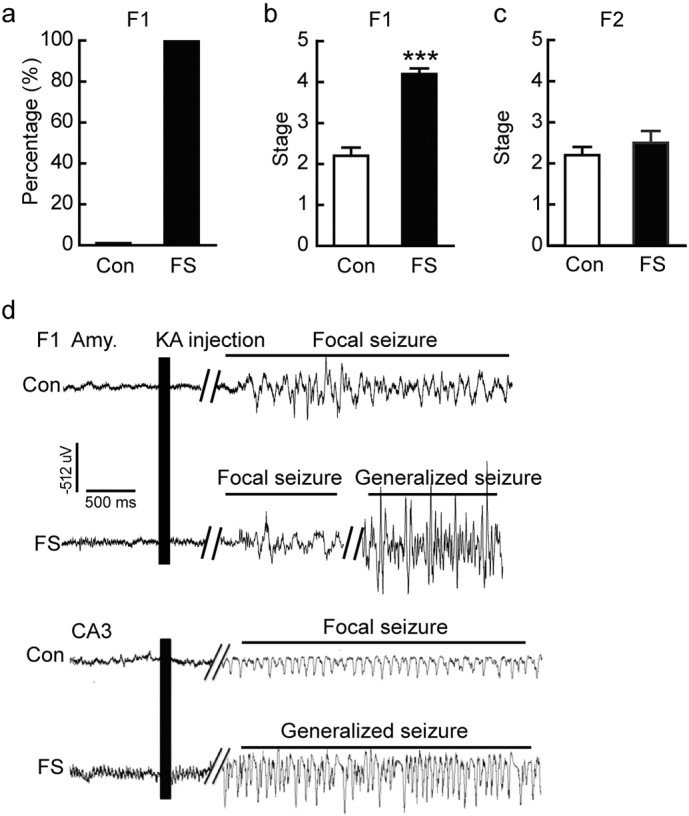
Transgenerational transmission of enhanced susceptibility to kainate (KA)-induced seizures. (a) Percentage of stage 4 and 5 seizures induced by KA injection in the offspring of control and FS rats. (b) Average seizure stage after intrahippocampal KA injection in the 90-day-old offspring of control and FS rats (n = 10 for the FS group, n = 6 for the control group). (c) Average seizure stage after intrahippocampal KA injection in 90-day-old F2 generation rats (n = 6 for the FS group, n = 6 for the control group). (d) Representative EEG traces in amygdala and CA3 of offspring of control and FS rats. Error bars indicate SEM. ***P < 0.001.
3.6. DNA Methylation Involved in Transgenerational Transmission
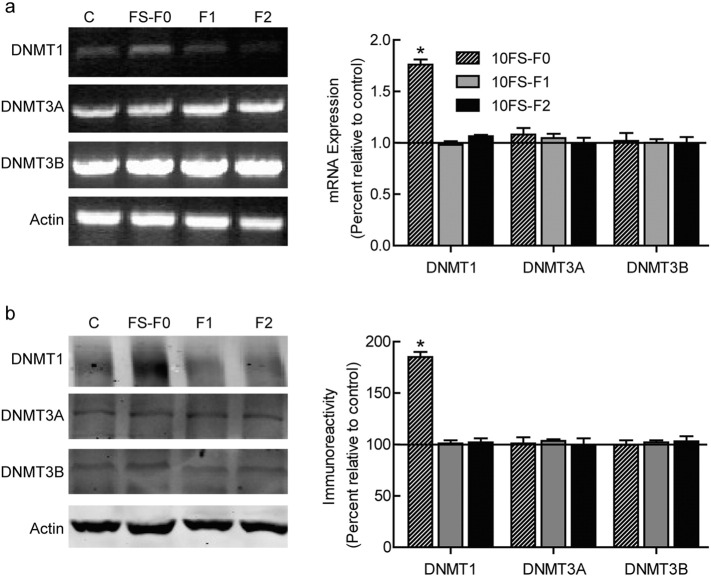
So far, our results showed that the transgenerational transmission seemed had epigenetic characteristics. So we further asked whether FS induced changes in DNA methylation, which is the main epigenetic component. We first investigated whether the transcription and translation of DNA methyltransferases (DNMTs) (Siedlecki & Zielenkiewicz, 2006) in the hippocampus were altered by FS. Then we assayed the levels of three DNMT subtypes, DNMT 1, 3A, and 3B and found that, at both the transcriptional and translational levels, FS rats (F0 group) and their offspring (F1 group, whose mother experienced FS) displayed an increase in DNMT 1 in the hippocampus compared to control rats, whereas the F2 and control groups showed similar expression (Fig. 4). The expression levels of DNMT 3A and 3B did not differ in these groups. These results demonstrated that FS lead to an increase in DNA methylation, which can inherit to the F1 generation only.
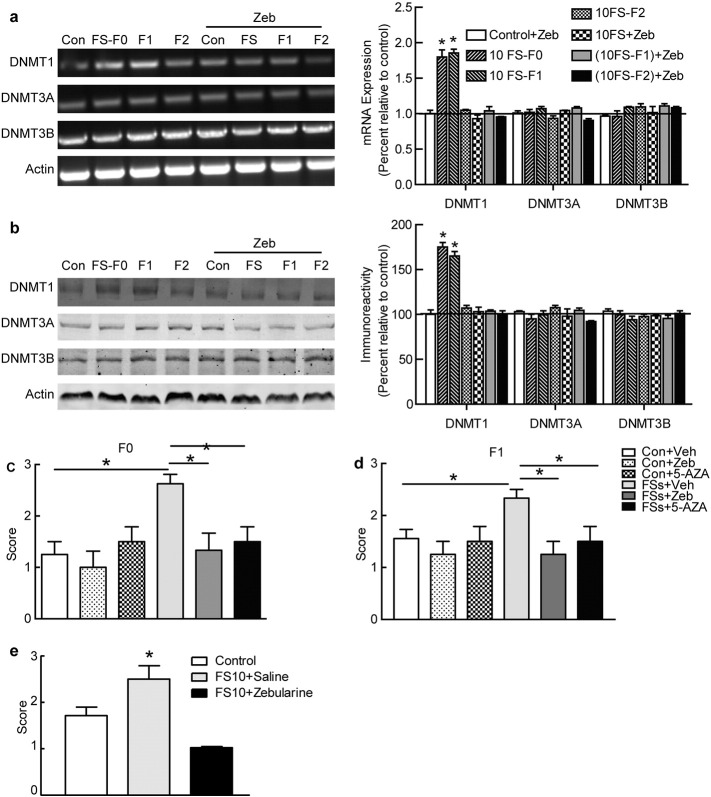
Fig. 4.
DNA methylation participated in the transgenerational transmission. (a) DNMT 1 mRNA levels were up-regulated in the hippocampus of FS rats and their F1offspring. DNMT 1 mRNA level did not change in F2 rats, the F0 generation of FS rats treated with zebularine, and FS rat offspring treated with zebularine. Zebualrine treatment did not change DNMT 1 mRNA levels in control rats. DNMT 3A and 3B mRNA levels did not differ among these groups (n = 6 for each group). (b) DNMT 1 protein expression was up-regulated in the hippocampus of FS rats and their future offspring but not in the F2 generation. Zebualrine treatment inhibited the up-regulation of DNMT 1 protein levels in F0 FS rats and the F1 offspring of FS rats but not in control and F2 rats (n = 6 for each group). DNMT 3A and 3B protein levels did not differ among them (n = 6 for each group, P > 0.05). (c) MES-induced seizure scores in adult FS rats treated with DNMT inhibitors (zebularine or 5-AZA) or saline immediately after FSs and once daily for the following 5 days in infant (n = 8 for each group). (d) MES-induced seizure scores in offspring of FS rats treated with DNMT inhibitors or saline in infant (n = 6 for each group). (e) MES-induced seizure scores in FS rats treated with DNMT inhibitors or saline in adult. C, control group; FS, 10 FS group; F1, F1 generation of FS group; F2, F2 generation of FS group; Con + Veh: control group treated with vehicle; FS + Veh: FS group treated with vehicle; (FS + Veh)-F1:offspring of FS group treated with vehicle; Error bars indicate SEM. *P < 0.05.
Next, we asked whether the up-regulated DNA methylation resulted in enhanced seizure susceptibility. We administered two DNMT inhibitors, zebularine and 5-AZA, to rat pups immediately after FS and once daily for 5 consecutive days. The susceptibility to MES-induced seizures was measured when these rats reached 60 days of age. There were no differences in seizure susceptibility between the animals treated with 5-AZA or zebularine, nor were there differences between their respective vehicle groups. Thus, the zebularine and 5-AZA data were merged into a DNMT inhibitor group. As shown in Fig. 4c, compared with vehicle treatment, the DNMT inhibitor-treated control group showed no difference in the susceptibility to MES, suggesting that DNMT inhibitors themselves did not influence the baseline level of seizure induction. However, FS rats treated with DNMT inhibitors were less susceptible to MES than those treated with vehicle, but rather similar to control rats (Fig. 4c). The offspring of DNMT inhibitor-treated FS rats were less susceptible to MES than those of vehicle-treated FS rats, but similar to those of control rats (Fig. 4d). In addition, zebularine-treated FS rats and their offspring showed DNMT 1 expression similar to the control group and DNMT 1 in the F2 generation, with or without zebularine, showed expression similar to the control group. On the other hand, DNMT 3A and 3B did not show differences in any groups. Furthermore, we treated FS 10-F0 dams with Zeb after the development of the seizure phenotype, we found that Zeb treatment could prevent the development of seizure susceptibility in adult FS10-F0 dams (Fig. 4e).
Furthermore, both mRNA and protein of DNMT1, DNMT 3A and 3B were not up-regulated in the offspring (both F1 and F2 generations) of 10-FS male rats comparing with the normal controls (Fig. 5). This was in accordance with that the enhanced susceptibility was passed on to subsequent generations through the mother, suggesting that the mother and father may influence offspring gene expression differently, which means that transgenerational transmission of DNA methylation was through the mother.
Fig. 5.
Transgenerational transmission of DNA methylation was through the mother. (a) DNMT1 mRNA level was up-regulated in the hippocampus of FS rats. DNMT1 mRNA was not upregulated in offspring of 10-FS male rats and control female rats (n = 6 for F0 and F1 group, n = 6 for F2 group). DNMT 3A and 3B did not show differences in these groups (n = 6 for each group, P > 0.05). (b) DNMT1 protein expression was up-regulated in the hippocampus of FS rats. DNMT1 protein was not upregulated in male offspring of 10-FS male rats and control female rats (n = 6 for F0 and F1 group, n = 6 for F2 group). DNMT 3A and 3B protein levels were similar in these groups (n = 6 for each group, P > 0.05). C: control male rats; FS:10-FS male rats; F1: F1 male generation of 10-FS father and control mother; F2: Offspring of F1 generation; Error bars indicated SEM. *P < 0.05.
In addition, we assessed whether zebularine interfered with the onset of FS. Zebularine was administered to rat pups 30 min before exposure to hyperthermia. The threshold and latency of FS in the pretreated rats were 41.0 ± 0.3 °C and 31.5 ± 0.5 min, respectively; both were comparable to FS rats pretreated with vehicle: 40.8 ± 0.3 °C and 31.9 ± 0.5 min. This excluded the possibility that zebularine influenced the generation of FS.
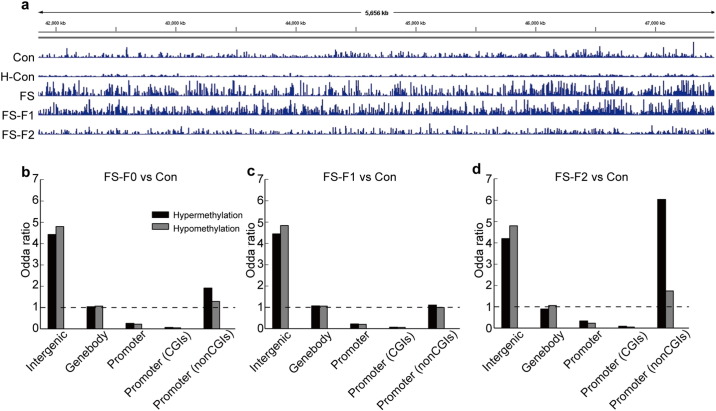
Further immunoprecipitation of methylated DNA followed by massively parallel sequencing (MeDIP-seq) analysis reveals a large number of differentially-methylated regions (DMRs). To identify focal methylation differences, we calculated the read density in 500-bp overlapping windows over the complete genome, and compared 4 samples for each group (see Fig. 6a for a characteristic hypermethylated DMR in the region of chr16: 41,848,235–47,512,328 by MeDIP-seq).
Fig. 6.
Generation and validation of genome-wide CpG methylation maps of normal, hyperthermia-only (H), FS, F1, and F2 rats. Distribution of differentially-mediated Regions (DMRs) in different subgenomic compartments. (a) IGV (Integrative Genomics Viewer) view of sequenced DNA methylation tracks of 5 rats from different groups in the 41,848,235–47,512,328 region of chromosome 16. (b), (c) and (d) Odds ratios (fraction of experimentally observed DMRs divided by relative size of subgenomic compartment) of hyper- and hypo-methylation within intergenic, genebody, promoters (all) and promoters that contain or do not contain CpG island (CGI) regions. Dashed lines demarcate over- versus under-representation. Comparisons between FS and control groups (b), F1 generation of FS and control groups (c), and F2 generation of FS and control groups (d).
Using odds ratio calculation showed that the methylation changes were unevenly distributed over the genome. Compared with the control group, the F0 generation of FS rats showed that both hyper- and hypomethylated DMRs were highly enriched in intergenic and promoter regions (Fig. 6b). In the F1 generation, both hyper- and hypomethylated DMRs were highly enriched in intergenic regions (Fig. 6c). In contrast, the F2 generation showed hyper- and hypomethylated DMRs highly enriched in intergenic and promoter (non-CGI) regions (Fig. 6d).
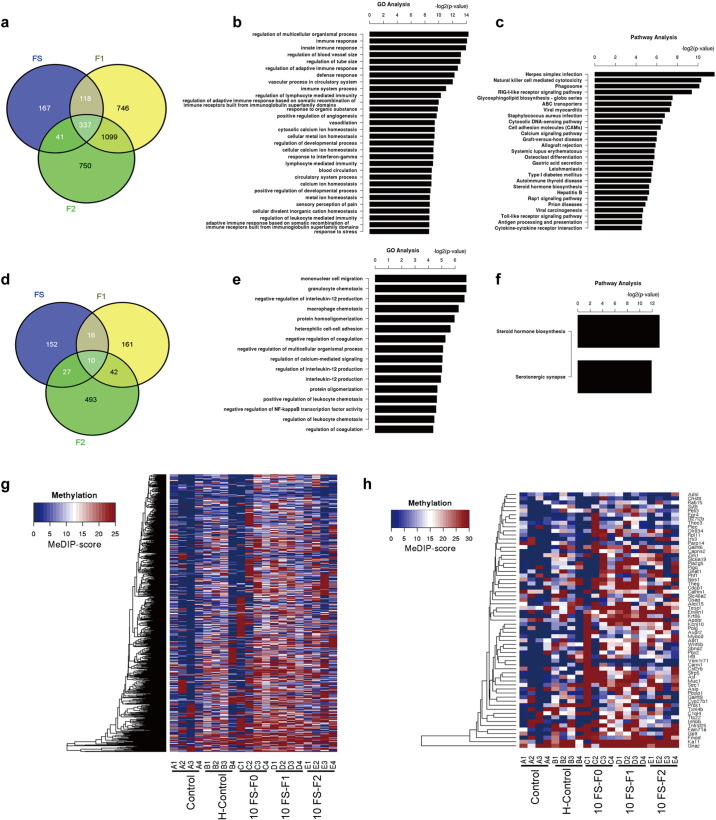
Significant GO and pathway categories were designated as those with a P value < 0.05. In order to compare across all the treatment groups, we first picked out the transcriptionally down-regulated genes in FS group comparing to control groups (the blue circle in Fig. 7a, number: 167 + 118 + 337 + 41 = 663), and then picked out the down-regulated genes in F1 generation comparing to control groups (the yellow circle in Fig. 7a, number: 746 + 118 + 337 + 1099 = 2302), further picked out the overlapping genes (118 + 337 = 455), finally we excluded the down-regulated genes in F2 generation comparing to control groups (the green circle in Fig. 7a, number: 750 + 41 + 337 + 1099 = 2227) out of these overlapping genes, the remaining genes were that included in GO and pathway analysis (the brown fraction in Fig. 7a, number: 118).
Fig. 7.
GO and Pathway Analysis for RNA down-regulation and DNA methylation up-regulation in the transgenerational transmission of enhanced seizure susceptibility. (a) Venn diagram showing the overlapped transcriptionally down-regulated genes of F0 and F1 generations but not F2 generation comparing to control group. (b) and (c) GO and pathways with the overlapped transcriptionally down-regulated genes. (d), Venn diagram showing overlapped hypermethylated genes of F0 and F1 generations but not F2 generation comparing to control group by DNA methylation analysis. (e) and (f) GO and pathway analysis of the overlapped hypermethylated genes. (g) and (h) Heat maps of the differentially methylation analysis among groups. (g) Heat map of the differential DNA methylation among the F0 and F1 generations of FS and the F2 generation of the FS and control groups. (h) Heat map of the differential DNA methylation among the F0 and F1 generations of FS, and the F2 generation of FS and control groups only when log10LR ≥ 150. A, control group; B, hyperthermia only group; C, 10FS group; D, F1 group; E, F2 group.
For DNA methylation analysis (Fig. 7d), we first picked out the hypermethylated genes in FS groups comparing to control groups (the blue circle in Fig. 7d, number: 152 + 16 + 10 + 27 = 205), and then picked out the hypermethylated genes in the F1 generation comparing to control groups (the yellow circle in Fig. 7d, number: 161 + 16 + 10 + 42 = 229), further picked out the overlapping genes (16 + 10 = 26), finally we excluded the hypermethylated genes in F2 generation comparing to control groups (the green circle in Fig. 7d, number: 493 + 10 + 27 + 42 = 572) out of these overlapping genes, the remaining genes (the brown fraction in Fig. 7d, number: 16) were that included in GO and pathway analysis (Fig. 7e and f). For our results, there is an overall net increase in methylation in the FS and F1 groups.
Further pathway analysis of genes with up-regulated methylation and down-regulated mRNA showed that they mainly belong to the steroid hormone biosynthesis and the serotonergic synapse pathways. Heat maps of the differential DNA methylation were shown in Fig. 7g and h. The condition of not only significant differential expression between the FS and control groups but also between the F1 generation of the 10 FS group and the control group was shown in Fig. 7g. Data with log10LR ≥ 150 were shown in Fig. 7h.
4. Discussion
Here, we established a modified model of FS that enhanced adult seizure susceptibility in the long-term without inducing spontaneous seizures. Based on this model, we found that FS not only enhanced the susceptibility to seizures in themselves, but also enhanced susceptibility in their non-hyperthermia-exposed offspring. Thus, this study provides the evidence that acquired susceptibility of seizures can be transmitted to the next generation.
In our study, we were surprised to find that the transmission is limited to F1 generation. One possible explanation for the limited transmission for enhancement of LTP is that the phenotype ends at a younger age in the offspring of FSs rats than in their parents, such that it is no longer present and thus not transferable to offspring by the time the F1 generation are old enough to reproduce (Arai et al., 2009). But the phenomenon described here is clearly distinct from the studies described above. In our study, the enhancement in F0 generation maintained for at least 360 days, within which the susceptibility of F2 generation were tested. Defining why the effect ends in F1 generation will require further experimentation.
Traditional research on the combined effects of the environment and genetics on individual variation in disease risk highlighted the importance of genotype in human diseases. However, it is now becoming clear that a full understanding of environmental interactions with the genome will require epigenetic mechanisms (Anway & Skinner, 2006). Epigenetic changes encompass both DNA and chromatin modification, the most extensively studied is DNA methylation, which changes the chromatin packaging of DNA and finally inhibits gene expression (Feinberg, 2007). Our results showed that increased DNMT1 expression were induced by FSs. DNMT1 has preferential activity for hemimethylated DNA and is traditionally considered as a maintenance methyltransferase in DNA replication (Siedlecki & Zielenkiewicz, 2006). The increased DNMT1 may maintain the hyper-methylation status to the adult. Indeed, both of the seizure susceptibility and DNMT1expression in these two generations can be reversed by DNA methylation inhibitor. With the results presented here, we now found the existence of FSs induced changes in DNA methylation across one generation. Thus, our finding raises the intriguing speculation that some interventions, such as treatment with DNMT inhibitor, might be proved useful as therapeutic strategies for reversing persisting effects of FSs on both generations with enhancement in susceptibility.
Notably, we found that enhanced susceptibility was passed on to the next generation through the mother, suggesting that the parents may differently influence the gene expression of offspring (Badcock & Crespi, 2008), which is consistent with the finding that transgenerational transmission of DNA methylation was through the mother. This phenomenon also exists in enriched environment induced enhancement of LTP (Arai et al., 2009). A possible explanation is that this transgenerational transmission of enhanced seizure susceptibility induced by FSs may be due to cytoplasmic inheritance, in which mitochondrial DNA played an important role (Luo et al., 2013). With regard to clinical significance, patients with epilepsy need to be asked not only whether they have relatives who suffer from epilepsy, but also whether patient's mother had experienced complex febrile convulsions. Therefore, these findings may affect the subsequent evaluation of patients with epilepsy.
Overall, this study revealed the powerful transgenerational transmission of enhanced susceptibility to seizures after acquired FS. Thus, our study highlights the power of environmental stimulation during youth to influence the composition of signaling networks that are passed on through subsequent generations, suggesting epigenetic transgenerational transmission in the neurobiology of disease.
Author Contributions
D.C.W., B.F., and Y.J.D. performed most of the experiments, acquired most of the data presented, and performed the statistical analyses. Y.J.D., Y.S.T., and B.C. performed western blots and RT-PCR. C.L.X., S.W., K.W., S.H.Z., and B.Y.L. assisted with statistical analyses and in vivo electrophysiological recordings. D.C.W. and Z.C. designed experiments and co-wrote the manuscript. Z.C. supervised the research. All authors discussed the results and implications and commented on the manuscript at all stages.
Completing Financial Interests
The authors declare no competing financial interests.
Acknowledgements and Funding Sources
This work was funded by the National Natural Science Foundation of China (91332202, 81630098, 81521062, and 81503045) and the Fundamental Research Funds for the Central Universities (2016QNA7015). We thank Dr. FD Shi (Tianjin Medical University General Hospital, China) and Dr. C Chen (Louisiana State University Health New Orleans Sciences Center, USA) for helpful discussions, and we are grateful to Dr. IC Bruce for reading the manuscript.
References
- Anway M.D., Skinner M.K. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147:S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- Arai J.A., Li S., Hartley D.M., Feig L.A. Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J. Neurosci. 2009;29:1496–1502. doi: 10.1523/JNEUROSCI.5057-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock C., Crespi B. Battle of the sexes may set the brain. Nature. 2008;454:1054–1055. doi: 10.1038/4541054a. [DOI] [PubMed] [Google Scholar]
- Baram T.Z., Gerth A., Schultz L. Febrile seizures: an appropriate-aged model suitable for long-term studies. Brain Res. Dev. Brain Res. 1997;98:265–270. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J., Gapp K., Saab B.J., Mansuy I.M. Transgenerational epigenetic effects on brain functions. Biol. Psychiatry. 2013;73:313–320. doi: 10.1016/j.biopsych.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Bough K.J., Mott D.D., Dingledine R.J. Medial perforant path inhibition mediated by mGluR7 is reduced after status epilepticus. J. Neurophysiol. 2004;92:1549–1557. doi: 10.1152/jn.00315.2004. [DOI] [PubMed] [Google Scholar]
- Bragin A., Azizyan A., Almajano J., Wilson C.L., Engel J., Jr. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Cendes F., Andermann F., Dubeau F., Gloor P., Evans A., Jones-Gotman M., Olivier A., Andermann E., Robitaille Y., Lopes-Cendes I. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- Chen K., Baram T.Z., Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat. Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Feng B., Tang Y., You Y., Wang Y., Hou W., Hu W., Chen Z. Blocking GluN2B subunits reverses the enhanced seizure susceptibility after prolonged febrile seizures with a wide therapeutic time-window. Exp. Neurol. 2016;283:29–38. doi: 10.1016/j.expneurol.2016.05.034. [DOI] [PubMed] [Google Scholar]
- Dube C., Chen K., Eghbal-Ahmadi M., Brunson K., Soltesz I., Baram T.Z. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann. Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Dube C., Richichi C., Bender R.A., Chung G., Litt B., Baram T.Z. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C.M., Ravizza T., Hamamura M., Zha Q., Keebaugh A., Fok K., Andres A.L., Nalcioglu O., Obenaus A., Vezzani A. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J. Neurosci. 2010;30:7484–7494. doi: 10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A.P. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;24:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Feng B., Tang Y., Chen B., Xu C., Wang Y., Dai Y., Wu D., Zhu J., Wang S., Zhou Y. Transient increase of interleukin-1beta after prolonged febrile seizures promotes adult epileptogenesis through long-lasting upregulating endocannabinoid signaling. Sci. Rep. 2016;6:21931. doi: 10.1038/srep21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D., Obana K., Olson J. Hyperthermia-induced seizures in the rat pup: a model for febrile convulsions in children. Science. 1981;213:1034–1036. doi: 10.1126/science.7268407. [DOI] [PubMed] [Google Scholar]
- Jirtle R.L., Skinner M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., Capaldi D.M., Pears K.C., Kerr D.C., Owen L.D. Intergenerational transmission of internalising and externalising behaviours across three generations: gender-specific pathways. Crim. Behav. Ment. Health. 2009;19:125–141. doi: 10.1002/cbm.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.G., Zhang X.L., Luo Z.D., Gold M.S. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain. 2010;151:633–643. doi: 10.1016/j.pain.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S.M., Ge Z.J., Wang Z.W., Jiang Z.Z., Wang Z.B., Ouyang Y.C., Hou Y., Schatten H., Sun Q.Y. Unique insights into maternal mitochondrial inheritance in mice. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13038–13043. doi: 10.1073/pnas.1303231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Schuchmann S., Schmitz D., Rivera C., Vanhatalo S., Salmen B., Mackie K., Sipila S.T., Voipio J., Kaila K. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat. Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P.A. Mechanisms of brain plasticity: from normal brain function to pathology. Int. Rev. Neurobiol. 2001;45:1–15. doi: 10.1016/s0074-7742(01)45004-5. [DOI] [PubMed] [Google Scholar]
- Shinnar S. Prolonged febrile seizures and mesial temporal sclerosis. Ann. Neurol. 1998;43:411–412. doi: 10.1002/ana.410430402. [DOI] [PubMed] [Google Scholar]
- Siedlecki P., Zielenkiewicz P. Mammalian DNA methyltransferases. Acta Biochim. Pol. 2006;53:245–256. [PubMed] [Google Scholar]
- Sillanpaa M., Jalava M., Kaleva O., Shinnar S. Long-term prognosis of seizures with onset in childhood. N. Engl. J. Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- Skinner M.K., Manikkam M., Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterba S.K., Prinstein M.J., Cox M.J. Trajectories of internalizing problems across childhood: heterogeneity, external validity, and gender differences. Dev. Psychopathol. 2007;19:345–366. doi: 10.1017/S0954579407070174. [DOI] [PubMed] [Google Scholar]
- Wu D.C., Zhu-Ge Z.B., Yu C.Y., Fang Q., Wang S., Jin C.L., Zhang S.H., Chen Z. Low-frequency stimulation of the tuberomammillary nucleus facilitates electrical amygdaloid-kindling acquisition in Sprague-Dawley rats. Neurobiol. Dis. 2008;32:151–156. doi: 10.1016/j.nbd.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Wu D.C., Xu Z.H., Wang S., Fang Q., Hu D.Q., Li Q., Sun H.L., Zhang S.H., Chen Z. Time-dependent effect of low-frequency stimulation on amygdaloid-kindling seizures in rats. Neurobiol. Dis. 2008;31:74–79. doi: 10.1016/j.nbd.2008.03.007. [DOI] [PubMed] [Google Scholar]