Abstract
We previously reported that insufficiency of antithrombin III (ATIII), the major anti-coagulation molecule in vivo, exacerbated renal ischemia-reperfusion injury in animal models and possibly humans. In the present study, we investigated the relationship between ATIII level and contrast induced nephropathy (CIN) in patients and examined therapeutic effect of ATIII on CIN in Sprague-Dawley rats. Patients with low ATIII activity presented a higher incidence of acute kidney injury (AKI) following coronary angiography. ATIII (500 μg/kg) was intravenously injected before or after the induction of AKI in rats. Our data demonstrated ATIII significantly attenuated the elevation of serum creatinine, blood urea nitrogen, and renal histological injury. The beneficial effects of ATIII were accompanied by diminished renal inflammatory response, oxidative stress, cell apoptosis and improved renal blood flow in rats. In conclusion, ATIII appears to attenuate CIN through inhibiting inflammation, oxidative stress, apoptosis and improving renal blood flow. ATIII administration may represent a promising strategy for the prevention and treatment of contrast-induced AKI.
Keywords: Antithrombin III, Acute kidney injury, Contrast induced nephropathy, Inflammation
Highlights
-
•
Patients with low ATIII activity presented a higher incidence of acute kidney injury following coronary angiography.
-
•
ATIII supplementation attenuated renal injury in animal models of contrast induced nephropathy.
-
•
ATIII exerted renoprotective effect by inhibiting inflammation, oxidative stress, apoptosis and improving renal blood flow.
Antithrombin III (ATIII), a potent anti-coagulation molecule in vivo, has been reported that it can exert reno-protective effects in ischemia-reperfusion model. Nevertheless, whether exogenous ATIII administration can protect against contrast induced nephropathy (CIN) in animal models remains unclear. This study revealed that ATIII administration has therapeutic effects against CIN in Sprague-Dawley Rats. Furthermore, the reno-protection conferred by ATIII might be mediated by inhibition of inflammation, oxidative stress, apoptosis and improving renal blood flow. ATIII supplementation represents a promising prophylactic and treatment strategies for contrast induced AKI.
1. Introduction
Acute kidney injury (AKI) is a severe condition with high morbidity and mortality. AKI is associated with a > 2.2–4 times increased likelihood of death in hospitalized patients (Zeng et al., 2014, Wang et al., 2012). Contrast-induced nephropathy (CIN) is the third leading cause of hospital acquired AKI due to iodinated contrast media used in diagnostic and interventional procedures (Nash et al., 2002, Feldkamp and Kribben, 2008). Despite prevention strategies are improved, the increasing incidence of CIN is still challenging. Novel prophylactic and treatment strategies to decrease CIN are urgently needed.
Up to now, the exact underlying mechanism of CIN remains unclear. It has been generally suggested that renal hypoperfusion, direct toxic tubular injury, inflammatory response and oxidative stress are involved in the pathogenesis of CIN (Solomon and Dauerman, 2010, Zhao et al., 2016, Wang et al., 2016).
Among numerous candidates that possess reno-protective effects, Antithrombin III (ATIII) is not only a powerful anticoagulant but also possesses anti-inflammatory properties (Dunzendorfer et al., 2001, Kellner et al., 2014). It has been reported that administration of exogenous ATIII could protect against organ ischemia/reperfusion injury (IRI) (Ozden et al., 2001, Harada et al., 2004). Importantly, our recent study showed that patients with low ATIII activity were more vulnerable to AKI after cardiac surgery and endogenous ATIII insufficiency exacerbated renal injury in an animal model (Wang et al., 2015). However, the role of ATIII in contrast induced AKI has not been previously investigated.
In the current study, we examined whether low plasma ATIII activity increased the risk of CIN and whether exogenous ATIII administration had reno-protective effects in CIN. In addition, the underlying mechanism of ATIII's protective effects against contrast induced AKI was investigated.
2. Materials and Methods
2.1. ATIII Activities and the Incidence of AKI in Patients Undergoing CAG
This study was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Patients undergoing CAG in our hospital were eligible for inclusion while patients with endocarditis, hepatic diseases, and baseline Scr levels > 106 μmol/l were excluded. For the diagnosis of AKI, we adopted KDOQI 2012 criteria for acute kidney injury (Palevsky et al., 2013). Plasma ATIII activities were measured before CAG. ATIII activities were measured using an automatic coagulation analysis machine (Sysmex CA7000, SIEMENS, Munich, Germany). Low or normal ATIII activity was defined as previously described (Wang et al., 2015).
2.2. Animals and Reagents
Male Sprague-Dawley rats, weighing 250 ± 20 g, were purchased from Shanghai Science Academy animal center. This study was approved by the Animal Care and Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. All the procedures were conducted in accordance with the policies for the Care and Use of Laboratory Animals of our institution.
Loversol was purchased from Hengrui Corp. (Jiansu, China). N-nitro-L-arginine methyl ester (L-NAME), indomethacin, sodium taurocholate and ATIII recombinant protein were from Sigma-Aldrich (St Louis, Mo, USA).
2.3. Animal Study Protocols
Study was designed to determine whether exogenous ATIII administration could protect against contrast induced AKI. CIN model was established as described previously (Zhao et al., 2015). Briefly, rats were given a tail vein injection of indomethacin (10 mg/kg), followed by Ioversol (3 g/kg organically bound iodine) and L-NAME. 48 rats were randomly divided into four groups (n = 6 in each group): sham-operated group with injection of normal saline (sham + vehicle), CIN group with injection of normal saline (CIN + vehicle), CIN group with pre-injection of ATIII (CIN + pre-ATIII), CIN group with post-injection of ATIII (CIN + post-ATIII). ATIII (500 μg/kg) or an equivalent volume of vehicle was intravenously delivered 30 min before or after CIN. The ATIII dose used to reduce kidney injury was chosen based on a previous study (Hagiwara et al., 2009). The animals were killed 24 h or 36 h after induction of CIN. Blood and renal tissues were harvested for analysis.
2.4. Kidney Sonography Examinations
Kidney sonography was performed using an Esote Mylab Twice device (Esaote, XVISION, Italy) with 2.5 MHz transducer as previously described (Thalhammer et al., 2006). Doppler ultrasound examinations and calculation of intrarenal resistance index (RI) were performed on the left kidney. RI values were calculated automatically by the device using a standard formula (RI = [peak systolic velocity-peak diastolic velocity/peak systolic velocity). RI assessments were made 24 h after CIN.
2.5. Measurement of Biochemistry Parameters and Oxidative Stress Markers
Scr and BUN were measured by an automatic biochemical analyzer 7600 (Hitachi, Tokyo, Japan) to assess the alteration of renal function. The levels of MDA and SOD in renal tissue homogenate were measured using commercial kits following the manufacturer's protocol (Nanjing Jiancheng, Nanjing, Jiangsu, China).
2.6. Histological Injury Assessment and Immunohistochemical Staining
The paraformaldehyde-fixed kidney was embedded in paraffin and then was cut into 3 μm sections. Histological alterations were evaluated by Periodic acid–Schiff (PAS) staining. The renal injury was evaluated using a scoring system grading tubular necrosis, tubular dilatation, loss of brush border, and cast formation in 10 randomly chosen, non-overlapping fields. The severity of renal injury was semiquantified by the following criteria: 0, none; 1, 0–10%; 2, 11–25%; 3, 26–45%; 4, 46–75%; and 5, 76–100%, as described previously (Melnikov et al., 2002).
Kidney sections were stained with an anti-F4/80 antibody (Abcam, Cambridge, MA, USA) to detect macrophage infiltration in renal tissue as described previously (Yin et al., 2016a, Yin et al., 2016b).
2.7. Quantitative Real-Time PCR
Total RNA from HK2 cells or kidney tissues was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) and was reverse transcribed into cDNA with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). Real-time PCR was performed with Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) as described previously. (Wang et al., 2014) The specific primers were as following: rat TNFα: 5′GTCTGTGCCTCAGCCTCTTC3′ (forward) and 5′TGGAACTGATGAGAGGGAGC3′ (reverse); rat MCP-1: 5′CCCCACTCACCTGCTGCTAC3′ (forward) and 5′CCTGCTGCTGGTGATTCTCTT3’ (reverse); rat ICAM-1:5′GAGACCCCGTTGCCTAAA3′ (forward) and 5′CCGCAGGTCCAGTTCAGT-3’ (reverse); human MCP-1: 5′TGCAGAGGCTCGCGAGCTA3’ (forward) and 5′CAGGTGGTCCATGGAATCCTGA3’ (reverse); human ICAM-1: 5′GGCCTCAGTCAGTGTGA3’ (forward) and 5′AACCCCATTCAGCGTCA3’ (reverse); Quantitation was normalized to internal control 18S rRNA and 2− ΔΔCT method was used to determine relative gene expression level.
2.8. Western Blot Analysis
The primary antibodies, rabbit anti-caspase-3, anti-bcl-2 and mouse anti-Tubulin were both from Cell Signaling Technology (Danvers, MA, USA). Horseradish peroxidase–conjugated secondary antibodies (Beyotime, Suzhou, Jiangsu, China) were used and bands were visualized by Image Quant LAS 4000 Mini System (GE Healthcare, Pittsburgh, PA, USA).
2.9. Statistical Analysis
The statistical software SPSS (Ver. 19.0) (IBM, Armonk, NY, USA) was used for data analysis. All the data were expressed as mean ± standard deviation. One-way ANOVA with Sidak compensation was employed for parametric data comparison. Statistical significance was defined as P < 0.05.
3. Results
3.1. Patients With Low ATIII Activity Presented a Higher Risk for Developing AKI After Coronary Angiography
We assessed whether low plasma ATIII activity increased the risk of AKI following coronary angiography. All 209 eligible patients undergoing CAG in our hospital from July 1, 2014 to Dec 31, 2015 were examined. Of these 209 cases, 31 had low ATIII activity before CAG procedure, among which, 12 (or 38.7%) developed AKI after CAG. Of the 178 cases with normal ATIII activity, 10 (or 5.6%) developed AKI after CAG. The incidence of AKI was significantly higher in patients with low ATIII activity (Fisher's exact test, P < 0.05, Table 1).
Table 1.
Incidences of AKI following CAG in patients with low or normal ATIII activities.
| Normal ATIII (n = 178) | Low ATIII (n = 31) | P | |
|---|---|---|---|
| Gender (male/female) | 94/84 | 17/14 | > 0.05 |
| Age (years) | 69.3 ± 13.1 | 68.8 ± 17.6 | > 0.05 |
| ATIII activity, % (median, range) | 95.6, 78.4–115 | 69.8 (52.1–74.8) | < 0.05 |
| AKI, % | 10, 5.6% | 12, 38.7% | < 0.05 |
| Baseline Scr (μmol/l) | 83.3 ± 22.7 | 78.2 ± 14.7 | > 0.05 |
| Peak Scr of AKI patients (μmol/l) | 204.4 ± 33.1 | 217 ± 35.9 | > 0.05 |
| Proteinuria (n, %) | 12, 6.7% | 3, 9.7% | > 0.05 |
| Diabetes (n, %) | 32, 18.0% | 6, 19.3% | > 0.05 |
| Hb (g/L) | 115.2 ± 13.1 | 123.6 ± 23.8 | > 0.05 |
| D-dimer (mg/L) | 2.0 ± 0.9 | 1.8 ± 1.0 | > 0.05 |
| ALT (U/L) | 69.1 ± 35.2 | 54.3 ± 19.6 | > 0.05 |
| Heart failure (n, %) | 20, 11.2% | 5, 16.1% | > 0.05 |
| AMI (n, %) | 37, 20.8% | 8, 25.8% | > 0.05 |
Abbreviations: CAG, coronary angiography; AKI, acute kidney injury; ATIII, antithrombin III; Scr, serum creatinine; Hb, hemoglobin; ALT, alanine aminotransferase; Scr, serum creatinine; AMI: acute myocardial infraction.
There was no significant difference in diabetes incidence, proteinuria, baseline serum creatinine (Scr), peak Scr, hemoglobin, D-dimer, alanine aminotransferase, and heart failure between the two groups (Table 1). There was no nephrotoxic drug use except necessary anticoagulatory agents and diuretics. Moreover, we divided these patients into quartiles based on ATIII activities and observed that the lowest quartile presented a significantly higher incidence of subsequent AKI (Table 2), consistent with the analysis based on clinically defined low and normal ATIII activities. Thus, Patients with low ATIII activity presented a higher risk for developing AKI after CAG.
Table 2.
AKI incidences in CAG patients divided into quartiles based on ATIII activities.
| Group | N | ATIII activity | AKI incidence |
|---|---|---|---|
| Quartile 1 | 53 | 69.3–88% | 17/53, 32%# |
| Quartile 2 | 56 | 89–99% | 2/56, 3.6% |
| Quartile 3 | 48 | 101–107% | 1/48, 2.1% |
| Quartile 4 | 52 | 108–115% | 2/52, 3.8% |
Abbreviations: CAG, coronary angiography; AKI, acute kidney injury; ATIII, antithrombin III.
P < 0.05, versus group quartiles 2, 3, and 4.
3.2. Antithrombin III Attenuated Contrast Induced Renal Dysfunction and Renal Histological Injury
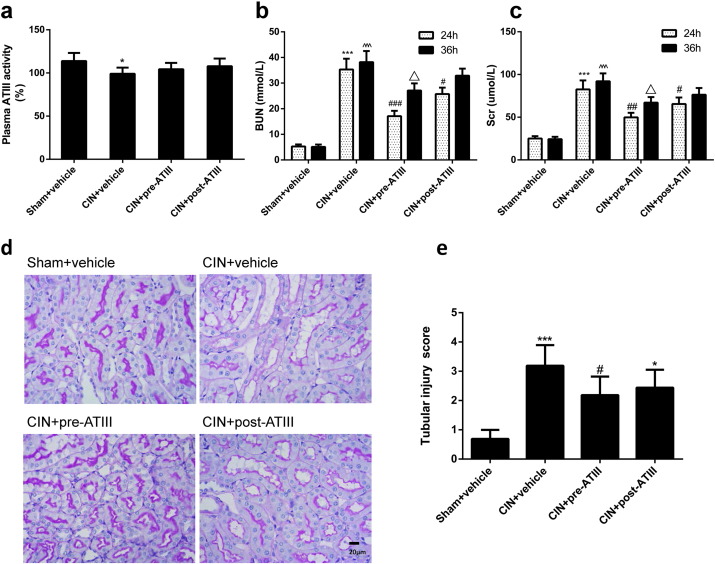
We used an experimental model to confirm whether exogenous ATIII administration had beneficial effects on CIN and underlying mechanisms. ATIII (500 μg/kg) or an equivalent volume of vehicle was intravenously infused 30 min before or after CIN. Administration of ATIII significantly attenuated the elevation of serum creatinine, blood urea nitrogen (BUN) in the CIN model at 24 h (Fig. 1). Moreover, the reno-protective effects of ATIII were sustained to 36 after contrast infusion in ATIII pre-administered rats whereas post-administration of ATIII could not decrease the elevation of Scr and BUN in CIN rats at 36 h.
Fig. 1.
Antithrombin III attenuated renal injury in contrast induced nephropathy (CIN). Contrast induced nephropathy model was induced by intravenous injection of Ioversol and L-NAME in Sprague-Dawley rats. Blood and kidney tissues were collected 24 h or 36 h after CIN, respectively. (a) Plasma AT III activity at 24 h after CIN. (b) Blood urea nitrogen. (c) Serum creatinine. (d) Renal histological alterations by Periodic acid–Schiff (PAS) staining (representative pictures, 400 ×, PAS) at 24 h. (e) Quantitative analysis of tubular injury scoring at 24 h. Sham + vehicle, sham-operated group with normal saline injection; CIN + vehicle, contrast induced nephropathy group with normal saline injection; CIN + pre-ATIII, contrast induced nephropathy group following ATIII injection; CIN + post-ATIII, contrast induced nephropathy group with ATIII post-injection. N = 6, ⁎P < 0.05, ⁎⁎⁎P < 0.001 versus Sham + vehicle at 24 h; #P < 0.05, ###P < 0.001 versus CIN + vehicle at 24 h. ^^^P < 0.001 versus Sham + vehicle at 36 h; △P < 0.05 versus CIN + vehicle at 36 h.
Consistent with the Scr and BUN data, histological analysis demonstrated that the renal tubular detachment, brush border loss, and necrosis of tubular cells were improved in ATIII pre-administered CIN groups compared with CIN + vehicle group (Fig. 1d and e). Therefore, exogenous ATIII administration could attenuate contrast induced renal dysfunction and renal histological injury.
3.3. Antithrombin III Attenuated Renal Inflammation and Oxidative Stress in CIN Rats
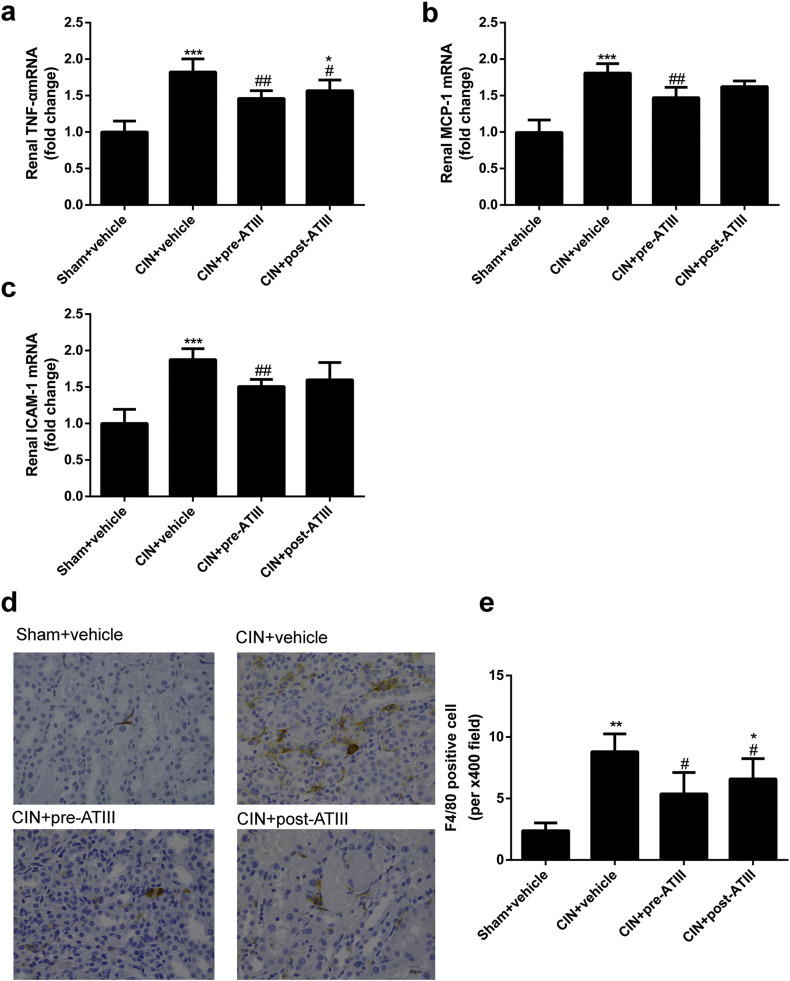
To explore the reno-protective mechanisms of ATIII in CIN, the effects of ATIII on inflammation and oxidative stress were examined. As shown in Fig. 2a, CIN rats showed substantially increased the mRNA expression level of renal tumor necrosis factor α (TNFα) concentration, while both pre- and post-administration of ATIII significantly decreased renal TNFα expression. The mRNA expression levels of renal monocyte chemotactic protein 1 (MCP-1) and intercellular cell adhesion molecule 1 (ICAM-1) in CIN rats were significantly increased compared with sham rats, and pre-administration but not post-administration of ATIII inhibited MCP-1 and ICAM-1 expression in CIN rats (Fig. 2b and c). Immunostaining analysis showed that CIN led to increased intrarenal F4/80-positive cells infiltration, which was mitigated by post- or pre-administration of ATIII (Fig. 2d and e).
Fig. 2.
Antithrombin III treatment mitigated renal inflammation in CIN rats. Kidney tissues were collected at 24 h after CIN. (a) Renal TNFα mRNA expression. (b) Renal MCP-1 mRNA expression. (c) Renal ICAM-1 mRNA expression. (d) Representative images of immunostaining of F4/80 (400 ×). (e) Quantitative analysis of F4/80 positive cells. N = 6, ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001 versus Sham + vehicle; #P < 0.05, ##P < 0.01 versus CIN + vehicle.
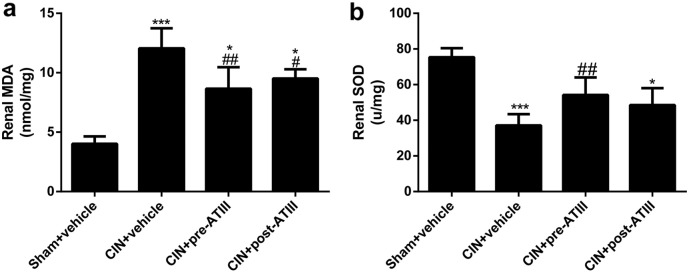
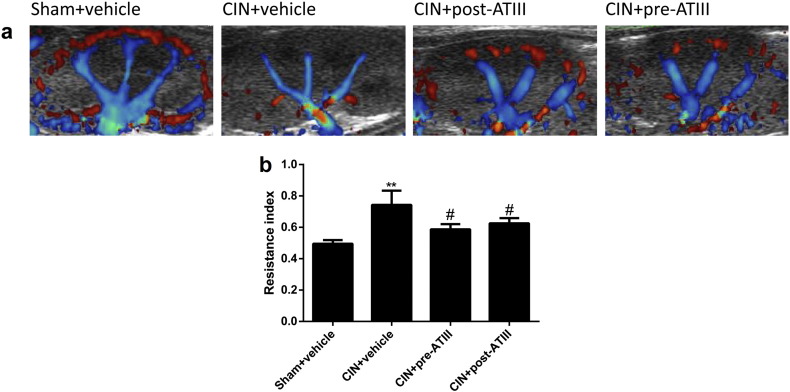
Furthermore, ATIII administration decreased renal oxidative stress levels in CIN rats (Fig. 3). Renal malondialdehyde (MDA) levels were increased in CIN rats, which were attenuated by pre- or post- administration of ATIII (Fig. 3a). Renal superoxide dismutase (SOD) activity was decreased in CIN rats, which was significantly attenuated by pre-administration of ATIII, but not post-administration (Fig. 3b). In addition, ATIII treatment effectively restored renal cortical blood supply and reduced intrarenal resistance index in CIN rats (Fig. 4). These data indicated that ATIII might elicit its beneficial effects by inhibiting inflammatory response, oxidative stress and improving renal blood flow.
Fig. 3.
Antithrombin III treatment decreased renal oxidative stress in CIN rats. Kidney tissues were collected 24 h after CIN. (a) Renal MDA. (b) Renal SOD. N = 6, ⁎P < 0.05, ⁎⁎⁎P < 0.001 versus Sham + vehicle; #P < 0.05, ##P < 0.01 versus CIN + vehicle.
Fig. 4.
Antithrombin III administration improved renal blood flow in CIN rats. Ultrasonography was performed at 24 h after contrast infusion. (a) Representative pictures of distribution of renal blood flow. (b) Quantitative analysis of intrarenal resistance index (RI). N = 6, ⁎⁎P < 0.01 versus Sham + vehicle; #P < 0.05 versus CIN + vehicle.
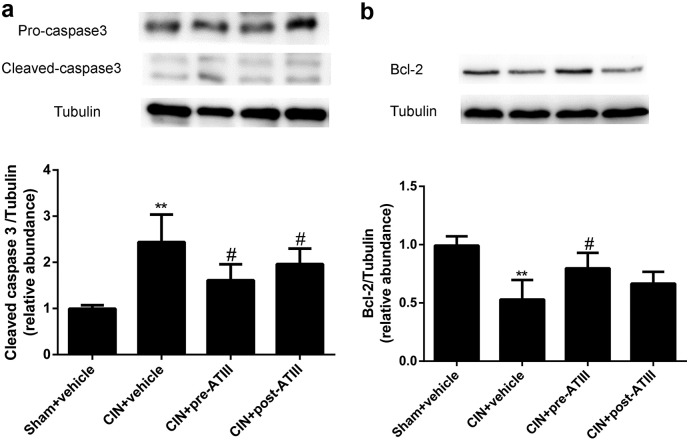
3.4. Effects of Antithrombin III on Renal Cell Apoptosis in CIN Rats
To determine whether ATIII was involved in cell apoptosis, expression of caspase-3 and bcl-2 was analyzed. As shown in Fig. 5, CIN led to substantial increase in cleaved caspases-3 expression, which was attenuated by pre- or post- administration of ATIII (P < 0.05). The expression of anti-apoptosis bcl-2 was significantly increased in ATIII pre-treated CIN group compared with un-treated CIN rats (P < 0.05). In summary, ATIII administration mitigated renal cell apoptosis following CIN.
Fig. 5.
Renal cells apoptosis was diminished after antithrombin III administration in CIN rats. Tissues were collected 24 h after CIN. (a) Caspase-3 protein abundance in renal cortex. (b) Bcl-2 protein abundance in renal cortex. N = 6, ⁎⁎P < 0.01 versus Sham + vehicle; #P < 0.05 versus CIN + vehicle.
4. Discussion
This study demonstrated an important role of ATIII in the endogenous defense mechanisms against medium contrast induced AKI and provided molecular mechanisms of ATIII's reno-protective properties. We found that lower plasma ATIII activity was associated with higher incidence of AKI following CAG. Furthermore, the prophylactic and therapeutic administration of ATIII could effectively improve renal functional and structural damage in contrast induced AKI. The reno-protective effects of ATIII might be mediated by inhibition of inflammation, oxidative stress, apoptosis and improving renal blood flow.
The clinical study results indicate that plasma ATIII could be a useful predictor of AKI in patients received coronary angiography and more proactive prophylactic measures should be taken to prevent the occurrence of AKI in patients with low ATIII activity.
ATIII is not only an inhibitor of thrombin and other serine proteases of the clotting cascade, but also possesses anti-inflammatory and cyto-protective effects (Sen et al., 2011, Lu et al., 2017a, Lu et al., 2017b). In accordance with our recent studies (Wang et al., 2015) and other studies (Ozden et al., 2001), ATIII supplementation appears to attenuate contrast induced renal dysfunction through inhibiting inflammation, oxidative stress, and apoptosis.
It has been recognized that hypoxia, endotoxins, and multiple drugs can directly activate renal resident leukocytes and prime intrarenal inflammation (Rabb et al., 2015). Compared with untreated CIN rats, ATIII administration alleviated intrarenal inflammation and F4/80-positive cells infiltration. ATIII-treated CIN rats exhibited less expression of renal TNFα, MCP-1, and ICAM-1. It appears that ATIII is a crucial anti-inflammatory mediator against local renal inflammation. Nevertheless, how ATIII exerts its anti-inflammatory effects remains elusive. It has been reported that the mechanisms underlying anti-inflammatory properties of ATIII include inhibiting neutrophils recruitment and leukocytes rolling and infiltration (Ostrovsky et al., 1997, Yamashiro et al., 2001), pro-inflammatory cytokines secretion (Souter et al., 2001) and protection in vascular endothelium (Oelschlager et al., 2002). Besides, ATIII can directly inhibit nuclear factor (NF)-κB pathways and leucocytes activation (Zuo et al., 1999, Oelschlager et al., 2002). Thus, ATIII might elicit its beneficial effects on contrast induced AKI by inhibiting inflammatory response.
Excess production of oxygen radical and cell apoptosis is involved in the pathogenesis of CIN. Our data showed that ATIII had inhibitory effects on renal oxidative stress in AKI following contrast infusion. Moreover, tubular cell apoptosis was mitigated in ATIII-administered rats.
Continued renal hypoperfusion plays a pivotal role in the development of CIN (Ince, 2014). Due to their high energy demand and complicated microvascular network, kidneys are especially vulnerable to ischemic injury. Contrast media infusion can directly decrease renal blood flow leading to subsequent renal I/R injury. Our results showed that ATIII administration significantly restored renal blood flow in ATIII–treated CIN rats compared with untreated CIN rats. This hemodynamic alteration may be attributed to increased levels of prostaglandin I2 (PGI2), which is a powerful vasodilator. Our previous study also found blunted compensatory elevation of PGI2 due to ATIII insufficiency might be responsible for renal IRI (Wang et al., 2015). These results indicate that ATIII might indirectly increase renal blood flow via upregulating PGI2.
Clinical studies have demonstrated that the ATIII activity is considerably decreased in critically ill patient with sepsis and pancreatitis (Afshari et al., 2008). Our study also found that ATIII activity was decreased in experimental model of CIN. ATIII deficiency might be caused by increased ATIII consumption and impaired synthesis. In this study, administration of ATIII did not increase the activity of ATIII. This phenomenon could be explained as follows: First, the dose of ATIII was too minimal to influence the anticoagulation activity of ATIII and low dose of ATIII might exert its beneficial effects via its anti-inflammation properties; Second, ATIII might have been metabolized by 24 h after surgery due its consumption.
The present also had some limitations. First, clinical data indicated that insufficiency of endogenous ATIII increased the risk of AKI after coronary angiography. Although confounding factors were partially adjusted by using control group, there still exists a limitation due to the failure to control all factors simultaneously in observational studies. By the way, the animal study only investigated the effect of exogenous ATIII on CIN rats; further study should focus on the role of the endogenous ATIII in the development of CIN. Besides, we used F4/80-positive cells to represent macrophage, however, F4/80-positive cell also includes monocyte. Because there is still no more specific antibody for macrophage so far, it would be more accurate to examine the macrophage infiltration by using double or triple-staining flow cytometry in isolated renal immune cells.
The findings of this study provided novel insights into the mechanisms of ATIII's protection against CIN. ATIII deficiency due to chronic diseases or genetic variations may predispose patients to AKI. Whether prophylactic and therapeutic administration of ATIII can effectively prevent CIN incidence and promote survival in patients also warrants further investigation. This study will give us some new views on antithrombin function and prevention of kidney injury (Lu et al., 2017a, Lu et al., 2017b, Yin et al., 2016a, Yin et al., 2016b).
Author Contributions
W.F., Y.J., and L.Z. designed the experiments. Y.J., L.Z., R.W. and Z.G. performed the experiments. W. F., C.D., Z.Q., and W.N. analyzed and interpreted the data. Y.J., C.D., and W.F., draft the manuscript and L.M. revised the paper. All authors read and approved the final version of manuscript.
Conflicts of Interests
The authors declare no conflict of interests.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (81570603), the New-100 talent Plan of Shanghai Jiao Tong University School of Medicine, Shanghai Talents Development Fund (201350) and Shanghai Pujiang Talent Projects (15PJ1406700). The authors wish to thank Marc Casati of Medical College of Wisconsin for his advice and revision of the manuscript. Part of this work was submitted to ASN 2016 Kidney Week as an abstract.
Contributor Information
Zeyuan Lu, Email: luzeyuan9376@foxmail.com.
Dongsheng Cheng, Email: 191284172@qq.com.
Jianyong Yin, Email: yinjianyong09@163.com.
Rui Wu, Email: 1547846219@qq.com.
Guangyuan Zhang, Email: zgy0879@qq.com.
Qing Zhao, Email: zhaoqingcardiology@163.com.
Niansong Wang, Email: wangniansong2008@163.com.
Feng Wang, Email: zyzwq1030@hotmail.com.
Mingyu Liang, Email: mliang@mcw.edu.
References
- Afshari A., Wetterslev J., Brok J., Moller A.M. Antithrombin III for critically ill patients. Cochrane Database Syst. Rev. 2008;16(3) doi: 10.1002/14651858.CD005370.pub2. [DOI] [PubMed] [Google Scholar]
- Dunzendorfer S., Kaneider N., Rabensteiner A., Meierhofer C., Reinisch C., Romisch J., Wiedermann C.J. Cell-surface heparan sulfate proteoglycan-mediated regulation of human neutrophil migration by the serpin antithrombin III. Blood. 2001;97:1079–1085. doi: 10.1182/blood.v97.4.1079. [DOI] [PubMed] [Google Scholar]
- Feldkamp T., Kribben A. Contrast media induced nephropathy: definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99:177–196. [PubMed] [Google Scholar]
- Hagiwara S., Iwasaka H., Shingu C., Matsumoto S., Uchida T., Noguchi T. Antithrombin III prevents cerulein-induced acute pancreatitis in rats. Pancreas. 2009;38:746–751. doi: 10.1097/MPA.0b013e3181aba9fa. [DOI] [PubMed] [Google Scholar]
- Harada N., Okajima K., Uchiba M., Kushimoto S., Isobe H. Antithrombin reduces ischemia/reperfusion-induced liver injury in rats by activation of cyclooxygenase-1. Thromb. Haemost. 2004;92:550–558. doi: 10.1160/TH03-07-0460. [DOI] [PubMed] [Google Scholar]
- Ince C. The central role of renal microcirculatory dysfunction in the pathogenesis of acute kidney injury. Nephron Clin. Pract. 2014;127:124–128. doi: 10.1159/000363203. [DOI] [PubMed] [Google Scholar]
- Kellner P., Nestler F., Leimert A., Bucher M., Czeslick E., Sablotzki A., Raspe C. Antithrombin III, but not C1 esterase inhibitor reduces inflammatory response in lipopolysaccharide-stimulated human monocytes in an ex-vivo whole blood setting. Cytokine. 2014;70:173–178. doi: 10.1016/j.cyto.2014.07.253. [DOI] [PubMed] [Google Scholar]
- Lu Z., Wang F., Liang M. Serpin C1/antithrombin III in kidney related diseases. Clin. Sci. 2017 doi: 10.1042/CS20160669. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Yin J., Zhang G., Wu R., Zhao Q., Wang N., Yan C., Wang F. Underestimated incidence of kidney disease in nonrenal outpatient. Ren. Fail. 2017;39:328–332. doi: 10.1080/0886022X.2017.1279551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikov V.Y., Faubel S., Siegmund B., Lucia M.S., Ljubanovic D., Edelstein C.L. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J. Clin. Invest. 2002;110:1083–1091. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash K., Hafeez A., Hou S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- Oelschlager C., Romisch J., Staubitz A., Stauss H., Leithauser B., Tillmanns H., Holschermann H. Antithrombin III inhibits nuclear factor kappaB activation in human monocytes and vascular endothelial cells. Blood. 2002;99:4015–4020. doi: 10.1182/blood.v99.11.4015. [DOI] [PubMed] [Google Scholar]
- Ostrovsky L., Woodman R.C., Payne D., Teoh D., Kubes P. Antithrombin III prevents and rapidly reverses leukocyte recruitment in ischemia/reperfusion. Circulation. 1997;96:2302–2310. doi: 10.1161/01.cir.96.7.2302. [DOI] [PubMed] [Google Scholar]
- Ozden A., Sarioglu A., Demirkan N.C., Bilgihan A., Duzcan E. Antithrombin III reduces renal ischemia-reperfusion injury in rats. Res. Exp. Med. (Berl.) 2001;200:195–203. [PubMed] [Google Scholar]
- Palevsky P.M., Liu K.D., Brophy P.P., Chawla L.S., Parikh C.R., Thakar C.V., Tolwani A.J., Waikar S.S., Weisbord S.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013;61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- Rabb H., Griffin M.D., Mckay D.B., Swaminathan S., Pickkers P., Rosner M.H., Kellum J.A., Ronco C., Acute Dialysis Quality Initiative Consensus, X. W. G Inflammation in AKI: current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 2015;27:371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen O., Sonmez E., Cekinmez M., Ozen O., Caner H. Antithrombin III and enoxaparin treatment inhibit contusion-triggered cell death, inflammation, hemorrhage and apoptosis after severe traumatic brain injury in rats. Turk. Neurosurg. 2011;21:203–209. doi: 10.5137/1019-5149.JTN.3646-10.1. [DOI] [PubMed] [Google Scholar]
- Solomon R., Dauerman H.L. Contrast-induced acute kidney injury. Circulation. 2010;122:2451–2455. doi: 10.1161/CIRCULATIONAHA.110.953851. [DOI] [PubMed] [Google Scholar]
- Souter P.J., Thomas S., Hubbard A.R., Poole S., Romisch J., Gray E. Antithrombin inhibits lipopolysaccharide-induced tissue factor and interleukin-6 production by mononuclear cells, human umbilical vein endothelial cells, and whole blood. Crit. Care Med. 2001;29:134–139. doi: 10.1097/00003246-200101000-00027. [DOI] [PubMed] [Google Scholar]
- Thalhammer C., Aschwanden M., Mayr M., Koller M., Steiger J., Jaeger K.A. Duplex sonography after living donor kidney transplantation: new insights in the early postoperative phase. Ultraschall Med. 2006;27:141–145. doi: 10.1055/s-2006-926560. [DOI] [PubMed] [Google Scholar]
- Wang F., Cai H., Zhao Q., Xing T., Li J., Wang N. Epinephrine evokes renalase secretion via alpha-adrenoceptor/NF-kappaB pathways in renal proximal tubular epithelial cells. Kidney Blood Press. Res. 2014;39:252–259. doi: 10.1159/000355802. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang G., Lu Z., Geurts A.M., Usa K., Jacob H.J., Cowley A.W., Wang N., Liang M. Antithrombin III/SerpinC1 insufficiency exacerbates renal ischemia/reperfusion injury. Kidney Int. 2015;88:796–803. doi: 10.1038/ki.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Yin J., Lu Z., Zhang G., Li J., Xing T., Zhuang S., Wang N. Limb ischemic preconditioning protects against contrast-induced nephropathy via renalase. EBioMedicine. 2016;9:356–365. doi: 10.1016/j.ebiom.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.E., Muntner P., Chertow G.M., Warnock D.G. Acute kidney injury and mortality in hospitalized patients. Am. J. Nephrol. 2012;35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K., Kiryu J., Tsujikawa A., Honjo M., Nonaka A., Miyamoto K., Honda Y., Tanihara H., Ogura Y. Inhibitory effects of antithrombin III against leukocyte rolling and infiltration during endotoxin-induced uveitis in rats. Invest. Ophthalmol. Vis. Sci. 2001;42:1553–1560. [PubMed] [Google Scholar]
- Yin J., Chen W., Ma F., Lu Z., Wu R., Zhang G., Wang N., Wang F. Sulodexide pretreatment attenuates renal ischemia-reperfusion injury in rats. Oncotarget. 2016;8(6):9986–9995. doi: 10.18632/oncotarget.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Lu Z., Wang F., Jiang Z., Lu L., Miao N., Wang N. Renalase attenuates hypertension, renal injury and cardiac remodelling in rats with subtotal nephrectomy. J. Cell. Mol. Med. 2016;20:1106–1117. doi: 10.1111/jcmm.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Mcmahon G.M., Brunelli S.M., Bates D.W., Waikar S.S. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin. J. Am. Soc. Nephrol. 2014;9:12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Zhao Q., Li J., Xing T., Wang F., Wang N. Renalase protects against contrast-induced nephropathy in Sprague-Dawley rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Yin J., Lu Z., Kong Y., Zhang G., Zhao B., Wang F. Sulodexide protects contrast-induced nephropathy in Sprague-Dawley rats. Cell. Physiol. Biochem. 2016;40:621–632. doi: 10.1159/000452575. [DOI] [PubMed] [Google Scholar]
- Zuo X.J., Okada Y., Nicolaidou E., Toyoda T., Marchevsky A., Matloff J.M., Jordan S.C. Antithrombin III inhibits T and B lymphocyte activation in vitro and improves parameters of inflammation in a rat model of acute lung allograft rejection. Transplant. Proc. 1999;31:847–848. doi: 10.1016/s0041-1345(98)01800-4. [DOI] [PubMed] [Google Scholar]