Abstract
Purpose
This study was conducted to examine the dynamic distribution of polo-like 1 kinase (Plk1) and the possible role it plays in first mitotic division during early porcine embryo development.
Methods
Indirect immunofluorescence and confocal microscopy imaging techniques combined with western blot analyses were used to study the dynamic expression and subcellular localization of Plk1 protein in pig parthenogenetic embryos. Finally, a selective Plk1 inhibitor, GSK461364, was used to evaluate the potential role of Plk1 during this special stage.
Results
The results showed that Plk1 upon expression exhibited specific dynamic intracellular localization, which closely correlated with the α-tubulin distribution during the first mitotic division. GSK461364 treatment resulted in cleavage failure, with majority of the GSK461364-treated embryos being arrested in prometaphase. Further results of the subcellular structure examination showed that GSK461364 treatment led to a significantly higher proportion of the treated embryos having abnormal spindles and misarranged chromosomes at the prometaphase stage.
Conclusions
Thus, these results indicated that Plk1 is essential for porcine embryos to complete the first mitotic division. Furthermore, Plk1 regulation was associated with effects on spindle assembly and chromosome arrangement.
Keywords: Pig, Polo-like 1, Embryo, Mitotic division, Spindle
Introduction
Polo-like 1 kinase (Plk1), an important member of the family of serine/threonine protein kinases, has been related to various mitotic functions in the regulation of multiple processes in mitosis [1, 2]. The multi-functionality of Plk1 is coupled with its dynamic subcellular localization during mitotic division [3, 4]. Plk1, primarily located in the centrosome and kinetochore during prophase, shifts to the spindle intermediate region in meta-anaphase and then concentrates at the equatorial plate during cytokinesis [5, 6]. Plk1 consists of an N-terminal kinase domain and a phosphorylation sequence of the C-terminal Polo box domain (PBD) [7, 8]. It is mainly located in different subcellular structures based on the PBD and phosphorylation of Thr210 [1, 5]. It is involved in the regulation of Mps1, Bub3, Mad1, and other proteins [9]. In human somatic cells, inhibited Plk1 leads to multiple mitotic defects, including the formation of abnormal spindles, misaligned chromosomes and improper chromosome condensation [10]. Additionally, up- or downregulation of Plk1 can induce a variety of human tumor cells or mouse embryonic cell mitotic defects, causing aneuploidy or cancer [11–13]. This study shows that Plk1 might play a critical role in the process of bipolar spindle assembly and chromosome arrangement during mitosis in somatic cells.
Mammalian early embryo development mechanisms have attracted increasing attention in research worldwide. It is arguably the most important field in developmental biology, especially the exact mechanism of transition from 1-cell to 2-cell stages. In mammalian early embryo development, the conversion from 1-cell to 2-cell stages and the successful completion of first mitotic division are essential for embryo development [1]. However, data on failure of embryos to successfully finish their first cleavage in vitro are very limited. During the first mitotic division, successful chromosome separation and cytokinesis requires multiple spatial biological events, such as accurate bipolar spindle assembly and correct chromosome arrangement [14]; the failure of any of these processes can lead to developmental defects in early embryo development.
Plk1, as a characteristic regulator in mitosis, is mainly concentrated in the somatic or tumor cell models; however, the regulation of Plk1 in embryonic mitosis is not fully understood. Baran et al. found that Plk1-inhibited mouse embryos were arrested at the first metaphase and displayed aberrant chromosome arrangement in the metaphase plate and corrupt mitotic spindles. Plk1 homozygous null mice were embryonically lethal. Also, early depleted-Plk1 embryos failed to survive after the 8-cell stage [13]. In the present study, indirect immunofluorescence combined with western blot analyses were used to study the dynamic distribution and subcellular localization of the Plk1 protein. The specific inhibitor GSK461364 was used to examine the possible role of Plk1 in porcine embryo undergoing mitosis. We found that Plk1 expression exhibited specific dynamic intracellular localization pattern, which was associated with the distribution of α-tubulin. Plk1 inhibition by GSK461364 affected the mitotic division of embryos, resulting in most embryos being arrested in the prometaphase stage with abnormal spindles and misarranged chromosomes. These findings suggest that Plk1 may played an indispensable role in first mitotic division through the regulation of spindle assembly and chromosome arrangement during the prometaphase stage in porcine embryo development.
Materials and methods
Antibodies and chemicals
Mouse monoclonal anti-Plk1 was purchased from Abcam (Cambridge, UK), GSK641364 inhibitor from Selleck Chemicals, and anti-GAPDH mouse polyclonal antibody from Yi Feixue (Nanjing, China). All other chemicals and reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Oocyte collection and in vitro cultures
Porcine ovaries were collected from the Yuan Run (Nanjing) slaughterhouse, stored in sterile saline, and sent to the laboratory within 1 h. The cumulus oocyte complexes (COCs) were aspirated from follicles with 3–6-mm diameters; homogeneous COCs were selected for further development and transferred to TCM199 medium (Gibco BRL, Gaithersburg, MD, USA). Oocytes were then cultured at 38.5 °C in a 5% CO2 incubator for maturation [15].
Activation of porcine oocytes and in vitro cultures
After in vitro maturation for 44 h, 0.1% hyaluronidase was used to remove cumulus cells surrounding the oocytes. The oocytes with uniform ooplasms, intact cytoplasmic membranes, and visible first polar body in the perivitelline space were considered to have reached the second meiotic metaphase (MII) stage. MII oocytes were equilibrated in activation medium (0.3 M mannitol, 1 mM CaCl2, 0.1 mM MgCl2, and 0.1% bovine serum albumin, BSA) [15] for 3 min and activated with a single direct current (DC) pulse of 1.5 kV/cm for 80 μs using CRY-3 electric stimulation (Ningbo Xinzhi Co., Ltd., Ningbo, China). Activated oocytes were washed three times and moved to porcine zygote medium 3 (PZM-3) for embryo culture in vitro. According to the experimental design, 50 embryos of mitotic prophase, prometaphase, meta-anaphase, and telophase were collected for subsequent indirect immunofluorescent analysis.
Immunofluorescence staining and confocal microscopy
Collected samples were fixed in 4% paraformaldehyde in phosphate buffered solution (PBS) for 30 min at room temperature and then moved to 1% Triton X-100 at 37 °C for 8 h. Embryos were later placed into 1% BSA for 1 h and incubated overnight at 4 °C with a mouse monoclonal anti-Plk1 antibody (1:100) or anti-α-tubulin-FITC antibody (1:200), then washed three times in PBS containing 0.1% Tween 20. The embryos were then incubated with a Cy3-labeled goat anti-mouse IgG (H + L) (Beyotime) (1:100) at room temperature for 1 h. After washing three times, embryos were transferred to microfilament dye (phalloidin-TRITC) (1:200) at room temperature for 40 min. Finally, cells were stained with Hoechst 33342 for 10 min and mounted on slides for confocal laser scanning microscopy imaging (Zeiss LSM700 meta, Oberkochen, Germany).
Western blot analyses
One hundred embryos in prophase, prometaphase, meta-anaphase, and telophase were collected and transferred into 12 μL mercaptoethanol with SDS sample buffer. They were then placed in boiling water for 10 min to dissociate. After cooling, the samples were centrifuged and separated by 10% SDS-PAGE electrophoresis. Then, embryos were shifted onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA) and shaken in blocked liquid with 5% skim milk or BSA in Tris-buffered saline Tween 20 (TBST) at room temperature for 2 h. Membranes were incubated with mouse monoclonal anti-Plk1 (1:500) or mouse polyclonal anti-GAPDH antibody (1:3000) at 4 °C overnight. After washing three times in TBST, membranes were incubated with goat anti-mouse IgG (1:3000; Bioworld Technology, Nanjing, China) at room temperature for 2 h. The membranes were washed, and chemiluminescence reagent (1:1; Millipore, Billerica, MA) was used to soak them for visualization. Finally, the protein level was assessed by the ratio of protein and strategists (Plk1/GAPDH).
Treatment with the inhibitor GSK461364
The Plk1-selective inhibitor GSK461364 was prepared in stock solution of 5 mM DMSO and kept at –20 °C. To explore the effect of Plk1 inhibition on first mitotic division, the activated embryos were divided into four groups randomly (at least 50 embryos per group) and then placed into PZM-3 medium containing 0, 50, 100, or 200 nM GSK461364. After 48 h, cleaved embryos were examined under a stereomicroscope, and indirect immunofluorescence staining was used to observe and analyze the effects of Plk1inhibition on the subcellular structure of the cell skeleton.
Statistical analysis
Experiments were repeated at least three times. Most of the results were analyzed using one-way ANOVA followed by Duncan’s multiple comparisons test with GraphPad Prism 5 software; the Plk1 protein level was compared using Quantity One software. The results are given as means ± SEM. P <0.05 was seen as significant.
Results
Dynamic distribution of cytoskeleton
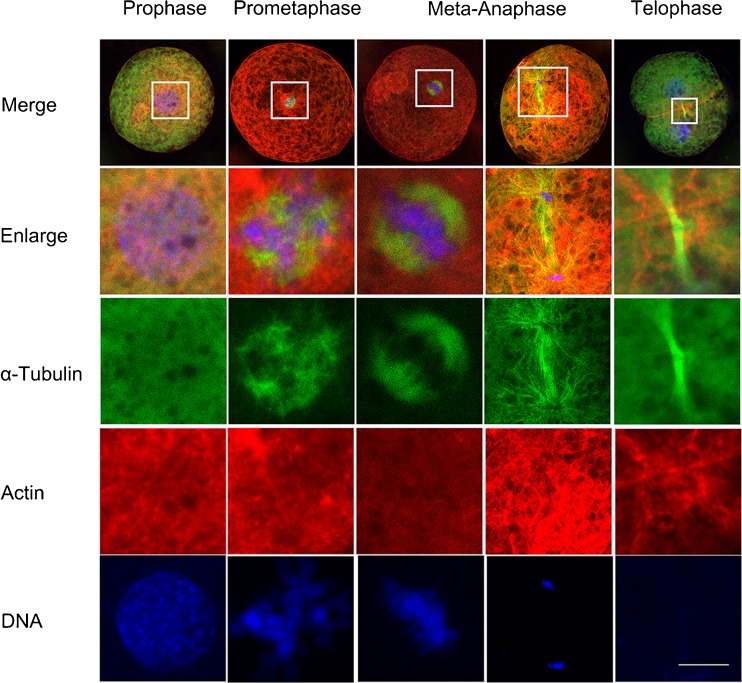
To explore the expression and regulation of Plk1 in porcine embryos during the first mitotic division, we systematically examined the dynamic distribution of cytoskeleton at different mitotic stages of the porcine embryo using immunofluorescent staining techniques. As shown in Fig. 1, microtubules demonstrated a relatively uniform distribution throughout the cytoplasm and nuclei with one to two nucleoli were at the central part of the embryo at prophase. However, microtubules were condensed around the mitotic spindle of the prometaphase, α-tubulin gradually gathered to form a network structure, and the nuclear envelope broke down. Chromatin content has become condensed and formed thick chromosomal structures. In metaphase, α-tubulin was observed as organized mitotic bipolar spindles that were symmetrical and barrel in shape and localized in the central region. Chromosomes were arranged on the equatorial plate as usual, followed by spindle traction. In anaphase, microtubules were observed around the chromosomal sets which were in the two polar regions. Although microfilaments were frequently located outside the chromosome-rich domain, they concentrated at the cortical cytoplasmic region before telophase. At telophase, α-tubulin was assembled as a midbody in the cleavage furrow and the chromosomes decondensed to form two new nuclei with two daughter cells containing microfilaments beneath the cell membrane and in the cleavage furrow. These results indicated that the dynamic and spatial distributions of microtubules and microfilaments are closely related to first mitotic division progression in porcine embryos.
Fig. 1.
Dynamic distribution of the cytoskeleton during the first mitosis in early porcine embryos. Samples were taken at prophase, prometaphase, meta-anaphase, and telophase. Microtubules had no obvious concentration, and the nuclear membrane maintained integrity at the prophase stage. α-Tubulin gathered to form a network structure, and chromatins became condensed and formed chromosome at the prometaphase. α-Tubulin was organized in a bipolar, symmetrical, barrel-shaped structure in the central region, and chromosomes were arranged on the equatorial plate at metaphase. At anaphase, sister chromatids were moved to two polar sites. Microfilaments were concentrated in the cytoplasm before telophase. α-Tubulin was concentrated in the cleavage furrow, chromosomes were shaped into two new nuclei, and microfilaments beneath the cleavage furrow at telophase. Green, microtubules (α-tubulin); red, microfilaments; blue, chromosomes. Scale bar, 10 μm
Dynamic expression and subcellular localization of Plk1
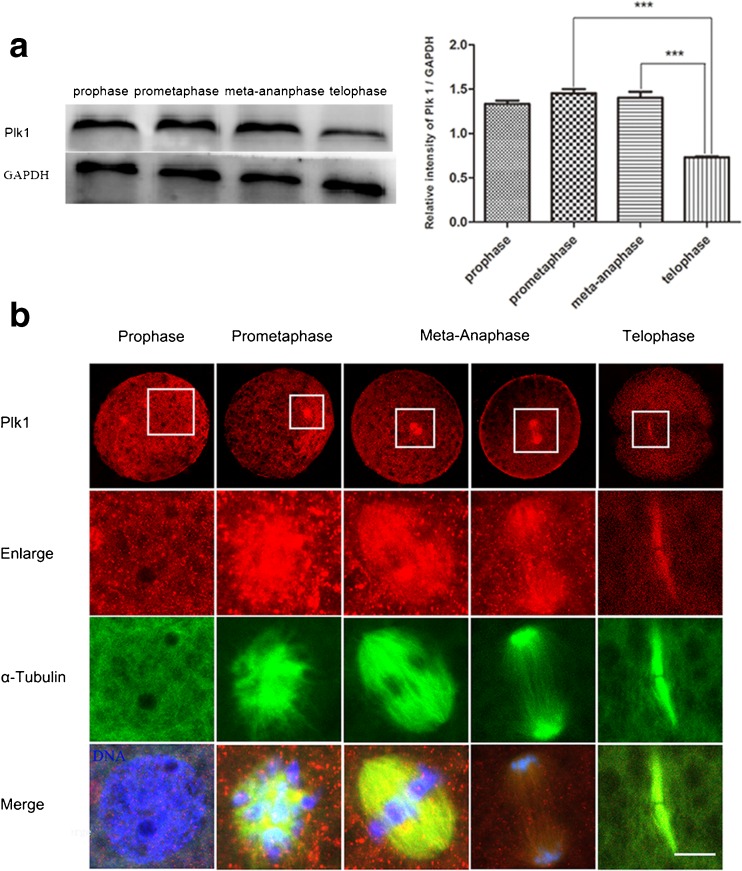
Based on the detection of the dynamic cytoskeleton distribution, we examined the expression of Plk1 at different stages of the first mitotic cycle using western blots. From Fig. 2a, Plk1 was expressed at different stages in porcine embryos, and the Plk1 protein showed a significantly higher level in prometaphase and meta-anaphase compared to that of telophase (P < 0.05), which indicated that Plk1 may play an important role in these two stages. The subcellular localization of Plk1 was explored at different mitotic stages using immunofluorescence staining. As shown in Fig. 2b, Plk1 was found scattered throughout the cytoplasm, with no obvious enrichment in the prophase stage. After prometaphase, Plk1 was associated significantly with the spindles. In metaphase, Plk1 was concentrated at the central region and symmetrically distributed around the chromosome, with similar subcellular localization as in the spindles. When embryos progressed to anaphase, Plk1 was moved to the polar sites, which followed by the sister chromatids. Plk1 and α-tubulin accumulated at the midbody in telophase. The above results showed that in the expression and subcellular localization processes, Plk1 had obvious periodic characteristics that closely correlated to the distribution of α-tubulin. This expression and localization pattern indicated that possibly Plk1 may be involved in regulating spindle assembling processes during the first mitotic division in porcine embryos.
Fig. 2.
Expression and subcellular localization of Plk1 in porcine embryos undergoing the first mitotic division. a Expression of Plk1 was examined using western blot analysis. Plk1 was expressed in porcine embryos during the first mitotic division, and a significantly higher Plk1 protein level was detected in prometaphase compared to telophase; *P < 0.05. b Subcellular localization of Plk1 in porcine embryos using immunofluorescent staining. Plk1 expression demonstrated no obvious enrichment in the prophase stage, but it appeared to accumulate at the chromosomes in prometaphase. After prometaphase, Plk1 was concentrated in the spindle region. Red, Plk1; green, spindle; blue, chromosome. Scale bar, 10 μm
GSK461364 treatment inhibits the first mitotic division of porcine embryos
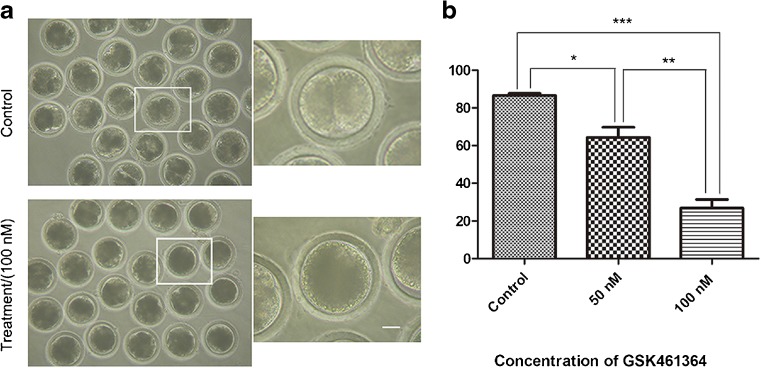
To further study the roles of Plk1 during the first mitotic division, embryos were treated with a Plk1 specific inhibitor, GSK461364, for 48 h to inhibit Plk1 activity, and the cleavage of embryos were examined under a stereomicroscope. As shown in Fig. 3a, compared with the control, a significantly large proportion of the treated embryos failed to complete the first cleavage, and the cleavage rates decreased in a dose-dependent manner in the treatment groups (Fig. 3b). After 48 h in culture, the cleavage rate in the control group was 86.52 ± 0.96% (n = 110), while that of the treated groups (50 or 100 nM GSK461364) was 64.31 ± 4.39% (n = 109, P < 0.05) and 26.85 ± 3.78% (n = 108, P < 0.001), respectively. The results showed that Plk1 inhibition can lead to a failure of embryonic cleavage, which suggested that Plk1 may play a critical role during the first mitotic division in porcine embryos.
Fig. 3.
Effect of GSK461364 treatment on the cleavage of porcine embryo. a After in vitro culture for 48 h, most of the control embryos had finished the first cleavage, and embryos failed to complete the first cleavage after treatment with GSK461364. Scale bar, 20 μm. b GSK461364 treatment decreased the cleavage rate for embryos in a concentration-dependent manner. The cleavage rate was significantly reduced after the 50 and 100 nM of GSK461364 treatments. *P < 0.05, **P < 0.01, ***P < 0.001
GSK461364 treatment disrupts the cell cycle during the first mitotic progression
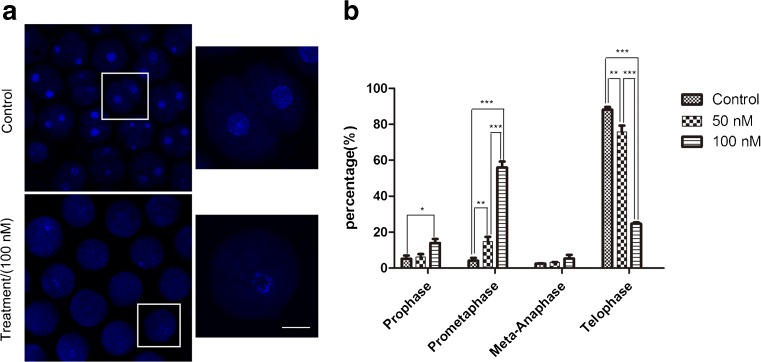
To analyze the reason why porcine embryos failed to complete the first cleavage after Plk1 inhibition and clarify the regulative role of Plk1, which may be involved in any events during the first mitosis in porcine embryos, proportions of porcine embryos at different mitotic stages were stuck after inhibiting Plk1 activity. From Fig. 4a, after culturing for 48 h with GSK461364, most control embryos finished the first cleavage and reached the cytokinesis stage, whereas most inhibitor-treated embryos were arrested at the prometaphase stage. In Fig. 4b, the percentage of embryos that reached the cytokinesis stage reduced rapidly after treatment with 50 or 100 nM GSK461364: 88.14 ± 1.23%, n = 92 vs. 75.75 ± 2.92%, n = 95 and 24.71 ± 0.59%, n = 100, respectively. This comparison was made with the control. The percentage of embryos that remained in the prometaphase stage obviously increased when treated with 50 and 100 nM GSK461364: 4.15 ± 1.30%, n = 92 vs. 14.80 ± 2.12%, n = 95 and 55.99 ± 2.68%, n = 100, respectively. The above results showed that Plk1 inhibition resulted in failure of embryonic mitotic progression and this blocked the cell cycle from progressing to the metaphase stage. This indicates that Plk1 might be required for the first mitotic division of normal porcine embryo during the prometaphase stage.
Fig. 4.
Effect of GSK461364 on cell cycle progression during the first mitosis in porcine embryos. a Immunofluorescent images were used in porcine embryo cultured with or without GSK461364. After in vitro culture for 48 h, most of the control embryos reached the cytokinesis stage, while the majority of Plk1-inhibited embryos were arrested at the prometaphase stage. Blue, chromosome. Scale bar, 25 μm. b The proportion of embryos at different stages. Most embryos were arrested at the prometaphase stage after Plk1 inhibition. Compared to the control group, the percentage of Plk1-inhibited embryos that finished the first cleavage was sharply decreased, whereas the proportion of embryos which were arrested at the prometaphase stage was significantly increased. *P < 0.05, **P < 0.01, ***P < 0.001
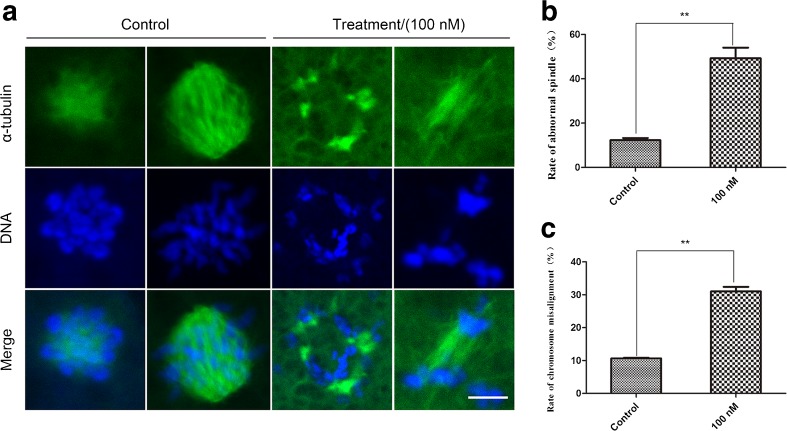
Disrupting Plk1 activity results in abnormal spindles and chromosome misalignment during the prometaphase stage
To ascertain why Plk1-inhibited embryos are arrested at the mitotic prometaphase stage, the subcellular structure of the cytoskeleton was examined from the prometaphase to metaphase stages. Immunofluorescence staining was used to analyze the variation in spindle and chromosome structures after treatment with 100 nM of GSK461364, and observations were made through a confocal microscope. In Fig. 5a, most of the microtubules were condensed around the mitotic spindle of the prometaphase and spindle showed a typical network structure in the control group. Chromosomes formed masses and exhibited regular arrangement; however, a large proportion of the inhibited embryos showed severe spindle morphology disruptions and chromosome misalignments. They exhibited irregular arrangements of the spindles, even without the appearance of a spindle phenomenon, and the chromosomes were randomly scattered in the cytoplasm. In Fig. 5b, the percentage of GSK461364-treated embryos with aberrant spindles (49.26 ± 3.94%, n = 122 for the control vs. 12.27 ± 0.83%, n = 100, P < 0.01) and misaligned chromosomes (31.05 ± 1.13%, n = 100, n = 122 for the control vs. 10.65 ± 0.08%, P < 0.01) were significantly increased compared to that of the control group. The above results showed that embryos without Plk1 activity had aberrant spindle assembly and chromosome arrangement as well as disrupted embryonic mitotic progression from the prometaphase stage. These results suggested that Plk1 might be involved in the regulation of the first mitotic division in porcine embryos through spindle assembly and chromosome alignment in the prometaphase stage.
Fig. 5.
Effect of GSK461364 on the morphology of spindles and chromosomes in early porcine embryonic cells. a GSK461364 treatment lead to aberrant spindles and misaligned chromosomes in porcine embryos. b The percentage of Plk1-inhibited embryos with aberrant spindles and misaligned chromosomes was obviously increased compared to the control embryos. Green, spindle; blue, chromosome. Scale bar, 5 μm. **P < 0.01
Discussion
Plk1 is a serine/threonine protein kinase that represents a mitotic target due to its important role in centrosome maturation [16], bipolar spindle formation [17], and other functions. Overexpression of Plk1 has been commonly detected in tumors. It is involved in regulating multiple biological events in cancer cell proliferation [14]. In recent years, Plk1 has been discovered to also play regulatory roles in germ cells and oocytes, such as mouse [18], porcine [19], and starfish [20]. The recent focus of research has been on some “experimental or model” animals, such as Drosophila melanogaster [21], zebrafish [2], and mice [1]. However, the detailed role of Plk1 in pig or other domestic animal embryos is not fully established yet.
In this study, we first examined the expression and subcellular localization of Plk1 in porcine embryos during the first cleavage. Previous studies have shown that Plk1 was distributed at the centromere during the prophase and prometaphase stages and then relocalized at spindles next to the equatorial plate in anaphase. It was also localized at the midbody in telophase in HeLa cells [7]. Haren et al. found that recruitment of γ-tubulin and AURKA (Aurora Kinase A) to the mitotic centrosome were Plk1 dependent before spindle formation in HeLa cells [22]. Petronczki et al. also found that Plk1 was localized to the centromere at the metaphase stage and involved in the stability of spindle assembly checkpoint (SAC)-dependent kinetochore-microtubule interactions. In telophase, Plk1 was concentrated at the middle area of the spindle from where it facilitated the sliding of microtubules. After chromosome separation, the Plk1 spindle was still concentrated in the midbody and was involved in the formation of cleavage furrow [23]. In a different study, Plk1 was accompanied by the expression of α-tubulin, after prometaphase, which indicated that Plk1 may be involved in spindle-related processes during mitosis of porcine embryos. These studies showed that Plk1 exhibited periodic and dynamic subcellular localization and played a conserved role in the regulation of spindle formation during the first mitosis in early embryos. The protein level of Plk1 fluctuated in a cell-cycle-dependent manner in mouse cells cultured in vitro, increasing at the S and G2/M phases, and swiftly diminished at the end of mitosis [24]. Our report also showed that expression of Plk1 gradually increased from prophase to meta-anaphase and reached its maximum at the prometaphase stage before it decreased in telophase of the first cleavage. These data are consistent with previous studies which indicated that Plk1 was differentially expressed at various stages and play a critical role in prometaphase stage.
To explore the specific role of Plk1 in porcine embryos undergoing first mitotic division, a highly selective inhibitor of Plk1, GSK461364, was used. Gilmartin et al. found that GSK461364 caused lung carcinoma cells to arrest at prometaphase, and at lower concentrations, mitotic cells exhibited abnormal spindles and chromatins that could not be arranged on the equatorial plate. At higher concentrations, a monopolar spindle was formed and the chromatin decondensed around the monopolar spindle [25]. Another study reported that null-Plk1 led to the activation of SAC which was arrested at the prometaphase stage, thus showing developmental defects in zebrafish embryonic cells [2]. In line with previous studies, our results showed that majority of treated embryos failed to complete the first cleavage and were arrested at the prometaphase stage, which demonstrated a failure of mitotic division in porcine embryos. These findings indicated that Plk1 is one of the essential regulatory factors during the first mitotic division in embryo development, especially in the prometaphase stage.
In addition, we studied the mechanism by which Plk1 inhibition affected pig embryo development during the first mitotic division, with most embryos arrested at the prometaphase stage. The transition from mitotic prometaphase to metaphase includes the breakdown of the nuclear envelope, arrangement of chromosomes at the equatorial plate, and the subsequent movement to the two polar sites. During prometaphase, chromosomes were interspersed in the spindle, and after chromosome condensation, normally, two centromere-driven chromosomes wait for arrangement in the equatorial plate [26]. During this process, dynamic chromosomal and spindle events are required for the successful transition through metaphase, such as proper chromosome alignment and correct bipolar spindle assembly. Consistent with the function of Plk1 in human somatic cells mitosis, Plk1-inhibited cells demonstrated abnormal spindle formation, eventually leading to the failure of cell mitosis [23]. Studies have shown that Drosophila Polo mutants exhibited highly condensed chromosomes, monopolar spindles, spindle disassembly, and abnormal chromosome segregation [27]. Another study indicated that a Plk1 gene mutant in yeast caused mitotic arrest due to chromosome misalignment and aberrant bipolar spindle formation [2]. This showed that the observed phenotype has aberrant spindle formation and abnormal chromosome alignment during this process. Our study found that inhibition of Plk1 induced misaligned chromosomes and aberrant spindle morphology in porcine embryonic cells, which exhibited monopolar or ring-shaped spindles, and ring-shaped or dispersive chromosomes. These defects may be due to the recently discovered role of PLK1 in MCAK stability [28]. Baran et al. concluded that PLK1 activity is not required for spindle bipolarization in mouse zygote but facilitates spindle formation partly by promoting γ-tubulin loading to microtubule-organizing centers (MTOCs) [26]. We also have shown that spindle formation is disturbed (Fig.5a, b) in pig embryos and then caused the failure of spindle bipolarization. These results suggest that Plk1 may facilitate the onset of spindle formation. According to a recent report, LRRK1 regulates mitotic spindle orientation downstream of PLK1 through CDK5RAP2-dependent centrosome maturation [29]. This report showed that Plk1 may control the onset of spindle formation through regulating phosphorylation of LRRK1. Taken together, these studies show that Plk1 might play an indispensable role in the regulation of accurate spindle assembly and chromosome arrangement during the first mitotic division. Further studies are required to determine how Plk1 can regulate the onset of spindle formation during the first mitotic division in pig embryos.
In conclusion, the results of this study indicate that Plk1 is indispensable for first mitotic division of porcine embryos. The regulation of Plk1 is associated with spindle assembly and chromosome arrangement during the prometaphase stage of the first mitosis in pig embryo.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (31572589 and 31201967), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20130097110020), the Natural Science Foundation (BK2012369), and Priority Academic Program Development (PAPD) of Jiangsu Province. We are thankful to Hao Zhang for providing porcine ovaries. We also express our gratitude to Guoqing Huang for his help with using confocal laser-scanning microscopy.
Compliance with ethical standards
Funding
This work was supported by the Natural Science Foundation of China (31572589 and 31201967), the Specialized Research Fund for Doctoral Program of Higher Education of China (20130097110020), the Natural Science Foundation (BK2012369), and Priority Academic Program Development (PAPD) of Jiangsu Province.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed were in accordance with institutional, national, and/or international guidelines with comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Reference
- 1.Baran V, Solc P, Kovarikova V, Rehak P, Sutovsky P. Polo-like kinase 1 is essential for the first mitotic division in the mouse embryo. Mol Reprod Dev. 2013;80:522–34. doi: 10.1002/mrd.22188. [DOI] [PubMed] [Google Scholar]
- 2.Jeong K, Jeong JY, Lee HO, Choi E, Lee H. Inhibition of Plk1 induces mitotic infidelity and embryonic growth defects in developing zebrafish embryos. Dev Biol. 2010;345:34–48. doi: 10.1016/j.ydbio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Lu LY, Yu X. The balance of Polo-like kinase 1 in tumorigenesis. Cell Div. 2009;4:4. doi: 10.1186/1747-1028-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Weerdt BC, Medema RH. Polo-like kinases: a team in control of the division. Cell Cycle. 2006;5:853–64. doi: 10.4161/cc.5.8.2692. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa H, Hyodo T, Asano E, Ito S, Maeda M, Kuribayashi H, et al. The role of PLK1-phosphorylated SVIL in myosin II activation and cytokinetic furrowing. J Cell Sci. 2013;126:3627–37. doi: 10.1242/jcs.124818. [DOI] [PubMed] [Google Scholar]
- 6.Wachowicz P, Fernandez-Miranda G, Marugan C, Escobar B, de Carcer G. Genetic depletion of Polo-like kinase 1 leads to embryonic lethality due to mitotic aberrancies. BioEssays: News Rev Mole, Cell Dev Biol. 2016;38(Suppl 1):S96–S106. doi: 10.1002/bies.201670908. [DOI] [PubMed] [Google Scholar]
- 7.Maia AR, Garcia Z, Kabeche L, Barisic M, Maffini S, Macedo-Ribeiro S, et al. Cdk1 and Plk1 mediate a CLASP2 phospho-switch that stabilizes kinetochore-microtubule attachments. J Cell Biol. 2012;199:285–301. doi: 10.1083/jcb.201203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaoka M, Saito H, Takenaka K, Miki Y, Nakanishi A. BRCA2 phosphorylated by PLK1 moves to the midbody to regulate cytokinesis mediated by nonmuscle myosin IIC. Cancer Res. 2014;74:1518–28. doi: 10.1158/0008-5472.CAN-13-0504. [DOI] [PubMed] [Google Scholar]
- 9.von Schubert C, Cubizolles F, Bracher JM, Sliedrecht T, Kops GJ, Nigg EA. Plk1 and Mps1 cooperatively regulate the spindle assembly checkpoint in human cells. Cell Rep. 2015;12:66–78. doi: 10.1016/j.celrep.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Schmit TL, Nihal M, Ndiaye M, Setaluri V, Spiegelman VS, Ahmad N. Numb regulates stability and localization of the mitotic kinase PLK1 and is required for transit through mitosis. Cancer Res. 2012;72:3864–72. doi: 10.1158/0008-5472.CAN-12-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang GJ, Zhang Z, Liu ZG. Polo-like kinase 1 is overexpressed in renal cancer and participates in the proliferation and invasion of renal cancer cells. Tumor Biol. 2013;34:1887–94. doi: 10.1007/s13277-013-0732-0. [DOI] [PubMed] [Google Scholar]
- 12.Liu XQ. Targeting Polo-like kinases: a promising therapeutic approach for cancer treatment. Transl Oncol. 2015;8:185–95. doi: 10.1016/j.tranon.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu LY, Wood JL, Minter-Dykhouse K, Ye L, Saunders TL, Yu XC, et al. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870–6. doi: 10.1128/MCB.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu YY, Wang C, Lan JP, Yu J, Jin CJ, Huang H. Phosphorylation of Tara by Plk1 is essential for faithful chromosome segregation in mitosis. Exp Cell Res. 2012;318:2344–52. doi: 10.1016/j.yexcr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Lai L, Prather RS. Production of cloned pigs by using somatic cells as donors. Cloning Stem Cells. 2003;5:233–41. doi: 10.1089/153623003772032754. [DOI] [PubMed] [Google Scholar]
- 16.Chen YJ, Lai KC, Kuo HH, Chow LP, Yih LH, Lee TC. HSP70 colocalizes with PLK1 at the centrosome and disturbs spindle dynamics in cells arrested in mitosis by arsenic trioxide. Arch Toxicol. 2014;88:1711–23. doi: 10.1007/s00204-014-1222-x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Kim EJ, Oh JS, Park IC, Hwang SG. CIP2A modulates cell-cycle progression in human cancer cells by regulating the stability and activity of Plk1. Cancer Res. 2013;73:6667–78. doi: 10.1158/0008-5472.CAN-13-0888. [DOI] [PubMed] [Google Scholar]
- 18.Sun SC, Liu HL, Sun QY. Survivin regulates Plk1 localization to kinetochore in mouse oocyte meiosis. Biochem Bioph Res Co. 2012;421:797–800. doi: 10.1016/j.bbrc.2012.04.089. [DOI] [PubMed] [Google Scholar]
- 19.Anger M, Klima J, Kubelka M, Prochazka R, Motlik J, Schultz RM. Timing of Plk1 and MPF activation during porcine oocyte maturation. Mol Reprod Dev. 2004;69:11–6. doi: 10.1002/mrd.20151. [DOI] [PubMed] [Google Scholar]
- 20.Okano-Uchida T, Okumura E, Iwashita M, Yoshida H, Tachibana K, Kishimoto T. Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles. Embo J. 2003;22:5633–42. doi: 10.1093/emboj/cdg535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenton B, Glover DM. A conserved mitotic kinase active at late anaphase-telophase in syncytial Drosophila embryos. Nature. 1993;363:637–40. doi: 10.1038/363637a0. [DOI] [PubMed] [Google Scholar]
- 22.Haren L, Stearns T, Luders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. Plos One. 2009;4:e5976. doi: 10.1371/journal.pone.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petronczki M, Lenart P, Peters JM. Polo on the rise—from mitotic entry to cytokinesis with Plk1. Dev Cell. 2008;14:646–59. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Su WH, Feng C, Yu DH, Cui C, Xu XY, et al. Polo-like kinase 1 may regulate G2/M transition of mouse fertilized eggs by means of inhibiting the phosphorylation of Tyr 15 of Cdc2. Mol Reprod Dev. 2007;74:1247–54. doi: 10.1002/mrd.20703. [DOI] [PubMed] [Google Scholar]
- 25.Gilmartin AG, Bleam MR, Richter MC, Erskine SG, Kruger RG, Madden L, et al. Distinct concentration-dependent effects of the polo-like kinase 1-specific inhibitor GSK461364A, including differential effect on apoptosis. Cancer Res. 2009;69:6969–77. doi: 10.1158/0008-5472.CAN-09-0945. [DOI] [PubMed] [Google Scholar]
- 26.Baran V, Brzakova A, Rehak P, Kovarikova V, Solc P. PLK1 regulates spindle formation kinetics and APC/C activation in mouse zygote. Zygote. 2016;24:338–45. doi: 10.1017/S0967199415000246. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc Natl Acad Sci U S A. 2002;99:8672–6. doi: 10.1073/pnas.132269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanhaji M, Ritter A, Belsham HR, Friel CT, Roth S, Louwen F, et al. Polo-like kinase 1 regulates the stability of the mitotic centromere-associated kinesin in mitosis. Oncotarget. 2014;5:3130–44. doi: 10.18632/oncotarget.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanafusa H, Kedashiro S, Tezuka M, Funatsu M, Usami S, Toyoshima F, et al. PLK1-dependent activation of LRRK1 regulates spindle orientation by phosphorylating CDK5RAP2. Nat Cell Biol. 2015;17:1024–35. doi: 10.1038/ncb3204. [DOI] [PubMed] [Google Scholar]