Abstract
Purpose
In an attempt to improve in vitro embryo production, we investigated the effect of fibroblast growth factor 10 (FGF10) during in vitro maturation on the developmental capacity of bovine oocytes.
Material and methods
Cumulus–oocyte complexes (COCs) were aspirated from follicles of 3–8 mm diameter. After selection, the COCs were matured in medium with or without 0.5 ng/mL of FGF10. The effect of FGF10 during in vitro maturation (IVM) on nuclear maturation kinetics and expansion of the cumulus cells was investigated. Oocyte competence was assessed by the production and development speed of embryos and the relative expression of genes associated with embryo quality.
Results
FGF10 delayed the resumption of meiosis from 8 h onwards, but did not affect the percentage of oocytes reaching metaphase II, nor did it increase cumulus expansion at 22 h of maturation. We found no difference between treatments regarding embryo production, developmental speed, and gene expression.
Conclusion
In conclusion, the presence of FGF10 during IVM had no effect on embryo production, developmental speed, and gene expression.
Keywords: Cumulus expansion, Maturation, Blastocyst, Oocyte, Cattle
Introduction
The production of viable female gametes is dependent on the interactions between oocyte and somatic cells during folliculogenesis and oogenesis, which are regulated by hormones and local factors [1]. In farm animals such as cattle, oocytes are usually removed prematurely from their follicles to be used in assisted reproductive techniques (ARTs). In this case, they are deprived of cellular events that occur at the end of folliculogenesis and have to be in vitro matured, which may be one of the reasons why they are less competent than those that remain in the follicle until ovulation.
Oocyte maturation is a very complex process that depends on the oocyte quality, which can be strongly influenced by extrinsic factors. Therefore, supplementation of culture media during in vitro maturation (IVM) could provide additional factors to improve the developmental competence of bovine oocytes. Fibroblast growth factors (FGFs) are involved in various cellular processes, including chemotaxis, cell migration, differentiation, survival, apoptosis, embryonic development, and angiogenesis [2, 3]. In addition, they have been reported as having critical involvement in the regulation of folliculogenesis and oogenesis [4–8]. Therefore, FGFs are potential factors to improve in vitro embryo production.
Among the members of the FGF family, fibroblast growth factor 10 (FGF10) is one of particular interest since its expression was detected in ovarian stromal cells, granulosa cells, theca cells and oocytes in humans [9, 10], and theca cells, luteal cells, and oocytes in bovine [5, 11]. In addition, FGF10 receptor (FGFR) expression is regulated by FSH, and its two main receptors, FGFR1B and FGFR2B, are present in oocyte and cumulus cells (CCs) [12], suggesting that it has an important role in oocyte maturation [13, 14].
Recently, the beneficial effect of FGF10 supplementation during IVM on embryo production has been reported [12, 15], although the mechanism of action is mostly unknown. Furthermore, Caixeta [14] reported that FGF10 enhanced cumulus expansion by involving an increase in glucose uptake by cumulus cells associated with the upregulation of the expression of key genes involved in hyaluronic acid production. Considering the importance of FGF10 in the regulation of folliculogenesis and its effects during IVM, more studies are needed to confirm the beneficial effects of this growth factor in vitro.
In an attempt to improve in vitro embryo production, we investigate the effect of FGF10 during in vitro maturation on the developmental capacity of bovine oocytes.
Material and methods
Unless otherwise indicated, the reagents/chemicals used for the preparation of the maturation, fertilization, and in vitro culture media were purchased from Sigma (St. Louis, MO, USA).
Oocyte recovery and selection
Ovaries from crossbred females (Bos indicus × Bos taurus) were collected from local abattoirs immediately after slaughter and transported in saline solution (0.9% NaCl) supplemented with antibiotics (100 mg/mL streptomycin and 100 IU/mL G penicillin) at temperatures of 35–36 °C. Cumulus–oocyte complexes (COCs) were aspirated from follicles of 3–8 mm diameter using an 18-gauge needle attached to a 10-mL syringe. Follicular fluid was used for searching and selection, and only COCs presenting homogenous cytoplasm and at least three layers of cumulus cells were used.
In vitro maturation
Immediately after selection, the COCs were washed and transferred in groups of 20–30 to a 200-μL droplet of maturation medium with or without 0.5 ng/mL of FGF10 according to the experiment. The maturation medium used was composed of TCM-199 with Earl’s salts (Invitrogen, Carlsbad, CA, USA) supplemented with 0.075 mg/mL amikacin, 0.4% fatty acid-free bovine serum albumin (BSA), 0.68 mM l-glutamine, 0.2 mM pyruvate, 0.1 mM cysteamine, and 10−1 IU/mL of recombinant FSH (Gonal-F®, Serono, Rockland, MA, USA). Culture was performed for 24 h at 38.5 °C and 5% CO2 in air.
Nuclear maturation
To assess the stage of meiosis at different times, the oocytes were removed from the drops, denuded by repeated pipetting until the complete removal of CCs, fixed for 48 h in ethanol and acetic acid (3:1), and stained with 45% lacmoid in glacial acetic acid. The evaluation of meiotic stage was performed under a phase-contrast microscope (Nikon Eclipse E200, 1.000X) and classified into: germinal vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (MI), anaphase I (AI), telophase I (TI), and metaphase II (MII), with alignment of chromosomes on the metaphase plate and extrusion of the first polar body. Any oocyte that had diffuse or undefined chromatin or had some chromosomal aberration was considered degenerate or abnormal.
In vitro fertilization and in vitro embryo culture
After maturation, groups of 20–30 COCs were transferred to 200 μL drops of fertilization medium, which consisted of Tyrode’s albumin lactate pyruvate supplemented with penicillamine (2 mM), hypotaurine (1 mM), epinephrine (250 mM), and heparin (10 mg/mL) [16]. Frozen semen from a bull of proven fertility that had been used for several years as the reference bull for in vitro embryo production in our laboratory was used for all treatments and replicates. Motile spermatozoa were obtained using the Percoll (GE Healthcare, Piscataway, NJ, USA) gradient method in microtubes [17] and were added to the fertilization drop at a final concentration of 1 × 106 spermatozoa per milliliter. Spermatozoa and oocytes were co-incubated for 18 h at 39 °C with 5% CO2 in air. The day of in vitro insemination was considered to be day 0. Eighteen hours after insemination, the presumptive zygotes were washed and transferred to 200 μL drops of synthetic oviduct fluid medium with amino acids, citrate, and inositol [18] supplemented with 5% FCS and incubated at 38.5 °C in an atmosphere of 5% CO2 in air. Embryos were evaluated on day 2 for cleavage and on days 6 and 7 for blastocyst.
Cumulus expansion
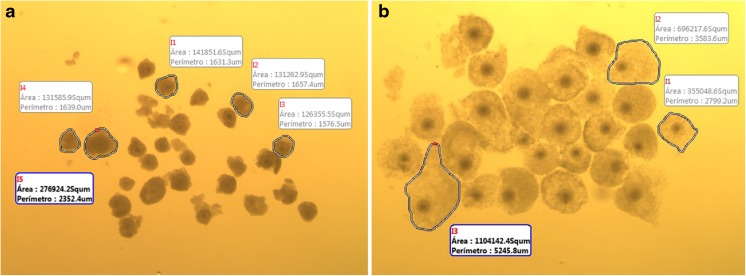
To determine CC expansion, each COC was measured after selection and after maturation using the Motic Image Plus 2.0 program (Motic China Group Co, Ltd., Xia-men, China). The CC expansion during IVM was determined by the difference between the mean area of all COCs from each treatment before and after in vitro maturation (Fig. 1).
Fig. 1.
Representative image illustrating the cumulus cell expansion measurements using the program Motic Image Plus 2.0 before (a) and after (b) maturation
RNA extraction and cDNA synthesis
Three pools of 10–12 expanded blastocysts (D7) for each treatment were collected. Embryos were stored in RNA Latter at −20 °C until the RNA extraction.
Total RNA of embryos was isolated using the RNeasy Plus Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, with minor modifications. cDNA synthesis was performed using SuperScriptIII (200 U/μL, Invitrogen®) and Oligo dT primer (0.5 μg/mL, Invitrogen®) in a final volume of 25 μL. Reactions were performed at 65 °C for 5 min and 42 °C for 60 min, followed by inactivation of the enzyme at 70 °C for 15 min.
Real-time RT-PCR
The genes quantified by qPCR are genes involved in embryo quality. The genes referred are: [placenta-specific 8 (PLAC8), keratin proteins 8 (KRT8)]; heat stress [heat shock 27-kDa protein 1 (HSPB1)]; pregnancy [Tetraspanin 9 (CD9), pregnancy-associated glycoprotein 2 (PAG2)]; DNA repair [DNA mismatch repair protein MSH, mutS homolog 6 (MSH6)], which were quantified for qPCR.
Real-time RT-PCR analysis was performed using 7500 Fast Real-Time PCR System (Applied Biosystem, Foster City, CA, USA). The primer sequences, fragment size, annealing temperature, and primer concentrations for each gene are listed in Table 1. Reactions were performed in duplicate for each gene, with an amplification efficiency of >90%. The levels of expression of three housekeeping genes—glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin (ACTB), and peptidylprolyl isomerase A (PPIA)—were subjected to GeNorm software [19], which indicated the ACTB as the most stable gene. This was used as a reference for data normalization. The relative abundance of each gene was calculated using the ΔΔC t method with efficiency correction [20].
Table 1.
Information of specific primers used for amplification of gene fragment analysis by real-time PCR
| Gene | Primer sequence | Size (bp) | Primer concentration (nM) | Genbank no./reference |
|---|---|---|---|---|
| B-actin | F: GGC ACC CAG CAC AAT GAA GAT CAA R: ATC GTA CTC CTG CTT GCT GAT CCA |
126 | 300 | XM_010845770.1 |
| KRT8 | F: TGT GAA GAA GAT TGA GAC CCG CGA R: AAA CCT CAG GTC TCC TGT GCA GAT |
160 | 300 | X12877 [31] |
| CD9 | F: CAC ATC AGT CCA ACC CAG AC R: AAT CGG AGC CAT AGT CCA AC |
146 | 300 | NM_173900 [31] |
| PLAC8 | F: GAC TGG CAG ACT GGC ATC TT R: CTC ATG GCG ACA CTT GAT CC |
140 | 300 | NM_016619 [31] |
| HSPB1 | F: CTG GAC GTC AAC CAC TTC R: GGA CAG AGA GGA GGA GAC |
180 | 250 | NM_001025569.1 |
| PAG2 | F: GAA CAC AAA CAA GCC AGA G R: TTG GGC CGT AGA TCA TTG |
208 | 200 | NM_176614 |
| MSH6 | F: CCC AGG TGC TTA AAG GTA TG R: GGA CCA TGT CAG AAT CCA AG |
186 | 300 | NM_001192737.1 |
F: forward primer; R: reverse primer
Experimental design
Experiment 1: effect of FGF10 on oocyte maturation and cumulus expansion
This experiment aimed to evaluate whether the addition of FGF10 during IVM of bovine oocytes affects meiosis progression and cumulus expansion. The COCs were selected and distributed into two groups, a control group and a group in which the maturation medium was supplemented with 0.5 ng/mL of FGF10. Initially, 244 COCs were used for evaluation of nuclear maturation kinetics at 0, 8, and 22 h. At each of these time intervals, the oocytes were denuded, fixed, and stained to determine the stage of meiosis. A total of 250 COCs were measured before and after in vitro maturation to determine CC expansion.
Experiment 2: effect of FGF10 during in vitro maturation of bovine oocytes on embryo production
Here, we proposed to verify whether adding FGF10 into the maturation media would improve subsequent embryo production. Then, we observed cleavage rate on D2 and blastocyst rates on D6 and D7. The COCs used for CC expansion measurement were fertilized in vitro and cultured up to D7.
Experiment 3: effect of FGF10 during in vitro maturation of bovine oocytes on speed of developmental and genic expression of embryos
This experiment was conducted to investigate the effect of the addition of FGF10 during IVM on the quality of embryos, as measured by the speed of development, obtained by the rate of each stage of embryonic development, and the expression of genes related to embryo quality: KRT8, PLAC8, CD9, PAG2, HSPB1, and MSH6.
Statistical analysis
Nuclear maturation and embryo development data were analyzed by chi-square test (P < 0.05). The comparison between treatments for the measurement of CC expansion and gene expression was performed by analysis of variance. All data concerning the expansion have a normal distribution. To compare CC expansion of the first experiment, the t test was used. The relative expression of each gene was calculated using the ΔΔC t method of correction efficiency by the Pfaffl method and compared with the t test (Welch–Satterthwaite approximation). P < 0.05 was considered statistically significant. Data are presented as the mean ± standard deviation. All analyses were performed by the Prophet, version 5.0 (BBN Systems and Technologies, 1996).
Results
The presence of FGF10 did not affect the percentage of oocytes reaching MII (Table 2). However, the presence of FGF10 in the maturation medium delayed the resumption of meiosis, since at 8 h of maturation a lower percentage of oocytes showed GVBD and a higher percentage was still at the GV stage compared to the oocytes from the control group.
Table 2.
Meiotic stage of oocytes cultured in maturation medium supplemented or not with 0.5 ng/mL of FGF10 at 0, 8, and 22 h
| Treatments | N | Meiosis stage | |||
|---|---|---|---|---|---|
| GV (%) | GVBD (%) | MI, AI, and TI (%) | MII (%) | ||
| Control 0 h | 41 | 41 (100)a | 0 (0)c | 0 (0)c | 0 (0)b |
| Control 8 h | 87 | 3 (3.4)c | 53 (60.9)a | 31 (35.6)a | 0 (0)b |
| FGF10 8 h | 33 | 9 (27.3)b | 11 (33.3)b | 13 (39.4)a | 0 (0)b |
| Control 22 h | 39 | 0 (0)d | 0 (0)c | 5 (12.8)b | 34 (87.2)a |
| FGF10 22 h | 44 | 0 (0)d | 0 (0)c | 4 (9.1)b | 40 (90.9)a |
Values in the same column with different lowercase letters differ significantly by chi-square test (P < 0.05)
GV germinal vesicle, GVBD germinal vesicle broke down, MI metaphase 1, AI anaphase 1, TI telophase 1, MII metaphase II (N = 3 replicates)
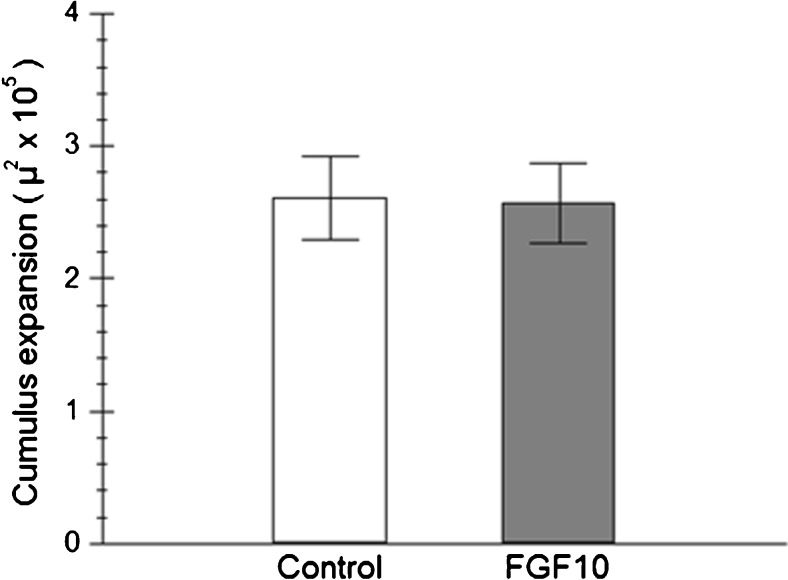
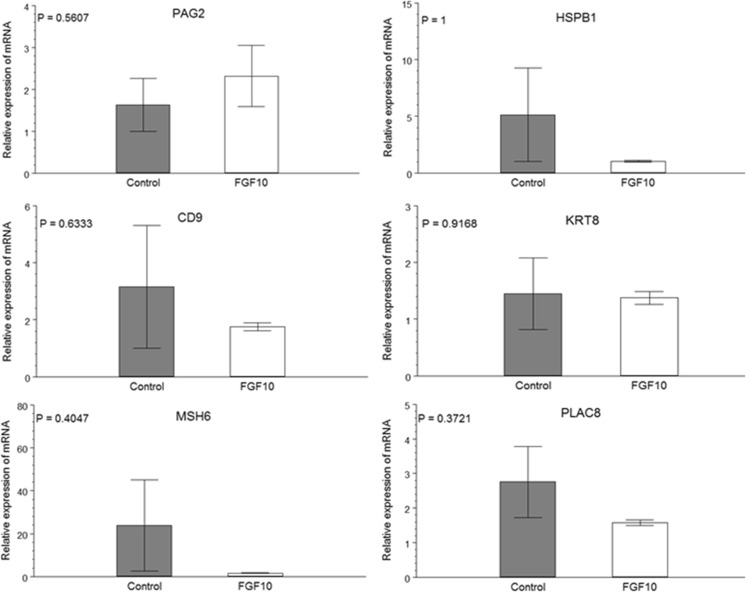
When cumulus expansion was evaluated, no difference was observed between treatments (Fig. 2). Similarly, no effect of the presence of FGF10 during in vitro maturation was observed on cleavage and blastocyst rates on D6 and D7 (Table 3). When the speed of development of the embryos was considered, it was observed that FGF10 supplementation during IVM also had no effect on embryo developmental kinetics. For both treatments, the majority of the embryos were expanded blastocysts on D7 (Table 4). Those embryos also showed similar expressions of all genes evaluated (Fig. 3).
Fig. 2.
Area of cumulus–oocyte complexes matured for 22 h in media supplemented (FGF10) or not (Control) with 0.5 ng/mL of FGF10, measured before and after maturation (N = 5 replicates)
Table 3.
Effect of FGF10 during in vitro maturation of cumulus–oocyte complexes (IVM + FGF10) in cleavage rate on day 2 and blastocyst rate on days 6 and 7 of development of bovine embryos
| Treatment | No. of oocytes | Cleavage day 2 (%) | Blastocysts day 6 (%) | Blastocysts day 7 (%) |
|---|---|---|---|---|
| Control | 127 | 109 (85.8) | 56 (44.9) | 62 (48.8) |
| IVM + FGF10 | 123 | 114 (92.6) | 45 (36.6) | 59 (47.9) |
Data analyzed by chi-square test (P < 0.05, N = 5 replicates)
Table 4.
Effect of FGF10 during in vitro maturation of cumulus–oocyte complexes (IVM + FGF10) on speed of development of bovine embryos at days 6 and 7 of culture
| Treatment | Day 6 | Day 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Bi (%) | Bl (%) | Bx (%) | Total (%) | Bi (%) | Bl (%) | Bx (%) | Bh (%) | Total (%) | |
| Control | 127 | 15 (26.7) | 17 (30.3) | 24 (42.8) | 56 (44.09) | 1 (1.6) | 12 (19.3) | 37 (59.6) | 12 (19.3) | 62 (48.8) |
| IVM + FGF10 | 123 | 11 (24.4) | 15 (33.3) | 19 (42.2) | 45 (36.6) | 0 (0) | 11 (18.6) | 38 (64.4) | 10 (16.9) | 59 (47.9) |
Data analyzed by chi-square test (P < 0.05)
N number of oocytes, Bi initial blastocyst, Bl blastocyst, Bx expanded blastocyst, Bh hatched blastocyst (N = 5 replicates)
Fig. 3.
Relative expression of mRNA of various genes by qPCR of expanded blastocysts on D7 of development originated from oocytes in vitro matured with (FGF10) or without FGF10 (Control) (P < 0.05)
Discussion
Considering the importance of in vitro maturation in ARTs in humans and animals, several research groups have conducted studies in order to improve the in vitro maturation system. In the present study, we investigated the presence of FGF10 during maturation on the yield and quality of in vitro produced embryos using evaluation parameters such as blastocyst development and gene expression.
Before evaluating the effects of FGF10 on IVP, it was assessed whether its presence during IVM had any effect on the progression of meiosis. The results showed that the presence of 0.5 ng/mL FGF10 caused a delay in the resumption of meiosis in the first 8 h, but did not affect the rate of oocytes in MII at 22 h. Zhang [12] and Pomini [15] had also found that FGF10 affected the nuclear maturation rate in a dose-dependent manner. However, in Zhang’s study, they only observed an effect on the first polar body’s extrusion rate, but not on the MII rate, similar to the results obtained in our work.
The biological activities of FGF proteins are exerted through specific high-affinity binding to receptor tyrosine kinases [21]. However, there is a body of evidence that suggests that FGFs are unstable proteins with limited half-life in vivo, which affects FGF signaling [22]. Activated FGFR phosphorylates adaptor proteins for four major intracellular signaling pathways—RAS-MAPK, PI3K-AKT, and PLCγ—and signal transducer and activator of transcription [3]. The MAPK pathway is a major activation pathway in oocyte development [23, 24], whereas PI3K-AKT is required at the end of maturation [25]. In our study, it is possible that the MAPK pathway was delayed in some unknown way. The action of FGF10 was probably not by the PI3K-AKT pathway since there were no differences in MII proportion at the end of maturation. We hypothesized that the delay in the transient effect of FGF10 on the resumption of meiosis could be caused by lack of binding stability and/or fast degradation in the culture medium in the early hours of culture, but more studies are needed to elucidate this finding.
Contrasting with other reports, we did not find any effect of FGF10 on CC expansion. Caixeta [14] found that expansion of bovine COCs was enhanced by FGF10, but the concentration of FGF10 used in their study was considerably higher (10 ng/mL) than the concentration used in our study (0.5 ng/mL). Using the same concentration as in the present study, Zhang [12] noted an increase in the expansion of CCs (0.5 ng/mL). It is important to highlight that the maturation medium used by those authors contained only PVA with no protein source, contrasting to ours wherein the maturation medium was supplemented with BSA. In addition, the source of FSH, which also induces CC expansion, differed between the studies. We know that FSH increases FGF10 receptors in cumulus cells, and FGF10 does not stimulate the expansion of CCs at high doses, probably for a receptor saturation effect. So it is possible that, in our study, the presence of BSA and recombinant FSH provided the ideal conditions for CC expansion, masking the FGF10 effect, even though possibly increasing their receptors. This hypothesis can be supported by Caixeta’s [14] results, which only observed increasing effect with a higher FGF10 concentration (10 ng/mL).
Supplementing the maturation medium with FGF10 also did not affect any parameters of embryo production. Not only the blastocyst rates at D6 and D7 but also the speed of development of those embryos were similar between treatments. These results are in contrast with those reported by Zhang [12]. One of the factors that could be responsible for these differences could be the composition of the maturation media, as stated above. We believe that an increase in FGF10 during IVM in embryo production is dependent on the concentration used and the cultivation conditions. It is well known that different protein supplementations and sources of FSH during IVM may interfere in CC expansion, nuclear maturation, and embryo development [26, 27]. PVA is a synthetic polymer which generally produces a lower embryo rate (D7) than BSA because it does not provide any of the embryonic requirements. Thus, it is possible that in the study by Zhang [12], the beneficial effects of FGF10 could be noticeable for the oocytes. In fact, the beneficial effect of FGF10 was confirmed by the same group blocking FGFR activity during IVM and interfering in embryo development [13]. Under the conditions of IVM in our study, either the FGF10 produced by the oocytes, by cumulus cells, or by the other sources that were sequestered within COCs was sufficient to cause the expected effect, or the concentration used was not enough to enhance embryo production. The lack of response observed in our study has already been noted in CC expansion, which is the main target for FGF10, and so an effect on embryo production could hardly be expected. Pomini Pinto [15] also observed no increase in embryo production, but did not evaluate the expansion of cumulus cells.
To evaluate the quality of embryos produced from oocytes matured in the presence of FGF10, we chose to use two approaches: the speed of development and gene expression. It is also well established that embryos that develop earlier are of better quality than those that develop later, which have been widely used to predict embryo quality. In contrast to the results of Zhang [12], we found no difference between treatments regarding embryo developmental speed, with both groups showing the same percentage of expanded and hatched blastocysts at D7.
The other assessment we used to evaluate embryo quality was gene expression, which has been widely used to predict the ability of the bovine IVP to establish a pregnancy [28–34]. The genes evaluated in the present study were chosen for their correlation with pregnancy outcome and for their involvement with stress and DNA repair. KRT8 is one of the first genes activated after activation of the embryonic genome [35] and is related to post-hatching development [36]. HSPB1 [37] and MSH6 [38] are implicated in cell survival under stress conditions. PLAC8 [39], PAG2 [40, 41], and CD9 [42] are genes related to the development of the placenta, which are essential for successful pregnancy. Differences in the expressions of these genes were not observed between treatments. Indeed, Pomini Pinto [15] only found increases in PLAC8 expression with a higher concentration than that used in this study (50 ng/mL), and, moreover, higher expression was only a tendency (P < 0.10).
In conclusion, the presence of FGF10 during IVM had no effect on embryo production, developmental speed, and gene expression.
Acknowledgements
The authors thank the Brazilian Agricultural Research Corporation (EMBRAPA, 01.13.06.001.04.00) and Coordination for Improvement of Higher Education Personnel (CAPES, 564376/2010-8) for their financial support and the Qualimax (Luziânia-GO) slaughterhouse for providing the necessary biological materials for this experiment.
Footnotes
Capsule Use of FGF 10 during IVM of bovine oocytes does not affect embryonic development.
Contributor Information
Mateus Nunes Diógenes, Email: mateusnunes563@hotmail.com.
Ana Luiza Silva Guimarães, Email: guimaraes.analuiza24@gmail.com.
Ligiane Oliveira Leme, Email: ligileme@gmail.com.
Margot Alves Nunes Dode, Phone: +55 61 34484659, Email: margot.dode@embrapa.br.
References
- 1.Webb R, et al. Intra-ovarian regulation of follicular development and oocyte competence in farm animals. Theriogenology. 2007;68(Suppl 1):S22–9. doi: 10.1016/j.theriogenology.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 2.Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26(1):63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- 3.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–66. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasperin BG, et al. FGF10 inhibits dominant follicle growth and estradiol secretion in vivo in cattle. Reproduction. 2012;143(6):815–23. doi: 10.1530/REP-11-0483. [DOI] [PubMed] [Google Scholar]
- 5.Castilho AC, et al. Expression of fibroblast growth factor 10 and cognate receptors in the developing bovine ovary. Theriogenology. 2014;81(9):1268–74. doi: 10.1016/j.theriogenology.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Portela VM, et al. The role of fibroblast growth factor-18 in follicular atresia in cattle. Biol Reprod. 2015;92(1):14. doi: 10.1095/biolreprod.114.121376. [DOI] [PubMed] [Google Scholar]
- 7.Cho JH, et al. Fibroblast growth factor 7 stimulates in vitro growth of oocytes originating from bovine early antral follicles. Mol Reprod Dev. 2008;75(12):1736–43. doi: 10.1002/mrd.20912. [DOI] [PubMed] [Google Scholar]
- 8.Price CA. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J Endocrinol. 2016;228(2):R31–43. doi: 10.1530/JOE-15-0414. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi F, et al. Aberrant expression of keratinocyte growth factor receptor in ovarian surface epithelial cells of endometrioma. Fertil Steril. 2008;89(2):478–80. doi: 10.1016/j.fertnstert.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Oron G, et al. Fibroblast growth factor 10 in human ovaries. Reprod Biomed Online. 2012;25(4):396–401. doi: 10.1016/j.rbmo.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Buratini J, Jr, et al. Expression and function of fibroblast growth factor 10 and its receptor, fibroblast growth factor receptor 2B, in bovine follicles. Biol Reprod. 2007;77(4):743–50. doi: 10.1095/biolreprod.107.062273. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K, Hansen PJ, Ealy AD. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction. 2010;140(6):815–26. doi: 10.1530/REP-10-0190. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K, Ealy AD. Disruption of fibroblast growth factor receptor signaling in bovine cumulus-oocyte complexes during in vitro maturation reduces subsequent embryonic development. Domest Anim Endocrinol. 2012;42(4):230–8. doi: 10.1016/j.domaniend.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Caixeta ES, et al. Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake, and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus-oocyte complexes. Reproduction. 2013;146(1):27–35. doi: 10.1530/REP-13-0079. [DOI] [PubMed] [Google Scholar]
- 15.Pomini Pinto RF, et al. Effects of FGF10 on bovine oocyte meiosis progression, apoptosis, embryo development and relative abundance of developmentally important genes in vitro. Reprod Domest Anim. 2015;50(1):84–90. doi: 10.1111/rda.12452. [DOI] [PubMed] [Google Scholar]
- 16.Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology. 1995;44(6):859–69. doi: 10.1016/0093-691X(95)00271-9. [DOI] [PubMed] [Google Scholar]
- 17.Machado GM, et al. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology. 2009;71(8):1289–97. doi: 10.1016/j.theriogenology.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Holm P, et al. Developmental kinetics of the first cell cycles of bovine in vitro produced embryos in relation to their in vitro viability and sex. Theriogenology. 1998;50(8):1285–99. doi: 10.1016/S0093-691X(98)00227-1. [DOI] [PubMed] [Google Scholar]
- 19.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139–49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Buchtova M, et al. Instability restricts signaling of multiple fibroblast growth factors. Cell Mol Life Sci. 2015;72(12):2445–59. doi: 10.1007/s00018-015-1856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang CG, et al. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21(9):2037–55. doi: 10.1210/me.2006-0408. [DOI] [PubMed] [Google Scholar]
- 24.Salhab M, et al. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol Reprod Dev. 2013;80(2):166–82. doi: 10.1002/mrd.22148. [DOI] [PubMed] [Google Scholar]
- 25.Tomek W, Smiljakovic T. Activation of Akt (protein kinase B) stimulates metaphase I to metaphase II transition in bovine oocytes. Reproduction. 2005;130(4):423–30. doi: 10.1530/rep.1.00754. [DOI] [PubMed] [Google Scholar]
- 26.Ali A, Sirard MA. Effect of the absence or presence of various protein supplements on further development of bovine oocytes during in vitro maturation. Biol Reprod. 2002;66(4):901–5. doi: 10.1095/biolreprod66.4.901. [DOI] [PubMed] [Google Scholar]
- 27.Ali A, Sirard MA. The effects of 17beta-estradiol and protein supplement on the response to purified and recombinant follicle stimulating hormone in bovine oocytes. Zygote. 2002;10(1):65–71. doi: 10.1017/S0967199402002095. [DOI] [PubMed] [Google Scholar]
- 28.Ghanem N, et al. Bovine blastocysts with developmental competence to term share similar expression of developmentally important genes although derived from different culture environments. Reproduction. 2011;142(4):551–64. doi: 10.1530/REP-10-0476. [DOI] [PubMed] [Google Scholar]
- 29.Machado GM, et al. Post-hatching development of in vitro bovine embryos from day 7 to 14 in vivo versus in vitro. Mol Reprod Dev. 2013;80(11):936–47. doi: 10.1002/mrd.22230. [DOI] [PubMed] [Google Scholar]
- 30.El-Halawany N, et al. Quantitative expression analysis of blastocyst-derived gene transcripts in preimplantation developmental stages of in vitro-produced bovine embryos using real-time polymerase chain reaction technology. Reprod Fertil Dev. 2005;16(8):753–62. doi: 10.1071/RD04041. [DOI] [PubMed] [Google Scholar]
- 31.El-Sayed A, et al. Large-scale transcriptional analysis of bovine embryo biopsies in relation to pregnancy success after transfer to recipients. Physiol Genomics. 2006;28(1):84–96. doi: 10.1152/physiolgenomics.00111.2006. [DOI] [PubMed] [Google Scholar]
- 32.Wrenzycki C, Herrmann D, Niemann H. Messenger RNA in oocytes and embryos in relation to embryo viability. Theriogenology. 2007;68:S77–83. doi: 10.1016/j.theriogenology.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Hoelker M, et al. Molecular signatures of bovine embryo developmental competence. Reprod Fertil Dev. 2013;26(1):22–36. doi: 10.1071/RD13255. [DOI] [PubMed] [Google Scholar]
- 34.Loren, P., et al. Effect of short-term exposure of cumulus–oocyte complex to 3-morpholinosydnonimine on in vitro embryo development and gene expression in cattle. Reproduction in Domestic Animals. 2016; 51(6):1010–19. [DOI] [PubMed]
- 35.Jackson BW, et al. Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation. 1980;17(3):161–79. doi: 10.1111/j.1432-0436.1980.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 36.Machado GM, et al. Post-hatching development of bovine embryos in vitro: the effects of tunnel preparation and gender. Zygote. 2012;20(2):123–34. doi: 10.1017/S0967199411000086. [DOI] [PubMed] [Google Scholar]
- 37.Skouri-Panet F, et al. Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: role of the in vitro hetero-complex formation in chaperone activity. Biochimie. 2012;94(4):975–84. doi: 10.1016/j.biochi.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Habraken Y, et al. Binding of insertion/deletion DNA mismatches by the heterodimer of yeast mismatch repair proteins MSH2 and MSH3. Curr Biol. 1996;6(9):1185–7. doi: 10.1016/S0960-9822(02)70686-6. [DOI] [PubMed] [Google Scholar]
- 39.Galaviz-Hernandez C, et al. Plac8 and Plac9, novel placental-enriched genes identified through microarray analysis. Gene. 2003;309(2):81–9. doi: 10.1016/S0378-1119(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 40.Touzard E, et al. Specific expression patterns and cell distribution of ancient and modern PAG in bovine placenta during pregnancy. Reproduction. 2013;146(4):347–62. doi: 10.1530/REP-13-0143. [DOI] [PubMed] [Google Scholar]
- 41.Green JA, et al. The establishment of an ELISA for the detection of pregnancy-associated glycoproteins (PAGs) in the serum of pregnant cows and heifers. Theriogenology. 2005;63(5):1481–503. doi: 10.1016/j.theriogenology.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Xiang W, MacLaren LA. Expression of fertilin and CD9 in bovine trophoblast and endometrium during implantation. Biol Reprod. 2002;66(6):1790–6. doi: 10.1095/biolreprod66.6.1790. [DOI] [PubMed] [Google Scholar]