Abstract
AIM
To evaluate the importance of endoscopic ultrasonography (EUS) for small (≤ 10 mm) rectal neuroendocrine tumor (NET) treatment.
METHODS
Patients in whom rectal NETs were diagnosed by endoscopic resection (ER) at the Pusan National University Yangsan Hospital between 2008 and 2014 were included in this study. A total of 120 small rectal NETs in 118 patients were included in this study. Histologic features and clinical outcomes were analyzed, and the findings of endoscopy, EUS and histology were compared.
RESULTS
The size measured by endoscopy was not significantly different from that measured by EUS and histology (r = 0.914 and r = 0.727 respectively). Accuracy for the depth of invasion was 92.5% with EUS. No patients showed invasion of the muscularis propria or metastasis to the regional lymph nodes. All rectal NETs were classified as grade 1 and demonstrated an L-cell phenotype. Mean follow-up duration was 407.54 ± 374.16 d. No patients had local or distant metastasis during the follow-up periods.
CONCLUSION
EUS is not essential for ER in the patient with small rectal NETs because of the prominent morphology and benign behavior.
Keywords: Neuroendocrine tumor, Small, Rectal, Endoscopic ultrasonography, Histology, Endoscopy
Core tip: Small rectal neuroendocrine tumors (NETs; ≤ 10 mm) that are confined to the mucosa or submucosa can be managed by endoscopic resection because of their low risk of metastatic spread. According to the 2015 guidelines of the National Comprehensive Cancer Network, when we evaluate rectal NET, endorectal magnetic resonance or endoscopic ultrasonography (EUS) is recommended. However, EUS may not be essential for evaluation of small rectal NET because of its prominent morphology and benign behavior.
INTRODUCTION
Rectal neuroendocrine tumors (NETs) have rapidly increased in incidence, with more than a 10-fold increase occurring over the last 30 years[1]. However, the rectum remains one of the most frequent sites of digestive NETs[1,2]. The treatment of rectal NETs depends on the tumor size and depth of invasion[1]. Recent consensus guidelines on the management of rectal NETs suggest that small tumors (≤ 10 mm) that are confined to the mucosa or submucosa can be managed by endoscopic resection (ER) because of their low risk of metastatic spread[3,4].
Endoscopic ultrasonography (EUS) was found to be useful for measuring the size and local staging of rectal NETs, which is essential information for determining appropriate treatment[5-8]. Endoscopic size measurement using forceps or other accessories is also possible because the majority of rectal NETs are located in the mucosa or submucosa and have the easily recognized features of a nodular shape with a yellowish color. According to a previous study of 237 patients with rectal NETs < 10 mm, none had metastasis to the regional lymph node (LN) or invasion of the muscularis propria[9]. Furthermore, EUS with miniprobe, which is commonly used for the evaluation of small rectal NETs, offers limited assessment of regional LNs because of its low penetration depth. Thus, endoscopic evaluation without EUS may be sufficient in the management of small rectal NETs, but this has not been well established.
This study was designed to evaluate the biologic behavior of small NETs, to analyze the accuracy of endoscopy and EUS as compared with pathologic findings, and to determine the clinical impact of EUS in the choice of treatment strategy for small rectal NETs.
MATERIALS AND METHODS
Study design
This retrospective study was performed at a single tertiary referral center. Patients in whom rectal NETs were diagnosed by ER at the Pusan National University Yangsan Hospital between 2008 and 2014 were considered for study inclusion. Among the 132 rectal NETs that were treated with ER, 118 patients with a total of 120 small (≤ 10 mm) rectal NETs were enrolled in this study. Two of the patients had two small rectal NETs each. Twelve rectal NETs were excluded from study, and these included 5 patients without EUS examination, 4 patients with no visualization by EUS, and 3 patients with a tumor size > 10 mm. All rectal NETs were found incidentally on a screening colonoscopy, and all patients underwent computed tomography (CT) to assess the presence of metastasis to the perirectal lymph nodes or liver. CT investigations revealed that none of the tumors was associated with either regional LN or distant metastasis.
Rectal NETs were defined as tumors located within 15 cm of the anal verge, while tumors located more than 15 cm above the anal verge were regarded as colonic NETs. Rectal NETs were treated by ER, including endoscopic submucosal dissection, endoscopic mucosal resection (EMR) and EMR with suction methods, and were diagnosed by histologic analysis.
The study protocol was approved by the Institutional Review Board at the Pusan National University Yangsan Hospital (IRB number 05-2015-043).
Measurements
Medical records were reviewed retrospectively to extract clinical information, such as endoscopic, EUS and histologic findings. We consistently described the size, color and shape characteristics of the tumors according to the endoscopic record; however, if the endoscopic description of the tumor size was vague, it was retrospectively re-estimated.
The size and depth of invasion for all NETs was examined by endoscopy, EUS and histologic examination. The NET size was estimated by measuring the diameter of the lesion, and using an open or closed biopsy forceps as a size reference on endoscopic examination. The biopsy forceps was then closed for use in determining the consistency and mobility of the mass.
Immediately after the endoscopic examination, EUS examination was performed by the UM-DP20-25R miniature ultrasonic probe (Olympus Medical Systems Corp, Tokyo, Japan) with a frequency of 20 MHz and the EU-M2000 sonogram processing equipment (Olympus). On EUS examination, the cross-sectional size of the lesion was measured electronically and the location of the lesion was identified as the EUS layer of origin. We also examined the appearance of the NET on EUS (hypoechoic or hyperechoic, homogenous or inhomogenous), and determined whether or not tumor invasion of the proper muscle layer had occurred. After EUS examination, ER was performed. The type of ER was determined by the size of the NET and by the endoscopists’ experience and preference. All endoscopy, EUS and ER procedures were performed by two experienced endoscopists (Kim HW, Choi CW).
We did not perform biopsy before ER because biopsy can induce fibrosis and complicate removal of the submucosal lesion. After endoscopic removal, all resected specimens were evaluated histologically using light microscopy at both low and high power magnifications. Histologic examination of the NETs was performed, and included determination of tumor size, mitotic count, Ki-67 index, presence of lymphovascular invasion and margin status. The grading system used for NETs was that of the European Neuroendocrine Tumor Society[10]. NETs were classified as grade 1, grade 2 or grade 3 according to the mitotic count and the Ki-67 index. The three tumor categories were defined as follows[11]: grade 1, mitotic count < 2 per 10 high-power fields (HPFs) and/or ≤ 2% on the Ki-67 index; grade 2, mitotic count 2-20 per 10 HPFs and/or 3%-20% on the Ki-67 index; and grade 3, mitotic count > 20 per 10 HPFs and/or > 20% on the Ki-67 index.
Examination following ER
Medical records were reviewed to determine the clinical outcomes. The first visit to the outpatient clinic was usually performed within 1 to 2 wk after endoscopic treatment and included a confirmation of the pathologic report as well as an assessment for any complications, such as bleeding and perforation. All patients were recommended surveillance colonoscopies and abdominal CT, with the first follow-up at 6 mo and the second follow-up at 30 mo after endoscopic treatment. All patients underwent the first 6-mo follow-up examination, but some patients did not undergo the second follow-up examination.
Statistical analysis
Continuous variables were reported as mean ± SD or as median (range), and categorical variables were reported as frequency (i.e., %). The diagnostic accuracy of EUS was compared with that of histology in the subset of cases where tissue was obtained. The association among the size estimates by endoscopy, size measurements by EUS and histology was evaluated by calculating the Pearson correlation coefficients with unadjusted significance levels (correlation analysis, r value > 0.7: considered to be strongly correlated). Comparison of diagnostic certainty by endoscopy, EUS and histology was performed by using the Wilcoxon signed-rank test (Wilcoxon signed-rank test, P > 0.10: not statistically significant). All data analyses were performed by the Statistical Package for the Social Sciences (SPSS) software (version 18.0; SPSS, Chicago, IL, United States).
RESULTS
One hundred and eighteen patients [76 men and 42 women, with a mean age of 50.7 ± 11.4 years (range 18-77 years)] with a total of 120 rectal NETs were enrolled in this study. Two patients had two rectal NETs each. For most of the tumors, endoscopic morphology showed sessile or slightly elevated lesions (n = 110, 91.7%), with the others being flat lesions (n = 10, 8.3%). Some tumors had central depression (n = 8, 6.7%). The types and proportions of ERs were conventional EMR (n = 3, 2.5%), EMR with suction methods (n = 70, 58.3%) and endoscopic submucosal dissection (n = 47, 39.2%).
On histologic evaluation, all tumors were classified as grade 1 and as either enteroglucagon type or L-cell type. Microscopic invasion was observed in the histologic findings for 1 case [both lymphatic and vascular invasion (n = 1)]. Lymphovascular invasion was found in 1 patient who had a 6-mm tumor that required additional surgical therapy of low anterior resection. There was no recurrence during the follow-up periods. The mean follow-up period was 407.54 ± 374.16 d (range 154-2148 d) for all patients. Of the 120 lesions evaluated, 23 had follow-up at ≥ 24 mo. The demographics of the lesions are shown in Table 1.
Table 1.
Clinical data of the small rectal neuroendocrine tumors n (%)
| Parameter | Total (n = 120) |
| Tumor size in mm, mean ± SD | |
| Endoscopy | 5.47 ± 1.78 |
| EUS | 5.53 ± 1.76 |
| Histology | 5.54 ± 2.15 |
| Endoscopic morphology | |
| Sessile or slightly elevated | 110 (91.7) |
| Flat | 10 (8.3) |
| Central depression | 8 (6.7) |
| Resection method | |
| Conventional EMR | 3 (2.5) |
| EMR with suction methods | 70 (58.3) |
| ESD | 47 (39.2) |
| Histologic grade | |
| 1 | 120 (100) |
| 2 | 0 |
| 3 | 0 |
| Histologic type | |
| Enteroglucagon or L-cell | 120 (100) |
| Enterochromaffin or enterochromaffin-like cell | 0 |
| Microscopic invasion | |
| Lymphatic and vascular | 1 (0.8) |
| Lymphatic | 0 |
| Vascular | 0 |
| Follow-up duration | |
| 6-12 mo | 84 (70.00) |
| 12-24 mo | 13 (10.83) |
| 24-36 mo | 16 (13.33) |
| ≥ 36 mo | 7 (5.83) |
| Follow-up in day, median (range) | 196 (154-2148) |
| Follow-up in day, mean ± SD | 407.54 ± 374.16 |
EUS: Endoscopic ultrasound; EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection.
Size and depth measurements
The sizes of the rectal NETs were estimated by endoscopy, EUS and histologic findings, and the results were 5.47 ± 1.78 mm, 5.53 ± 1.76 mm and 5.54 ± 2.15 mm respectively. Overall, the sizes estimated by the three different methods were similar and not statistically different (Table 1). The mean size differences between the endoscopic and EUS measurements, between endoscopy and histology, and between EUS and histology were 0.065 ± 0.650 mm (maximum error range 1.5 mm), 0.071 ± 1.407 mm (maximum error range 3.0 mm) and 0.006 ± 1.393 mm (maximum error range 3.5 mm) respectively. None of the size differences were statistically significant (Table 2).
Table 2.
Comparison among the sizes measured by endoscopy, endoscopic ultrasonography and histology
| Measurement technique | Wilcoxon signed-rank test |
| Endoscopy and EUS | P = 0.215 |
| Endoscopy and histology | P = 0.540 |
| EUS and histology | P = 0.933 |
EUS: Endoscopic ultrasonography.
There was very good correlation between the sizes estimated by endoscopy and by EUS (r = 0.914, P < 0.001), and the size measurements of both endoscopy and histology, and EUS and histology, were well correlated (r = 0.727, P < 0.001 and r = 0.727, P < 0.001 respectively) (Figure 1 and Table 3).
Figure 1.
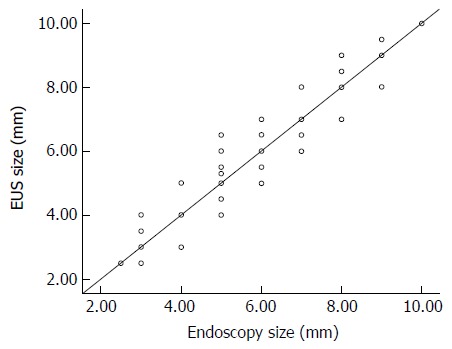
Correlation between the sizes of neuroendocrine tumors measured by endoscopy and endoscopic ultrasonography (r = 0.914). EUS: Endoscopic ultrasonography.
Table 3.
Correlation coefficient among the sizes measured by endoscopy, endoscopic ultrasonography and histology
| Measurement technique | Correlation coefficient |
| Endoscopy and EUS | 0.914 (P < 0.01) |
| Endoscopy and histology | 0.727 (P < 0.01) |
| EUS and histology | 0.727 (P < 0.01) |
EUS: Endoscopic ultrasonography.
The locations of the rectal NETs estimated by EUS were found at the second layer (n = 9, 7.5%) and the third layer (n = 111, 92.5%), but none were found at the fourth layer. The accuracy of EUS as compared to histology was 92.5% (Table 4). Involvement of the muscularis propria was not observed by either EUS or histology in any of the cases.
Table 4.
Comparison of depth of invasion measured by endoscopic ultrasonography and histology n (%)
| Depth of invasion | EUS | Histology |
| 2nd layer (muscularis mucosa) | 9 (7.5) | 2 (1.7) |
| 3rd layer (submucosa) | 111 (92.5) | 118 (98.3) |
| 4th layer (muscularis propria) | 0 (0) | 0 (0) |
| EUS accuracy | 111 (92.5) |
EUS: Endoscopic ultrasonography.
DISCUSSION
The overall incidence of rectal NETs is rapidly increasing[1], and the incidence in South Korea, in particular, has reached 48% among gastroenteropancreatic NETs[12]. Since most small rectal NETs will follow an indolent course with very infrequent local and distant metastases, endoscopic treatment is usually recommended. Endoscopic treatment for rectal NETs is considered a curative treatment for lesions ≤ 10 mm in diameter and without lymphovascular invasion or metastasis. However, metastasis was reportedly detected in 9.7% of cases of ≤ 10 mm rectal NETs[13], and the reported range of rates of distant metastasis in patients with rectal NETs is 2%-8%[10,14,15]. In our study, lymphovascular invasion was found in 1 patient. However, more studies are needed to verify the low metastasis rate in recent studies.
Risk factors for metastasis include tumor size, muscularis propria invasion, histologic grade (Ki-67 index and mitotic count), lymphovascular invasion, neural invasion, and atypical endoscopic features[10,16]. In particular, presence of a > 10-mm tumor size and lymphatic invasion strongly corresponded to LN metastasis, with the rate of LN metastasis increasing to 16% with either one of these two risk factors, and further increased to as high as 77% in patients with both risk factors[17].
In order to ascertain the presence of local or distant metastasis before the endoscopic treatment of rectal NETs, EUS and CT scan are routinely performed. EUS is especially useful for measuring the size and performing local staging of rectal NETs, which is essential for determining appropriate treatment[5-8]. Yet, the clinical value of EUS, particularly of EUS with miniprobe used for small rectal NETs, decreases in conjunction with the very low risk of LN metastasis, limited scope of evaluation for regional LNs, and ease of endoscopic measurement because of the well-demarcated margins of rectal NETs. Moreover, the predictive ability of EUS regarding other risk factors, such as histologic grade and lymphovascular invasion, is minimal. Considering these issues, an effective evaluation of regional LN metastasis is possible using a combination of endoscopy and histology.
Recent reports have suggested that L-cell phenotype and small tumor size predict a favorable clinical outcome for rectal NETs[18,19]. In a Korean study, the frequency of the L-cell phenotype as detected by tumor immunoreactivity for L-cell markers (i.e., glucagon-like peptide 1, pancreatic peptide and peptide YY) was 79%[18]. In our study, the frequency of enteroglucagon or L-cell phenotype, determined by histologic pattern and chromogranin A immunoreactivity, was 100%. We did not evaluate tumor immunoreactivity for L-cell markers, since performance of these studies involves high costs and some previous studies have suggested that about 80% of rectal NETs are L-cell type with typical trabecular pattern and reduced/absent chromogranin A immunoreactivity[20]. Therefore, given the highly frequent detection of the L-cell phenotype, EUS evaluation for regional LN metastasis and invasion of the muscularis propria is not essential for small rectal NETs.
Several studies have examined the utility of EUS in the assessment and management of rectal NETs[2,5-8,21]. However, no previous studies have compared the size measurement of rectal NETs by endoscopy to either measurements by EUS or by histology; although, one study compared endoscopy and EUS in the evaluation of gastrointestinal subepithelial masses, including carcinoid tumor[22].
Our study examined the accuracy of endoscopy and EUS in evaluating 120 rectal NETs in 118 patients. The purpose of this study was to determine whether endoscopy could (1) measure the size of tumors as accurately as EUS; (2) estimate the invasion of the muscularis propria or metastasis to regional LNs; and (3) perform an adequate evaluation of small rectal NETs prior to ER.
In our study, the endoscopic size estimation was similar to the measurements of EUS and histology, with no greater than a 3.5 mm difference between them. The measuring methods were also strongly correlated (Table 3 and Figure 1), and these findings showed the accuracy of endoscopy as well as the accuracy of EUS for small rectal NETs. Although previous reports on endoscopic size estimation have suggested that this method is inaccurate, primarily due to underestimation of the lesion size[23-25], the findings of our study and of Hwang et al[22] have suggested that endoscopy is quite accurate in determining the size of subepithelial masses if the size is estimated using a known size reference, such as an open biopsy forceps. We found a strong correlation between size measurements by endoscopy and EUS (r = 0.914, P < 0.001), with a mean difference in size measurement of 0.065 ± 0.650 mm. The correlation between size measurements by endoscopy and histology was also significant (r = 0.727, P < 0.001). Therefore, the size of small rectal NETs could be as accurately estimated by endoscopic examination as by EUS, if used with a known size reference.
As shown in Table 4, the diagnostic accuracy of EUS for invasion depth was 111/120 (92.5%), and invasion of the muscularis propria was not present in any of the rectal NETs. Therefore, although the evaluation of invasion depth by EUS is very effective, these findings showed that using EUS to evaluate invasion depth is not essential for small rectal NETs.
According to Kasuga et al[26], factors such as a tumor size of 10 mm or more, the presence of central depression, depth of tumor invasion, lymphatic invasion and venous invasion were all significantly associated with a higher incidence of LN metastasis on univariate analysis. Multivariate analysis revealed that a tumor size of 10 mm or more and the presence of venous invasion were independently predictive of LN metastasis.
Our study has some limitations. First, the patients’ information could be inaccurate because of the selection bias related to the retrospective nature of the study. Second, the accuracy of endoscopic measurement differs depending on the endoscopists’ level of experience. Third, the number of rectal NETs in our study is relatively small. Since there is generally a very low risk of LN metastasis and invasion of the muscularis propria, a study with a greater number of small rectal NETs is necessary to capture more accurate results. Fourth, the short follow-up interval may have influenced the results of our study because rectal NETs progress very slowly.
In conclusion, though the EUS estimation of tumor size and depth of invasion in small rectal NETs was very accurate and useful, the endoscopic estimation was equivalent. Overall, the accuracy of the endoscopic measurement, along with the lack of muscularis propria involvement in all of the rectal NETs of our study, suggested that histologic evaluation after ER is more significant than EUS evaluation before ER. Therefore, EUS may not be an essential factor in deciding the treatment strategy for small rectal NETs.
COMMENTS
Background
Endoscopic ultrasonography (EUS) is commonly used in cases of neuroendocrine tumors (NETs) to evaluate the size of the tumor, invasion of the muscularis propria and metastasis of regional lymph nodes. This study was designed to investigate the biologic behavior of small (≤ 10 mm) rectal NETs and the clinical impact of EUS for endoscopic resections (ER).
Research frontiers
No previous studies have compared the size measurement of rectal NETs obtained by using endoscopy to measurements from either EUS or histology; although, one study compared endoscopy and EUS in the evaluation of gastrointestinal subepithelial masses. The size measured by endoscopy was not significantly different from that measured by EUS and histology. Moreover, the small rectal NETs had an indolent course and a low metastasis rate, compared with a few prior reports. The results of this study suggest the utility of performing EUS in small rectal NETs selectively.
Innovations and breakthroughs
EUS is helpful for measuring accurate tumor size and depth of invasion; however, it is not always necessary for deciding the treatment strategy. In this report, small rectal NETs were evaluated carefully by endoscopy and, then, if the tumor was deemed appropriate for endoscopic removal and no risk factors were present, it could be removed without EUS. It is important to identify risk factors and histologic results after ER.
Applications
This study suggests that EUS may not be necessary in deciding the treatment strategy for small rectal NETs ≤ 10 mm. If the lesion is strongly suspected with small rectal NET, ER can be chosen based on endoscopic features and size measurement without evaluating EUS.
Terminology
EUS is an endoscopic procedure that enables observation of the chest and abdominal organs, including the gastrointestinal tract.
Peer-review
The authors of this paper highlight the importance of EUS for deciding treatment strategy of small rectal NETs. EUS may not be essential for ER in the patient with small rectal NETs because of the relatively exact size measurement and prominent morphology obtained through endoscopy and the benign behavior of this tumor type. EUS can be applied selectively in patients with risk factors, and further clinical trials in a large population of patients with small rectal NETs will be valuable.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study protocol was approved by the Institutional Review Board at the Pusan National University Yangsan Hospital (IRB number 05-2015-043).
Informed consent statement: Patients were not required to give informed consent for study participation because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Peer-review started: December 9, 2016
First decision: January 10, 2017
Article in press: February 17, 2017
P- Reviewer: Attaallah W, Chiba H S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
References
- 1.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18, vii. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen HT, Xu GQ, Teng XD, Chen YP, Chen LH, Li YM. Diagnostic accuracy of endoscopic ultrasonography for rectal neuroendocrine neoplasms. World J Gastroenterol. 2014;20:10470–10477. doi: 10.3748/wjg.v20.i30.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O’Dorisio TM, Warner RR, Wiseman GA, Benson AB, Pommier RF. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- 4.Choi HH, Kim JS, Cheung DY, Cho YS. Which endoscopic treatment is the best for small rectal carcinoid tumors? World J Gastrointest Endosc. 2013;5:487–494. doi: 10.4253/wjge.v5.i10.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Mizutani K, Nakamura T. Carcinoid tumors of the gastrointestinal tract: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1993;39:375–383. doi: 10.1016/s0016-5107(93)70109-1. [DOI] [PubMed] [Google Scholar]
- 6.De Angelis C, Carucci P, Repici A, Rizzetto M. Endosonography in decision making and management of gastrointestinal endocrine tumors. Eur J Ultrasound. 1999;10:139–150. doi: 10.1016/s0929-8266(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 7.Ishii N, Horiki N, Itoh T, Maruyama M, Matsuda M, Setoyama T, Suzuki S, Uchida S, Uemura M, Iizuka Y, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24:1413–1419. doi: 10.1007/s00464-009-0791-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou FR, Huang LY, Wu CR. Endoscopic mucosal resection for rectal carcinoids under micro-probe ultrasound guidance. World J Gastroenterol. 2013;19:2555–2559. doi: 10.3748/wjg.v19.i16.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, Jeen YT, Lee SH, Ji JS, Han DS, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43:790–795. doi: 10.1055/s-0030-1256414. [DOI] [PubMed] [Google Scholar]
- 10.Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88–97. doi: 10.1159/000335594. [DOI] [PubMed] [Google Scholar]
- 11.Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–762. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 12.Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park DY, Lee JH, et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157–165. doi: 10.4143/crt.2012.44.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587–1595. doi: 10.1002/cncr.20939. [DOI] [PubMed] [Google Scholar]
- 14.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 15.McDermott FD, Heeney A, Courtney D, Mohan H, Winter D. Rectal carcinoids: a systematic review. Surg Endosc. 2014;28:2020–2026. doi: 10.1007/s00464-014-3430-0. [DOI] [PubMed] [Google Scholar]
- 16.de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039–1046. doi: 10.1055/s-0033-1344794. [DOI] [PubMed] [Google Scholar]
- 17.Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H; Japanese Society for Cancer of the Colon and Rectum. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007;56:863–868. doi: 10.1136/gut.2006.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Kim BC, Chang HJ, Sohn DK, Han KS, Hong CW, Lee EJ, Lee JB, Lee DS, Lee IT, et al. Rectal neuroendocrine and L-cell tumors: diagnostic dilemma and therapeutic strategy. Am J Surg Pathol. 2013;37:1044–1052. doi: 10.1097/PAS.0b013e3182819f0f. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Kim KS, Kim KJ, Park IJ, Lee JL, Myung SJ, Park Y, Park YS, Yu CS, Kim JC, et al. Non-L-cell immunophenotype and large tumor size in rectal neuroendocrine tumors are associated with aggressive clinical behavior and worse prognosis. Am J Surg Pathol. 2015;39:632–643. doi: 10.1097/PAS.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 20.Rindi G, Inzani F, Solcia E. Pathology of gastrointestinal disorders. Endocrinol Metab Clin North Am. 2010;39:713–727. doi: 10.1016/j.ecl.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Moon W, Park SJ, Park MI, Kim SE, Ku KH, Lee GW, Choi YJ. Clinical impact of endoscopic ultrasonography for small rectal neuroendocrine tumors. Turk J Gastroenterol. 2014;25:657–660. doi: 10.5152/tjg.2014.6647. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–208. doi: 10.1016/s0016-5107(05)01567-1. [DOI] [PubMed] [Google Scholar]
- 23.Margulies C, Krevsky B, Catalano MF. How accurate are endoscopic estimates of size? Gastrointest Endosc. 1994;40:174–177. doi: 10.1016/s0016-5107(94)70162-8. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg A, Giger M, Kern L, Noll C, Study K, Weber KB, Blum AL. How reliable is determination of ulcer size by endoscopy? Br Med J. 1979;2:1322–1324. doi: 10.1136/bmj.2.6201.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vakil N. Measurement of lesions by endoscopy: an overview. Endoscopy. 1995;27:694–697. doi: 10.1055/s-2007-1005790. [DOI] [PubMed] [Google Scholar]
- 26.Kasuga A, Chino A, Uragami N, Kishihara T, Igarashi M, Fujita R, Yamamoto N, Ueno M, Oya M, Muto T. Treatment strategy for rectal carcinoids: a clinicopathological analysis of 229 cases at a single cancer institution. J Gastroenterol Hepatol. 2012;27:1801–1807. doi: 10.1111/j.1440-1746.2012.07218.x. [DOI] [PubMed] [Google Scholar]


