Abstract
The characteristics of the cycles of activity and rest stand out among the most intensively investigated aspects of circadian rhythmicity in humans and experimental animals. Alterations in the circadian patterns of activity and rest are strongly linked to cognitive and emotional dysfunctions in severe mental illnesses such as Alzheimer’s disease (AD) and major depression (MDD). The proinflammatory cytokine interleukin 6 (IL-6) has been prominently associated with the pathogenesis of AD and MDD. However, the potential involvement of IL-6 in the modulation of the diurnal rhythms of activity and rest has not been investigated. Here, we set out to study the role of IL-6 in circadian rhythmicity through the characterization of patterns of behavioral locomotor activity in IL-6 knockout (IL-6 KO) mice and wild-type littermate controls. Deletion of IL-6 did not alter the length of the circadian period or the amount of locomotor activity under either light-entrained or free-running conditions. IL-6 KO mice also presented a normal phase shift in response to light exposure at night. However, the temporal architecture of the behavioral rhythmicity throughout the day, as characterized by the quantity of ultradian activity bouts, was significantly impaired under light-entrained and free-running conditions in IL-6 KO. Moreover, the assessment of clock gene expression in the hippocampus, a brain region involved in AD and depression, revealed altered levels of cry1, dec2, and rev-erb-beta in IL-6 KO mice. These data propose that IL-6 participates in the regulation of ultradian activity/rest rhythmicity and clock gene expression in the mammalian brain. Furthermore, we propose IL-6-dependent circadian misalignment as a common pathogenetic principle in some neurodegenerative and neuropsychiatric disorders.
Keywords: interleukin 6, circadian activity, mouse, behavior, clock gene, hippocampus
Introduction
Changes in the diurnal oscillations of the periods of activity and rest are in the spotlight of basic and applied biomedical research on circadian rhythms in humans and other animals (1). The interest in analyzing these changes in active wakefulness and quiescent rest rhythmicity relates to the fact that alterations of these rhythmic fluctuations are associated with a wide spectrum of pathologies, ranging from metabolic and cardiovascular dysfunctions to tumorigenesis and cancer. In the neurosciences, the consequences of circadian disruptions and chronic misalignments have been most prominently studied with regards to their effects on cognitive and emotional functions within the framework of some of the most severe neurological and psychiatric illnesses. Specifically, strong clinical and experimental evidence supports a link between disturbances of the sleep–wake cycle and other physiological functions regulated by the circadian system in the pathophysiology of Alzheimer’s disease (AD) and major depression (MDD). These dysfunctions include interruptions of the wakefulness during the day and bursts of activity during the night in individuals suffering from AD (2–6).
In addition, it has been described that part of the clinical symptomatology in AD patients is exacerbated at particular periods of the day, most commonly in the early evening (2, 7–10). In addition, a derangement in the circadian rhythmicity of several physiological functions (including the regulation of body temperature and hormone release) is frequently observed (11–15).
Similarly, MDD patients often report disrupted sleep–wake cycles and impairments in the diurnal patterns of other physiological processes [as reviewed in Ref. (16)]. In parallel to the reported “sun downing” in AD, MDD patients often also show significant diurnal mood swings with depressive symptoms usually being strongest in the morning (1).
At the molecular level, polymorphisms and expressional changes in several clock genes, the genetic elements constituting the molecular machinery organizing endogenous circadian rhythmicity, have been identified in postmortem samples of AD and MDD patients and animal models thereof (15, 17–29). Together with the shared involvement of circadian disruptions, both MDD and AD have been associated with altered inflammatory states (30, 31). The pro-inflammatory cytokine interleukin 6 (IL-6) (32), which is linked to circadian clock-related inflammation (33), is considered to play a central role in the pathophysiology of MDD and AD (30, 31, 34–39). Indeed, IL-6 has been proposed as a molecular bridge between circadian and inflammatory processes in a chronobiological animal model of depression (40) and is implicated in circadian rhythmicity (41) and in the circadian regulation of sleep drive (42, 43). Moreover, its secretion is determined by a marked diurnal pattern (44–46), and several clock genes are known as regulator of its production (47, 48).
However, the specific relationship between IL-6 and the diurnal rhythms of activity and rest remain poorly understood as varying observations regarding IL-6 levels under physiological and pathology conditions emerge from literature. These apparent discrepancies may be a consequence of species-specific effects and/or depend on the sample type or methodological approaches employed (31, 44–46, 49). Hence, further investigations using specific, genetically engineered animals are warranted. We here, therefore, set out to examine the involvement of IL-6 in the regulation of behavioral circadian rhythms by studying the changes in the diurnal patterns of locomotor activity in constitutive IL-6 knockout mice (IL-6 KO) in comparison with their wild-type (WT) littermate controls. To determine the impact of IL-6 deletion on the orchestration of circadian rhythmicity at the molecular level, the expression of 19 clock and clock-controlled genes was analyzed in the hippocampus, a brain region importantly implicated in the pathophysiology of MDD and AD.
Materials and Methods
Animals
Experiments were carried out in male adult IL-6 KO (B6.129S2-Il6tm1Kopf/J) and WT littermate control mice (Jackson Laboratories, Bar Harbor, ME, USA) (n = 9–11 per group). All mice were 8- to 10-week old at the time of experiments. Mice were housed individually in Nalgene cages equipped with running wheels (15 cm in diameter; Actrimetrics, Evanston, IL, USA) in a sound-attenuated room with constant temperature of 22 ± 2°C. Before experimental assessment of the circadian activity all animals were kept on a light/dark (LD) cycle of 12:12 h with lights on at 6 a.m. and off at 6 p.m. During the light phase, mice were exposed to a light intensity of ~200 lux. During conditions of constant darkness [dark/dark (DD)] defined as LD cycle of 0:24 h, the cage cleaning and animal care taking was carried out under dim red light (15 W). Mice were supplied with food and tap water ad libitum throughout the experimental period. All experiments were designed to minimize animal suffering and the number of animals used. Animal procedures were approved by the Austrian ethical committee (BMWF-66.009/0069-II/36/2011) on animal care and use conducted in accordance with international laws and policies.
Assessment of Circadian Wheel-Running Activity
Acquisition
Wheel revolutions were recorded using the ClockLab computer software, with sampling epochs of 1 min (Actimetrics, Evanston, IL, USA). After 1 week of habituation to the vivarium, the light-entrained daily activity was assessed for 14 days during LD followed by the evaluation of the free-running circadian activity during DD. On day 29, DD was briefly interrupted by a light pulse (30 min, 300 lux) at circadian time (CT) 16 (4 h after activity onset) for the induction of a phase-shift response to evaluate the response of the endogenous circadian pacemakers to external zeitgebers. After 7 additional days of DD, all mice were exposed to LD for 7 days before sacrifice on day 46 (Figure 1).
Figure 1.
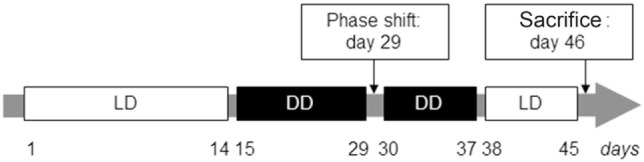
Experimental paradigm for the evaluation of light-entrained and free-running circadian rhythms in interleukin (IL)-6 knockout (IL-6 KO) and wild-type (WT) mice. Illustration of the temporal course (in days) for the analysis of circadian behavioral locomotor activity in IL-6 KO and WT mice under light-entrainment [light/dark (LD): 12 h light and 12 h dark phase; white boxes] and during settings of free-running rhythms [dark/dark (DD): 24 h constant darkness, black boxes].
Analysis
Wheel-running activity was analyzed using the ClockLab software package (Actimetrics, Evanston, IL, USA) as previously described (27, 50). The default software settings were used to determine the activity onsets, which were manually edited when appropriate. Measures of the entrainment period (T) in LD and circadian period (tau) in DD and the total activity were derived from regression lines fit to the activity onsets. Activity bouts were defined as periods during which activity never reached less than 1 count per minute (bout threshold) for longer than 18 min (maximum gap length) at a time. All parameters were determined for each animal under LD and DD conditions. Phase-shift responses were evaluated by comparing the predicted activity onset for the day after light pulse treatment from extrapolated lines of the activity onsets of the days preceding the light pulse and 7 days after the pulse.
Gene Expression Analysis
Brain Dissection
All brain dissections were carried out during the light phase of the circadian cycle (between 9 a.m. and 11 a.m.). Mice were sacrificed by neck dislocation, and brains were rapidly dissected over ice and total hippocampi were bilaterally collected and stored in RNA later® (Ambion, Austria, Austin, TX, USA) at −20°C until used for RNA isolation or kept at −80°C for protein expression studies.
RNA Isolation, cDNA Synthesis, and Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
RNA was isolated from hippocampal tissues using the miRNeasy kit (Qiagen®, USA, Hilden, Germany) following the instructions of the manufacturer. Briefly, 900 ng of total RNA was used for cDNA synthesis using the MMLV reverse transcriptase first-strand cDNA synthesis kit G1 (Biozym®, Hessisch Oldendorf, Germany) following the manufacturer’s instructions. The resulting cDNA reaction mix (1:10 dilution) was used for PCR amplification using the Fast SYBR Green Mastermix (Applied Biosystems, Foster City, CA, USA) on a StepOnePlus real-time PCR system (serial no. 271000455; Applied Biosystems, Foster City, CA, USA). All reactions were carried out in duplicates. Primer sequences for all clock were analyzed: brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (bmal1), circadian locomotor output cycles kaput (clock), cryptochrome 1/2 (cry1/2), deleted in esophageal cancer 1/2 (dec1/2), neuroD1, neuronal PAS domain-containing protein 2 (npas2), period 1–3 (per1–3), reverse erythroblastosis virus α/β (rev-erbα/β) and RAR (retinoic acid receptor)-related orphan receptor α-γ (rorα-γ) and clock-controlled genes D site of albumin promoter (albumin D-box) binding protein (dbp), E4 promoter-binding protein 4 (e4bp4), inhibitor of DNA binding 2 (id2), and neuronal differentiation 1 are listed in the Supplementary Table 1 of Ref. (27)
The C(t) values of β-actin were used for calculation of ΔC(t), representing the relative quantification of mRNA amounts in each sample. This further allowed the calculation of ΔΔC(t), subtracting mean ΔC(t) value of the WT from the mean ΔC(t) value for the KO. ΔΔC(t) was then used to express the fold change of mRNA levels observed between WT and KO mice, using the formula 2−ΔΔC(t).
Statistical Analysis
BioStat software (AnalystSoft Inc., Alexandria, VA, USA) was used for statistical analysis. Comparisons between two groups were determined using unpaired two-tailed Student’s t-test. In addition, two-way analysis of variance (ANOVA) (light condition × genotype) was employed for statistical evaluation of locomotor activity (alpha, rho, and total) and for bout analysis (number of bouts/day, bout length and counts/bout). The level of significance was set at p < 0.05 in all instances.
Results
IL-6 KO Mice Present with Fragmented Daily Activity Patterns under LD and DD Conditions
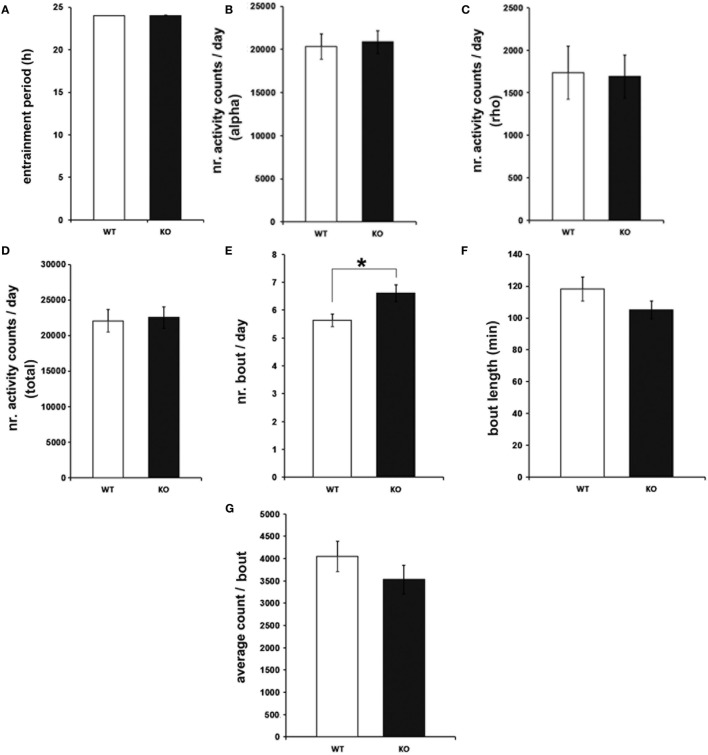
To characterize the effects of genetic IL-6 deficiency on behavioral rhythms of rest and activity, wheel-running activity was monitored in IL-6 KO and WT littermate control mice. The investigation of light-entrained rhythms under LD conditions indicated unaltered length of the entrainment period (T) (Figure 2A) in IL-6 KO mice. Similarly, the amount of wheel-running activity was comparable between IL-6 KO and WT mice during periods of inactivity (rho) and activity (alpha) within the circadian cycle (Figures 2B–D). IL-6 deletion, however, was associated with an increased quantity of activity bouts (p < 0.05) with unchanged duration and amount of activity/bout (Figures 2E–G). Calculations of activity onsets and offsets revealed no differences between genotypes, and the duration of the active period was not statistically different between groups under LD conditions (Figure S1 in Supplementary Material).
Figure 2.
Entrainment period (T), wheel-running activity, and bout analysis in under light-entrained [light/dark] conditions in interleukin (IL)-6 knockout (IL-6 KO) and wild-type (WT) mice. Analysis of the light-entrained circadian behavioral locomotor activity in IL-6 KO and WT mice (n = 9–11 per group) demonstrating comparable (A) T and wheel-running activity during the (B) alpha and (C) rho phase and in (D) total amounts. (E) Significantly increased quantity of activity bouts in IL-6 KO compared with WT mice with unaltered (F) bout length and (G) activity counts/bout. All data are displayed as mean ± SEM; *p < 0.05.
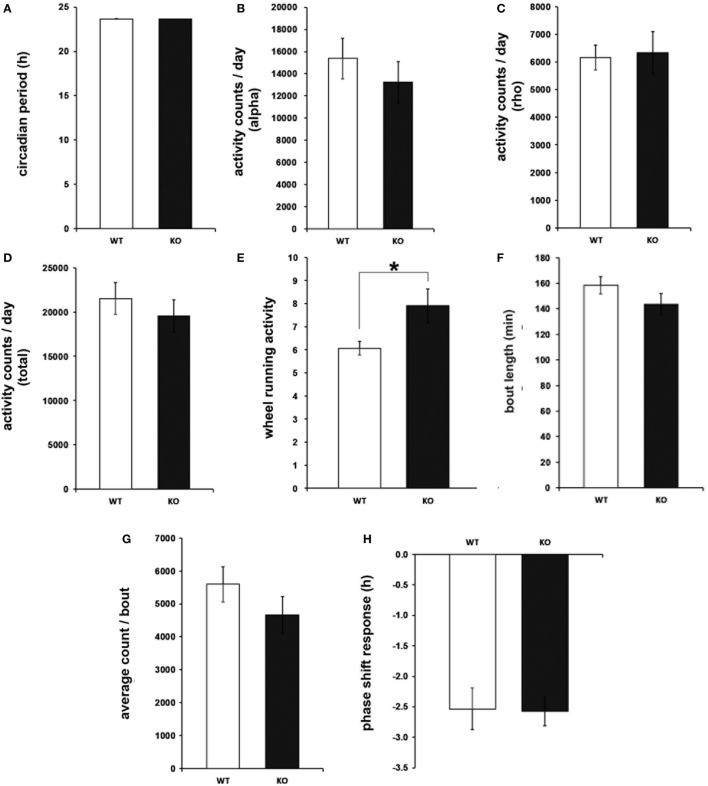
To determine circadian locomotor patterns during free-running rhythms, daily behavioral activity was further analyzed under DD conditions. In the same way as for the light-entrained rhythms, the circadian period, as well as the amount of wheel-running activity, was undistinguishable between IL-6 KO and WT mice (Figures 3A–D). Consistent with the results from the LD paradigm, the number of activity bouts was enhanced in IL-6 KO mice under DD conditions (p < 0.05), whereas no differences were seen in the duration and quantity of activity/bout or in the phase shift response in comparison with WT controls (Figures 3E–H). In addition, the duration of the active period was shorter in IL-6 KO mice under DD conditions (p < 0.05) (Figure S1 in Supplementary Material).
Figure 3.
Circadian period (tau), wheel-running activity, bout analysis, and phase shift response under free-running (dark/dark) conditions in interleukin (IL)-6 knockout (IL-6 KO) and wild-type (WT) mice. Analysis of the free-running circadian behavioral locomotor activity in IL-6 KO and WT mice (n = 9–11 per group) demonstrating comparable (A) tau and wheel-running activity during the (B) alpha and (C) rho phase and in (D) total amounts. (E) Significantly increased quantity of activity bouts in IL-6 KO compared with WT mice with unaltered (F) bout length and (G) activity counts/bout. (H) Unaltered phase shift response to a brief light pulse at CT14 is in IL-6 KO mice. All data are displayed as mean ± SEM; *p < 0.05.
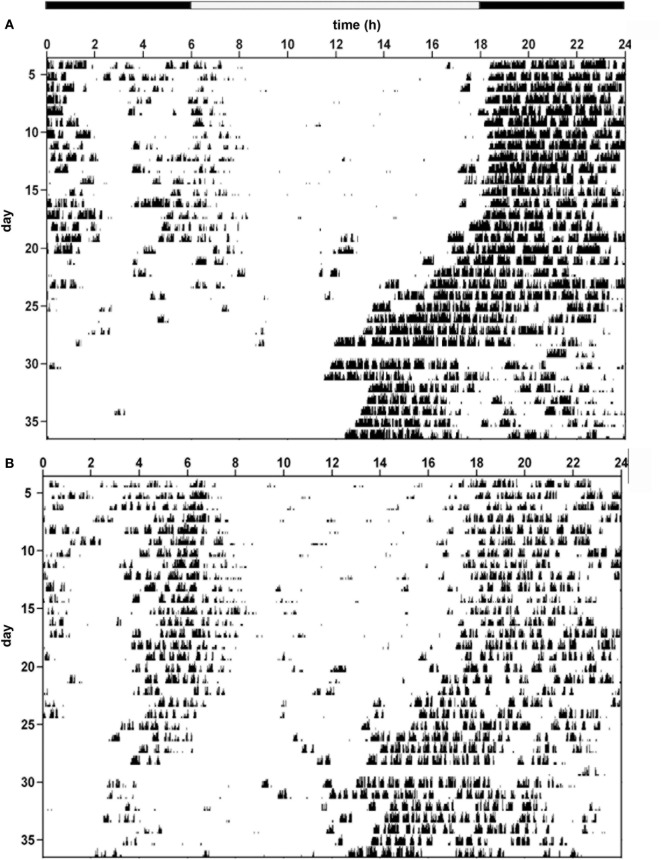
Hence, the temporal architecture of the ultradian rhythms is disrupted in IL-6 KO mice under both LD and DD conditions as illustrated in the respective actograms of the two genotypes (Figures 4A,B). Further examples of representative actograms are provided in Figure S2 in Supplementary Material.
Figure 4.
Behavioral actograms exemplifying circadian locomotor activity patterns in interleukin (IL)-6 knockout (IL-6 KO) and wild-type (WT) mice. Sample actograms illustrating wheel-running activity in (A) WT and (B) IL-6 KO mice.
In addition, two-way ANOVA analysis (light condition × genotype) has been carried out to examine the possible effect of the light condition and its interaction with the genotype. The following main effects have been observed: for overall activity significant main effects of light condition for alpha: F(3,43) = 88.54, p < 0.001 and rho: F(3,43) = 178.17, p < 0.001. The characterization of the bouts revealed a significant main effect of genotype [F(3,43) = 10.47, p < 0.01] for bouts per day and significant main effects of light condition for bout length: F(3,43) = 29.98, p < 0.001 and counts/bout: F(3,43) = 8.57, p < 0.01. The duration of the active periods revealed a significant main effect of genotype [F(3,43) = 7.17, p < 0.05]. No other significant main effects or interactions were found.
Aberrant mRNA Expression of Cry1, Dec2, and Rev-erb-Beta in the IL-6 KO Mouse Hippocampus
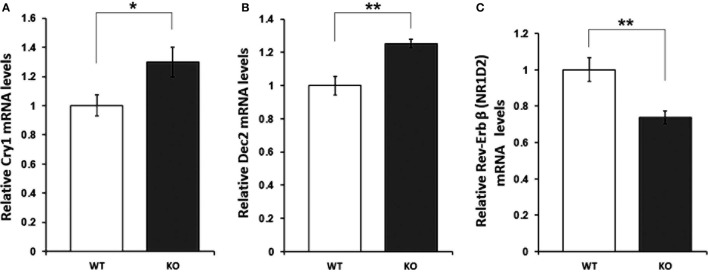
With regard to the molecular mediators of the observed alterations in the rhythmic oscillation of rest and activity patterns, mRNA levels of 19 clock (clock, cry1/2, npas2, per1–3, rev-erbα/β, and rorα-γ) and clock-controlled genes (dbp, e4bp4, id2, and neuroD1) were assessed in the hippocampus of IL-6 KO and WT mice. qRT-PCR analysis revealed a significant increase in levels of cry1 (p < 0.05) and dec2 (p < 0.01), whereas expression of rev-erb-beta (p < 0.01) was reduced in IL-6 KO compared with WT controls (Figure 5). No differences in the mRNA of any of the other clock genes investigated were found (Table 1).
Figure 5.
mRNA levels of clock genes with significantly different expression in hippocampal tissue of interleukin (IL)-6 knockout (IL-6 KO) compared with wild-type (WT) mice. Relative expression of (A) cry1, (B) dec2, and (C) rev-erb beta in hippocampal tissue of IL-6 KO compared with WT mice (n = 6–9 per group). All data are data displayed as mean ± SEM. *p < 0.05, **p < 0.01.
Table 1.
Clock and clock-controlled genes with comparable mRNA levels in hippocampal tissue of knockout (KO) and wild-type (WT) mice.
| Gene name | WT (rel. expression) | KO (rel. expression) | p Value |
|---|---|---|---|
| clock | 1.000 ± 0.1293 | 1.0197 ± 0.0038 | 0.6 |
| cry2 | 1.000 ± 0.1332 | 0.9949 ± 0.0168 | 0.9 |
| dbp | 1.000 ± 0.0916 | 1.0272 ± 0.0140 | 0.4 |
| dec1 | 1.000 ± 0.1414 | 0.9893 ± 0.0406 | 0.8 |
| e4bp4 | 1.000 ± 0.1375 | 0.9952 ± 0.0084 | 0.8 |
| id2 | 1.000 ± 0.0902 | 1.0385 ± 0.0371 | 0.4 |
| neuroD1 | 1.000 ± 0.0673 | 1.0045 ± 0.0038 | 0.7 |
| npas2 | 1.000 ± 0.0759 | 0.9993 ± 0.0192 | 0.9 |
| per1 | 1.000 ± 0.0841 | 1.0516 ± 0.0468 | 0.3 |
| per2 | 1.000 ± 0.1055 | 1.0291 ± 0.0105 | 0.2 |
| per3 | 1.000 ± 0.2047 | 1.0170 ± 0.0206 | 0.7 |
| rev-erbα/β | 1.000 ± 0.0740 | 1.0367 ± 0.0181 | 0.2 |
| ror-α | 1.000 ± 0.1685 | 0.9714 ± 0.0344 | 0.5 |
| ror-β | 1.000 ± 0.0731 | 0.9714 ± 0.0191 | 0.2 |
| ror-γ | 1.000 ± 0.1034 | 0.9829 ± 0.0092 | 0.2 |
| bmal1 | 1.000 ± 0.1180 | 1.0225 ± 0.0131 | 0.3 |
Fold change values in KO mice (normalized to WT means for each transcript) of clock and clock-controlled (gray) genes are displayed as mean ± SEM (n = 6–9 per group). p Values represent results of statistical analyses using two-tailed Student’s t-tests.
Discussion
Most species living on the surface of earth have evolved under conditions of rhythmically changing daily variations in fundamental environmental constituents, such as light. To anticipate and respond to these oscillating physical properties, organisms have developed systems to accordingly fit their physiology. Hence, the most essential functions of the body, including those of the nervous and the immune systems, are determined by these intrinsic timing regulations. Thus, the association between disruption in “biological clocks” and pathologies of the brain (31, 51–53) and the immune response is unsurprising [see for review Ref. (54)]. Indeed, the circadian regulation of the behavioral states of activity/rest (as fundamental output of brain function) is well described. Similarly, evidence for the impact of the endogenous clockwork on the most pivotal elements of the body’s defense mechanisms, such as the release of immune modulatory substances, is augmenting (55–58) [see for review Ref. (59)].
The current report is, to the best of our knowledge, the first comprehensive, long-term assessment of the impact of a genetic deficiency in a central element of the immune response (the proinflammatory cytokine IL-6) on circadian wheel-running activity rhythms in the mouse. This interrelationship is particularly noteworthy within the framework of diseases and disorders in which all these functions are of pathophysiological relevance, as is the case for the neurodegenerative AD and the neuropsychiatric MDD, where the involvement of the circadian and the immune systems have been extensively demonstrated (31). In the case of both these mental illnesses, frequent presentations of aberrant diurnal oscillations of behavioral activity have been reported in patients and in subjects of the respective experimental animal models (15–29, 31, 60–62).
In the herein studied IL-6 KO mice, traditional parameters of diurnal behavioral rhythmicity were unaltered under light-entrained and free-running conditions, as tau and the amount of activity during active and inactive phase were comparable with those of WT controls but were determined by the light conditions (LD versus DD) for both genotypes. Interestingly, the duration of the active period was shortened in IL-6 KO mice. In a previous short-term evaluation of home cage behavior, higher activity of IL-6 KO compared with WT mice has been reported (63). However, the analysis of home cage activity does characterize a behavioral output distinct from circadian wheel-running activity (64). Although home cage activity reflects the baseline activity, wheel running is an elective action, which is driven by additional endogenous factors, such as motivation (64). However, it is the only system to reliably address some distinct features of the internal timekeeping system, such as the modulation of the endogenous circadian machinery by environmental stimuli. Indeed, an unaltered phase-shift response in IL-6 KO mice indicated an intact responsivity of the endogenous CT keeping system to an external zeitgeber. Hence, the 24-h structure of the behavioral locomotor rhythm seemed largely preserved IL-6 KO mice. However, a close examination of the activity bouts as indicators of units of ultradian activity revealed a significant difference in the number of bouts between genotypes, independent of the external lighting conditions: IL-6 KO mice presented with an augmentation in the number of bouts/circadian day, while the bout length and activity/bout remained unchanged. This result is also reflected in the two-way ANOVA analysis, which revealed a significant main effect of genotype for the number of bouts, whereas interestingly the bout length and activity/bout were significantly dependent on the light conditions for both WT and KO mice.
The nature and regulation of ultradian rhythms and activity bouts is less well described than is the case for the classical indicators of diurnal rhythms, e.g., length of the circadian period tau and activity onsets and offsets, which are largely dependent on the suprachiasmatic nucleus (SCN) of the hypothalamus as a central circadian pacemaker (65–70). The SCN also orchestrates rhythmic activities in other regions of the brain and peripheral parts of the body with synchronization of clock gene expression as a pivotal molecular event.
To examine potential neurobiological mechanisms contributing to the observed phenotype of IL-6 KO mice, we decided to focus on the hippocampus, a brain region involved in the pathophysiology of AD (71–73) and MDD (74, 75). Examination of the expression of major clock genes as molecular mediators of circadian rhythmicity revealed a selective effect of genetic IL-6 deficiency on the hippocampal mRNA levels of cry1, dec2, and rev-erb-beta.
Although the statistically significant expressional differences between IL-6 KO and WT mice were modest in magnitude, they may be well of biological relevance considering the role of these genes in the tightly controlled feedback loops of transcription–translation from which circadian rhythms are generated at the molecular level (20, 24, 28, 76). The increased levels of cry1 in IL-6 KO are paralleling observations in plasma levels of sepsis patients were an increase in IL-6 was associated with a decrease in cry1 mRNA (77). A modulatory influence of several immune mediators on the expression of dec2, which is here to be reported significantly reduced in the hippocampal tissue of IL-6 KO mice, has been described. Interestingly, IL-6 is a direct activator of AMP-activated protein kinase (78), which has been found to mediate the regulatory effects of dec2 in several tissues (79).
Previous work reports that rev-erb expression in peripheral blood leukocytes of human subjects, together with several other clock genes (including cry1), is dampened by endotoxin treatment, which leads to a concomitant increase in circulating levels of IL-6. This description is in line with our observation on augmented rev-erb-beta and cry1 levels in IL-6 KO.
Alternatively or additionally to a mechanistic involvement of clock gene expression, the alteration in the ultradian architecture of behavioral activity in IL-6 KO mice may relate to the direct regulatory effect of IL-6 on the serotonin transporter (SERT) (80). Indeed, multifaceted interactions between the circadian and the serotonergic systems have been demonstrated with a proposed role of these interrelationships for several mental illnesses, including MDD [see for review Ref. (81, 82)]. However, although a defined role for dopamine and the dopamine transporter in the regulation of ultradian rhythms of locomotor behavior have been proposed (83), a potential involvement of SERT in the control of ultradian activity architecture remains to be examined in future studies.
Some conceptual restrictions, which were imposed by the study design, such as the determination of clock gene expression at a single time of the day in a priori selected brain region of interest have to be considered for the interpretation of the results obtained. Hence, the observed differences in clock gene expression between IL-6 KO and WT mice do not allow for conclusions regarding the diurnal oscillation in the expression of these genes in the two genotypes, an important mechanistic insight that will be addressed in follow-up investigations. Within this framework, however, this study allows for the deduction of three major conclusions: first, IL-6 is not required for diurnal time keeping of the circadian period under either light-entrained or free-running conditions; second, genetic IL-6 deficiency is associated with aberrant ultradian activity patterns as reflected in an increased number of activity bouts with unaltered length and activity counts per bout, independent of the external light conditions; and third, a selective modulation of hippocampal clock gene expression proposes an involvement of disrupted mRNA levels of cry1, dec2, and rev-erb-beta in the circadian phenotype of IL-6 KO mice.
Collectively these data suggest a potential pathophysiological involvement of the pro-inflammatory cytokine IL-6 in the circadian alterations associated with severe neurological and psychiatric disorders and invite further investigations on the underlying molecular mechanisms.
Author Contributions
FM co-designed experiments, analyzed data, and co-wrote the manuscript; AC and JA analyzed data; IE and OH conducted behavioral experiments; WD carried out gene expression analysis; and DP and MG conceived the study, analyzed data, and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
FM is supported by the Austrian Science Fund (FWF): P 27551. DP is supported by the Austrian Science Fund (FWF): P 22424, P 27520, P 28683, and W1205.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fneur.2017.00099/full#supplementary-material.
Duration of the active period (alpha) and activity onsets and offsets in interleukin-6 (IL-6) and wild-type (WT) mice. Analysis of the length of the active period in IL-6 and WT mice (n = 9–11 per group) under (A) light/dark and (B) dark/dark conditions. (C) Activity onsets and (D) offsets in circadian hours in IL-6 compared with WT mice. All data are displayed as mean ± SEM; *p < 0.05.
Behavioral actograms exemplifying circadian locomotor activity patterns in interleukin-6 (IL-6) and wild-type (WT) mice. Sample actograms illustrating wheel-running activity in (A) WT and (B) IL-6 mice.
References
- 1.Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry (1995) 3(1):18–35. 10.3109/10673229509017161 [DOI] [PubMed] [Google Scholar]
- 2.Klaffke S, Staedt J. Sundowning and circadian rhythm disorders in dementia. Acta Neurol Belg (2006) 106(4):168–75. [PubMed] [Google Scholar]
- 3.Lee JH, Bliwise DL, Ansari FP, Goldstein FC, Cellar JS, Lah JJ, et al. Daytime sleepiness and functional impairment in Alzheimer disease. Am J Geriatr Psychiatry (2007) 15(7):620–6. 10.1097/JGP.0b013e3180381521 [DOI] [PubMed] [Google Scholar]
- 4.Martin J, Marler M, Shochat T, Ancoli-Israel S. Circadian rhythms of agitation in institutionalized patients with Alzheimer’s disease. Chronobiol Int (2000) 17(3):405–18. 10.1081/CBI-100101054 [DOI] [PubMed] [Google Scholar]
- 5.Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int (1999) 16(4):505–18. 10.3109/07420529908998724 [DOI] [PubMed] [Google Scholar]
- 6.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry (1990) 27(6):563–72. 10.1016/0006-3223(90)90523-5 [DOI] [PubMed] [Google Scholar]
- 7.Coogan AN, Schutová B, Husung S, Furczyk K, Baune BT, Kropp P, et al. The circadian system in Alzheimer’s disease: disturbances, mechanisms, and opportunities. Biol Psychiatry (2013) 74(5):333–9. 10.1016/j.biopsych.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 8.Moe KE, Vitiello MV, Larsen LH, Prinz PN. Symposium: cognitive processes and sleep disturbances: sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res (1995) 4(1):15–20. 10.1111/j.1365-2869.1995.tb00145.x [DOI] [PubMed] [Google Scholar]
- 9.Vitiello MV, Prinz PN. Alzheimer’s disease. Sleep and sleep/wake patterns. Clin Geriatr Med (1989) 5(2):289–99. [PubMed] [Google Scholar]
- 10.Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer’s disease: a review. Int J Alzheimers Dis (2010) 2010:716453. 10.4061/2010/716453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahlberg R, Kunz D, Sutej I, Kühl KP, Hellweg R. Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer disease: an open-label pilot study using actigraphy. J Clin Psychopharmacol (2004) 24(4):456–9. 10.1097/01.jcp.0000132443.12607.fd [DOI] [PubMed] [Google Scholar]
- 12.Satlin A, Teicher MH, Lieberman HR, Baldessarini RJ, Volicer L, Rheaume Y. Circadian locomotor activity rhythms in Alzheimer’s disease. Neuropsychopharmacology (1991) 5(2):115–26. [PubMed] [Google Scholar]
- 13.Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging (1995) 16(5):765–71. 10.1016/0197-4580(95)00059-N [DOI] [PubMed] [Google Scholar]
- 14.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry (2001) 158(5):704–11. 10.1176/appi.ajp.158.5.704 [DOI] [PubMed] [Google Scholar]
- 15.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol (2003) 38(1–2):199–206. 10.1016/S0531-5565(02)00198-5 [DOI] [PubMed] [Google Scholar]
- 16.Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry (2013) 73(12):1164–71. 10.1016/j.biopsych.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 17.Cermakian N, Lamont EW, Boudreau P, Boivin DB. Circadian clock gene expression in brain regions of Alzheimer’s disease patients and control subjects. J Biol Rhythms (2011) 26(2):160–70. 10.1177/0748730410395732 [DOI] [PubMed] [Google Scholar]
- 18.Lim AS, Srivastava GP, Yu L, Chibnik LB, Xu J, Buchman AS, et al. 24-hour rhythms of DNA methylation and their relation with rhythms of RNA expression in the human dorsolateral prefrontal cortex. PLoS Genet (2014) 10(11):e1004792. 10.1371/journal.pgen.1004792 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol (2013) 70(12):1544–51. 10.1001/jamaneurol.2013.4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J (2006) 20(11):1874–6. 10.1096/fj.05-4446fje [DOI] [PubMed] [Google Scholar]
- 21.Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein period2. Proc Natl Acad Sci U S A (2005) 102(11):4180–4. 10.1073/pnas.0500901102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segall LA, Milet A, Tronche F, Amir S. Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci Lett (2009) 457(1):58–60. 10.1016/j.neulet.2009.03.083 [DOI] [PubMed] [Google Scholar]
- 23.Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res (2010) 1337:21–31. 10.1016/j.brainres.2010.03.113 [DOI] [PubMed] [Google Scholar]
- 24.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A (2013) 110(24):9950–5. 10.1073/pnas.1305814110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savalli G, Diao W, Schulz S, Todtova K, Pollak DD. Diurnal oscillation of amygdala clock gene expression and loss of synchrony in a mouse model of depression. Int J Neuropsychopharmacol (2014) 18(5):yu095. 10.1093/ijnp/pyu095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savalli G, Diao W, Berger S, Ronovsky M, Partonen T, Pollak DD. Anhedonic behavior in cryptochrome 2-deficient mice is paralleled by altered diurnal patterns of amygdala gene expression. Amino Acids (2015) 47(7):1367–77. 10.1007/s00726-015-1968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griesauer I, Diao W, Ronovsky M, Elbau I, Sartori S, Singewald N, et al. Circadian abnormalities in a mouse model of high trait anxiety and depression. Ann Med (2014) 46(3):148–54. 10.3109/07853890.2013.866440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partonen T. Clock gene variants in mood and anxiety disorders. J Neural Transm (Vienna) (2012) 119(10):1133–45. 10.1007/s00702-012-0810-2 [DOI] [PubMed] [Google Scholar]
- 29.Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci (2010) 30(15):5357–67. 10.1523/JNEUROSCI.5017-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer J, Strauss S, Volk B, Berger M. IL-6-mediated events in Alzheimer’s disease pathology. Immunol Today (1991) 12(11):422. 10.1016/0167-5699(91)90148-M [DOI] [PubMed] [Google Scholar]
- 31.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev (1995) 21(2):195–218. 10.1016/0165-0173(95)00011-9 [DOI] [PubMed] [Google Scholar]
- 32.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature (1986) 324(6092):73–6. 10.1038/324073a0 [DOI] [PubMed] [Google Scholar]
- 33.Carter SJ, Durrington HJ, Gibbs JE, Blaikley J, Loudon AS, Ray DW, et al. A matter of time: study of circadian clocks and their role in inflammation. J Leukoc Biol (2016) 99(4):549–60. 10.1189/jlb.3RU1015-451R [DOI] [PubMed] [Google Scholar]
- 34.Baranowska-Bik A, Bik W, Wolinska-Witort E, Martynska L, Chmielowska M, Barcikowska M, et al. Plasma beta amyloid and cytokine profile in women with Alzheimer’s disease. Neuro Endocrinol Lett (2008) 29(1):75–9. [PubMed] [Google Scholar]
- 35.Mondin TC, de Azevedo Cardoso T, Moreira FP, Wiener C, Oses JP, de Mattos Souza LD, et al. Circadian preferences, oxidative stress and inflammatory cytokines in bipolar disorder: a community study. J Neuroimmunol (2016) 301:23–9. 10.1016/j.jneuroim.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 36.Voderholzer U, Fiebich BL, Dersch R, Feige B, Piosczyk H, Kopasz M, et al. Effects of sleep deprivation on nocturnal cytokine concentrations in depressed patients and healthy control subjects. J Neuropsychiatry Clin Neurosci (2012) 24(3):354–66. 10.1176/appi.neuropsych.11060142 [DOI] [PubMed] [Google Scholar]
- 37.Rasmuson S, Nasman B, Olsson T. Increased serum levels of dehydroepiandrosterone (DHEA) and interleukin-6 (IL-6) in women with mild to moderate Alzheimer’s disease. Int Psychogeriatr (2011) 23(9):1386–92. 10.1017/S1041610211000810 [DOI] [PubMed] [Google Scholar]
- 38.Jehn CF, Kühnhardt D, Bartholomae A, Pfeiffer S, Schmid P, Possinger K, et al. Association of IL-6, hypothalamus-pituitary-adrenal axis function, and depression in patients with cancer. Integr Cancer Ther (2010) 9(3):270–5. 10.1177/1534735410370036 [DOI] [PubMed] [Google Scholar]
- 39.Martínez M, Fernández-Vivancos E, Frank A, De la Fuente M, Hernanz A. Increased cerebrospinal fluid fas (Apo-1) levels in Alzheimer’s disease. Relationship with IL-6 concentrations. Brain Res (2000) 869(1–2):216–9. 10.1016/S0006-8993(00)02363-5 [DOI] [PubMed] [Google Scholar]
- 40.Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BRs, et al. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J Neurosci (2011) 31(25):9075–83. 10.1523/JNEUROSCI.1537-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motzkus D, Albrecht U, Maronde E. The human PER1 gene is inducible by interleukin-6. J Mol Neurosci (2002) 18(1–2):105–9. 10.1385/JMN:18:1-2:105 [DOI] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation (2005) 12(3):131–40. 10.1159/000084844 [DOI] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab (1999) 84(8):2603–7. 10.1210/jcem.84.8.5894 [DOI] [PubMed] [Google Scholar]
- 44.Guan Z, Vgontzas AN, Omori T, Peng X, Bixler EO, Fang J. Interleukin-6 levels fluctuate with the light-dark cycle in the brain and peripheral tissues in rats. Brain Behav Immun (2005) 19(6):526–9. 10.1016/j.bbi.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 45.Izawa S, Miki K, Liu X, Ogawa N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain Behav Immun (2013) 27(1):38–41. 10.1016/j.bbi.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 46.Agorastos A, Hauger RL, Barkauskas DA, Moeller-Bertram T, Clopton PL, Haji U, et al. Circadian rhythmicity, variability and correlation of interleukin-6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology (2014) 44:71–82. 10.1016/j.psyneuen.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 47.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A (2012) 109(2):582–7. 10.1073/pnas.1106750109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugimoto T, Morioka N, Zhang FF, Sato K, Abe H, Hisaoka-Nakashima K, et al. Clock gene Per1 regulates the production of CCL2 and interleukin-6 through p38, JNK1 and NF-kappaB activation in spinal astrocytes. Mol Cell Neurosci (2014) 59:37–46. 10.1016/j.mcn.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 49.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology (2002) 27(8):921–31. 10.1016/S0306-4530(02)00006-9 [DOI] [PubMed] [Google Scholar]
- 50.Schaufler J, Ronovsky M, Savalli G, Cabatic M, Sartori SB, Singewald N, et al. Fluoxetine normalizes disrupted light-induced entrainment, fragmented ultradian rhythms and altered hippocampal clock gene expression in an animal model of high trait anxiety- and depression-related behavior. Ann Med (2016) 48(1–2):17–27. 10.3109/07853890.2015.1122216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai Y, Liu S, Li N, Xu S, Zhang Y, Chan P. Postnatal ontogenesis of molecular clock in mouse striatum. Brain Res (2009) 1264:33–8. 10.1016/j.brainres.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 52.Onishi H, Yamaguchi S, Yagita K, Ishida Y, Dong X, Kimura H, et al. Rev-erbalpha gene expression in the mouse brain with special emphasis on its circadian profiles in the suprachiasmatic nucleus. J Neurosci Res (2002) 68(5):551–7. 10.1002/jnr.10226 [DOI] [PubMed] [Google Scholar]
- 53.Wongchitrat P, Mukda S, Phansuwan-Pujito P, Govitrapong P. Effect of amphetamine on the clock gene expression in rat striatum. Neurosci Lett (2013) 542:126–30. 10.1016/j.neulet.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 54.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity (2014) 40(2):178–86. 10.1016/j.immuni.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 55.Kizaki T, Sato S, Shirato K, Sakurai T, Ogasawara J, Izawa T, et al. Effect of circadian rhythm on clinical and pathophysiological conditions and inflammation. Crit Rev Immunol (2015) 35(4):261–75. 10.1615/CritRevImmunol.2015014925 [DOI] [PubMed] [Google Scholar]
- 56.Labrecque N, Cermakian N. Circadian clocks in the immune system. J Biol Rhythms (2015) 30(4):277–90. 10.1177/0748730415577723 [DOI] [PubMed] [Google Scholar]
- 57.Ohdo S. Chronotherapeutic strategy: rhythm monitoring, manipulation and disruption. Adv Drug Deliv Rev (2010) 62(9–10):859–75. 10.1016/j.addr.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 58.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris (2011) 105(4–6):170–82. 10.1016/j.jphysparis.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 59.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature (2016) 535(7610):65–74. 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- 60.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci (2000) 25(5):446–58. [PMC free article] [PubMed] [Google Scholar]
- 61.Lamont EW, Coutu DL, Cermakian N, Boivin DB. Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatry Relat Sci (2010) 47(1):27–35. [PubMed] [Google Scholar]
- 62.Lamont EW, James FO, Boivin DB, Cermakian N. From circadian clock gene expression to pathologies. Sleep Med (2007) 8(6):547–56. 10.1016/j.sleep.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 63.Aniszewska A, Szymanski J, Winnicka MM, Turlejski K. Interleukin 6 deficiency affects spontaneous activity of mice in age- and sex-dependent manner. Acta Neurobiol Exp (Wars) (2014) 74(4):424–32. [DOI] [PubMed] [Google Scholar]
- 64.Bains RS, Cater HL, Sillito RR, Chartsias A, Sneddon D, Concas D, et al. Analysis of individual mouse activity in group housed animals of different inbred strains using a novel automated home cage analysis system. Front Behav Neurosci (2016) 10:106. 10.3389/fnbeh.2016.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hastings MH, Brancaccio M, Maywood ES. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J Neuroendocrinol (2014) 26(1):2–10. 10.1111/jne.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honma S, Ono D, Suzuki Y, Inagaki N, Yoshikawa T, Nakamura W, et al. Suprachiasmatic nucleus: cellular clocks and networks. Prog Brain Res (2012) 199:129–41. 10.1016/B978-0-444-59427-3.00029-0 [DOI] [PubMed] [Google Scholar]
- 67.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms (2012) 27(5):339–52. 10.1177/0748730412456367 [DOI] [PubMed] [Google Scholar]
- 68.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci (2012) 35:445–62. 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore RY. The suprachiasmatic nucleus and the circadian timing system. Prog Mol Biol Transl Sci (2013) 119:1–28. 10.1016/B978-0-12-396971-2.00001-4 [DOI] [PubMed] [Google Scholar]
- 70.Rosenwasser AM, Turek FW. Neurobiology of circadian rhythm regulation. Sleep Med Clin (2015) 10(4):403–12. 10.1016/j.jsmc.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 71.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med (2009) 15(3):331–7. 10.1038/nm.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shipton OA, Leitz JR, Dworzak J, Acton CE, Tunbridge EM, Denk F, et al. Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J Neurosci (2011) 31(5):1688–92. 10.1523/JNEUROSCI.2610-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature (2002) 416(6880):535–9. 10.1038/416535a [DOI] [PubMed] [Google Scholar]
- 74.Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry (2013) 18(5):595–606. 10.1038/mp.2012.33 [DOI] [PubMed] [Google Scholar]
- 75.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci (2007) 10(9):1110–5. 10.1038/nn1969 [DOI] [PubMed] [Google Scholar]
- 76.Musiek ES. Circadian clock disruption in neurodegenerative diseases: cause and effect? Front Pharmacol (2015) 6:29. 10.3389/fphar.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li CX, Liang DD, Xie GH, Cheng BL, Chen QX, Wu SJ, et al. Altered melatonin secretion and circadian gene expression with increased proinflammatory cytokine expression in early-stage sepsis patients. Mol Med Rep (2013) 7(4):1117–22. 10.3892/mmr.2013.1331 [DOI] [PubMed] [Google Scholar]
- 78.Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes (2009) 58(9):1953–60. 10.2337/db08-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato F, Muragaki Y, Kawamoto T, Fujimoto K, Kato Y, Zhang Y. Rhythmic expression of DEC2 protein in vitro and in vivo. Biomed Rep (2016) 4(6):704–10. 10.3892/br.2016.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kong E, Sucic S, Monje FJ, Savalli G, Diao W, Khan D, et al. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci Rep (2015) 5:9009. 10.1038/srep09009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ciarleglio CM, Axley JC, Strauss BR, Gamble KL, McMahon DG. Perinatal photoperiod imprints the circadian clock. Nat Neurosci (2011) 14(1):25–7. 10.1038/nn.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ciarleglio CM, Resuehr HE, McMahon DG. Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience (2011) 197:8–16. 10.1016/j.neuroscience.2011.09.036 [DOI] [PubMed] [Google Scholar]
- 83.Blum ID, Zhu L, Moquin L, Kokoeva MV, Gratton A, Giros B, et al. A highly tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. Elife (2014) 29:3. 10.7554/eLife.05105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Duration of the active period (alpha) and activity onsets and offsets in interleukin-6 (IL-6) and wild-type (WT) mice. Analysis of the length of the active period in IL-6 and WT mice (n = 9–11 per group) under (A) light/dark and (B) dark/dark conditions. (C) Activity onsets and (D) offsets in circadian hours in IL-6 compared with WT mice. All data are displayed as mean ± SEM; *p < 0.05.
Behavioral actograms exemplifying circadian locomotor activity patterns in interleukin-6 (IL-6) and wild-type (WT) mice. Sample actograms illustrating wheel-running activity in (A) WT and (B) IL-6 mice.