IgE antibodies have a critical importance in the pathophysiology of allergic diseases (Oettgen, 2016), even though their presence in tissues and blood is not sufficient to elicit clinical allergy, as shown by the substantial rates of subjects with positive tests to allergens but no symptoms. The goal to block the effects of IgE by anti-IgE antibodies was long pursued and finally achieved in 2001 with the introduction of the monoclonal humanized anti-IgE antibody omalizumab (Schulman, 2001). This biologic agent was approved for use in patients with severe allergic asthma uncontrolled by conventional drug treatment. Its ability to reduce the circulating IgE antibodies and consequently the IgE-mediated manifestations made possible its application in a number of other diseases, also including apparently non-IgE mediated disorders (Incorvaia et al., 2008). Still, research continued for developing other kind of anti-IgE agents able to expand the therapeutic performance of omalizumab.
In 2012, the first attempt was based on suppressing the B-cell receptor signaling (and thus the IgE production) by the inhibitory IgG Fc receptor FcγRIIb obtained by a therapeutic antibody co-engaging FcγRIIb and IgE B-cell receptor. From a murine anti-IgE antibody an humanized antibody (XmAb7195) with increased IgE binding affinity for FcγRIIb was developed. Compared with omalizumab, XmAb7195 showed a 5-fold higher affinity for human IgE and more than 400-fold higher affinity for FcγRIIb (Chu et al., 2012).
Two years later, based on the exclusion from omalizumab treatment of patients with too high total IgE levels or body mass, two novel high affinity anti-IgE antibodies, QGE031 (ligelizumab) and MEDI4212, were developed with the aim of targeting a larger patient population with severe uncontrolled asthma. QGE031, a monoclonal antibody with greater affinity for IgE than omalizumab, was investigated in two controlled, double-blind clinical trials, the first administering single doses (up to 10 mg/kg) or placebo intravenously, while the second trial administering two to four doses of QGE031 (0.2–4 mg/kg) or placebo subcutaneously at 2-week intervals. The trials were completed by 82% and 87% of subjects, respectively. QGE031 showed a dose- and time-dependent suppression of free IgE, basophil FcεRI and basophil surface IgE higher than omalizumab, also inducing the development of negative skin prick tests to allergens in > 95% of treated, compared with 41% in subjects treated with omalizumab (Arm et al., 2014). In a recent trial, ligelizumab was found to be more effective than omalizumab in a small group of patients with mild allergic asthma (Gauvreau et al., 2016).
The generation of MEDI4212 antibody was based on phage display technology, using protein crystallography to determine the details of the interaction between MEDI4212 and IgE. MEDI4212 was shown to target residues in the IgE Cε3 domain, that is essential for interaction with FcεRI, to prevent the binding of IgE to CD23 and, in ex vivo experiments at identical concentration, to deplete free-IgE from human sera to levels ~ 1 log lower than omalizumab (Cohen et al., 2014). MEDI4212 was further investigated by Nyborg et al., who assessed for each MEDI4212 variant the affinity with human IgE. All variants bound similarly to IgE at the membrane surface of IgE expressing cells, but demonstrated enhanced affinity for FcγRIIIa, leading to increased effector function in cell-based assays. According to the authors, the major advantages of MEDI4212 over omalizumab should be the ability to neutralize high levels of soluble IgE and to eliminate, by antibody-dependent cell-mediated cytotoxicity, IgE-expressing B cells before differentiation in IgE-secreting plasma cells (Nyborg et al., 2016). In a phase 1 study, atopic subjects with baseline IgE ≥ 30 IU/mL were randomized to a single subcutaneous dose (up to 300 mg) or intravenous (300 mg) MEDI4212, subcutaneous omalizumab, or placebo. MEDI4212 rapidly suppressed free serum IgE more than omalizumab; however, recovery of free IgE to baseline in MEDI4212-dosed subjects was rapid when compared with the slow and gradual recovery seen in omalizumab-dosed individuals, this suggesting a limited potential for dosing schedule advantages over omalizumab (Sheldon et al., 2016). In all these studies no serious adverse events were reported.
In the present issue of EBioMedicine, Lupinek et al. address by a different approach the limit of too high total IgE levels or body mass precluding the use of omalizumab in severe asthma (Lupinek et al., 2017). They used IgEnio (Fresenius Medical Care, Bad Homburg, Germany) a device that consist of a extracorporeal immunoadsorber column for selective removal of IgE from patients' plasma by the use of ScFv12, a recombinant single-chain variable fragment obtained by E. coli that selectively removes IgE. Fifteen patients with seasonal allergic asthma and IgE levels up to 4344 U/ml, i.e. largely exceeding the limit for omalizumab treatment, were randomly assigned to treatment with IgEnio (10 patients), based on 3 cycles of immune adsorptions with a 4 week interval, or to the control group (5 patients). The two groups had comparable total IgE levels. At the end of the last cycle, IgE immune adsorption depleted 86.2% of IgE and also depleted immune complexes IgE-omalizumab (generated by introducing in vitro omalizumab in patients' plasma). Also, a reduction of allergen skin test and basophil sensitivity, as well as of clinical symptoms, was observed. The treatment was well-tolerated. According to authors, IgE immune adsorption can be proposed in patients with severe pollen-induced asthma and in patients with severe asthma who cannot be treated to omalizumab because of too high IgE levels. The first suggestion must be confirmed by clinical trials on large populations of pollen-allergic patients, while the second could be an option to the future use of the new anti-IgE monoclonal antibodies (Fig. 1). In fact, omalizumab has an acknowledged role in severe uncontrolled allergic asthma but is significantly restricted by the limit of too high IgE levels or body mass. It seems reasonable to expect that this barrier could be overcome by a pre-treatment with IgEnio, to allow to excluded patients to be admitted to omalizumab treatment.
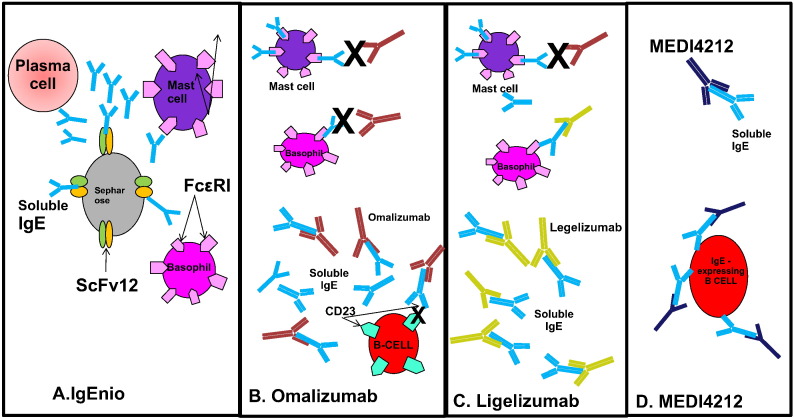
Fig. 1.
A. Sepharose-bound ScFv12 removes IgE produced by plasma cells so that it does not bind to basophils and mast cells. B. Omalizumab binds soluble IgE and prevents IgE binding to the high affinity receptor for IgE on mast cells and basophils. Omalizumab also prevents IgE binding to CD23 and thus inhibits binding to CD23-expressing B cells. C. Ligelizumab binds soluble IgE with higher affinity than Omalizumab and also neutralizes IgE on basophil surface. D. MEDI1412 neutralizes soluble IgE and eliminates IgE-expressing B-cells through ADCC.
Disclosure
The authors declared no conflicts of interest.
References
- Arm J.P., Bottili I., Skerianec A. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin. Exp. Allergy. 2014;44:1371–1385. doi: 10.1111/cea.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S.Y., Horton H.M., Pong E. Reduction of total IgE by targeted co-engagement of IgE B-cell receptor and FcγRIib with FC-engineered antibody. J. Allergy Clin. Immunol. 2012;129:1102–1115. doi: 10.1016/j.jaci.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Cohen E.S., Dobson C.L., Kack H. A novel IgE-neutralizing antibody for the treatment of severe uncontrolled asthma. MAbs. 2014;6:756–764. doi: 10.4161/mabs.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau G.M., Arm J.P., Boulet L.P. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J. Allergy Clin. Immunol. 2016;138:1051–1059. doi: 10.1016/j.jaci.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Incorvaia C., Mauro M., Riario-Sforza G.G. Current and future applications of the anti-IgE antibody omalizumab. Biologics. 2008;2:67–73. doi: 10.2147/btt.s1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupinek C., Derfler K., Lee S. Extracorporeal IgE immunoadsorption in allergic asthma: safety and efficacy. EBioMedicine. 2017;17:119–133. doi: 10.1016/j.ebiom.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyborg A.C., Zacco A., Ettinger R. Development of an antibody that neutralizes soluble IgE and eliminates IgE expressing B cells. Cell. Mol. Immunol. 2016;13:391–400. doi: 10.1038/cmi.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen H.C. Fifty years later: emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. J. Allergy Clin. Immunol. 2016 doi: 10.1016/j.jaci.2016.04.009. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman E.S. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am. J. Respir. Crit. Care Med. 2001;164:S6–11. doi: 10.1164/ajrccm.164.supplement_1.2103025. [DOI] [PubMed] [Google Scholar]
- Sheldon E., Schwickart M., Li J. Pharmacokinetics, pharmacodynamics, and safety of MEDI4212, and anti-IgE monoclonal antibody, in subjects with atopy: a phase 1 study. Adv. Ther. 2016;33:225–251. doi: 10.1007/s12325-016-0287-8. [DOI] [PubMed] [Google Scholar]