Abstract
To examine the mechanism of ocular axial elongation in myopia, guinea pigs (age: 2–3 weeks) which either underwent unilateral or bilateral lens-induced myopization (group 1) or which were primarily myopic at baseline (group 2) received unilateral intraocular injections of amphiregulin antibody (doses: 5, 10, or 15 μg) three times in intervals of 9 days. A third group of emmetropic guinea pigs got intraocular unilateral injections of amphiregulin (doses: 0.25, 0.50 or 1.00 ng, respectively). In each group, the contralateral eyes received intraocular injections of Ringer's solution. In intra-animal inter-eye comparison and intra-eye follow-up comparison in groups 1 and 2, the study eyes as compared to the contralateral eyes showed a dose-dependent reduction in axial elongation. In group 3, study eyes and control eyes did not differ significantly in axial elongation. Immunohistochemistry revealed amphiregulin labelling at the retinal pigment epithelium in eyes with lens-induced myopization and Ringer's solution injection, but not in eyes with amphiregulin antibody injection. Intraocular injections of amphiregulin-antibody led to a reduction of lens-induced axial myopic elongation and of the physiological eye enlargement in young guinea pigs. In contrast, intraocularly injected amphiregulin in a dose of ≤ 1 ng did not show a significant effect. Amphiregulin may be one of several essential molecular factors for axial elongation.
Keywords: Amphiregulin, Epithelial growth factor, Experimental myopia, Axial elongation, Myopia
Highlights
-
•
Intraocular injections of amphiregulin-antibody led to a reduction of lens-induced axial myopic elongation in guinea pigs.
-
•
Intraocular injections of amphiregulin-antibody also led to a reduction of the physiological eye growth in guinea pigs.
-
•
Amphiregulin may be one of several essential molecular factors for axial elongation in young guinea pigs.
Due to an increase in its prevalence, myopia has been feared to become one of the most common causes of irreversible visual impairment worldwide. Although staying indoors in childhood has been identified as the most important factor for the development of myopia, the underlying mechanism leading to myopia has remained elusive so far. In the present experimental study, young guinea pigs which were myopized by a lens, developed less myopia if they simultaneously received intraocular injections of an antibody of amphiregulin, a member of the epithelial growth factor family. It suggests that amphiregulin is associated with axial elongation in myopia.
1. Introduction
The prevalence of high myopia among the young generation has markedly increased within the last three decades (Morgan et al., 2012, He et al., 2004, Congdon et al., 2008, Wu et al., 2013, Rudnicka et al., 2017). Since high myopia in adults is strongly associated with myopic retinopathy and glaucomatous optic neuropathy, myopia has become one of the leading causes of irreversible visual impairment and blindness (Morgan et al., 2012, Xu et al., 2006, Xu et al., 2007, Chang et al., 2013, Ohno-Matsui et al., 2015). Procedures to prevent development or progression of myopia are needed. Although the influence of lifestyle on the development of myopia in children and teenagers has been demonstrated, the basic mechanisms leading to axial myopia as an overshooting in the process of emmetropization have not yet been fully explained (Jones et al., 2007, Rose et al., 2008, He et al., 2015).
The process of emmetropization describes the adaptation of the length of the ocular optical axis to the refractive power of the anterior segment including cornea and lens. This process, occurring after the end of the second year of life in humans, mainly involves the sagittal axis of the eye while the horizontal diameter and the vertical diameter elongate by a lower amount (Heine, 1899). Until recently, sclera or choroid were thought to be the primary tissues leading to axial elongation of the eye (Chen et al., 2013, Frost and Norton, 2012, He et al., 2014, Guo et al., 2014, Li et al., 2015, McBrien et al., 2000, Nickla and Wallman, 2010, Siegwart and Strang, 2007, Tao et al., 2013, Wang et al., 2011, Zou et al., 2014). Recent investigations however gave hints that Bruch's membrane might be the structure which primarily increased in length and elongated the eye in the process of myopization (Wei et al., 2013, Jonas et al., 2014, Shen et al., 2016, Jonas et al., 2016, Jonas et al., 2017a, Jonas et al., 2015a, Jonas et al., 2015b, Jonas et al., 2017b). Reasons for these assumptions were 1) that the choroid got thinner with increasing axial length (if the sclera was the primary elongating tissue, the distance between sclera and Bruch's membrane (i.e. thickness of the choroidal space) would become larger) (Wei et al., 2013); 2) that the optical axis ended at the photoreceptor outer segments in close vicinity to the retinal pigment epithelium and Bruch's membrane, and not at the sclera, which is separated from the photoreceptor outer segments by the spongy choroid with a physiologically fluctuating thickness of about 250 μm; 3) that the volume of the sclera was independent of axial length in individuals with an age of more than two years, so that it was unlikely that an active tissue growth of the sclera was primarily involved in axial elongation (Shen et al., 2016); 4) that retinal thickness and retinal pigment epithelium density in the macular region were independent of axial length (Jonas et al., 2017a); 5) that the length of the macular Bruch's membrane in any direction was independent of axial length (Jonas et al., 2015a); and 6) that, subsequently, the axial elongation associated increase in the disc-fovea distance was due to the development and enlargement of parapapillary gamma zone (Jonas et al., 2015b). These findings led to the hypothesis that axial elongation might occur by production of Bruch's membrane in the retro-equatorial region leading to a decreased retinal pigment epithelium cell density and retinal thinning in that region and a more tube-like than spherical enlargement of the globe, without compromise in the density of the macular retinal pigment epithelium and in macular retinal thickness (Jonas et al., 2017b).
Bruch's membrane is produced by the retinal pigment epithelium. We here postulate that the molecule directing the retinal pigment epithelium to produce more Bruch's membrane is amphiregulin. Amphiregulin, also known as AREG, is a member of the epidermal growth factor family and it is a ligand of the epidermal growth factor receptor (EGFR), a widely expressed transmembrane tyrosine kinase (Shoyab et al., 1989, Berasain and Avila, 2014). Encoded by the AREG gene, amphiregulin is synthesized as a membrane-anchored precursor protein that can engage in juxtacrine signaling on adjacent cells. After proteolytic processing by cell membrane proteases amphiregulin is secreted and becomes an autocrine or paracrine factor. The expression of the amphiregulin gene and the release of amphiregulin are induced by many different stimuli such as inflammatory lipids, cytokines, hormones, growth factors and xenobiotics. If amphiregulin binds to the epidermal growth factor receptor, major intracellular signaling cascades are activated which govern cell survival, proliferation and motility (Shoyab et al., 1989, Berasain and Avila, 2014). Amphiregulin has been detected in many normal tissues including the retinal pigment epithelium, among other tissues such as ovary, testis, placenta, and heart (Shoyab et al., 1989, Berasain and Avila, 2014, Yan et al., 2007). Amphiregulin also plays a role in the process of corneal epithelial wound repair (Zieske et al., 2000).
One has to bear in mind, that the potential role of Bruch's membrane in the process of emmetropization and axial elongation is only at the start of being explored and that studies by Norton, McBrien and others strongly suggested that it is a stretching of the sclera, caused by changes in the biomechanical properties of the scleral stroma without a change in scleral mass and volume, that produces axial globe enlargement in myopia (Chen et al., 2013, Frost and Norton, 2012, He et al., 2014, Guo et al., 2014, Li et al., 2015, McBrien et al., 2000). If Bruch's membrane may not be involved in the regulation of axial elongation, the assessment of a potential role of amphiregulin in axial elongation may still be interesting since amphiregulin-related pathways in ocular growth regulation may also include the sclera (Shelton & Rada, 2009).
In a first attempt to test the hypothesis, we performed an experimental study to assess the ability of amphiregulin to mediate ocular growth. In the experiment, young guinea pigs underwent lens-induced myopization and the study eyes received intraocular injections of amphiregulin antibody in various doses. A second group of guinea pigs which were primarily myopic with a refractive error of at least − 2 diopters at baseline of the study received intraocular injections of amphiregulin antibody without lens-induced myopization. Finally, we injected amphiregulin into the eyes of a third group of guinea pigs to assess its effect on the physiological growth of the eyes.
2. Material and Methods
2.1. Experimental Design
2.1.1. Animals
The experimental study included guinea pigs with an age of 2–3 weeks and a body weight of 100–150 g at baseline. The study was approved by the Ethics committee of the Eye Institute of the Shandong University of Traditional Chinese Medicine.
The whole batch of animals was divided into several groups:
A-I - Animals without lens-induced myopization and without any intervention (n = 8 animals).
B-II - Animals with bilateral lens-induced myopization and without any intraocular intervention (n = 5).
C-III - Animals with bilateral lens-induced myopization and with intraocular injection of amphiregulin antibody in a dose of 5 μg into the right eyes and intraocular injection of Ringer's solution into the left eyes (n = 10).
C-IV - Animals with bilateral lens-induced myopization and with intraocular injection of amphiregulin antibody in a dose of 10 μg into the right eyes and intraocular injection of Ringer's solution into the left eyes (n = 13).
C-V - Animals with bilateral lens-induced myopization and with intraocular injection of amphiregulin antibody in a dose of 15 μg into the right eyes and intraocular injection of Ringer's solution into the left eyes (n = 10).
D-VI - Animals with lens-induced myopization of the right eyes and with intraocular injection of Ringer's solution into the right eyes (n = 5).
D-VII - Animals with lens-induced myopization of the right eyes and with intraocular injection of amphiregulin antibody in a dose of 5 μg into the right eyes (n = 5).
D-VIII - Animals with lens-induced myopization of the right eyes and with intraocular injection of amphiregulin antibody in a dose of 10 μg into the right eyes (n = 5).
D-IX - Animals with lens-induced myopization of the right eyes and with intraocular injection of amphiregulin antibody in a dose of 15 μg into the right eyes (n = 5).
E-X - Animals which were primarily myopic at baseline with a refractive error of at least − 2 diopters, without additional lens-induced myopization, and with unilateral intraocular injection of amphiregulin antibody in a dose of 20 μg into the right eyes and injection of Ringer's solution into the left eyes (n = 6).
F-XI - Animals without lens-induced myopization and with intraocular injection of amphiregulin in a dose of 0.25 ng into the right eyes and intraocular injection of Ringer's solution into the left eyes (n = 6).
F-XII - Animals without lens-induced myopization and with intraocular injection of amphiregulin in a dose of 0.50 ng into the right eyes and intraocular injection of Ringer's solution into the left eyes (n = 6).
F-XIII - Animals without lens-induced myopization and with intraocular injection of amphiregulin in a dose of 1.00 ng into the right eyes and intraocular injection of Ringer's solution into the left eyes (n = 6).
At baseline of the study and each follow-up examination, the animals underwent measurement of body weight and sonographic ocular biometry (A/B-mode scan; oscillator frequency: 11 MHz; Quantel Co., Les Ulis, France) for measurement of the anterior chamber depth, lens thickness, vitreous cavity length and total sagittal length.
For animals undergoing lens-induced myopization, goggles with a refractive power of − 10 diopters were glued onto the orbital rim of both eyes in guinea pigs with bilateral myopization or on the right eyes in guinea pigs with unilateral myopization. Two weeks later, the goggles were removed, and ofloxacin containing antibiotic eye drops (Chen Xin, Chen Xin Pharmaceutical Co. Ltd., Jining, China) were applied three times per day. The next day, biometry for both eyes was repeated and the intravitreal injections were performed. These injections either contained amphiregulin antibody (in doses of 5 μg, 10 μg, or 15 μg, respectively; volume: 5 μ) (Catalog Number: AF989; Accession # P31955; R&D Systems, Bio-Techne Co., Minneapolis, Minnesota, USA) or Ringer's solution (volume: 5 μ) (Otsuka, China Otsuka Pharmaceutical Co., Ltd., Tianjin, China). The antibody preparation to amphiregulin was carried out in goats, by immunization with the mouse amphiregulin-precursor sequence, Ser94 à Lys191. According to the supplier's brochure, it neutralized the biological activity of amphiregulin, with the amino acid sequences of mice and guinea pigs amphiregulins being similar. In sandwich immunoassays, < 1% cross-reactivity with recombinant human Amphiregulin was observed. The injections were performed using Hamilton micro needles (Hamilton® Microliter™ syringe, removable needle 701 ASRN, volume 10 μL, needle size 23s–26s ga (cone tip), needle L 43 mm; Sigma-Aldrich, St. Louis, MO, USA) after the periorbital region and the ocular surface had been disinfected by an iodine containing solution and after the periorbital region had been covered with a sterile drape. The injections were performed between the corneal limbus and the equator of the eyes and were aimed towards the posterior pole of the eyes. At the first day after the injection, the same antibiotic eye drops were re-applied three times per day, before the goggles were re-fixed. After an interval of 9 days, the goggles were removed and antibiotic eyes drops were again applied three times per day, before the next day, 9 days after the last injection, biometry and the same injection were repeated. The following day, antibiotic eyes drops were again given three times, before the goggles were re-applied for another period of 9 days. Applying and removal or wearing of the goggles did not lead to an infection or inflammation.
Another group of animals did not undergo lens-induced myopization and received intraocular injections either of amphiregulin antibody (dose of 20 μg) or of amphiregulin (Catalog Number: 989-AR, R&D Systems, Bio-Techne Co., Minnesota, U.S.A.) into the right eyes and intraocular injections of Ringer's solution into the left eyes. The amphiregulin protein preparation had a purity of > 97% and the effective dose ED50 was 5–20 ng/mL as measured in a cell proliferation assay using Balb/3T3 mouse embryonic fibroblast cells. The source was E. coli-derived Ser94-Lys191, and the predicted molecular mass was 11.3 kDa. The applied doses of amphiregulin were 0.25 ng (concentration: 50 ng/mL; volume: 5 μL), 0.50 ng (concentration: 100 ng/mL; volume: 5 μL), and 1.00 ng (concentration: 200 ng/mL; volume: 5 μL), respectively. The injections were started at 2 weeks after baseline and were repeated three times in an interval of 7 days. The animals were sacrificed 7 days after the fourth injection.
To explore the change of the thickness of sclera, a final biometry was carried out and the animals were sacrificed at 9 days after the last injection. The globes were harvested and three eyes of each group were prepared for light microscopical examination with Periodic Acid-Schiff (PAS) staining. For the light microscopical examination, the eyes were fixed in a solution containing 20% formaldehyde, 28.5% alcohol and 20% acetic acid. The globes were dehydrated in 50%, 70%, 80% alcohol, each for 1 h once, and 95% alcohol for 30 min twice, 100% alcohol for 20 min twice, xylene for 20 min twice, and then embedded in paraffin. Slides of a thickness of 8 μm were obtained which were stained by PAS according to the producer's manual (Solarbio Co., Beijing, China). Briefly, sections were treated with 0.5% periodic acid solution for 6 min and rinsed with distilled water for 5 min. In a dark chamber, sections were treated with Schiff solution for 10–20 min. After rinsing with distilled water, sections were counterstained with hematoxylin for 1–2 min and then rinsed with acidic ethanol solution for 2–5 s. After rinsing with distilled water again, dehydrated in alcohol and rinsed in xylene, and then solided with neutral balsam.
2.1.2. Quantitative Real-time PCR
Total RNA was extracted from tissues or cultured cells using Trizol reagent (Invitrogen, 15596026, CA, USA), and 1–2 μg of RNA was used for reverse transcription using a Reverse Transcript kit (Takara, 6210A, Japan). Quantitative real-time PCR (qRT-PCR) analysis was performed using the SYBR Green I Master kit (Roche, 4707516001, Switzerland). Diluted cDNA was used in a 20 μL real-time PCR reaction in duplicate for each gene. Cycle parameters were 95 °C for 5 min hot start and 45 cycles of 95 °C for 5 s, 55 °C for 10 s and 72 °C for 20 s. Blank controls with no cDNA templates were performed to rule out contamination. The specificity of the PCR product was confirmed by melting curve analysis. Primers for endogenous amphiregulin were: forward seq., 5′-ACGGGGAGTGCAAATACCTG-3′, reverse seq., 5′-TTCACCGAAAGGAGACCAGC-3′. Primers for endogenous EGFR were: forward seq., 5′-CACAACTCATGCCCTTTGGC-3′, reverse seq., 5′-TGACTCCGTAGCTCCAGACA-3′. Primers for endogenous Beta-actin were: forward seq., 5′-ACCCCAAGGCCAACCGTGAGAAGATG-3′, reverse seq., 5′-CTCGGCCGTGGTGGTGAAACTGTAGC-3′. The expression levels of all genes were normalized to that of the house keeping gene beta-actin. Relative gene expression levels were calculated by the formula 2−△Ct, where △Ct (Critical threshold) = Ct of genes of interest – Ct of beta-actin. For analysis of retina tissues, fold changes of gene expression levels in test groups relative to corresponding normal control groups were calculated by 2−△Ct method as previously described (Guo et al., 2014).
2.1.3. Light-microscopical Histomorphometry
The slides prepared for light-microscopical examination were digitized and morphometrically analyzed using the planimetric program Motic Med 6.0 (Xiamen Motic Software Engineering Co. Ltd., Xiamen, China).
2.1.4. Immunohistochemistry
The eyes were embedded in cryo glue (SLEE Medical GmbH, Germany), stored at − 80 °C and sectioned vertically at 6 μm. The sections were blocked for 1 h in 5% bovine serum albumin (BSA) containing 0.5% Triton X-100 in PBS at room temperature, and then were incubated with the primary antibodies: rabbit anti-amphiregulin antibody (bs-3847R, Bioss, China), which diluted in the blocking solution overnight at 4 °C. Then the secondary antibodies were used for immunofluorescence for 2 h: goat anti-rabbit IgG H&L 555 (1:400 dilution; ab150078, Abcam, US). The nuclear staining was conducted with 40,6-diamidino-2-phenylindole (DAPI, 1 lg/mL) counterstaining for 20 min. The sections were observed using a LSM 780 laser confocal microscope (Zeiss, Germany) with a 63 oil-immersion objective. In each image, laser light levels and detector gain and offset were adjusted to avoid any saturated levels.
2.2. Statistical Analysis
A commercially available statistical software package (SPSS for Windows, version 22.0, IBM-SPSS, Chicago, IL) was used for the statistical analysis. We first calculated the mean and standard deviations of the main outcome parameters, i.e. the biometric parameters. The normal distribution of the values of the parameters was examined using the Kolmogorov-Smirnov-test. Applying student t-test for paired samples, we then compared the measurements obtained in the study eyes with the measurements made in the contralateral eyes. We used student-t-test for unpaired samples when we compared the measurements obtained in animals of different groups. All P-values were 2-sided and were considered statistically significant when the values were < 0.05.
3. Results
3.1. Changes in Axial Length
In animals without lens-induced myopization and without any intervention, mean axial length increased from 7.95 ± 0.11 mm (mean ± standard deviation) (treated (right) eyes) and from 7.92 ± 0.10 mm (untreated (left) eyes) at baseline to 8.66 ± 0.09 mm (treated eyes) and 8.67 ± 0.09 mm (untreated eyes) at the end of follow-up, without significant differences between treated eyes and untreated eyes, neither in the baseline measurements (P = 0.17) nor in the measurements obtained at the end of follow-up (P = 0.49) (Table 1). The mean increase in axial length was 0.72 ± 0.12 mm (treated eyes) and 0.76 ± 0.13 mm (untreated eyes) with no significant difference (P = 0.13) between treated eyes and untreated eyes.
Table 1.
Sonographic biometric measurements (mean ± standard deviation).
| Parameter | n | Vitreous cavity length (mm) | Anterior chamber depth (mm) | Lens thickness (mm) | Axial length (mm) | ||
|---|---|---|---|---|---|---|---|
| Animals without lens-induced myopization and without any intervention | Right eyes | Baseline | 8 | 3.47 ± 0.08 | 1.16 ± 0.06 | 3.31 ± 0.06 | 7.95 ± 0.11 |
| 1st injection | 8 | 3.56 ± 0.06 | 1.21 ± 0.06 | 3.51 ± 0.06 | 8.28 ± 0.09 | ||
| 2nd injection | 8 | 3.59 ± 0.04 | 1.21 ± 0.10 | 3.65 ± 0.11 | 8.45 ± 0.06 | ||
| 3rd injection | 8 | 3.60 ± 0.06 | 1.29 ± 0.07 | 3.69 ± 0.09 | 8.58 ± 0.09 | ||
| Study end | 8 | 3.66 ± 0.09 | 1.29 ± 0.08 | 3.72 ± 0.11 | 8.66 ± 0.09 | ||
| Left eyes | Baseline | 8 | 3.44 ± 0.07 | 1.16 ± 0.09 | 3.32 ± 0.08 | 7.92 ± 0.10 | |
| 1st injection | 8 | 3.55 ± 0.06 | 1.19 ± 0.07 | 3.54 ± 0.09 | 8.27 ± 0.09 | ||
| 2nd injection | 8 | 3.62 ± 0.07 | 1.21 ± 0.08 | 3.63 ± 0.09 | 8.46 ± 0.06 | ||
| 3rd injection | 8 | 3.63 ± 0.06 | 1.25 ± 0.05 | 3.71 ± 0.09 | 8.59 ± 0.10 | ||
| Study end | 8 | 3.64 ± 0.10 | 1.23 ± 0.09 | 3.80 ± 0.14 | 8.67 ± 0.09 | ||
| Animals wearing glasses on both eyes without any other intervention | Right eyes | Baseline | 5 | 3.50 ± 0.09 | 1.13 ± 0.05 | 3.31 ± 0.08 | 7.94 ± 0.13 |
| 1st injection | 5 | 3.67 ± 0.09 | 1.21 ± 0.08 | 3.55 ± 0.09 | 8.43 ± 0.15 | ||
| 2nd injection | 5 | 3.70 ± 0.08 | 1.19 ± 0.05 | 3.66 ± 0.18 | 8.55 ± 0.11 | ||
| 3rd injection | 5 | 3.77 ± 0.08 | 1.18 ± 0.03 | 3.78 ± 0.14 | 8.73 ± 0.14 | ||
| Study end | 5 | 3.80 ± 0.09 | 1.22 ± 0.06 | 3.85 ± 0.13 | 8.86 ± 0.10 | ||
| Left eyes | Baseline | 5 | 3.49 ± 0.08 | 1.11 ± 0.06 | 3.31 ± 0.22 | 7.90 ± 0.20 | |
| 1st injection | 5 | 3.61 ± 0.06 | 1.23 ± 0.05 | 3.55 ± 0.09 | 8.39 ± 0.12 | ||
| 2nd injection | 5 | 3.68 ± 0.03 | 1.20 ± 0.10 | 3.68 ± 0.14 | 8.57 ± 0.16 | ||
| 3rd injection | 5 | 3.74 ± 0.05 | 1.23 ± 0.03 | 3.74 ± 0.13 | 8.71 ± 0.14 | ||
| Study end | 5 | 3.76 ± 0.05 | 1.26 ± 0.07 | 3.86 ± 0.10 | 8.88 ± 0.08 | ||
| Animals wearing glasses on both eyes plus intraocular injection of 5 μg of amphiregulin antibody into the right eyes and of Ringer's solution into the left eyes | Right eyes | Baseline | 10 | 3.51 ± 0.09 | 1.09 ± 0.06 | 3.29 ± 0.10 | 7.89 ± 0.07 |
| 1st injection | 10 | 3.66 ± 0.15 | 1.14 ± 0.04 | 3.52 ± 0.14 | 8.33 ± 0.13 | ||
| 2nd injection | 10 | 3.74 ± 0.12 | 1.18 ± 0.04 | 3.56 ± 0.10 | 8.48 ± 0.05 | ||
| 3rd injection | 10 | 3.74 ± 0.08 | 1.21 ± 0.04 | 3.67 ± 0.09 | 8.62 ± 0.07 | ||
| Study end | 10 | 3.75 ± 0.03 | 1.21 ± 0.02 | 3.78 ± 0.07 | 8.73 ± 0.05 | ||
| Left eyes | Baseline | 10 | 3.52 ± 0.11 | 1.11 ± 0.04 | 3.27 ± 0.09 | 7.89 ± 0.08 | |
| 1st injection | 10 | 3.62 ± 0.10 | 1.19 ± 0.04 | 3.57 ± 0.06 | 8.38 ± 0.06 | ||
| 2nd injection | 10 | 3.71 ± 0.09 | 1.20 ± 0.03 | 3.57 ± 0.11 | 8.48 ± 0.11 | ||
| 3rd injection | 10 | 3.69 ± 0.06 | 1.26 ± 0.06 | 3.72 ± 0.07 | 8.67 ± 0.06 | ||
| Study end | 10 | 3.74 ± 0.07 | 1.25 ± 0.03 | 3.84 ± 0.09 | 8.82 ± 0.04 | ||
| Animals wearing glasses on both eyes plus intraocular injection of 10 μg of amphiregulin antibody into the right eyes and of Ringer's solution into the left eyes | Right eyes | Baseline | 13 | 3.48 ± 0.06 | 1.15 ± 0.10 | 3.28 ± 0.14 | 7.91 ± 0.14 |
| 1st injection | 13 | 3.62 ± 0.12 | 1.22 ± 0.07 | 3.52 ± 0.10 | 8.38 ± 0.08 | ||
| 2nd injection | 13 | 3.68 ± 0.12 | 1.23 ± 0.05 | 3.59 ± 0.10 | 8.50 ± 0.08 | ||
| 3rd injection | 13 | 3.71 ± 0.15 | 1.22 ± 0.06 | 3.72 ± 0.13 | 8.64 ± 0.10 | ||
| Study end | 13 | 3.69 ± 0.12 | 1.22 ± 0.08 | 3.80 ± 0.11 | 8.70 ± 0.12 | ||
| Left eyes | Baseline | 13 | 3.49 ± 0.07 | 1.14 ± 0.09 | 3.22 ± 0.14 | 7.86 ± 0.14 | |
| 1st injection | 13 | 3.65 ± 0.07 | 1.21 ± 0.07 | 3.52 ± 0.11 | 8.39 ± 0.10 | ||
| 2nd injection | 13 | 3.67 ± 0.10 | 1.21 ± 0.07 | 3.70 ± 0.13 | 8.57 ± 0.14 | ||
| 3rd injection | 13 | 3.70 ± 0.10 | 1.26 ± 0.06 | 3.79 ± 0.09 | 8.76 ± 0.11 | ||
| Study end | 13 | 3.78 ± 0.14 | 1.27 ± 0.07 | 3.85 ± 0.09 | 8.89 ± 0.16 | ||
| Animals wearing glasses on both eyes plus intraocular injection of 15 μg of amphiregulin antibody into the right eyes and of Ringer's solution into the left eyes | Right eyes | Baseline | 10 | 3.47 ± 0.12 | 1.27 ± 0.06 | 3.22 ± 0.18 | 7.96 ± 0.23 |
| 1st injection | 10 | 3.59 ± 0.12 | 1.27 ± 0.04 | 3.50 ± 0.07 | 8.36 ± 0.08 | ||
| 2nd injection | 10 | 3.64 ± 0.10 | 1.30 ± 0.03 | 3.62 ± 0.07 | 8.55 ± 0.09 | ||
| 3rd injection | 10 | 3.61 ± 0.12 | 1.27 ± 0.06 | 3.71 ± 0.09 | 8.59 ± 0.10 | ||
| Study end | 10 | 3.63 ± 0.13 | 1.28 ± 0.06 | 3.65 ± 0.12 | 8.56 ± 0.10 | ||
| Left eyes | Baseline | 10 | 3.45 ± 0.10 | 1.26 ± 0.07 | 3.23 ± 0.17 | 7.93 ± 0.20 | |
| 1st injection | 10 | 3.58 ± 0.09 | 1.27 ± 0.04 | 3.49 ± 0.10 | 8.34 ± 0.09 | ||
| 2nd injection | 10 | 3.62 ± 0.10 | 1.28 ± 0.02 | 3.64 ± 0.07 | 8.54 ± 0.09 | ||
| 3rd injection | 10 | 3.77 ± 0.18 | 1.31 ± 0.03 | 3.70 ± 0.13 | 8.78 ± 0.10 | ||
| Study end | 10 | 3.88 ± 0.28 | 1.32 ± 0.03 | 3.74 ± 0.09 | 8.94 ± 0.24 | ||
| Animals wearing glasses on the right eyes plus intraocular injection of Ringer's solution into the right eyes | Right eyes | Baseline | 5 | 3.42 ± 0.12 | 1.18 ± 0.07 | 3.37 ± 0.08 | 7.96 ± 0.14 |
| 1st injection | 5 | 3.62 ± 0.14 | 1.32 ± 0.07 | 3.46 ± 0.24 | 8.40 ± 0.28 | ||
| 2nd injection | 5 | 3.57 ± 0.06 | 1.27 ± 0.02 | 3.61 ± 0.09 | 8.45 ± 0.13 | ||
| 3rd injection | 5 | 3.54 ± 0.10 | 1.24 ± 0.06 | 3.79 ± 0.18 | 8.58 ± 0.18 | ||
| Study end | 5 | 3.68 ± 0.13 | 1.24 ± 0.07 | 3.78 ± 0.14 | 8.70 ± 0.18 | ||
| Left eyes | Baseline | 5 | 3.37 ± 0.08 | 1.16 ± 0.10 | 3.38 ± 0.08 | 7.91 ± 0.08 | |
| 1st injection | 5 | 3.51 ± 0.10 | 1.30 ± 0.05 | 3.47 ± 0.15 | 8.27 ± 0.17 | ||
| 2nd injection | 5 | 3.53 ± 0.08 | 1.31 ± 0.03 | 3.52 ± 0.17 | 8.37 ± 0.17 | ||
| 3rd injection | 5 | 3.58 ± 0.06 | 1.29 ± 0.07 | 3.62 ± 0.19 | 8.48 ± 0.15 | ||
| Study end | 5 | 3.57 ± 0.11 | 1.26 ± 0.07 | 3.75 ± 0.10 | 8.58 ± 0.09 | ||
| Animals wearing glasses on the right eyes plus intraocular injection of 5 μg of amphiregulin antibody into right eyes | Right eyes | Baseline | 5 | 3.48 ± 0.11 | 1.24 ± 0.09 | 3.37 ± 0.19 | 8.09 ± 0.09 |
| 1st injection | 5 | 3.72 ± 0.13 | 1.21 ± 0.06 | 3.68 ± 0.03 | 8.61 ± 0.16 | ||
| 2nd injection | 5 | 3.73 ± 0.13 | 1.27 ± 0.06 | 3.68 ± 0.03 | 8.67 ± 0.11 | ||
| 3rd injection | 5 | 3.66 ± 0.14 | 1.30 ± 0.08 | 3.76 ± 0.15 | 8.72 ± 0.09 | ||
| Study end | 5 | 3.70 ± 0.11 | 1.29 ± 0.02 | 3.86 ± 0.06 | 8.83 ± 0.12 | ||
| Left eyes | Baseline | 5 | 3.48 ± 0.07 | 1.23 ± 0.10 | 3.37 ± 0.19 | 8.08 ± 0.12 | |
| 1st injection | 5 | 3.52 ± 0.06 | 1.23 ± 0.05 | 3.76 ± 0.12 | 8.51 ± 0.10 | ||
| 2nd injection | 5 | 3.64 ± 0.12 | 1.28 ± 0.06 | 3.72 ± 0.07 | 8.64 ± 0.13 | ||
| 3rd injection | 5 | 3.58 ± 0.21 | 1.26 ± 0.08 | 3.86 ± 0.15 | 8.70 ± 0.17 | ||
| Study end | 5 | 3.57 ± 0.12 | 1.24 ± 0.12 | 3.92 ± 0.21 | 8.73 ± 0.08 | ||
| Animals wearing glasses on the right eyes plus intraocular injection of 10 μg of amphiregulin antibody into the right eyes | Right eyes | Baseline | 5 | 3.43 ± 0.11 | 1.24 ± 0.05 | 3.28 ± 0.10 | 7.96 ± 0.13 |
| 1st injection | 5 | 3.57 ± 0.12 | 1.30 ± 0.05 | 3.53 ± 0.21 | 8.40 ± 0.22 | ||
| 2nd injection | 5 | 3.57 ± 0.05 | 1.31 ± 0.04 | 3.55 ± 0.13 | 8.44 ± 0.11 | ||
| 3rd injection | 5 | 3.61 ± 0.06 | 1.15 ± 0.14 | 3.79 ± 0.08 | 8.56 ± 0.10 | ||
| Study end | 5 | 3.47 ± 0.12 | 1.23 ± 0.15 | 3.81 ± 0.30 | 8.51 ± 0.10 | ||
| Left eyes | Baseline | 5 | 3.39 ± 0.10 | 1.22 ± 0.04 | 3.31 ± 0.16 | 7.92 ± 0.10 | |
| 1st injection | 5 | 3.48 ± 0.03 | 1.33 ± 0.03 | 3.45 ± 0.18 | 8.26 ± 0.19 | ||
| 2nd injection | 5 | 3.55 ± 0.05 | 1.28 ± 0.02 | 3.51 ± 0.16 | 8.34 ± 0.15 | ||
| 3rd injection | 5 | 3.55 ± 0.13 | 1.23 ± 0.05 | 3.64 ± 0.13 | 8.42 ± 0.13 | ||
| Study end | 5 | 3.55 ± 0.09 | 1.28 ± 0.05 | 3.75 ± 0.19 | 8.57 ± 0.14 | ||
| Animals wearing glasses on the right eyes plus intraocular injection of 15 μg of amphiregulin antibody into the right eyes | Right eyes | Baseline | 5 | 3.43 ± 0.07 | 1.23 ± 0.06 | 3.32 ± 0.13 | 7.98 ± 0.07 |
| 1st injection | 5 | 3.62 ± 0.11 | 1.28 ± 0.06 | 3.56 ± 0.12 | 8.46 ± 0.22 | ||
| 2nd injection | 5 | 3.66 ± 0.09 | 1.30 ± 0.06 | 3.61 ± 0.11 | 8.57 ± 0.16 | ||
| 3rd injection | 5 | 3.67 ± 0.13 | 1.27 ± 0.05 | 3.67 ± 0.11 | 8.61 ± 0.12 | ||
| Study end | 5 | 3.38 ± 0.35 | 1.23 ± 0.13 | 3.88 ± 0.23 | 8.49 ± 0.22 | ||
| Left eyes | Baseline | 5 | 3.42 ± 0.06 | 1.21 ± 0.04 | 3.39 ± 0.13 | 8.02 ± 0.06 | |
| 1st injection | 5 | 3.50 ± 0.13 | 1.26 ± 0.09 | 3.56 ± 0.11 | 8.32 ± 0.14 | ||
| 2nd injection | 5 | 3.54 ± 0.09 | 1.29 ± 0.09 | 3.59 ± 0.13 | 8.42 ± 0.09 | ||
| 3rd injection | 5 | 3.61 ± 0.08 | 1.32 ± 0.11 | 3.60 ± 0.20 | 8.54 ± 0.03 | ||
| Study end | 5 | 3.62 ± 0.05 | 1.32 ± 0.03 | 3.70 ± 0.12 | 8.63 ± 0.08 | ||
| Primarily myopic animals (at baseline) without additional lens-induced myopization, with intraocular injection of amphiregulin antibody (20 μg) into the right eyes and injection of Ringer's solution into the left eye | Right eyes | Baseline | 6 | 3.66 ± 0.13 | 1.11 ± 0.05 | 3.33 ± 0.08 | 8.09 ± 0.09 |
| 1st injection | 6 | 3.72 ± 0.14 | 1.13 ± 0.06 | 3.46 ± 0.04 | 8.30 ± 0.14 | ||
| 2nd injection | 6 | 3.72 ± 0.19 | 1.15 ± 0.04 | 3.59 ± 0.07 | 8.46 ± 0.18 | ||
| 3rd injection | 6 | 3.69 ± 0.16 | 1.13 ± 0.08 | 3.70 ± 0.15 | 8.51 ± 0.20 | ||
| 4th injection | 6 | 3.79 ± 0.16 | 1.23 ± 0.06 | 3.82 ± 0.10 | 8.84 ± 0.20 | ||
| Left eyes | Baseline | 6 | 3.59 ± 0.15 | 1.16 ± 0.06 | 3.32 ± 0.08 | 8.06 ± 0.13 | |
| 1st injection | 6 | 3.66 ± 0.10 | 1.17 ± 0.05 | 3.51 ± 0.08 | 8.34 ± 0.14 | ||
| 2nd injection | 6 | 3.70 ± 0.14 | 1.20 ± 0.07 | 3.63 ± 0.04 | 8.52 ± 0.14 | ||
| 3rd injection | 6 | 3.69 ± 0.14 | 1.20 ± 0.06 | 3.74 ± 0.05 | 8.63 ± 0.15 | ||
| 4th injection | 6 | 3.77 ± 0.11 | 1.24 ± 0.06 | 3.88 ± 0.13 | 8.89 ± 0.10 | ||
| Animals without lens-induced myopization, with intraocular injection of amphiregulin (0.25 ng) into the right eyes and intraocular injection of Ringer's solution into the left eyes | Right eyes | Baseline | 6 | 3.58 ± 0.09 | 1.14 ± 0.04 | 3.24 ± 0.12 | 7.96 ± 0.11 |
| 1st injection | 6 | 3.54 ± 0.14 | 1.19 ± 0.09 | 3.47 ± 0.08 | 8.20 ± 0.08 | ||
| 2nd injection | 6 | 3.59 ± 0.11 | 1.24 ± 0.10 | 3.52 ± 0.16 | 8.35 ± 0.14 | ||
| 3rd injection | 6 | 3.62 ± 0.04 | 1.23 ± 0.06 | 3.62 ± 0.09 | 8.48 ± 0.08 | ||
| 4th injection | 6 | 3.64 ± 0.08 | 1.21 ± 0.03 | 3.80 ± 0.11 | 8.65 ± 0.13 | ||
| Left eyes | Baseline | 6 | 3.47 ± 0.08 | 1.17 ± 0.03 | 3.28 ± 0.12 | 7.93 ± 0–09 | |
| 1st injection | 6 | 3.55 ± 0.11 | 1.21 ± 0.07 | 3.41 ± 0.12 | 8.17 ± 0.08 | ||
| 2nd injection | 6 | 3.60 ± 0.11 | 1.18 ± 0.04 | 3.60 ± 0.06 | 8.37 ± 0.08 | ||
| 3rd injection | 6 | 3.62 ± 0.11 | 1.18 ± 0.05 | 3.71 ± 0.14 | 8.50 ± 0.12 | ||
| 4th injection | 6 | 3.72 ± 0.10 | 1.20 ± 0.06 | 3.77 ± 0.12 | 8.69 ± 0.13 | ||
| Animals without lens-induced myopization, with intraocular injection of amphiregulin (0.50 ng) into the right eyes and intraocular injection of Ringer's solution into the left eyes | Right eyes | Baseline | 6 | 3.49 ± 0.14 | 1.18 ± 0.08 | 3.31 ± 0.06 | 7.98 ± 0.12 |
| 1st injection | 6 | 3.64 ± 0.12 | 1.20 ± 0.05 | 3.46 ± 0.06 | 8.30 ± 0.12 | ||
| 2nd injection | 6 | 3.64 ± 0.10 | 1.19 ± 0.03 | 3.54 ± 0.08 | 8.36 ± 0.12 | ||
| 3rd injection | 6 | 3.61 ± 0.09 | 1.14 ± 0.04 | 3.67 ± 0.06 | 8.42 ± 0.09 | ||
| 4th injection | 6 | 3.71 ± 0.07 | 1.25 ± 0.05 | 3.71 ± 0.08 | 8.67 ± 0.09 | ||
| Left eyes | Baseline | 6 | 3.54 ± 0.16 | 1.19 ± 0.08 | 3.27 ± 0.12 | 8.00 ± 0.17 | |
| 1st injection | 6 | 3.66 ± 0.08 | 1.22 ± 0.11 | 3.40 ± 0.14 | 8.27 ± 0.08 | ||
| 2nd injection | 6 | 3.69 ± 0.04 | 1.20 ± 0.03 | 3.55 ± 0.05 | 8.44 ± 0.07 | ||
| 3rd injection | 6 | 3.66 ± 0.04 | 1.13 ± 0.02 | 3.72 ± 0.02 | 8.50 ± 0.06 | ||
| 4th injection | 6 | 3.73 ± 0.04 | 1.18 ± 0.02 | 3.75 ± 0.07 | 8.66 ± 0.09 | ||
| Animals without lens-induced myopization, with intraocular injection of amphiregulin (1 ng) into the right eyes and intraocular injection of Ringer's solution into the left eyes | Right eyes | Baseline | 6 | 3.49 ± 0.07 | 1.15 ± 0.02 | 3.33 ± 0.06 | 7.97 ± 0.09 |
| 1st injection | 6 | 3.63 ± 0.14 | 1.17 ± 0.07 | 3.48 ± 0.09 | 8.27 ± 0.13 | ||
| 2nd injection | 6 | 3.64 ± 0.14 | 1.24 ± 0.04 | 3.59 ± 0.05 | 8.46 ± 0.13 | ||
| 3rd injection | 6 | 3.69 ± 0.08 | 1.20 ± 0.01 | 3.65 ± 0.07 | 8.54 ± 0.08 | ||
| 4th injection | 6 | 3.74 ± 0.05 | 1.25 ± 0.07 | 3.80 ± 0.05 | 8.79 ± 0.05 | ||
| Left eyes | Baseline | 6 | 3.47 ± 0.06 | 1.17 ± 0.06 | 3.30 ± 0.06 | 7.94 ± 0.09 | |
| 1st injection | 6 | 3.60 ± 0.09 | 1.15 ± 0.09 | 3.47 ± 0.18 | 8.22 ± 0.12 | ||
| 2nd injection | 6 | 3.61 ± 0.10 | 1.23 ± 0.14 | 3.50 ± 0.17 | 8.33 ± 0.15 | ||
| 3rd injection | 6 | 3.67 ± 0.05 | 1.22 ± 0.04 | 3.60 ± 0.08 | 8.49 ± 0.11 | ||
| 4th injection | 6 | 3.75 ± 0.10 | 1.23 ± 0.03 | 3.75 ± 0.10 | 8.73 ± 0.15 |
In animals with bilateral lens-induced myopization without any other intervention, the mean increase in axial length was 0.92 ± 0.06 mm (treated eyes) and 0.98 ± 0.13 mm (untreated eyes) with no significant difference (P = 0.36) between treated eyes and untreated eyes. The increase in axial length from baseline to the end of follow-up was significantly larger in the group with lens-induced myopization than in the group with physiological growth of the eyes (treated eyes: P = 0.002; untreated eyes: P = 0.02).
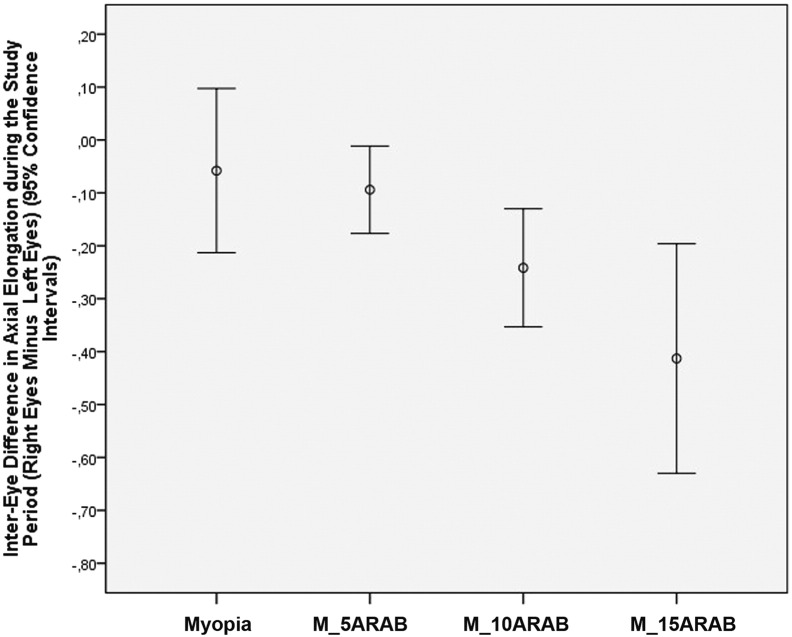
In animals with bilateral lens-induced myopization and intraocular injection of amphiregulin antibody in doses of 5 μg, 10 μg and 15 μg into the right (treated) eyes and intraocular injection of Ringer's solution into the left (untreated) eyes, the increase in axial length was significantly lower in the treated eyes than in the untreated eyes (0.84 ± 0.08 mm versus 0.94 ± 0.08 mm; P = 0.03; 0.79 ± 0.13 mm versus 1.03 ± 0.20 mm; P < 0.001; and 0.59 ± 0.22 mm versus 1.01 ± 0.33 mm; P = 0.002, respectively). The mean difference between treated eyes and untreated eyes in axial elongation at the end of the study was − 0.09 ± 0.12 mm (amphiregulin antibody dose 5 μg), − 0.24 ± 0.18 mm (dose: 10 μg) and − 0.41 ± 0.30 mm (dose: 15 μg) (Fig. 1), and it increased significantly (P < 0.001) with higher dose of amphiregulin antibody (Fig. 2).
Fig. 1.
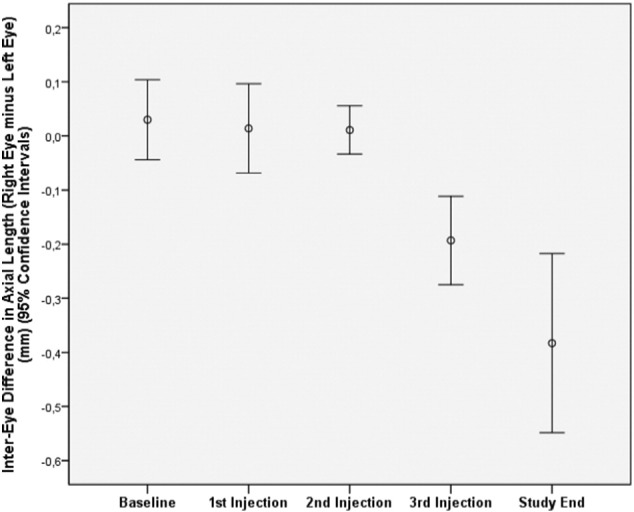
Changes of interocular difference in axial length (right eyes minus left eyes) after repeated intravitreal injections of amphiregulin antibody (15 μg) into right eyes and intraocular injection of Ringer's solution into left eyes in guinea pigs with bilateral lens-induced myopization at baseline, at 2 weeks after baseline (first injection), 9 days later at the second injection, 9 days later at the third injection, and 9 days later at study end.
Fig. 2.
Changes of interocular difference in axial length (right eyes minus left eyes) in guinea pigs with bilateral lens-induced myopization without any further intervention (Myopia) and in guinea pigs with bilateral lens-induced myopization and repeated intravitreal injections of amphiregulin antibody in doses of 5 μg (M_5ARAB), 10 μg (M_10ARAB) and 15 μg (M_15ARAB), respectively, into the right eyes and intraocular injection of Ringer's solution into left eyes.
In animals with unilateral lens-induced myopization of the treated eyes, intraocular injection of Ringer's solution or of amphiregulin antibody in doses of 5 μg, 10 μg or 15 μg into the treated eyes, and with intraocular injection of Ringer's solution into the untreated eyes, the inter-eye difference (treated eye minus untreated eye) in axial elongation during the study period decreased significantly (P = 0.01) with increasing dose of amphiregulin antibody, starting from 0.12 ± 0.14 mm for the animals with intraocular Ringer's solution injection (into the treated eyes) to 0.11 ± 0.05 mm (amphiregulin antibody dose 5 μg), − 0.06 ± 0.12 mm (amphiregulin antibody dose 10 μg), and − 0.15 ± 0.25 mm (amphiregulin antibody dose 15 μg).
In animals being primarily myopic at baseline of the study, without lens-induced myopization and with unilateral intraocular injection of amphiregulin antibody in a dose of 20 μg into the treated eyes and injection of Ringer's solution into the untreated eyes, axial elongation was significantly (P = 0.03) smaller in the treated eyes as compared with untreated eyes (0.41 ± 0.23 mm versus 0.57 ± 0.16 mm) (Fig. 3).
Fig. 3.
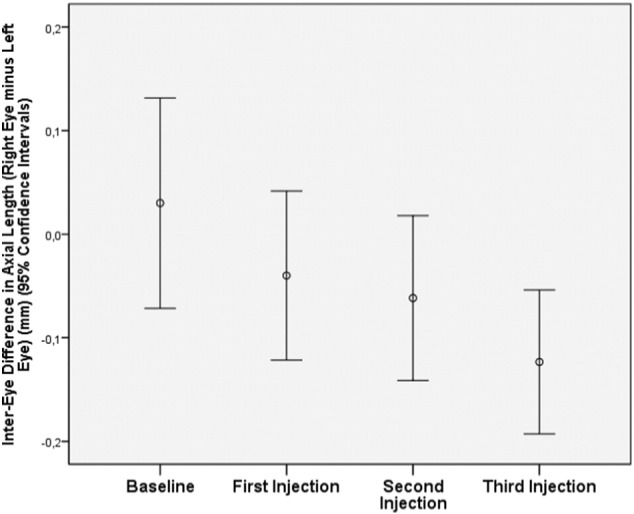
Changes of interocular difference in axial length (right eyes minus left eyes) in guinea pigs with primary myopia at baseline, without lens-induced myopization and with unilateral repeated intravitreal injections of amphiregulin antibody in a dose of 20 μg into right eyes and intraocular injection of Ringer's solution into left eyes, at baseline, at 2 weeks after baseline (first injection), 9 days later at the second injection, and 9 days later at the third injection.
In animals without lens-induced myopization and with intraocular injections of amphiregulin in doses of 0.25 ng, 0.50 ng and 1.00 ng, respectively, into the treated eyes and intraocular injections of Ringer's solution into the untreated eyes, inter-eye differences in axial elongation did not differ significantly (P = 0.11, P = 0.62, P = 0.56, respectively).
3.2. Light-microscopical Histomorphometry
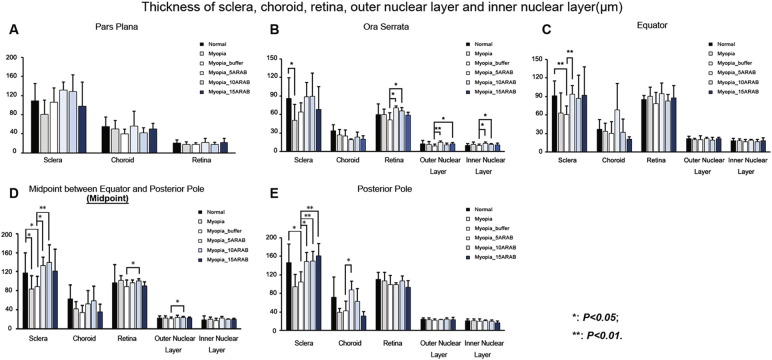
Histomorphometry revealed that the thickness of sclera, as measured at the posterior pole and at the midpoint between the equator and the posterior pole, was significant thinner in the eyes with lens-induced myopia without intravitreal amphiregulin antibody injection than in the eyes without intervention (P < 0.05, P < 0.05, respectively) (Fig. 4). Also, in the eyes with lens-induced myopia and with intravitreal amphiregulin antibody injection (5, 10, 15 μg) as compared to the eyes with lens-induced myopia without injection, the thickness of sclera was significantly thicker, as measured at the midpoint between the equator and the posterior pole (P < 0.05, P < 0.05, P < 0.01, respectively) and as measured at the posterior pole (P < 0.05, P < 0.01, P < 0.01, respectively). The light microscopical examination of histological slides of the eyes included into the study did not show any accumulation of inflammatory cells or any sign of edema in any part or tissue of the eyes, neither in the eyes with amphiregulin antibody injections or in the eyes with amphiregulin injection or in the eyes with intraocular injection of Ringer's solution.
Fig. 4.
Thickness of sclera, choroid, retina as a whole, outer nuclear retinal layer and inner nuclear retinal layer in young guinea pigs without intervention (“Normal”), with lens-induced myopization (“Myopia”), with lens-induced myopization and intraocular injection of Ringer's solution (“Myopia_buffer”), and with lens-induced myopization combined with intraocular injections of amphiregulin antibody in doses of 5 μg (“Myopia_5ARAB”), 10 μg (“Myopia_10ARAB”), and 15 μg (“Myopia_15ARAB”), respectively, in the pars plana region (A), at the ora serrata (B), at the equator (C), in the region of midpoint between equator and posterior pole (D), and at the posterior pole (E).
3.3. Expression of Amphiregulin
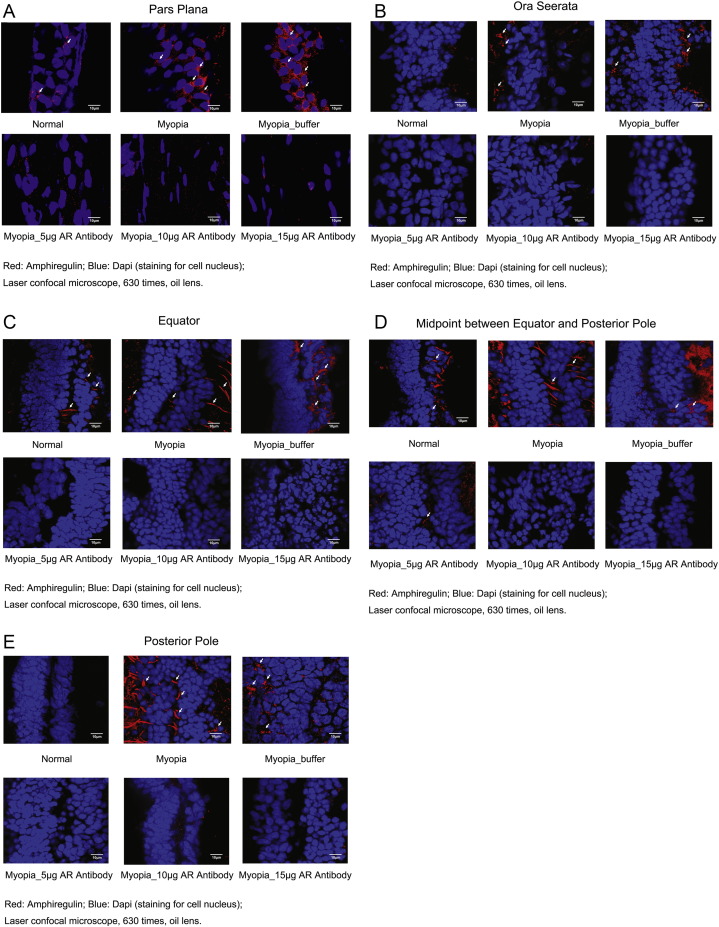
Immunohistochemistry revealed staining for amphiregulin mostly in the level of the retinal pigment epithelium and ciliary pigment epithelium in the eyes with lens-induced myopization without, and with, intraocular injection of Ringer's solution, while in the eyes with lens-induced myopization and with intraocular injection of amphiregulin antibody, staining of amphiregulin could not, or only in traces, be detected (Fig. 5).
Fig. 5.
Immunohistochemical staining of amphiregulin (red) in the level of the retinal pigment epithelium and ciliary pigment epithelium in young guinea pigs without intervention (“Normal”), with lens-induced myopization (“Myopia”), with lens-induced myopization and intraocular injection of Ringer's solution (“Myopia_buffer”), and with lens-induced myopization combined with intraocular injections of amphiregulin antibody in doses of 5 μg (“Myopia_5 μg AR Antibody”), 10 μg (“Myopia_10 μg AR Antibody”), and 15 μg (“Myopia_15 μg AR Antibody”), respectively, in the pars plana region (a), at the ora serrata (b), at the equator (c), in the region between equator and posterior pole (d), and at the posterior pole (e) (Confocal microscope (Zeiss, LSM780, Germany)). White arrows point at amphiregulin in the retina.
3.4. Expression of Endogenous Amphiregulin and EGFR in the Retina Tissues
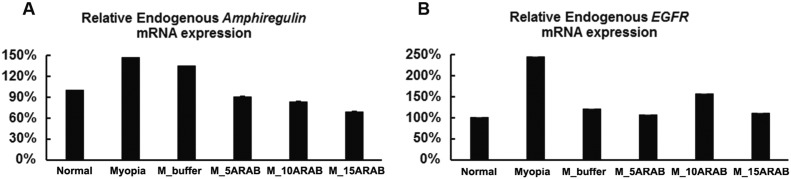
The mRNA expression of endogenous amphiregulin was significantly the highest in the eyes with lens-induced myopization without, and with, intraocular injection of Ringer's solution, followed by eyes without any intervention, and finally eyes with lens-induced myopization with intraocular injection amphiregulin antibody. The mRNA expression of endogenous amphiregulin decreased with increasing dose of amphiregulin antibody applied (Fig. 6). The expression of endogenous EGFR was the highest in the eyes with lens-induced myopization without any intraocular injection, while the other groups did not differ markedly (Fig. 6).
Fig. 6.
Effect of amphiregulin antibody on the mRNA expression of endogenous amphiregulin and epithelial growth factor receptor (EGFR) in young guinea pigs without intervention (“Normal”), with lens-induced myopization (“Myopia”), with lens-induced myopization and intraocular injection of Ringer's solution (“M_buffer”), and with lens-induced myopization combined with intraocular injections of amphiregulin antibody in doses of 5 μg (“M_5ARAB”), 10 μg (“M_10ARAB”), and 15 μg (“M_15ARAB”), respectively; (A) Effect of amphiregulin antibody on the mRNA expression of endogenous amphiregulin (n = 4 per group). (B) Effect of amphiregulin antibody on the mRNA expression of endogenous EGFR (n = 4 per group). The values are represented as mean ± SE.
4. Discussion
Our data are consistent with a role for amphiregulin in promoting myopia development in form-deprived guinea pig eyes, although amphiregulin itself did not promote myopization in eyes that were not form-deprived. The findings showed that in young guinea pigs with lens-induced myopization, the repeated intraocular application of amphiregulin antibody was associated with a dose dependent reduction in axial elongation (Fig. 1, Fig. 2). In a similar manner, animals with unilateral lens-induced myopization showed a significant dose dependent reduction in axial elongation after repeated intraocular injections of amphiregulin antibody. In eyes primarily myopic at baseline of the study and without additional lens-induced myopization, intraocular application of amphiregulin antibody in a dose of 20 μg was associated with a reduction of the physiological axial growth of the eyes. The intraocular application of amphiregulin in doses of 0.25 ng, 0.50 ng, and 1.00 ng, resp., was not correlated with a significant change in axial length in the study eyes as compared to the contralateral control eyes. One may infer that the inactivation of amphiregulin by the repeated intraocular application of its antibody resulted in a dose dependent reduction of lens-induced axial elongation, and to a minor degree, to a significant and dose dependent reduction in the physiological sagittal growth of the eyes. The intraocular injection of amphiregulin itself in a dose of up to 1 ng did not result in a significantly more pronounced axial elongation.
The results of our study cannot directly be compared with findings obtained in other investigations since our study was the first one to use intraocularly applied amphiregulin (or any other member of the epithelial growth factor family) to examine its influence on the physiological eye growth and on an externally induced additional myopization. In previous studies, other molecules had been used to study associations with axial elongation. In a study by Mao and colleagues, guinea pigs of an age of 4 weeks underwent form-deprived axial elongation and myopization (Mao et al., 2010). Repeated intraperitoneal injections of levodopa inhibited the axial elongation and significantly (P < 0.001) reduced the myopic shift from − 3.62 ± 0.98 diopters to − 1.50 ± 0.38 diopters. In eyes which were not occluded and did not undergo externally induced myopization, the application of levodopa did not influence axial length and refraction of the eyes. It remained unclear by which mechanism intraocularly applied levodopa led to a reduction in externally induced myopization. The study by Mao and our investigation differed in the substance examined (levodopa versus amphiregulin) and in the effect of the intraocularly injected substance on the natural growth of the globes. While in Mao's study, eyes without externally induced myopization did not show an effect by levodopa, our investigation demonstrated a reduction in the physiological growth of the eyes if a dose of 20 μg of amphiregulin antibody was applied (Fig. 3). In an investigation by Gao and associates, the right eyes of seven-days-old rabbits were sutured to induce a deprivation associated myopization (Gao et al., 2006). In animals in which the deprived eyes received four intravitreal injections of 20 μg of dopamine every 5 days, the myopic shift (− 0.06 ± 0.37 diopters versus − 2.70 ± 0.87 diopters) and the axial elongation (0.3 ± 0.2 mm versus 0.9 ± 0.3 mm) were significantly reduced at the end of the follow-up of 8 weeks. While Gao's study and our investigation both showed an effect of the substance injected, the amount of change in axial elongation cannot be compared between both studies since the studies included different species. In a study by Yan and coworkers, the effects of daily intraperitoneal injections versus a continuous subcutaneous infusion of apomorphine as a nonspecific dopamine agonist on axial elongation and refraction were examined in normal postnatal mice and mice with form-deprivation myopia (Yan et al., 2015). The procedures took place during postnatal days 28 to 56. The authors observed that in mice without externally induced form-deprivation myopia the application of apomorphine did not affect the normal postnatal development of axial length and refraction. In the mice with form-derived myopia however, the daily injection attenuated the ocular elongation in the study eyes as compared to the contralateral control eyes. The continuous subcutaneous infusion of apomorphine did not significantly affect axial length. The differences between Yan's study and our investigation are the difference in the substances used, the type of application, and that in our study the application of the substance decreased the axial elongation also in the animals without externally induced myopization. Lan et al. found that retinal dopamine release was severely reduced during the development of deprivation-associated myopia (Lan et al., 2016). The authors also found that illuminance of 15,000 lx partially rescued the drop in retinal dopamine release. This finding was in agreement with the notion that dopamine is involved in the light-induced inhibition of myopia. In a study by Mao and colleagues on deprivation-induced myopia in guinea pigs, the intraperitoneal injection of citicoline reduced the amount of myopia from − 3.25 ± 0.77 diopters to − 0.62 ± 0.47 diopters; (P < 0.001) and partially raised retinal dopamine levels in the form-deprived eyes (Mao et al., 2016). In contrast to some of the studies mentioned, Wu and colleagues measured whether and how retinal dopamine levels were changed in a C57BL/6 mouse model of experimental myopia (Wu et al., 2015). Interestingly, no significant changes in retinal dopamine, DOPAC (3,4-dihydroxyphenylacetic acid as primary metabolite of dopamine), DAT (dopamine transporter), and vitreal DOPAC levels were detected in deprived eyes, either in the daytime or at night. Furthermore, neither the number of dopaminergic amacrine cells, the area size occupied by the processes of these cells nor tyrosine hydroxylase expression in the retina was changed in the eyes with externally induced myopization.
The observation made in our study that the intraocular blockade of amphiregulin as a member of the epithelial growth factor family was associated with a reduction of axial elongation fits with the notion that Bruch's membrane, in addition to the sclera, may play a role in the process of emmetropization and myopization (Jonas et al., 2017b). The results of our study indirectly agree with the findings obtained in a recent genetic study in which three loci including AREG were significantly associated with education in Asian populations (Fan et al., 2016). Higher level of education is associated with the higher degree of axial myopia, supporting the notion of gene-environment interactions contributing to of myopia (Morgan et al., 2012, Jones et al., 2007). As also pointed out above, other studies, performed mainly in chicken, showed that substances other than amphiregulin may also be involved in the process of axial elongation. These studies may help to understand why signal-blockers such as dopamine antagonists or amphiregulin antibody, can influence the process of axial elongation. To cite an example, McCarthy and colleagues reported that intraocularly applied dopamine agonists restored a protective effect of light against form-deprivation induced myopia in chick, while this effect could be blocked by intraocularly injected dopamine antagonists (McCarthy et al., 2007). The findings obtained in our study may also be discussed parallel to the observations made in previous investigations on the potential association between transforming growth factor (TGF) and experimental myopia (Rohrer and Stell, 1994, Seko et al., 1995, Jobling et al., 2009). Rohrer and Stell reported that basic fibroblast growth factor (FGF) reduced the amount of form-derived myopia in a dose-dependent manner, with a 50% effective dose of 1.67 ng for intravitreal application (Rohrer & Stell, 1994). A similar effect could be obtained by acidic FBF, however with a potency approximately 160 times less than that of basic FGF. Interestingly, intraocular application of TGF-beta 1 did not result in induction of myopia in non-occluded eyes or an increase in myopia in occluded eyes. It was, however, a potent inhibitor of the basic FGF effect on reduction of myopia, if TGF was administered together with basic FGF in the subconjunctival space. The authors concluded that basic FGF and TGF influenced eye growth in a stop and go manner. Seko and colleagues found that the concentration of basic FGF were significantly lower in the sclera in the posterior region of eyes with form-derivation induced myopia than in control eyes, while the concentration in the retina-retinal pigment epithelium-choroid complex did not differ (Seko et al., 1995). In contrast, the concentration of TGF-beta 2 was significantly higher in the myopic eyes in both the retina-retinal pigment epithelium-choroid complex and in the sclera (Jobling et al., 2009).
The observation that the eyes with intraocular injections of amphiregulin antibody successfully suppressed the mRNA and protein expression of amphiregulin in the retina of guinea pigs, showed that the change of refractive error and axial length of experimental animals was significantly associated with the expression of amphireguin. It addressed a new question-why would an injection of exogenous amphiregulin antibody affect the mRNA expression of endogenous amphiregulin, though. A potential hypothesis is that the production of amphiregulin is a positive feedback process, and when amphiregulin interacted with EGFR or other receptors, it would active some intermediate products to induce the production of amphiregulin; and when the function of amphiregulin was blocked by the antibody, this positive feedback effect disappeared, and therefore the mRNA expression of endogenous amphiregulin reduced. In addition, endogenous EGFR mRNA expression was not found to be affected by the injection of exogenous amphiregulin antibody, while it might be affected by the injection of Ringer's solution or the behavior of injection itself, though. Also, the observation that the eyes with intraocular injections, in particular those which underwent intravitreal injection of amphiregulin antibody or of amphiregulin, did not show signs of inflammation may be of importance in that the observed changes were not due to secondary inflammation. These findings may also be of interest if at a later stage, amphiregulin antibody may be potentially be used for the prevention of progression of axial myopia.
Limitations of our study may be mentioned. First, as for any experimental study, caution has to be applied when data measured and conclusions drawn are transferred on humans. Second, the question is unresolved, whether the actions of amphiregulin observed in this study were specific to amphiregulin, since the preparation of the antibody to amphiregulin occurred in goats by immunization with the mouse amphiregulin-precursor sequence (i.e. Ser94 à Lys191). The manufacture stated however that amphiregulin antibody neutralized the biological activity of amphiregulin and that the amino acid sequences of mouse amphiregulin and guinea pig amphiregulin were similar. Future studies using guinea pigs may apply an amphiregulin antibody produced in guinea pigs. To strengthen the conclusion that the observed results were due to amphiregulin itself, future studies may therefore be performed applying an injection of non-immune goat IgG, at the same doses as those of anti-amphiregulin IgG, into eyes with lens-induced myopia to control for nonspecific effects of intravitreal goat IgG. Other studies may examine a potential preabsorption of the antibody with the amphiregulin peptide at concentrations in the 10− 3–10− 6 M range to determine whether this may abolish the immunolabeling of the amphiregulin-like material in ciliary epithelium, retina and RPE. It may control for the specificity of the antibody binding to endogenous amphiregulin. Third, when assessing the tissue dimensions in the histological sections, one has to take into account that dehydration, formaline treatment and paraffin embedding might have altered the volume of the cells and extracellular matrix during the preparation of the histologic sections. Frozen-sectioning or embedding in an aqueous medium would have given tissue measurements closer to the real, in vivo, measurements. Fourth, the blockade of amphiregulin by its antibody resulted in a significant decrease in further axial elongation. The intraocular application of amphiregulin, however, did not result in an increased axial elongation or myopization. The reason for the discrepancy may be that for the induction of axial elongation more than one factor may be necessary while the blockade of one of them, i.e., amphiregulin, was sufficient to reduce the process of axial elongation, or that a higher dose of amphiregulin or a higher frequency of its applications or a longer follow-up might have bene necessary to detect an axial length elongating effect of amphiregulin applied intraocularly. One may also discuss that a blockade of amphiregulin by the antibody affected only one amphiregulin-specific pathway, whereas amphiregulin itself could have affected multiple pathways (e.g., by penetrating through barriers that excluded the antibody). These aspects may be addressed in future studies. It could open up the possibility of a medical prevention of hyperopia. Fifth, instead of the use of Ringer's solution for intraocular application in the eyes of the control groups, one might have used an irrelevant antibody. We chose Ringer's solution since it was assumed that Ringer's solution had least likely an effect on the intraocular structures, while any, even irrelevant, protein might have changed the intraocular homeostasis. Correspondingly, the immunohistochemistry revealed that labelling for amphiregulin in the control groups with intraocular injection of Ringer's solution did not markedly differ from the labelling for amphiregulin in the groups without any intraocular injection (Fig. 4). It may suggest that the intraocular injection with Ringer's solution as procedure as such did not have a major influence on the findings obtained in the study. The strengths of the study are its novelty with investigating the role of amphiregulin in an environmental animal model of myopia, the application of biometry before and after experimental manipulations allowing the assessment of axial length changes over time, and the inclusion of a range of experimental groups including groups without intraocular injections, groups with intraocular injection of Ringer's solution, and groups with intraocular injection of amphiregulin antibody and with intraocular injection of amphiregulin. Sixth, at start of the study the guinea pigs had an age of 2–3 weeks and the investigation was conducted over a period about three times longer than the age of the guinea pigs at study baseline. Although one group of animals did not undergo lens-induced myopization or any other intervention and served as untouched control group, there was the possibility that the natural process of emmetropization physiologically undergoing in guinea pigs of that age might have mingled with the process of lens-induced myopization additionally influenced by the intraocular application of amphiregulin antibody or amphiregulin.
In conclusion, the intraocular and repeated application of amphiregulin-antibody was associated with a dose-related reduction of lens-induced axial elongation of eyes, while intraocularly injected amphiregulin did not show a significant effect on axial elongation. Amphiregulin may be an essential, but not the solely necessary, cytokine for externally induced myopic axial elongation in young guinea pigs. Blockade of amphiregulin may have potential for the prevention of axial myopia.
Funding Sources
This study was supported by the National Ministry of Science & Technology (2015BAI04B04), the National Natural Science Foundation of China (81603421, 81303081), the Shandong Science & Technology Department (2014GGH219004), and the Natural Science Foundation of Shandong Province (ZR2014HQ024). The funders had no role in study design, data collection and analysis, decision to publish, or preparation and writing of the manuscript.
Conflict of Interest
Jost B. Jonas - Consultant for Mundipharma Co., Patent holder with Biocompatible UK Ltd. (Title: Treatment of eye diseases using encapsulated cells encoding and secreting neuroprotective factor and/or anti-angiogenic factor; Patent number: 20120263794), and patent application with university of Heidelberg (Title: Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia; European Patent Number 3 070 101). All other authors none.
Author Contributions
Conceived and designed the experiments: WJJ, HSB, JBJ; Performed the experiments: WJJ, HXS, SYL, BG, JFW, GPL, DDG, DLS, HSB; Analyzed the data: WJJ, HXS, SYL, BG, JBJ; Contributed reagents/materials/analysis tools: HSB, JBJ; Wrote the paper: WJJ, HXS, SYL, BG, HSB, JBJ; Approved the manuscript: WJJ, HXS, SYL, BG, JFW, GPL, DDG, DLS, HSB, JBJ.
Contributor Information
Hong Sheng Bi, Email: hongshengbi@126.com.
Jost B. Jonas, Email: Jost.Jonas@medma.uni-heidelberg.de.
References
- Berasain C., Avila M.A. Amphiregulin. Semin. Cell Dev. Biol. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Chang L., Pan C.W., Ohno-Matsui K., Lin X., Cheung G.C., Gazzard G., Koh V., Hamzah H., Tai E.S., Lim S.C., Mitchell P., Young T.L., Aung T., Wong T.Y., Saw S.M. Myopia-related fundus changes in Singapore adults with high myopia. Am J. Ophthalmol. 2013;155:991–999. doi: 10.1016/j.ajo.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Chen B.Y., Wang C.Y., Chen W.Y., Ma J.X. Altered TGF-β2 and bFGF expression in scleral desmocytes from an experimentally-induced myopia guinea pig model. Graefes Arch. Clin. Exp. Ophthalmol. 2013;251:1133–1144. doi: 10.1007/s00417-013-2269-8. [DOI] [PubMed] [Google Scholar]
- Congdon N., Wang Y., Song Y., Choi K., Zhang M., Zhou Z., Xie Z., Li L., Liu X., Sharma A., Wu B., Lam D.S. Visual disability, visual function, and myopia among rural Chinese secondary school children: the Xichang Pediatric Refractive Error Study (X-PRES)–report 1. Invest. Ophthalmol. Vis. Sci. 2008;49:2888–2894. doi: 10.1167/iovs.07-1160. [DOI] [PubMed] [Google Scholar]
- Fan Q., Verhoeven V.J., Wojciechowski R., Barathi V.A., Hysi P.G., Guggenheim J.A., Höhn R., Vitart V., Khawaja A.P., Yamashiro K., Hosseini S.M., Lehtimäki T., Lu Y., Haller T., Xie J., Delcourt C., Pirastu M., Wedenoja J., Gharahkhani P., Venturini C., Miyake M., Hewitt A.W., Guo X., Mazur J., Huffman J.E., Williams K.M., Polasek O., Campbell H., Rudan I., Vatavuk Z., Wilson J.F., Joshi P.K., McMahon G., St Pourcain B., Evans D.M., Simpson C.L., Schwantes-An T.H., Igo R.P., Mirshahi A., Cougnard-Gregoire A., Bellenguez C., Blettner M., Raitakari O., Kähönen M., Seppala I., Zeller T., Meitinger T., Consortium for Refractive Error and Myopia, Ried J.S., Gieger C., Portas L., van Leeuwen E.M., Amin N., Uitterlinden A.G., Rivadeneira F., Hofman A., Vingerling J.R., Wang Y.X., Wang X., Tai-Hui Boh E., Ikram M.K., Sabanayagam C., Gupta P., Tan V., Zhou L., Ho C.E., Lim W., Beuerman R.W., Siantar R., Tai E.S., Vithana E., Mihailov E., Khor C.C., Hayward C., Luben R.N., Foster P.J., Klein B.E., Klein R., Wong H.S., Mitchell P., Metspalu A., Aung T., Young T.L., He M., Pärssinen O., van Duijn C.M., Jin Wang J., Williams C., Jonas J.B., Teo Y.Y., Mackey D.A., Oexle K., Yoshimura N., Paterson A.D., Pfeiffer N., Wong T.Y., Baird P.N., Stambolian D., Wilson J.E., Cheng C.Y., Hammond C.J., Klaver C.C., Saw S.M., Rahi J.S., Korobelnik J.F., Kemp J.P., Timpson N.J., Smith G.D., Craig J.E., Burdon K.P., Fogarty R.D., Iyengar S.K., Chew E., Janmahasatian S., Martin N.G., MacGregor S., Xu L., Schache M., Nangia V., Panda-Jonas S., Wright A.F., Fondran J.R., Lass J.H., Feng S., Zhao J.H., Khaw K.T., Wareham N.J., Rantanen T., Kaprio J., Pang C.P., Chen L.J., Tam P.O., Jhanji V., Young A.L., Döring A., Raffel L.J., Cotch M.F., Li X., Yip S.P., Yap M.K., Biino G., Vaccargiu S., Fossarello M., Fleck B., Yazar S., Tideman J.W., Tedja M., Deangelis M.M., Morrison M., Farrer L., Zhou X., Chen W., Mizuki N., Meguro A., Mäkelä K.M. Meta-analysis of gene-environment-wide association scans accounting for education level identifies additional loci for refractive error. Nat. Commun. 2016;7:11008. doi: 10.1038/ncomms11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost M.R., Norton T.T. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest. Ophthalmol. Vis. Sci. 2012;53:322–336. doi: 10.1167/iovs.11-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Liu Q., Ma P., Zhong X., Wu J., Ge J. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:1329–1335. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- Guo L., Frost M.R., Siegwart J.T., Jr., Norton T.T. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol. Vis. 2014;20:1643–1659. [PMC free article] [PubMed] [Google Scholar]
- He L., Frost M.R., Siegwart J.T., Jr., Norton T.T. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp. Eye Res. 2014;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Xiang F., Zeng Y., Mai J., Chen Q., Zhang J., Smith W., Rose K., Morgan I.G. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- He M., Zeng J., Liu Y., Xu J., Pokharel G.P., Ellwein L.B. Refractive error and visual impairment in urban children in southern China. Invest. Ophthalmol. Vis. Sci. 2004;45:793–799. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- Heine L. Beiträge zur Anatomie des myopischen Auges. Arch. Augenheilk. 1899;38:277–290. [Google Scholar]
- Jobling A.I., Wan R., Gentle A., Bui B.V., McBrien N.A. Retinal and choroidal TGF-beta in the tree shrew model of myopia: isoform expression, activation and effects on function. Exp. Eye Res. 2009;88:458–466. doi: 10.1016/j.exer.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Jonas J.B., Holbach L., Panda-Jonas S. Bruch's membrane thickness in high myopia. Acta Ophthalmol. 2014;92:e470–e474. doi: 10.1111/aos.12372. [DOI] [PubMed] [Google Scholar]
- Jonas J.B., Ohno-Matsui K., Holbach L., Panda-Jonas S. Retinal pigment epithelium cell density in relationship to axial length in human eyes. Acta Ophthalmol. 2017;95:e22–e28. doi: 10.1111/aos.13188. [DOI] [PubMed] [Google Scholar]
- Jonas J.B., Ohno-Matsui K., Jiang W.J., Panda-Jonas S. Bruch's membrane and the mechanism of myopization. A new theory. Retina. 2017 doi: 10.1097/IAE.0000000000001464. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Jonas R.A., Wang Y.X., Yang H., Li J.J., Xu L., Panda-Jonas S., Jonas J.B. Optic disc - fovea distance, axial length and parapapillary zones. The Beijing Eye Study 2011. PLoS One. 2015;10:e0138701. doi: 10.1371/journal.pone.0138701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas J.B., Wang Y.X., Zhang Q., Liu Y., Xu L., Wei W.B. Macular Bruch's membrane length and axial length. The Beijing Eye Study. PLoS One. 2015;10:e0136833. doi: 10.1371/journal.pone.0136833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas J.B., Xu L., Wei W.B., Pan Z., Yang H., Holbach L., Panda-Jonas S., Wang Y.X. Macular retinal thickness and axial length. The Beijing Eye Study 2011. Invest. Ophthalmol. Vis. Sci. 2016;57:1791–1797. doi: 10.1167/iovs.15-18529. [DOI] [PubMed] [Google Scholar]
- Jones L.A., Sinnott L.T., Mutti D.O., Mitchell G.L., Moeschberger M.L., Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest. Ophthalmol. Vis. Sci. 2007;48:3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Yang Z., Feldkaemper M., Schaeffel F. Changes in dopamine and ZENK during suppression of myopia in chicks by intense illuminance. Exp. Eye Res. 2016;145:118–124. doi: 10.1016/j.exer.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Li H., Cui D., Zhao F., Huo L., Hu J., Zeng J. BMP-2 is involved in scleral remodeling in myopia development. PLoS One. 2015;10:e0125219. doi: 10.1371/journal.pone.0125219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Liu S., Fu C. Citicoline retards myopia progression following form deprivation in guinea pigs. Exp. Biol. Med. (Maywood) 2016;241:1258–1263. doi: 10.1177/1535370216638773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Liu S., Qin W., Li F., Wu X., Tan Q. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom. Vis. Sci. 2010;87:53–60. doi: 10.1097/OPX.0b013e3181c12b3d. [DOI] [PubMed] [Google Scholar]
- McBrien N.A., Lawlor P., Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest. Ophthalmol. Vis. Sci. 2000;41:3713–3719. [PubMed] [Google Scholar]
- McCarthy C.S., Megaw P., Devadas M., Morgan I.G. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp. Eye Res. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Morgan I.G., Ohno-Matsui K., Saw S.M. Myopia. Lancet. 2012;379:1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- Nickla D.L., Wallman J. The multifunctional choroid. Prog. Retin. Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Matsui K., Kawasak R., Jonas J.B., Cheung C.M., Saw S.M., Verhoeven V.J., Klaver C.C., Moriyama M., Shinohara K., Kawasaki Y., Yamazaki M., Meuer S., Ishibashi T., Yasuda M., Yamashita H., Sugano A., Wang J.J., Mitchell P., Wong T.Y., META-analysis for Pathologic Myopia (META-PM) Study Group International classification and grading system for myopic maculopathy. Am J. Ophthalmol. 2015;159:877–883. doi: 10.1016/j.ajo.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Rohrer B., Stell W.K. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp. Eye Res. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- Rose K.A., Morgan I.G., Ip J., Kifley A., Huynh S., Smith W., Mitchell P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Rudnicka A.R., Kapetanakis V.V., Wathern A.K., Logan N.S., Gilmartin B., Whincup P.H., Cook D.G., Owen C.G. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2017 doi: 10.1136/bjophthalmol-2015-307724. (pii: bjophthalmol-2015-307724; Epub ahead of print; 2016 Jan 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko Y., Shimokawa H., Tokoro T. Expression of bFGF and TGF-beta 2 in experimental myopia in chicks. Invest. Ophthalmol. Vis. Sci. 1995;36:1183–1187. [PubMed] [Google Scholar]
- Shelton L., Rada J.A. Inhibition of human scleral fibroblast cell attachment to collagen type I by TGFBIp. Invest. Ophthalmol. Vis. Sci. 2009;50:3542–3552. doi: 10.1167/iovs.09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., You Q.S., Xu X., Gao F., Zhang Z., Li B., Jonas J.B. Scleral and choroidal volume in relation to axial length in infants with retinoblastoma versus adults with malignant melanomas or end-stage glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2016;254:1779–1786. doi: 10.1007/s00417-016-3345-7. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G.D., McDonald V.L., Bradley J.G., Todaro G.J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Siegwart J.T., Jr., Strang C.E. Selective modulation of scleral proteoglycan mRNA levels during minus lens compensation and recovery. Mol. Vis. 2007;13:1878–1886. [PubMed] [Google Scholar]
- Tao Y., Pan M., Liu S., Fang F., Lu R., Lu C., Zheng M., An J., Xu H., Zhao F., Chen J.F., Qu J., Zhou X. cAMP level modulates scleral collagen remodeling, a critical step in the development of myopia. PLoS One. 2013;8:e71441. doi: 10.1371/journal.pone.0071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhao G., Xing S., Zhang L., Yang X. Role of bone morphogenetic proteins in form-deprivation myopia sclera. Mol. Vis. 2011;17:647–657. [PMC free article] [PubMed] [Google Scholar]
- Wei W.B., Xu L., Jonas J.B., Shao L., Du K.F., Wang S., Chen C.X., Xu J., Wang Y.X., Zhou J.Q., You Q.S. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120:175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- Wu J.F., Bi H.S., Wang S.M., Hu Y.Y., Wu H., Sun W., Lu T.L., Wang X.R., Jonas J.B. Refractive error, visual acuity and causes of vision loss in children in Shandong, China. The Shandong Children Eye Study. PLoS One. 2013;8:e82763. doi: 10.1371/journal.pone.0082763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.H., Li Y.Y., Zhang P.P., Qian K.W., Ding J.H., Hu G., Weng S.J., Yang X.L., Zhong Y.M. Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Invest. Ophthalmol. Vis. Sci. 2015;56:967–977. doi: 10.1167/iovs.13-13362. [DOI] [PubMed] [Google Scholar]
- Xu L., Wang Y., Li Y., Wang Y., Cui T., Li J., Jonas J.B. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113:1134–1141. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Xu L., Wang Y., Wang S., Wang Y., Jonas J.B. High myopia and glaucoma susceptibility. The Beijing Eye Study. Ophthalmology. 2007;114:216–220. doi: 10.1016/j.ophtha.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Yan F., Hui Y.N., Li Y.J., Guo C.M., Meng H. Epidermal growth factor receptor in cultured human retinal pigment epithelial cells. Ophthalmologica. 2007;221:244–250. doi: 10.1159/000101926. [DOI] [PubMed] [Google Scholar]
- Yan T., Xiong W., Huang F., Zheng F., Ying H., Chen J.F., Qu J., Zhou X. Daily injection but not continuous infusion of apomorphine inhibits form-deprivation myopia in mice. Invest. Ophthalmol. Vis. Sci. 2015;56:2475–2485. doi: 10.1167/iovs.13-12361. [DOI] [PubMed] [Google Scholar]
- Zieske J.D., Takahashi H., Hutcheon A.E., Dalbone A.C. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest. Ophthalmol. Vis. Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- Zou L., Liu R., Zhang X., Chu R., Dai J., Zhou H., Liu H. Upregulation of regulator of G-protein signaling 2 in the sclera of a form deprivation myopic animal model. Mol. Vis. 2014;20:977–987. [PMC free article] [PubMed] [Google Scholar]