Abstract
Background
Plasmalogens (Pls) reportedly decreased in postmortem brain and in the blood of patients with Alzheimer's disease (AD). Recently we showed that intraperitoneal administration of Pls improved cognitive function in experimental animals. In the present trial, we tested the efficacy of oral administration of scallop-derived purified Pls with respect to cognitive function and blood Pls changes in patients with mild AD and mild cognitive impairment (MCI).
Methods
The study was a multicenter, randomized, double-blind, placebo-controlled trial of 24 weeks. Participants were 328 patients aged 60 to 85 years who had 20 to 27 points in Mini Mental State Examination-Japanese (MMSE-J) score and five or less points in Geriatric Depression Scale-Short Version-Japanese (GDS-S-J). They were randomized to receive either 1 mg/day of Pls purified from scallop or placebo. The patients and study physicians were masked to the assignment. The primary outcome was MMSE-J. The secondary outcomes included Wechsler Memory Scale-Revised (WMS-R), GDS-S-J and concentration of phosphatidyl ethanolamine plasmalogens (PlsPE) in erythrocyte membrane and plasma. This trial is registered with the University Hospital Medical Information Network, number UMIN000014945.
Findings
Of 328 patients enrolled, 276 patients completed the trial (140 in the treatment group and 136 in the placebo group). In an intention-to-treat analysis including both mild AD (20 ≤ MMSE-J ≤ 23) and MCI (24 ≤ MMSE-J ≤ 27), no significant difference was shown between the treatment and placebo groups in the primary and secondary outcomes, with no severe adverse events in either group. In mild AD patients, WMS-R improved significantly in the treatment group, and the between group difference was nearly significant (P = 0.067). In a subgroup analysis of mild AD patients, WMS-R significantly improved among females and those aged below 77 years in the treatment group, and the between-group differences were statistically significant in females (P = 0.017) and in those aged below 77 years (P = 0.029). Patients with mild AD showed a significantly greater decrease in plasma PlsPE in the placebo group than in the treatment group.
Interpretation
Oral administration of scallop-derived purified Pls may improve cognitive functions of mild AD.
Funding
The Japanese Plasmalogen Society.
Keywords: Plasmalogen, Scallop, Alzheimer's disease, Mild cognitive impairment, Cognitive function
Highlights
-
•
Plasmalogens (Pls), a kind of phospholipid, are reduced in the brain and blood of patients with Alzheimer’s disease (AD).
-
•
Scallop-derived purified Pls were orally administered to patients with mild AD and mild cognitive impairment by RCT.
-
•
Oral administration of scallop-derived purified Pls may improve cognitive functions of mild AD.
It is well known that Plasmalogens (Pls), a special class of glycerophospholipid, are decreased in the brain and blood of patients with Alzheimer’s disease (AD), and inhibit γ-secretase activity. Our recent studies showed that intraperitoneal administration of purified Pls improved cognitive function in the animal model of AD. We tested the efficacy of Pls for Alzheimer’s disease by a multicenter, randomized, double-blind, placebo-controlled trial and showed that oral administration of scallop-derived purified Pls may improve cognitive functions of patients with mild AD.
1. Introduction
Alzheimer's disease (AD) is an age-related neurodegenerative disorder that has increased in prevalence as people live longer. It is estimated that the prevalence of AD may reach > 74 million worldwide by 2030 (World Alzheimer Report, 2015). The cause and mechanism of AD is not fully elucidated, while progressive deposition of amyloid-β and Tau protein is considered to be a neuropathological hallmark of AD. On the other hand, the close connection between plasmalogens (Pls) and AD has been indicated by the observations of decreased phosphatidyl ethanolamine Pls (PlsPE) in the affected brain regions of AD patients, such as the hippocampus and frontal cortex (Ginsberg et al., 1995, Guan et al., 1999, Han et al., 2001). Decreased levels of PlsPE in the blood and cerebrospinal fluid of AD patients have been reported (Goodenowe et al., 2007, Wood et al., 2010, Wood et al., 2015, Oma et al., 2012, Yamashita et al., 2015). However, it is not clear whether the decrease of Pls in brain tissue and in plasma is the cause of the disease or merely a result of the disease. Recent studies of animal models of AD by our group indicated that intraperitoneal administration of purified Pls improved cognitive function (Katafuchi et al., 2012, Hossain et al., 2013, Hossain et al., 2016).
Pls are a special class of glycerophospholipids characterized by a vinyl ether bond at the sn-1 position of glycerol backbone, and they are sometimes called plasmenyl phospholipid or alkenyl acryl phospholipid. They are found in almost all mammalian tissues and constitute about 18–20% of the total phospholipids in cell membranes. Predominant Pls in mammalian tissues are PlsPE and choline plasmalogen. PlsPE is much more abundant than choline plasmalogen except in heart and skeletal muscle. It is reported that Pls are abundant in the brain, retina, leukocytes (immune cells), sperm, heart, and skeletal muscle in mammals. This characteristic distribution of Pls indicates the importance of Pls in mammals (Farooqui and Horrocks, 2001, Braverman and Moser, 2012).
Pls are not only a structural component of animal cell membranes and reservoir for secondary messengers, but may also be involved in membrane fusion, ion transport, and cholesterol efflux, and act as antioxidants in cell membranes. Recently, the inhibitory effects of Pls on γ-secretase activity have been reported (Onodera et al., 2015). This study aimed to assess whether oral administration of Pls extracted from scallops would improve the cognitive function in patients with mild AD and mild cognitive impairment (MCI).
2. Methods
2.1. Study Design and Participants
This study was a multicenter, randomized, double-blind, placebo-controlled trial to evaluate the memory-improving efficacy of scallop-derived Pls in patients with mild AD and MCI. The study period consisted of a 24-week administration period and a four-week post-treatment period without administration (28 weeks in total).
Patients were those who had 20 to 27 points of Mini Mental State Examination Japanese Version (MMSE-J) scores, i.e., patients with mild AD (20 ≤ MMSE-J ≤ 23) or those with MCI (24 ≤ MMSE-J ≤ 27) (Solfrizzi et al., 2004). All patients were required to meet the criteria for mild AD or MCI set out in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Patients were confirmed to have no arteriosclerotic dementia with MRI or CT scans performed within the previous six months. Patients were also ensured to have five or less points of Geriatric Depression Scale Short Version in Japanese (GDS-S-J) in order to exclude depressive pseudodementia. Patients taking anti-Alzheimer drugs had no change in the regimen during the previous three months. All caregivers were required to accompany patients to all visits throughout the study, and provided information on patients' daily life and health status. Patients were excluded if they were allergic to scallops, the raw materials for test substance. Written informed consent was obtained from either patients or their caregivers. The study protocol was approved by the Institutional Review Boards of Fukuoka University Hospital (Fukuoka), Nihonbashi Sakura Clinic (Tokyo), and BOOCS Clinic Fukuoka (Fukuoka). The study was implemented in compliance with the Declaration of Helsinki.
2.2. Randomization and Masking
A computer-generated random allocation list was created by an expert at CAC Croit Corporation (Tokyo) based on the blocked randomization method with each block consisting of two placebo allocations and two Pls allocations. Each study site was provided with test substance kits numbered according to the allocation list, which were handed directly to patients. At some study sites without cold storage space, patients received test kits by courier every month. Thus enrolled patients were randomly assigned to receive either 1.0 mg Pls/day or placebo. Test substance was jelly-like substance. Active substance and placebo were identical in appearance and taste. Patients, caregivers, study physicians, and clinical staff were masked to treatment allocation throughout the study period.
2.3. Procedures
Enrolled patients were provided with the test substance at baseline visit or within one week after the baseline visit, and were instructed to take it orally twice/day for 24 weeks. They received one month's extra test substance in case they missed the next scheduled visit. To confirm compliance, they were requested to return unconsumed test substance at their next visit. They were furthermore urged not to change the regimen during the study period as far as possible. If a patient changed his or her drug use, we terminated his or her observation at the point. We recorded any complications and adverse events reported by patients at each visit.
The primary outcome measure was MMSE-J. The secondary outcome measures included Wechsler Memory Scale-Revised (WMS-R), GDS-S-J, plasma PlsPE levels, and relative concentration of PlsPE in erythrocyte membrane, namely the percentage of Pls to the total phospholipids in erythrocyte membrane. Cognitive function was assessed at baseline, and at weeks 12, 24, and 28. Blood was drawn from patients in the fasting state at baseline, and at weeks 8, 16, 20, 24, and 28 for measuring erythrocyte PlsPE and plasma PlsPE. PlsPE measurement was performed using the previously reported method (Mawatari et al., 2007, Mawatari et al., 2016).
Safety assessment was conducted by recording adverse events and performing a physical examination and biochemical blood tests such as liver function, renal function, blood sugar, and lipid levels at each visit.
2.4. Statistical Analysis
To determine the sample size, we assumed that the MMSE-J score would improve by 5% in the placebo group and by 10% in the Pls treatment with SD of 15% in each. With a statistical power of 0.80 and a one-sided significance level of 0.01, the required sample size was estimated to be 181 in each group. The target number of enrollments was decided to be 200 in each with allowance for some extent of dropout.
The between-treatment difference was assessed by unpaired t-test, and the within-group change from the baseline was evaluated by paired t-test. In terms of after-treatment outcome of the cognitive function, the mean change from the baseline and 95% confidence interval (CI) were presented. Analyses were done with Stata version 13 (StataCorp, College Station, TX). The trial is registered at the University Hospital Medical Information Network as ID UMIN000014945.
2.5. Role of the Funding Source
B&S Corporation Co. Ltd. (Tokyo), one of the donors to The Japanese Plasmalogen Society, was involved in provision of Pls test substance and placebo and delivery to study sites, but it had no involvement in study design and planning, investigator training, data analysis, interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All co-investigators also had full access to the data.
3. Results
3.1. Study Participants
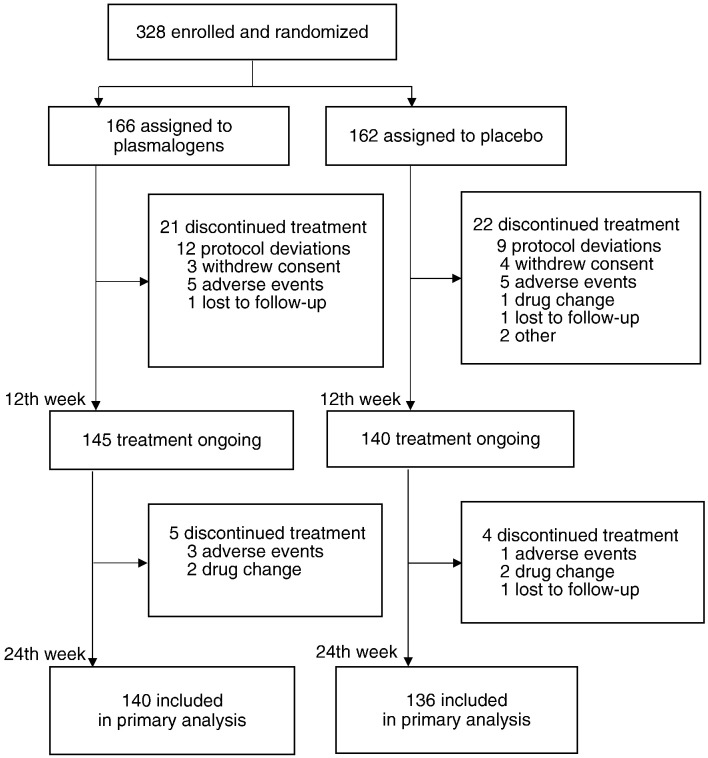
A total of 328 patients were enrolled at 25 hospitals or clinics in Kyushu, Kanto, and Kansai regions in Japan from November 15, 2014 to October 8, 2015. They were randomly assigned to either of the two treatment groups (166 to Pls group and 162 to placebo group). Of the 328 enrolled patients, 285 continued to participate in the study for 12 weeks, and 276 completed the 24 week study period. Those who completed the study included 11 patients (4.0%) who failed to use 80% or more of the provided test substance. Fig. 1 shows the trial profile.
Fig. 1.
Trial profile.
No significant difference was noted between the groups with respect to age, MMSE-J, GDS-S-J, WMS-R (0 min), WMS-R (30 min), erythrocyte PlsPE, and plasma PlsPE, at baseline, although the male-to-female ratio was significantly lower in the placebo group (Table 1).
Table 1.
Baseline characteristics.
| Variable | Pls group (n = 145) | Placebo group (n = 140) | P valuea |
|---|---|---|---|
| Male, n (%) | 65 (44.8) | 45 (32.1) | 0.03 |
| MCI, n (%) | 62 (42.8) | 59 (42.1) | 0.92 |
| Age in year | 76.4 (6.0) | 76.5 (5.6) | 0.94 |
| MMSE-J | 24.0 (2.4) | 24.2 (2.2) | 0.38 |
| GDS-S-J | 2.32 (1.54) | 2.36 (1.55) | 0.83 |
| WMS-R (0 min) | 4.21 (3.71) | 4.34 (4.03)b | 0.78 |
| WMS-R (30 min) | 2.50 (3.73) | 2.77 (4.07)b | 0.56 |
| Erythrocyte PlsPE | 8.03 (1.05) | 8.11 (0.96) | 0.49 |
| Plasma PlsPE | 3.65 (1.31) | 3.93 (1.28) | 0.08 |
Values are mean (SD) unless otherwise specified.
MMSE-J = Mini Mental State Examination-Japanese, GDS-S-J = Geriatric Depression Scale-Short Version-Japanese, WMS-R = Wechsler Memory Scale-Revised, min = minute, PlsPE = phosphatidyl ethanolamine plasmalogens.
Chi-square test for proportion and unpaired t-test for mean.
Number of the patients was 139.
3.2. Clinical Efficacy
3.2.1. Primary Analysis
In an intention-to-treat analysis, there was no significant difference between the treatment and placebo groups in the primary and secondary outcomes of cognitive function. The MMSE-J score showed a nearly significant improvement in the treatment group while no such improvement was observed in the placebo group, resulting no statistically significant between-group difference. The WMS-R (0 min) and WMS-R (30 min) score each showed a significant improvement in both groups, but there was no statistically significant between-group difference. Erythrocyte PlsPE increased and plasma PlsPE decreased to different degrees after treatment in both Pls and placebo groups. There was no statistically significant between-group difference at any points of time, while the decrease in plasma PlsPE seemed greater in the placebo group (Table 2).
Table 2.
Mean difference from the baseline.
| Variable | Week | Pls group |
Placebo group |
P value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | (95% CI) | n | Mean | (95% CI) | |||
| MMSE-J | 12 | 145 | 0.43 | (0.04; 0.80) | 140 | 0.33 | (− 0.08; 0.73) | 0.73 |
| 24 | 140 | 0.40 | (− 0.01; 0.81) | 136 | 0.32 | (− 0.14; 0.77) | 0.79 | |
| 28 | 81 | 0.69 | (0.12; 1.26) | 79 | 0.44 | (− 0.14; 1.03) | 0.54 | |
| GDS-S-J | 12 | 145 | 0.37 | (0.05; 0.68) | 140 | 0.39 | (0.06; 0.73) | 0.91 |
| 24 | 140 | 0.20 | (− 0.12; 0.52) | 136 | 0.18 | (− 0.16; 0.51) | 0.92 | |
| 28 | 81 | 0.17 | (− 0.32; 0.66) | 80 | 0.18 | (− 0.34; 0.69) | 1.00 | |
| WMS-R (0 min) | 12 | 145 | 0.66 | (0.21; 1.20) | 139 | 0.86 | (0.36; 1.36) | 0.55 |
| 24 | 140 | 1.41 | (0.91; 1.92) | 135 | 1.39 | (0.87; 1.92) | 0.95 | |
| 28 | 80 | 2.30 | (1.48; 3.12) | 78 | 2.46 | (1.67; 3.25) | 0.78 | |
| WMS-R (30 min) | 12 | 145 | 0.48 | (0.11; 0.83) | 139 | 0.35 | (− 0.05; 0.75) | 0.65 |
| 24 | 140 | 1.09 | (0.59; 1.59) | 135 | 1.10 | (0.61; 1.59) | 0.99 | |
| 28 | 80 | 2.09 | (1.19; 2.98) | 78 | 1.92 | (1.19; 2.66) | 0.78 | |
| Erythrocyte PlsPE | 8 | 145 | 0.13 | (− 0.03; 0.30) | 139 | 0.08 | (− 0.09; 0.24) | 0.64 |
| 16 | 140 | 0.24 | (0.10; 0.37) | 138 | 0.22 | (0.06; 0.37) | 0.86 | |
| 24 | 139 | 0.26 | (0.10; 0.41) | 135 | 0.35 | (0.19; 0.52) | 0.40 | |
| 28 | 139 | 0.27 | (0.12; 0.42) | 133 | 0.26 | (0.09; 0.43) | 0.95 | |
| Plasma PlsPE | 8 | 116 | − 0.48 | (− 0.70; − 0.27) | 116 | − 0.77 | (− 0.98; − 0.56) | 0.06 |
| 16 | 122 | − 0.59 | (− 0.80; − 0.39) | 118 | − 0.72 | (− 0.93; − 0.50) | 0.42 | |
| 24 | 140 | − 0.24 | (− 0.42; − 0.06) | 136 | − 0.38 | (− 0.60; − 0.17) | 0.31 | |
| 28 | 138 | − 0.59 | (− 0.77; − 0.40) | 131 | − 0.63 | (− 0.81; − 0.44) | 0.77 | |
MMSE-J = Mini Mental State Examination-Japanese, GDS-S-J = Geriatric Depression Scale-Short Version-Japanese, WMS-R = Wechsler Memory Scale-Revised, min = minute, PlsPE = phosphatidyl ethanolamine plasmalogens.
3.2.2. Subgroup Analysis
We examined the change in cognitive function in mild AD patients and MCI patients separately. No notable change was seen in MMSE-J in either group. WMS-R (0 min) and WMS-R (30 min) improved statistically significantly in the treatment group, and the improvement seemed greater in the Pls treatment group with respect to the 0 min value (P = 0.067) and the 30-min value (P = 0.078) (Table 3).
Table 3.
Mean difference from the baseline, in patients with MMSE-J score 20 to 23.
| Variable | Week | Pls group |
Placebo group |
P value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | (95% CI) | n | Mean | (95% CI) | |||
| MMSE-J | 12 | 55 | 0.31 | (− 0.44; 1.06) | 49 | 0.35 | (− 0.43; 1.12) | 0.94 |
| 24 | 50 | 0.06 | (− 0.76; 0.88) | 48 | 0.19 | (− 0.59; 0.96) | 0.82 | |
| 28 | 25 | − 0.12 | (− 1.48; 1.24) | 25 | 0.36 | (− 0.78; 1.50) | 0.58 | |
| GDS-S-J | 12 | 55 | 0.33 | (− 0.28; 0.94) | 49 | 0.67 | (0.02; 1.32) | 0.44 |
| 24 | 50 | 0.20 | (− 0.45; 0.85) | 48 | 0.25 | (− 0.28; 0.78) | 0.91 | |
| 28 | 25 | 0.72 | (− 0.66; 2.10) | 25 | 0.18 | (− 0.71; 1.03) | 0.48 | |
| WMS-R (0 min) | 12 | 55 | 0.53 | (− 0.13; 1.19) | 48 | 0.73 | (0.12; 1.34) | 0.66 |
| 24 | 50 | 1.24 | (0.49; 1.99) | 47 | 0.30 | (− 0.39; 0.99) | 0.067 | |
| 28 | 24 | 1.29 | (0.04; 2.55) | 24 | 1.08 | (0.46; 1.70) | 0.76 | |
| WMS-R (30 min) | 12 | 55 | 0.47 | (− 0.02; 0.96) | 48 | 0.13 | (− 0.25; 0.50) | 0.27 |
| 24 | 50 | 0.78 | (0.04; 1.52) | 47 | 0.02 | (− 0.37; 0.41) | 0.078 | |
| 28 | 24 | 0.96 | (− 0.33; 2.24) | 24 | 0.29 | (− 0.26; 0.84) | 0.33 | |
MMSE-J = Mini Mental State Examination-Japanese, GDS-S-J = Geriatric Depression Scale-Short Version-Japanese, WMS-R = Wechsler Memory Scale-Revised, min = minute.
3.2.3. Subgroup Analysis by Gender and Age
Furthermore, we analyzed the cognitive function in patients with mild AD patients by gender and age (Table 4). Mild AD patients were divided into two groups based on the median value, 77 years or younger and 78 years or older. In the patients aged 77 years or younger, WMS-R (30 min) increased statistically significantly in the treatment group, showing a significant between-group difference (P = 0.029). In female patients with mild AD, WMS-R (30 min) improved significantly in the treatment group, with a significant between-group difference (P = 0.017). There was no notable between-group difference in the analysis of MCI patients by gender and age.
Table 4.
Mean difference from the baseline, by gender and age, in patients with MMSE-J score 20 to 23.
| Gender/age | Week | Pls group |
Placebo group |
P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | (95% CI) | n | Mean | (95% CI) | ||||
| Female | WMS-R (0 min) | 12 | 27 | 1.04 | (0.02; 2.05) | 30 | 0.80 | (0.04; 1.56) | 0.70 |
| WMS-R (30 min) | 12 | 27 | 0.93 | (0.10; 1.75) | 30 | 0.17 | (− 0.37; 0.70) | 0.11 | |
| WMS-R (0 min) | 24 | 25 | 1.52 | (0.35; 2.69) | 29 | 0.48 | (− 0.47; 1.43) | 0.16 | |
| WMS-R (30 min) | 24 | 25 | 1.08 | (0.06; 2.10) | 29 | − 0.21 | (− 0.68; 0.26) | 0.02 | |
| Male | WMS-R (0 min) | 12 | 28 | 0.04 | (− 0.84; 0.92) | 18 | 0.61 | (− 0.53; 1.76) | 0.41 |
| WMS-R (30 min) | 12 | 28 | 0.04 | (− 0.51; 0.58) | 18 | 0.06 | (− 0.47; 0.58) | 0.96 | |
| WMS-R (0 min) | 24 | 25 | 0.96 | (− 0.06; 1.98) | 18 | 0.00 | (− 1.05; 1.05) | 0.19 | |
| WMS-R (30 min) | 24 | 25 | 0.48 | (− 0.66; 1.62) | 18 | 0.39 | (− 0.32; 1.10) | 0.90 | |
| 77 or younger | WMS-R (0 min) | 12 | 20 | 1.30 | (− 0.14; 2.74) | 21 | 1.19 | (0.37; 2.01) | 0.89 |
| WMS-R (30 min) | 12 | 20 | 1.15 | (0.05; 2.25) | 21 | 0.10 | (− 0.22; 0.41) | 0.06 | |
| WMS-R (0 min) | 24 | 19 | 2.21 | (0.61; 3.81) | 21 | 0.48 | (− 0.52; 1.47) | 0.06 | |
| WMS-R (30 min) | 24 | 19 | 1.84 | (0.10; 3.58) | 21 | 0.00 | (− 0.41; 0.41) | 0.03 | |
| 78 or older | WMS-R (0 min) | 12 | 35 | 0.09 | (− 0.57; 0.74) | 27 | 0.37 | (− 0.54; 1.28) | 0.60 |
| WMS-R (30 min) | 12 | 35 | 0.09 | (− 0.37; 0.54) | 27 | 0.15 | (− 0.50; 0.79) | 0.87 | |
| WMS-R (0 min) | 24 | 31 | 0.65 | (− 0.07; 1.36) | 26 | 0.15 | (− 0.86; 1.17) | 0.41 | |
| WMS-R (30 min) | 24 | 31 | 0.13 | (− 0.42; 0.68) | 26 | 0.04 | (− 0.61; 0.69) | 0.83 | |
WMS-R = Wechsler Memory Scale-Revised, min = minute.
3.2.4. Changes in Blood Plasmalogens
The changes in blood PlsPE levels among mild AD patients were examined (Table 5). Plasma PlsPE decreased in the placebo group, showing a significant between-group difference (P = 0.016). There was a statistically significant elevation of erythrocyte PlsPE at week 16 in the treatment group and at week 24 in placebo. However, the changes were not significantly different between the two groups.
Table 5.
Mean difference from the baseline, in patients with MMSE-J score 20 to 23.
| Variable | Week | Pls group |
Placebo group |
P value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | (95% CI) | n | Mean | (95% CI) | |||
| Erythrocyte PlsPE | 8 | 55 | 0.16 | (− 0.13; 0.46) | 50 | 0.28 | (− 0.03; 0.48) | 0.74 |
| 16 | 53 | 0.33 | (0.11; 0.57) | 48 | 0.19 | (− 0.07; 0.44) | 0.39 | |
| 24 | 49 | 0.24 | (− 0.03; 0.52) | 48 | 0.40 | (0.11; 0.69) | 0.42 | |
| 28 | 49 | 0.22 | (− 0.02; 0.46) | 46 | 0.26 | (− 0.02; 0.54) | 0.85 | |
| Plasma PlsPE | 8 | 41 | − 0.21 | (− 0.50; 0.08) | 39 | − 0.64 | (− 1.01; − 0.26) | 0.05 |
| 16 | 43 | − 0.39 | (− 0.68; − 0.11) | 40 | − 0.86 | (− 1.29; − 0.43) | 0.07 | |
| 24 | 50 | 0.16 | (− 0.07; 0.40) | 48 | − 0.34 | (− 0.69; 0.00) | 0.02 | |
| 28 | 49 | − 0.35 | (− 0.63; − 0.08) | 46 | − 0.85 | (− 1.13; − 0.57) | 0.01 | |
PlsPE = phosphatidyl ethanolamine plasmalogens.
3.3. Clinical Safety
Table 6 summarizes adverse events. There was no notable difference in the occurrence of adverse events between the two groups.
Table 6.
List of reported adverse events.
| Pls group (n = 169) | Placebo group (n = 167) | |
|---|---|---|
| Neoplasms | ||
| Esophageal cancer | 0 | 1 |
| Ovarian cancer | 0 | 1 |
| Bladder cancer | 1 | 0 |
| Nervous system | ||
| Parkinsonism | 1 | 0 |
| Transient ischaemic attack | 0 | 1 |
| Eye | ||
| Ophthalmecchymosis | 0 | 1 |
| Circulatory system | ||
| Hypertension | 2 | 1 |
| Ischaemic heart disease | 1 | 0 |
| Premature ventricular contraction | 0 | 2 |
| Stroke | 1 | 0 |
| Subarachnoid hemorrhage | 1 | 0 |
| Respiratory system | ||
| Cold | 2 | 3 |
| Aspiration pneumonia | 0 | 1 |
| Digestive system | ||
| Hepatic hemangioma | 1 | 0 |
| Stomatitis | 1 | 1 |
| Gastric ulcer | 1 | 1 |
| Intestinal obstruction | 1 | 0 |
| Acute hepatic disorder | 1 | 0 |
| Cholecystolithiasis | 1 | 0 |
| Skin and subcutaneous tissue | ||
| Herpes zoster | 0 | 1 |
| Oral herpes | 0 | 2 |
| Prurigo | 0 | 1 |
| Rash | 1 | 1 |
| Musculoskeletal system | ||
| Gouty attack | 1 | 0 |
| Genitourinary system | ||
| Renal failure | 1 | 0 |
| Symptoms and signs | ||
| Diarrhoea | 3 | 1 |
| Akathisia | 1 | 0 |
| Hand numbness | 0 | 1 |
| Ear pain | 1 | 0 |
| Sore throat | 1 | 0 |
| Constipation | 2 | 0 |
| Knee pain | 2 | 0 |
| Shoulder pain | 0 | 1 |
| Muscle pain | 1 | 0 |
| Cough | 1 | 1 |
| Vomiting | 0 | 1 |
| Dizziness | 0 | 3 |
| Stomach pain | 1 | 1 |
| Oedema | 1 | 3 |
| Injury | ||
| Contusion | 1 | 1 |
| Fracture | 4 | 4 |
| Traumatic subarachnoid hemorrhage | 1 | 0 |
| Meniscus injury | 1 | 0 |
4. Discussion
To our knowledge, this is the unprecedented trial that addressed efficacy on cognitive function and blood Pls changes by oral administration of Pls in patients with mild AD and MCI. The present results showed no significant difference in the primary outcome (MMSE-J score) between the treatment and placebo groups. However, among patients with mild AD, WMS-R score significantly improved in the treatment group, and the improvement seemed greater than in the placebo group. In a subgroup analysis of mild AD patients, improvement of WMS-R was more evident in female patients and in patients below 77 years. These results indicate that oral administration of scallop-derived purified Pls may be effective to improve memory function of patients with mild AD. This study did not find the efficacy of Pls in mild AD patients over 78 years or male AD patients. The reason for the lack of efficacy in these aged patients may be ascribed to age-related irreversible degenerative changes in the brain (Kou et al., 2011). It is unknown why the efficacy was evident in females but not in males.
The present study did not find the efficacy of Pls in MCI patients. It should be noted that WMS-R improved significantly even at end of the study (week 24) in the placebo group as well. These findings suggest that MCI patients may have exhibited a stronger placebo effect than AD patients. MCI patients may retain higher brain functions such as “expectation” and “hope”. AD patients are seemingly less affected by placebo effects because their cognitive function declines and the above-mentioned higher brain function diminishes more markedly than that of MCI patients. This line of conjecture indicates that the mental status of people can change Pls concentration in brain tissue.
Our previous studies showed that Pls in erythrocyte membrane significantly increased with orally ingested Pls, and another study also revealed that plasma PlsPE increased shortly after being ingested orally (Mawatari et al., 2012, Nishimukai et al., 2003). However, in the present study, only 1.0 mg/day of purified Pls showed an effect on memory function of mild AD patients. The physiological mechanism of this effect with such a small amount of Pls by oral administration is unclear. One hypothesis is that Pls may work through some receptors like hormones. Lipid rafts of cell membrane are considered to be associated with cell signaling (Hossain et al., 2016, New and Wong, 2007). There are reports that lipid rafts are rich in PlsPE (Pike et al., 2002). G-protein coupled receptors (GPCR) are also localized to lipid rafts and caveolae (Chini and Parenti, 2004). These may indicate the possibility that Pls work as a ligand of GPCR. Concentration of PlsPE in human plasma is about 100 μmol/l, but Pls in plasma may circulate as lipoproteins. Pls in the lipoproteins themselves are not likely to work as a ligand of receptors. However, it is possible that free Pls, which are derived from oral administration, even in a small amount, may work as a ligand of some receptors at intestinal cells before becoming lipoproteins. It is well known that there is close communication between the intestine and brain through neural, endocrine, and immune pathways (Yarandi et al., 2016).
On the other hand, there have been many reports that docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) relate closely to brain functions (Grimm et al., 2016, Hopperton et al., 2016, Ren et al., 2017). Scallop-derived Pls used in the present study presumably contained relatively large amounts of DHA and EPA (Kraffe et al., 2004, Hanuš et al., 2009). It is possible that these omega-3 polyunsaturated fatty acids of Pls may be effective for improvement of cognitive function in mild AD. Some studies suggest that DHA in the form of phospholipids passes through blood-brain barrier approximately ten times more efficiently than in the form of free fatty acid (Lagarde et al., 2001, Lagarde et al., 2015), although other studies report that a diet high in omega-3 fatty acids such as DHA and EPA resulted in negligible beneficial effect on cognition in AD patients (Quinn et al., 2010, Phillips et al., 2015).
Regarding the change of blood Pls levels after treatment, there were significant differences between the treatment and placebo groups in mild AD patients. Plasma PlsPE in the placebo group decreased significantly after treatment, whereas that of the treatment group remained unchanged. Some studies suggest that statins, a class of cholesterol-lowering drugs, raise blood level of PlsPE (Meikle et al., 2015). In the present study, 23 (23.5%) of 98 patients with mild AD used statins during the study period. However, there were still significant differences in the changes of plasma PlsPE between the treatment and placebo groups except for statin-users. Therefore, it is unlikely that statins protected the decrease of Plasma PlsPE in the treatment group. These results might indicate that Pls production in peroxisome was concurrently reduced with the progression of Alzheimer's disease during the 24-week treatment period, and suggest that orally administered Pls may contribute to maintain the production of Pls in peroxisome.
The present study has several problems and limitations. The study duration may be too short to detect the efficacy of Pls in MCI patients with MMSE-J score over 24, whose condition was ill-defined and may have little differed from that of healthy individuals. To resolve this problem, we should conduct additional research to ascertain whether it is possible to delay progression from MCI to AD in a long-term follow-up of MCI patients in the treatment group. Further randomized controlled trials for moderate and severe AD patients are also required.
Funding Sources
The study was funded by The Japanese Plasmalogen Society (Pls2014-01) (Fukuoka, Japan) as an investigator-initiated trial.
Conflicts of Interest
TF and SM have applied for patents on method for manufacturing ether phospholipid (patent application number: PCT/JP2015/63617, PCT/JP2015/63740). TY, TA, YT, CW, and SK declare that they have no conflicts of interest.
Author Contributions
TF was responsible for central management of the trial including literature search, study design, data collection, data interpretation and writing of the manuscript. TY, TA, and YT contributed to study design, recruitment and research monitoring. CW contributed to clinical management, data collection and writing of the manuscript. SM contributed to implementation of the study with laboratory measurements and writing of the manuscript. SK did study design, statistical analysis of the data and writing of the manuscript. All authors reviewed the manuscript and approved the final manuscript.
Acknowledgments
We thank all patients and caregivers who took part in the study. The following contributed to implementation of the trial as study physicians: E Nishikawa (Nishikawa-naika Clinic, Shimonoseki), H Fujino (Fujino Clinic, Yanagawa), H Matsuo (Matsuo Hospital, Fukuoka), H Nawata (Muta Hospital, Fukuoka), K Fukuyama (Hasami Hospital, Higashisonogi), K Irie (Hakujyuji Hospital, Fukuoka), K Saito, N Shinfuku (BOOCS Clinic Fukuoka, Fukuoka), K Serikawa (Monowasure Mental Clinic, Fukuoka), K Takasaki (Takasaki Neurosurgery Clinic, Kasuya), M Ichimaru (BOOCS Holistic Clinic Tokyo, Tokyo), M Kinoshita (Nagata Hospital, Yanagawa), M Munaka (Hayama Clinic, Munakata), N Araki (Utsunomiya Rehabilitation Hospital, Utsunomiya), S Nakamura (Sangenjaya Nakamura Mental Clinic, Tokyo), S Ouma (Fukuoka University Hospital, Fukuoka), S Nakano (Nihonbashi Sakura Clinic, Tokyo), T Asada (Olive Clinic Ochanomizu, Tokyo, Memory Clinic Ochanomizu, Tokyo), T Kaneko (Kaneko Hospital, Yanagawa), T Kinoshita (Nozomi Memory Clinic, Mitaka), T Yamada (Nishino Hospital, Kitakyushu, Tokorozawa Meisei Hospital, Tokorozawa), T Yosimatsu (Mito Hospital, Kasuya), Y Nakayama (Nakayoshi-Clinic, Kasuya), Y Sekine (Sekine Clinic, Hirakata).
References
- Braverman N.E., Moser A.B. Functions and biosynthesis of plasmalogen lipids in health and disease. Biochim. Biophys. Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Chini B., Parenti M. G-protein coupled receptors in lipid rats and caveolae; how, when and why do they go there? J. Mol. Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- Farooqui A.A., Horrocks L.A. Plasmalogens: workhorse lipids of membranes in normal and injured neurons and glia. Neuroscientist. 2001;7:232–245. doi: 10.1177/107385840100700308. [DOI] [PubMed] [Google Scholar]
- Ginsberg L., Rafique S., Xuereb J.H., Rapoport S.I., Gershfeld N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer's disease brain. Brain Res. 1995;698:223–226. doi: 10.1016/0006-8993(95)00931-f. [DOI] [PubMed] [Google Scholar]
- Goodenowe D.B., Cook L.L., Liu J., Lu Y., Jayasinghe D.A., Ahiahonu P.W., Heath D., Yamazaki Y., Flax J., Krenitsky K.F., Sparks D.L., Lerner A., Friedland R.P., Kudo T., Kamino K., Morihara T., Takeda M., Wood P.L. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer's disease and dementia. J. Lipid Res. 2007;48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- Grimm M.O., Mett J., Stahlmann C.P., Haupenthal V.J., Blümel T., Stötzel H., Grimm H.S., Hartmann T. Eicosapentaenoic acid and docosahexaenoic acid increase the degradation of amyloid-β by affecting insulin-degrading enzyme. Biochem. Cell Biol. 2016;94:534–542. doi: 10.1139/bcb-2015-0149. [DOI] [PubMed] [Google Scholar]
- Guan Z., Wang Y., Cairns N.J., Lantos P.L., Dallner G., Sindelar P.J. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999;58:740–747. doi: 10.1097/00005072-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Han X., Holtzman D.M., McKeel D.W., Jr. Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001;77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- Hanuš L.O., Levitsky D.O., Shkrob I., Dembitsky V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009;116:491–498. [Google Scholar]
- Hopperton K.E., Trépanier M.O., Giuliano V., Bazinet R.P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-β 1-40 in mice. J. Neuroinflammation. 2016;13:257. doi: 10.1186/s12974-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., Ifuku M., Take S., Kawamura J., Miake K., Katafuchi T. Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLoS One. 2013;8:e83508. doi: 10.1371/journal.pone.0083508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., Mineno K., Katafuchi T. Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling. PLoS One. 2016;11:e0150846. doi: 10.1371/journal.pone.0150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katafuchi T., Ifuku M., Mawatari S., Fujino T. Effects of plasmalogens on systemic lipopolysaccharide-induced glial activation and β-amyloid accumulation in adult mice. Ann. N. Y. Acad. Sci. 2012;1262:85–92. doi: 10.1111/j.1749-6632.2012.06641.x. [DOI] [PubMed] [Google Scholar]
- Kou J., Kovacs G.G., Höftberger R., Kulik W., Brodde A., Forss-Petter S., Hönigschnabl S., Gleiss A., Brügger B., Wanders R., Just W., Budka H., Jungwirth S., Fischer P., Berger J. Peroxisomal alterations in Alzheimer's disease. Acta Neuropathol. 2011;122:271–283. doi: 10.1007/s00401-011-0836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraffe E., Soudant P., Marty Y. Fatty acids of serine, ethanolamine, and choline plasmalogens in some marine bivalves. Lipids. 2004;39:59–66. doi: 10.1007/s11745-004-1202-x. [DOI] [PubMed] [Google Scholar]
- Lagarde M., Bernoud N., Brossard N., Lemaitre-Delaunay D., Thiès F., Croset M., Lecerf J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001;16:201–204. doi: 10.1385/JMN:16:2-3:201. [DOI] [PubMed] [Google Scholar]
- Lagarde M., Hachem M., Bernoud-Hubac N., Picq M., Véricel E., Guichardant M. Biological properties of a DHA-containing structured phospholipid (AceDoPC) to target the brain. Prostaglandins Leukot. Essent. Fat. Acids. 2015;92:63–65. doi: 10.1016/j.plefa.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Mawatari S., Okuma Y., Fujino T. Separation of intact plasmalogens and all other phospholipids by a single run of high-performance liquid chromatography. Anal. Biochem. 2007;370:54–59. doi: 10.1016/j.ab.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Mawatari S., Katafuchi T., Miake Y., Fujino T. Dietary plasmaalogen increases erythrocyte membrane plasmalogen in rats. Lipids Health Dis. 2012;11:161–168. doi: 10.1186/1476-511X-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawatari S., Hazeyama S., Fujino T. Measurement of ether phospholipids in human plasma with HPLC–ELSD and LC/ESI–MS after hydrolysis of plasma with phospholipase A1. Lipids. 2016;51:997–1006. doi: 10.1007/s11745-016-4170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle P.J., Wong G., Tan R., Giral P., Robillard P., Orsoni A., Hounslow N., Magliano D.J., Shaw J.E., Curran J.E., Blangero J., Kingwell B.A., Chapman M.J. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: potential relevance to statin-associated dysglycemia. J. Lipid Res. 2015;56:2381–2392. doi: 10.1194/jlr.P061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New D.C., Wong Y.H. Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J. Mol. Signal. 2007;2:2. doi: 10.1186/1750-2187-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimukai M., Wakisaka T., Hara H. Ingestion of plasmalogen markedly increased plasmalogen levels of blood plasma in rats. Lipids. 2003;38:1227–1235. doi: 10.1007/s11745-003-1183-9. [DOI] [PubMed] [Google Scholar]
- Oma S., Mawatari S., Saito K., Fujino T. Changes in phospholipid composition of erythrocyte membrane in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. Extra. 2012;2:298–303. doi: 10.1159/000341603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T., Futai E., Kan E., Abe N., Uchida T., Kamio Y., Kaneko J. Phosphatidylethanolamine plasmalogen enhances the inhibiting effect of phosphatidylethanolamine on γ-secretase activity. J. Biochem. 2015;157:301–309. doi: 10.1093/jb/mvu074. [DOI] [PubMed] [Google Scholar]
- Phillips M.A., Childs C.E., Calder P.C., Rogers P.J. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer's disease: a randomised controlled trial. Int. J. Mol. Sci. 2015;16:24600–24613. doi: 10.3390/ijms161024600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L.J., Han X., Chung K.N., Gross R.W. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- Quinn J.F., Raman R., Thomas R.G., Yurko-Mauro K., Nelson E.B., Van Dyck C., Galvin J.E., Emond J., Jack C.R., Jr., Weiner M., Shinto L., Aisen P.S. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Luo C., Feng Y., Yao X., Shi Z., Liang F., Kang J.X., Wan J.B., Pei Z., Su H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017;31:282–293. doi: 10.1096/fj.201600896. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V., Panza F., Colacicco A.M., D'Introno A., Capurso C., Torres F., Grigoletto F., Maggi S., Del Parigi A., Reiman E.M., Caselli R.J., Scafato E., Farchi G., Capurso A., Italian Longitudinal Study on Aging Working Group Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- Wood P.L., Mankidy R., Ritchie S., Heath D., Wood J.A., Flax J., Goodenowe D.B. Circulating plasmalogen levels and Alzheimer Disease Assessment Scale-Cognitive scores in Alzheimer patients. J. Psychiatry Neurosci. 2010;35:59–62. doi: 10.1503/jpn.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.L., Barnette B.L., Kaye J.A., Quinn J.F., Woltjer R.L. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects. Acta Neuropsychiatr. 2015;27:270–278. doi: 10.1017/neu.2015.18. [DOI] [PubMed] [Google Scholar]
- World Alzheimer Report . Alzheimer's Disease International; London: 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends.https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf Available from: [Google Scholar]
- Yamashita S., Kiko T., Fujiwara H., Hashimoto M., Nakagawa K., Kinoshita M., Furukawa K., Arai H., Miyazawa T. Alterations in the levels of amyloid-β, phospholipid hydroperoxide, and plasmalogen in the blood of patients with Alzheimer's disease: possible interactions between amyloid-β and these lipids. J. Alzheimers Dis. 2015;50:527–537. doi: 10.3233/JAD-150640. [DOI] [PubMed] [Google Scholar]
- Yarandi S.S., Peterson D.A., Treisman G.J., Moran T.H., Pasricha P.J. Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 2016;22:201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]