Abstract
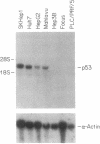
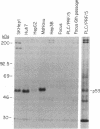
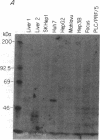
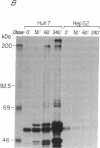
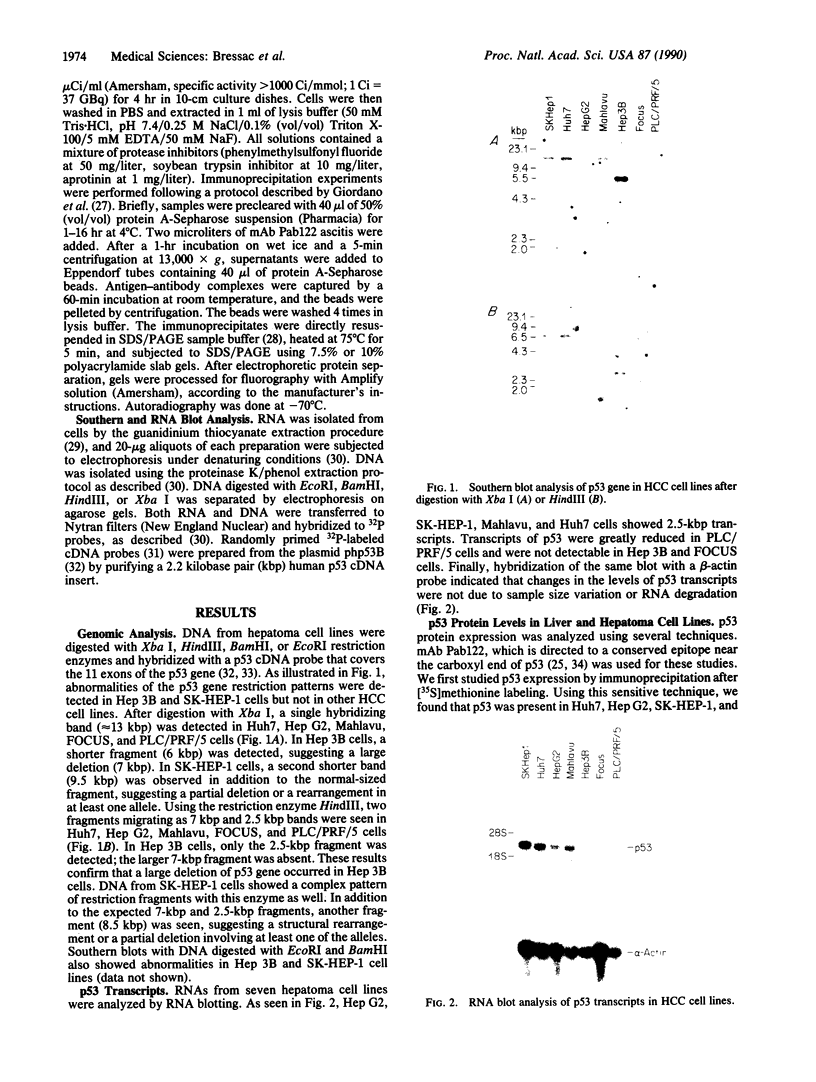
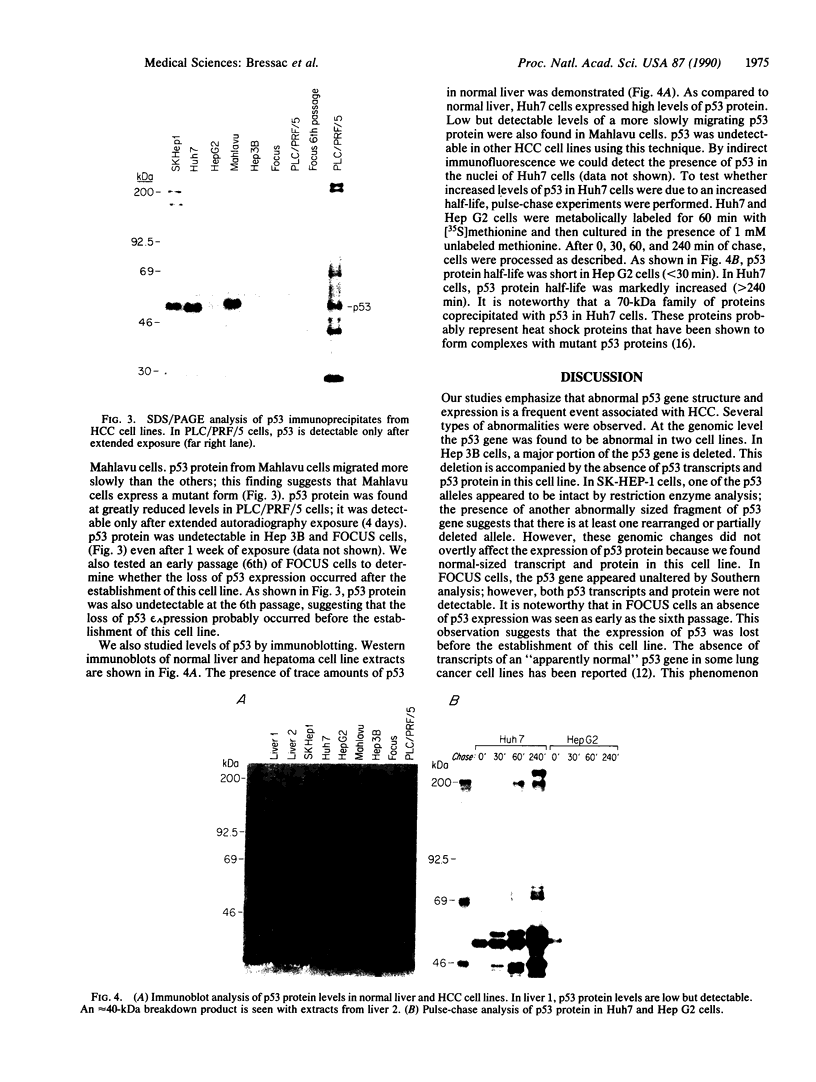
There is little information regarding the molecular mechanisms of hepatocarcinogenesis. We studied the p53 gene at the DNA, RNA, and protein level in seven human hepatocellular carcinoma (HCC)-derived cell lines; six of seven showed p53 abnormalities. By Southern blotting, the p53 gene was found to be partially deleted in Hep 3B and rearranged in SK-HEP-1 cells. Transcripts of the p53 gene were undetectable in Hep 3B as well as in FOCUS cells that had no apparent deletion or rearrangement of the p53 gene. Immunoprecipitation after [35S]methionine labeling of HCC cells demonstrated that p53 protein was absent in Hep 3B and FOCUS and reduced in concentration in PLC/PRF/5 cells. p53 synthesized by Mahlavu cells showed a slower migration on SDS/polyacrylamide gels suggesting it was an abnormal protein. In Huh7 cells, p53 protein had a prolonged half-life leading to its accumulation in the nuclei; increased levels of p53 protein were also found by immunoblotting. The p53 gene and its expression appeared to be unaltered in the hepatoblastoma-derived Hep G2 cell line. We found that the loss of p53 expression did not occur as a late in vitro event in the FOCUS cell line because p53 protein was also nondetectable at an early passage. We conclude that the loss of p53 expression or the presence of abnormal forms of the protein are frequently associated with HCC cell lines. These observations suggest that alterations in p53 may be important events in the transformation of hepatocytes to the malignant phenotype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Ahuja H., Bar-Eli M., Advani S. H., Benchimol S., Cline M. J. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Wilson S. H. Enzymes for modifying and labeling DNA and RNA. Methods Enzymol. 1987;152:94–110. doi: 10.1016/0076-6879(87)52013-4. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie A. M., Rustgi V. K., Hoofnagle J. H., Dusheiko G. M., Lotze M. T. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988 Mar;108(3):390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Grimm J. The influence of insulin on various enzyme activities in human and rat hepatoma cells. Eur J Biochem. 1976 Apr 15;64(1):249–253. doi: 10.1111/j.1432-1033.1976.tb10294.x. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Brill E., Shohat O., Prokocimer M., Wolf D., Arai N., Rotter V. Molecular basis for heterogeneity of the human p53 protein. Mol Cell Biol. 1986 Dec;6(12):4650–4656. doi: 10.1128/mcb.6.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Isselbacher K. J., Wands J. R., Goodman H. M., Shih C., Quaroni A. Establishment and characterization of a new human hepatocellular carcinoma cell line. In Vitro. 1984 Jun;20(6):493–504. doi: 10.1007/BF02619623. [DOI] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino O., Shows T. B., Rogler C. E. Hepatitis B virus integration site in hepatocellular carcinoma at chromosome 17;18 translocation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8338–8342. doi: 10.1073/pnas.83.21.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- MacNab G. M., Alexander J. J., Lecatsas G., Bey E. M., Urbanowicz J. M. Hepatitis B surface antigen produced by a human hepatoma cell line. Br J Cancer. 1976 Nov;34(5):509–515. doi: 10.1038/bjc.1976.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlashewski G. J., Tuck S., Pim D., Lamb P., Schneider J., Crawford L. V. Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol. 1987 Feb;7(2):961–963. doi: 10.1128/mcb.7.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Yamane T., Miyazaki M., Miyano K., Sato J. Phenotypical stability of a human hepatoma cell line, HuH-7, in long-term culture with chemically defined medium. Gan. 1984 Feb;75(2):151–158. [PubMed] [Google Scholar]
- Nakagama H., Ohnishi S., Imawari M., Hirai H., Takaku F., Sakamoto H., Terada M., Nagao M., Sugimura T. Identification of transforming genes as hst in DNA samples from two human hepatocellular carcinomas. Jpn J Cancer Res. 1987 Jul;78(7):651–654. [PubMed] [Google Scholar]
- Ochiya T., Fujiyama A., Fukushige S., Hatada I., Matsubara K. Molecular cloning of an oncogene from a human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):4993–4997. doi: 10.1073/pnas.83.14.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk M., Motté P., Takahashi H., Frohlich M., Wilson B., Hill L., Bressac B., Wands J. R. Identification and characterization of a Mr 50,000 adrenal protein in human hepatocellular carcinoma. Cancer Res. 1989 Dec 1;49(23):6764–6773. [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Popper H., Shafritz D. A., Hoofnagle J. H. Relation of the hepatitis B virus carrier state to hepatocellular carcinoma. Hepatology. 1987 Jul-Aug;7(4):764–772. doi: 10.1002/hep.1840070425. [DOI] [PubMed] [Google Scholar]
- Prokocimer M., Shaklai M., Bassat H. B., Wolf D., Goldfinger N., Rotter V. Expression of p53 in human leukemia and lymphoma. Blood. 1986 Jul;68(1):113–118. [PubMed] [Google Scholar]
- Rotter V., Abutbul H., Ben-Ze'ev A. P53 transformation-related protein accumulates in the nucleus of transformed fibroblasts in association with the chromatin and is found in the cytoplasm of non-transformed fibroblasts. EMBO J. 1983;2(7):1041–1047. doi: 10.1002/j.1460-2075.1983.tb01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Burke K., Chou M. J., Zeldis J. B., Yang C. S., Lee C. S., Isselbacher K. J., Wands J. R., Goodman H. M. Tight clustering of human hepatitis B virus integration sites in hepatomas near a triple-stranded region. J Virol. 1987 Nov;61(11):3491–3498. doi: 10.1128/jvi.61.11.3491-3498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Searls D. B., Cao Y., Sun K., Knowles B. B. Chromosomal site of hepatitis B virus (HBV) integration in a human hepatocellular carcinoma-derived cell line. Cytogenet Cell Genet. 1985;39(2):116–120. doi: 10.1159/000132118. [DOI] [PubMed] [Google Scholar]
- T'Ang A., Varley J. M., Chakraborty S., Murphree A. L., Fung Y. K. Structural rearrangement of the retinoblastoma gene in human breast carcinoma. Science. 1988 Oct 14;242(4876):263–266. doi: 10.1126/science.3175651. [DOI] [PubMed] [Google Scholar]
- Takada S., Koike K. Activated N-ras gene was found in human hepatoma tissue but only in a small fraction of the tumor cells. Oncogene. 1989 Feb;4(2):189–193. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tokino T., Fukushige S., Nakamura T., Nagaya T., Murotsu T., Shiga K., Aoki N., Matsubara K. Chromosomal translocation and inverted duplication associated with integrated hepatitis B virus in hepatocellular carcinomas. J Virol. 1987 Dec;61(12):3848–3854. doi: 10.1128/jvi.61.12.3848-3854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twist E. M., Clark H. F., Aden D. P., Knowles B. B., Plotkin S. A. Integration pattern of hepatitis B virus DNA sequences in human hepatoma cell lines. J Virol. 1981 Jan;37(1):239–243. doi: 10.1128/jvi.37.1.239-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Evans A., Jenkins J. R. Precise epitope mapping of the murine transformation-associated protein, p53. EMBO J. 1985 Mar;4(3):699–706. doi: 10.1002/j.1460-2075.1985.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut-Houri R., Bienz-Tadmor B., Givol D., Oren M. Human p53 cellular tumor antigen: cDNA sequence and expression in COS cells. EMBO J. 1985 May;4(5):1251–1255. doi: 10.1002/j.1460-2075.1985.tb03768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Z., Slagle B. L., Donehower L. A., vanTuinen P., Ledbetter D. H., Butel J. S. Structural analysis of a hepatitis B virus genome integrated into chromosome 17p of a human hepatocellular carcinoma. J Virol. 1988 Nov;62(11):4224–4231. doi: 10.1128/jvi.62.11.4224-4231.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]