Abstract
We propose an alternative GMO based strategy to obtain Saccharomyces cerevisiae mutant strains with a slight reduction in their ability to produce ethanol, but with a moderate impact on the yeast metabolism. Through homologous recombination, two truncated Pdc2p proteins Pdc2pΔ344 and Pdc2pΔ519 were obtained and transformed into haploid and diploid lab yeast strains. In the pdc2Δ344 mutants the DNA-binding and transactivation site of the protein remain intact, whereas in pdc2Δ519 only the DNA-binding site is conserved. Compared to the control, the diploid BY4743pdc2Δ519 mutant strain reduced up to 7.4% the total ethanol content in lab scale-vinifications. The residual sugar and volatile acidity was not significantly affected by this ethanol reduction. Remarkably, we got a much higher ethanol reduction of 10 and 15% when the pdc2Δ519 mutation was tested in a native and a commercial wine yeast strain against their respective controls. Our results demonstrate that the insertion of the pdc2Δ519 mutation in wine yeast strains can reduce the ethanol concentration up to 1.89% (v/v) without affecting the fermentation performance. In contrast to non-GMO based strategies, our approach permits the insertion of the pdc2Δ519 mutation in any locally selected wine strain, making possible to produce quality wines with regional characteristics and lower alcohol content. Thus, we consider our work a valuable contribution to the problem of high ethanol concentration in wine.
Keywords: Yeast, Ethanol, Metabolism, Genetic engineering, Wine
Introduction
Nowadays there is a growing demand for softer wines with reduced ethanol content. However, during the last twenty years there has been an increment in the alcohol concentration of wine of about 2% (v/v) (Kutyna et al. 2010; Tilloy et al. 2014). Current viticultural practices favor the harvest of very mature grapes to obtain wines with sweet tannins, as demanded by the consumers. The biosynthesis of polyphenols is usually delayed with respect to sugar production, leading to a harvest of grapes with high sugar amounts. This sugar excess in turn, produces wines with high ethanol. As a consequence of global warming, this effect is particularly exacerbated in regions with hot summers (Mira de Orduña 2010). A high concentration of alcohol in wine can have many negative consequences. The quality of the product may be compromised, e.g. the perception of viscosity and hotness could be enhanced, in detriment of acidity, aroma, intensity of flavors, sweetness and other organoleptic properties (Gawel et al. 2007a, b). Production costs may rise in countries where taxes are applied according to the ethanol content. Sluggish or stuck fermentations might also happen as a result of yeast inhibition provoked by high ethanol levels (Buescher et al. 2001). In addition, consumption of wine with high ethanol content can also have a negative impact on health. This combination of quality, economic and health problems associated with high alcohol in wine, has promoted a significant interest in developing technologies to reduce ethanol, but conserving all desirable sensory characteristics of wine (Kutyna et al. 2010). Indeed, this problem represents a major challenge for the wine industry in its attempt to counteract some of the negative effects of global warming. Different approaches have been tried, such as the application of alternative viticultural practices (Kontoudakis et al. 2011), or the implementation of physical methods like dealcoholization (Bogianchini et al. 2011). Nevertheless, the microbiological approach is probably the most attractive because of its easy implementation and low costs (Kutyna et al. 2010). The microbiological approach includes the use of non-GMO (genetically modified organisms) strategies like sequential inoculations and co-inoculations of S. cerevisiae with non-Saccharomyces yeasts, as well as GMO-based strategies with the use of genetically modified yeasts. Considering the first case, sequential inoculations and co-inoculations have become quite popular in recent years (Comitini et al. 2011; Magyar and Tóth 2011; Di Maio et al. 2012; Sadoudi et al. 2012 and reviewed in Ciani et al. 2016). Unfortunately, low yields of ethanol are usually the result of wines with high residual sugar concentrations (Ciani and Ferraro 1996; Ciani et al. 2006). In some cases, reduction in the ethanol concentration varied only between 0.2 and 0.7% (v/v) (Benito et al. 2011; Gobbi et al. 2013). However, in recent works, ethanol reduction between 1.5 and 2.2% (v/v) has been achieved through different strategies like sequential fermentation with immobilized non-Saccharomyces yeasts (Canonico et al. 2016) and the use of non-Saccharomyces yeasts combined with S. cerevisiae under controlled aeration conditions (Contreras et al. 2015; Morales et al. 2015). With regards to GMO-based strategies, most of the studies have concentrated in redirecting part of the carbon flux from the ethanol metabolic pathway to other secondary products like glycerol. The overexpression of GPD1 and/or GDP2 genes, which encode the glycerol-3-phosphate dehydrogenase isozymes, reduce the ethanol and enhance the glycerol production (Remize et al. 1999; Lopes et al. 2000). However, the concentrations of some undesirable by-products, particularly acetic acid, are also increased. There were some efforts to inhibit the formation of acetic acid by deleting the ALD6 gene, but this increased the synthesis of other oxidized compounds like acetoin, which has negative organoleptic properties (Cambon, et al. 2006; Eglinton, et al. 2002). In a previous study, a large number of genetic modifications were generated and evaluated in order to reduce ethanol concentration in wine (Varela et al. 2012). Using the same genetic background, 41 different alterations in different combinations were tested. Of all the strategies tried, the most successful to reduce ethanol were those designed to increase glycerol formation. Still, like in previous works, the increase of glycerol formation was accompanied with high levels of oxidized by-products like acetic acid, acetoin and acetaldehyde, and this effect could not be completely neutralized with additional genetic modifications.
In the present work, we propose an alternative GMO based strategy to obtain S. cerevisiae mutant strains with a slight reduction in their ability to produce ethanol, but with a moderate impact on the yeast metabolism. For this purpose, we designed two functional strategic mutations at the C-terminus of the PDC2 gene in order to alter the structure of the encoded protein. PDC2 encodes a transcription factor (Hohmann 1993) that regulates the availability of the pyruvate decarboxylase (PDC) isozymes Pdc1p and Pdc5p, which catalyze the reaction of pyruvate to acetaldehyde in the ethanol biosynthetic pathway [the main active form during glucose catabolism is PDC1 and PDC5 is a secondary form which is only expressed under thiamine starvation (Mojzita and Hohmann 2006)]. The full Δpdc2 deletion has already been tested to study the genetic factors affecting the glycerol formation and its overproduction (Nevoigt and Stahl 1996). Although the glycerol formation is enhanced without affecting the acetic acid concentration, the Δpdc2 deletion exhibits a phenotype incompatible for yeasts with oenological purposes (drastic reduction of PDC specific activity and ethanol concentration, as well as strong inhibition of growth in aerobic conditions). A structurally altered version of the Pdc2p transcription factor may display a reduced positive regulatory activity of PDC1 and PDC5, leading to a moderate reduction of the PDC enzymatic activity and consequently a reduction in ethanol production. Through homologous recombination, two truncated Pdc2p proteins lacking 344 (pdc2Δ344) or 519 (pdc2Δ519) amino acids at the C-terminus were obtained and transformed into lab yeast strains. In the case of pdc2Δ344 the DNA-binding and transactivation site are both intact, whereas in pdc2Δ519 only the DNA-binding site is conserved. Subsequently, these mutants were tested in lab-scale vinifications to select low-ethanol yeasts. The selected mutation was then tested in both native and commercial wine yeast strains.
Materials and methods
Strains, media and growth conditions
Saccharomyces cerevisiae laboratory strains BY4741 (haploid, MAT a ; his3Δ 1; leu2Δ 0; met15Δ 0; ura3Δ 0) and BY4743 (diploid, MAT a /MAT α his3Δ 1/his3Δ 1; leu2Δ0/leu2Δ 0; met15Δ 0/MET15; LYS2/lys2Δ 0; ura3Δ 0/ura3Δ 0) were used to construct the mutants and as control strains during the vinification experiments. The commercial wine yeast EC1118 (Lallemand, Denmark) and the native Mab2C strain previously selected in our laboratory, were used to test the pdc2Δ519 mutation in native genetic backgrounds. All yeast strains were grown at 30 °C and 150 rpm in YPD medium (2% glucose, 2% peptone and 1% yeast extract). YPD supplemented with 200 mg/L of G-418 was used for selection and maintenance of transformants.
Construction and genomic integration of the pdc2Δ344 and pdc2Δ519 mutations
The pdc2Δ344 and pdc2Δ519 mutations were integrated into the yeast genome through homologous recombination following the method described by Güldener et al. (1996). The disrupting fragment was obtained by PCR amplification of the KanMx resistance cassette present in the pUG6 plasmid (Güldener et al. 1996). The primers used were, forward 5′-ACAGAATACTGTTGATAATAGTACCAAAA CAGGTAACCCTTGAAGCTGAAGCTCGTACGC-3 for pdc2Δ344 or 5′-TTGGGAT GATATACCCGTTGATGCTATCAAAGCAAATTGGTGAAGCTGAAGCTTCGTACGC-3 for pdc2Δ519 with the reverse primer 5′-CTAAAAAAAGCCTGTGT TACCAGGTAAGTGTAAGTTATTAGCATAGGCCACTAGTGGATCTG-3′. A specific PDC2 homologous sequence was inserted at the 5′-end of each primer to generate the recombination event at the expected C-terminal region of the PDC2 gene. Besides, two stop codons were placed downstream to the homologous region of each forward primer to generate the specific truncated proteins at the C-terminus. After the PCR reaction, the disrupting fragments were transformed by a slightly modified version of the lithium acetate method (Gietz and Woods 2002) selecting the G-418 resistant transformants. The correct insertion of the fragment was confirmed by PCR using the forward primer 5′-GCGTGGTCGACTCAAAACCAATAGCTGCTTAAAAA-3′ which binds upstream of the PDC2 gene and the reverse 5′-GGATGTATGGGCTAAATG-3′ which binds inside the KanMx resistance cassette.
Growth curves in YPD rich medium
Yeast cultures were inoculated into 10 mL YPD and grown overnight. These cultures were then used to inoculate 50 mL of YPD in 100 mL Erlenmeyer flasks at an initial OD600 of 0.2 (Spectrophotometer UV–visible T60U PG Instruments, Leicestershire, UK). The cultures were grown with 150 rpm at 30 °C and aliquots of 100 µL were taken at different intervals for the measurement of the OD600.
Laboratory scale vinifications
Following the recommendation of Vazquez et al. (2001), Lab-scale vinifications were carried out using concentrated white must as substrate, diluted to a final sugar concentration of 20°Bx and supplemented with 1 g/L of yeast extract. The vinifications were performed in 500 mL flasks plugged with glass fermentation traps so that only CO2 could evolve from the system, and they were kept at 28 °C without agitation (Vaughan-Martini and Martini 1998). All vinification experiments were performed under the described conditions but comparing different strains (V1, V2, V3 and V4). Fermentation kinetics was monitored by measuring the daily CO2 weight loss. Alcohol concentration, acetic acid and residual sugar were measured according to standard methods (OIV 2015), whereas glycerol was measured with spectrophotometry (WinescanTM Foss, Hillerød, Denmark). Several derived fermentative parameters such as carbon balance, glucose and ethanol yield were calculated (Vazquez et al. 2001). Carbon balance was calculated as the ratio between carbon moles of fermentation by-products and carbon moles of glucose. Meanwhile, glucose yield results from the amount of glucose required (g) to produce 1% (v/v) of ethanol, and ethanol yield, from the ratio between grams of produced ethanol and grams of consumed glucose. All assays were performed at least in triplicates (three independent cultures).
Statistical analysis of data
An ANOVA and a LSD Fisher test with a p value <0.05 was performed for the analysis and media comparison of the growth, fermentative and kinetics parameters. Growth parameters as well as kinetic parameters were estimated using the reparameterized Gompertz equation as proposed by Zwietering et al. (1990):
where y = ln (ODt/OD0) OD0 is the initial OD and ODt is the OD at time t; D = ln (ODmax/DO0) is the curve maximum asymptotic, μmax is the maximum specific growth rate (1/h), and λ is the lag phase period (h). Growth and kinetics data were fitted by nonlinear regression procedure, minimizing the sum of squares of the difference between the experimental data and the fitted model.
Results
Growth of haploid and diploid pdc2Δ519 and pdc2Δ344 mutants in aerobic conditions
The Δpdc2 complete deletion causes a drastic reduction of the PDC specific activity, an accumulation of pyruvate and a strong inhibition of growth in aerobic conditions (Hohmann 1993; Nevoigt and Stahl 1996). The ability to grow in aerobic conditions is essential for a yeast strain to be selected to develop wine yeast starters. Although the conditions of wine fermentation are predominantly anaerobic, the biomass production is performed in aerobic conditions. Hence, before quantifying the fermentative parameters of each strain we wanted to test the growth capability of the pdc2Δ519 and pdc2Δ344 mutants under aerobic conditions with glucose as carbon source. Figure 1 shows the growth curves in YPD medium for the haploid mutant strains BY4741pdc2Δ344 and BY4741pdc2Δ519 as well as the diploid BY4743pdc2Δ344 and BY4743pdc2Δ519 with their respective BY4741 and BY4743 controls. Remarkably, all mutants displayed growth pattern similar to the controls, and consequently no statistical difference was detected among the kinetic parameters analyzed (λ, and µmax) (data not shown). This result demonstrates that all mutant strains grow as good as the controls in aerobic conditions, and therefore they are suitable for further experimentation.
Fig. 1.
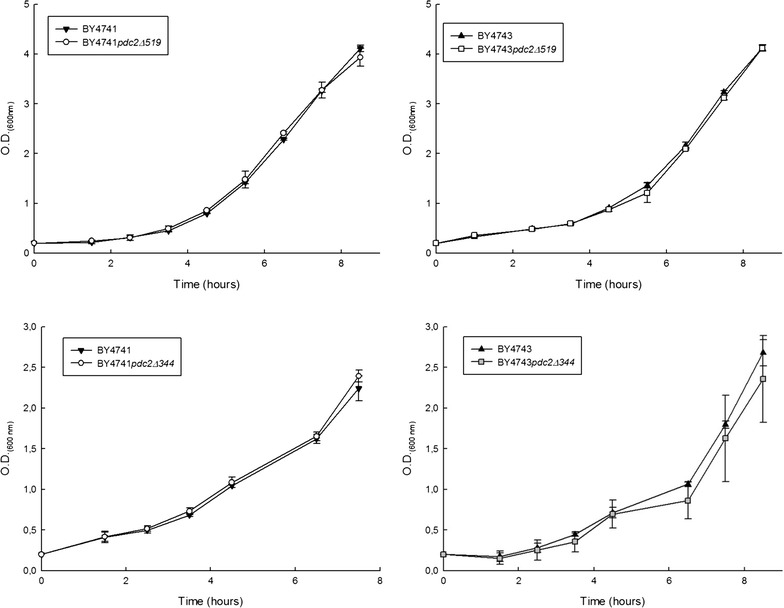
Growth of parental and mutant laboratory strains in YPD at 30 °C and 150 rpm shaking. Each point represents the average value of two independent cultures
Quantification of fermentative parameters from lab-scale vinifications and selection of low ethanol pdc2 mutants
In order to select low ethanol pdc2 mutant strains, we performed a series of microvinifications with concentrated must diluted to 20°Bx and supplemented with 0.1% (w/v) of yeast extract (Vazquez et al. 2001). The concentration of ethanol, acetic acid, glycerol and residual sugar were determined, and other derived fermentative parameters like carbon balance and glucose yield were calculated. We searched for a mutant strain with a slight inefficiency to produce ethanol in order to reduce the alcohol content of wine between 1 and 2% (v/v). During the first vinification experiment (V1) the haploid and diploid pdc2Δ519 mutants were assayed against their respective BY4741 and BY4743 controls (Table 1). Considering the ethanol production, BY4741pdc2Δ519 showed no statistical difference when compared to the control, whereas BY4743pdc2Δ519 displayed a statistically significant ethanol reduction (p < 0.05) of around 7%. The moderate reduction observed in BY4743pdc2Δ519 was within the expected, however, it is surprising we didn’t observe any phenotype for the haploid BY4741pdc2Δ519, which carries only the mutant copy of the PDC2 gene. In correspondence with the observed ethanol reduction, BY4743pdc2Δ519 also presented the lowest value of carbon balance and it was the least efficient to produce ethanol. It is important to note that this ethanol reduction didn’t cause an increase of the volatile acidity. Taken together, this was a promising result and as a consequence BY4743pdc2Δ519 was selected for further experimentation. Continuing with the mutant’s characterization we performed a second vinification experiment (V2), with the selected BY4743pdc2Δ519, the still uncharacterized BY4741pdc2Δ344, BY4743pdc2Δ344d strains, and both haploid BY4741 and BY4743 diploid controls (Table 1). The same trend was observed for the mutant BY4743pdc2Δ519, which again is the least efficient to produce ethanol, showing statistically significant lower values of glucose yield compared with the control and the rest of the strains. With regards to the ethanol production of the pdc2Δ344 mutants, the haploid produced significantly less ethanol than the BY4741 control, whereas the mutant diploid showed no statistical difference comparing with the diploid BY4743 control. Nonetheless, the ethanol reduction observed for BY4741pdc2Δ344 was not reflected in the efficiency of the mutant strains, since its glucose yield was not significantly different from the control. This compensation in the glucose yield production is explained by the fact that BY4741pdc2Δ344 displayed considerably higher values of residual sugar compared with BY4741. Up to this point, the BY4743pdc2Δ519 mutant showed a consistent phenotype of ethanol reduction being the least efficient in both vinifications. According to the value obtained for the glucose yield, BY4743pdc2Δ519 would reduce the ethanol almost 1 degree (0.85) for a wine with a prediction of 15.5% (v/v). The BY4743pdc2Δ344 mutant showed an intermediate phenotype producing 4% less ethanol than the control, but there was no statistically significant difference in the glucose yield. In summary, the vinification experiments showed that BY4743pdc2Δ519 is the most promising mutant strain displaying a small but consistent reduction of the ethanol concentration, as a result of a slight inefficiency for its production. Importantly, this ethanol reduction did not cause an increment of the concentration of acetic acid, which is perhaps the most undesirable by-product of the wine fermentation.
Table 1.
Fermentative parameters of lab-scale vinification assays in 20°Bx white must by parental and mutant laboratory yeast strains
| Parameter | BY4741 | BY4741 pdc2Δ519 |
BY4743 | BY4743 pdc2Δ519 |
BY4741 | BY4741 pdc2Δ344 |
BY4743 | BY4743 pdc2Δ344 |
BY4743 pdc2Δ519 |
|---|---|---|---|---|---|---|---|---|---|
| Main compounds (g/Liter) | V1 | V1 | V1 | V1 | V2 | V2 | V2 | V2 | V2 |
| Consumed sugar | 153.95 ± 0.87A | 146.49 ± 0.63B | 147.22 ± 0.00B | 143.84 ± 1.66C | 172.04 ± 4.64B,C | 154.90 ± 4.22D | 179.56 ± 3.08A | 170.98 ± 1.98C | 176.05 ± 4.71A,B |
| CO2 | 71.11 ± 1.92A | 73.33 ± 5.77A | 66.67 ± 0.00A,B | 62.22 ± 3.85B | 81.88 ± 3.89A,B | 72.48 ± 2.52C | 83.45 ± 1.60A | 78.68 ± 2.84B | 83.75 ± 2.20A |
| Ethanol | 75.61 ± 0.91A | 75.35 ± 2.23A | 74.56 ± 1.58A | 69.04 ± 0.79B | 87.58 ± 5.69A | 76.007 ± 2.28C | 87.74 ± 1.41A | 84.27 ± 2.58A,B | 81.27 ± 1.48B |
| Acetate | 1.11 ± 0.16A | 0.91 ± 0.06A,B | 0.88 ± 0.24A,B | 0.78 ± 0.07B | 1.11 ± 0.11A | 0.63 ± 0.06B,C | 0.58 ± 0.12C | 0.83 ± 0.22B | 0.60 ± 0.09C |
| Glycerolb | ND | ND | ND | ND | 2.67 ± 0.20B | 2.44 ± 0.074B,C | 2.58 ± 0.14B | 3.16 ± 0.11A | 2.24 ± 0.18C |
| Balance (%)a | |||||||||
| Carbon | 97.18 ± 1.27B | 101.43 ± 3.18A | 97.64 ± 0.72A,B | 95.26 ± 1.13B | 99.32 ± 2.50A | 97.33 ± 1.27A,B | 96.40 ± 0.96A,B | 96.67 ± 2.66A,B | 95.18 ± 2.37B |
| Yield | |||||||||
| Ethanol production (%[v/v]) | 9.58 ± 0.12A | 9.55 ± 0.20A | 9.45 ± 0.20A | 8.80 ± 0.10B | 11.1 ± 0.72A | 9.63 ± 0.29C | 11.12 ± 0.18A | 10.68 ± 0.33A,B | 10.30 ± 0.19B |
| EtOH (g/g glucose consumed) | 0.49 ± 0.008A | 0.51 ± 0.011A | 0.51 ± 0.009A | 0.48 ± 0.009A | 0.51 ± 0.02A | 0.49 ± 0.02A,B | 0.49 ± 0.008B | 0.49 ± 0.019A,B | 0.46 ± 0.012C |
| Glucose (g) required for 1% (v/v) ethanol production | 16.07 ± 0.28A,B | 15.34 ± 0.27C | 15.58 ± 0.33B,C | 16.35 ± 0.32A | 15.52 ± 0.62B | 16.09 ± 0.59B | 16.15 ± 0.25B | 16.02 ± 0.62B | 17.09 ± 0.42A |
| Glucose (g) required for 1 g/L glycerol Residual sugar (g/Liter) |
ND 62.65 ± 0.87C |
ND 70.11 ± 0.63B |
ND 69.38 ± 0.00B |
ND 72.76 ± 1.66A |
65.64 ± 4.21B
44.56 ± 4.64B,C |
69.49 ± 2.30A,B
61.7 ± 4.22A |
64.56 ± 3.83B
37.04 ± 3.08D |
54.57 ± 1.99C
45.62 ± 1.98B |
73.64 ± 3.38A
40.55 ± 4.71C,D |
ND not determined
Distinct letters correspond to statistical significant difference for a Fischer test with p < 0.05
aCarbon balance represents the ratio between carbon moles of fermentation by-products and carbon moles of glucose
bGlycerol was measured in an independent experiment
Most of the efforts to deviate the carbon flux away from ethanol have concentrated in the production of glycerol, a desirable secondary metabolite of fermentation. Therefore, the glycerol produced in lab-scale vinifications was also measured (Table 1). The quantification of glycerol could give as a clue of what is happening to the carbon flux of the pdc2Δ mutants, particularly BY4743pdc2Δ519 which showed a consistent ethanol reduction. Surprisingly, the glycerol production of BY4743pdc2Δ519 was not increased but reduced, showing statistically significant differences with the control in the final concentration and the glucose yield for glycerol. In contrast, BY4743pdc2Δ344 which previously showed no ethanol reduction produced more glycerol than the control with statistically significant higher values. At least for the Δpdc2 diploid mutants, there seems to be no clear correlation between ethanol and glycerol production and the ethanol reduction observed in BY4743pdc2Δ519 is not a consequence of an increment of glycerol concentration.
Kinetics analysis of the vinifications by CO2 weight loss
The progression of the vinifications was daily monitored by measuring the CO2 weight loss. Figure 2 shows the curves of accumulated CO2 weight loss obtained for each vinification experiment. As seen in the graphs (panels a, b), all fermentations were very slow, lasting around three weeks (an industrial fermentation performed with wine yeasts usually last 7–10 days). Nevertheless, this result is not surprising considering we used lab strains, which are not specialized for wine fermentation. We calculated for each curve the mean value of the three main kinetic parameters (Zwietering et al. 1990) lag phase (λ), maximum CO2 weight loss speed (μmax) and total accumulated CO2 weight loss (A, for asymptote). After performing an ANOVA, the mean values of the kinetic parameters were compared (Table 2). With few exceptions, the kinetics of the fermentations was very similar between the mutants and their controls. In the first vinification (V1) there was no statistical difference between the haploid strains BY4741pdc2Δ519h and BY4741, but they lost more CO2 than the diploid strains. The diploid mutant BY4743pdc2Δ519 lost less CO2 than its control but the difference was not statistically significant. Considering that this strain also produces less ethanol, we expected a higher CO2 reduction for BY4743pdc2Δ519, which was not the case. In the second vinification, BY4743pdc2Δ519 showed again similar values of CO2 and μmax when compared to the control. It is interesting to note that despite the ethanol reduction observed for BY4743pdc2Δ519, there was little (vinification 1) or no difference (vinification 2) in the total CO2 weight loss comparing with the BY4743 control. Apparently, another decarboxylation reaction is compensating the CO2 formed during the ethanol biosynthesis, and this is a clue which could help us to reveal how the carbon flux has been modified in the BY4743pdc2Δ519 mutant strain.
Fig. 2.
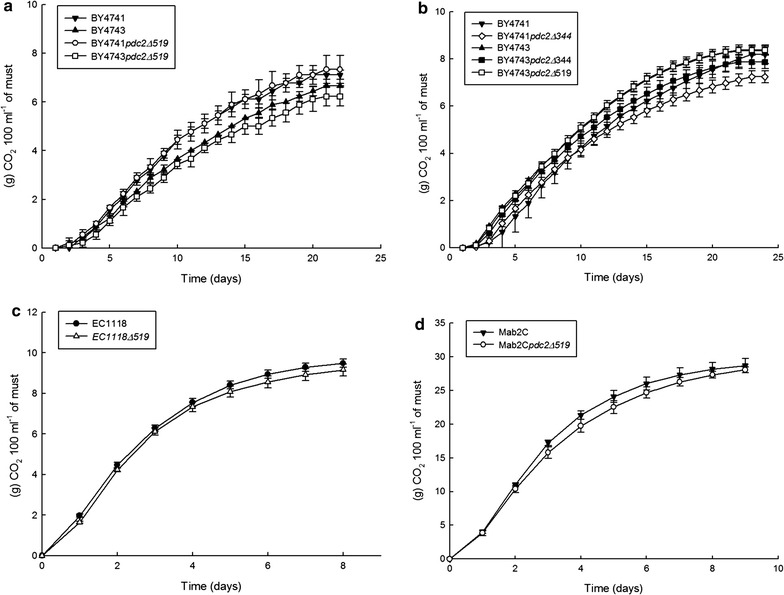
Accumulated CO2 weight loss curves along the experiments of vinifications 1 (a), 2 (b), 3 (c) and 4 (d) with parental and mutant strains. The evolution of each vinification was daily monitored by measuring the CO2 weight loss. Each point represents the average value of three independent cultures
Table 2.
Quantification and statistical analyses of fermentation parameters
| Parameter | BY4741 | BY4741 pdc2Δ519 |
BY4743 | BY4743 pdc2Δ519 |
|---|---|---|---|---|
| Vinification 1 | ||||
| A | 21.96 ± 0.59A,B | 22.83 ± 1.57A | 21.31 ± 0.52A,B | 19.79 ± 1.74B |
| µmax | 1.8 ± 0.05A | 1.74 ± 0.13A | 1.44 ± 0.05B | 1.39 ± 0.01B |
| λ | 2.66 ± 0.26A | 2.38 ± 0.01A | 2.47 ± 0.39A | 2.86 ± 0.40A |
| BY4741 | BY4741 pdc2Δ344 |
BY4743 | BY4743 pdc2Δ344 |
BY4743 pdc2Δ519 |
|
|---|---|---|---|---|---|
| Vinification 2 | |||||
| A | 25.24 ± 1.10A,B | 22.05 ± 0.68C | 26.11 ± 0.49A | 24.38 ± 0.88B | 26.07 ± 0.74A |
| µmax | 1.78 ± 013B,C | 1.64 ± 0.09C | 1.91 ± 0.06A,B | 1.80 ± 0.09B | 1.98 ± 0.06A |
| λ | 2.97 ± 1.11A | 2.30 ± 0.17A,B | 1.80 ± 0.05B | 1.97 ± 0.05A,B | 2.04 ± 0.23B |
A = Asymptote, µ max = maximum specific growth rate and (λ) = lag time, for the three vinifications
Distinct letters correspond to statistical significant difference for a Fischer test with p < 0.05
Insertion of the pdc2Δ519 mutation in the commercial EC1118 and native Mab2C wine yeast strains
The BY4743pdc2Δ519 mutant strain showed a phenotype according to our aim of reducing around 1–2% (v/v) the ethanol content of wine, without significantly affecting the concentration of residual sugar and acetic acid. This is already a positive result, but we should bear in mind that all the previous experiments were performed with laboratory yeast strains which are genetically quite different from wine yeasts. Therefore, our next challenge was to check whether this phenotype could be reproduced or even improved in a wine yeast genetic background. This way, we would obtain wine yeasts suitable for industrial fermentation and capable of reducing the ethanol content of wine. To test this, the pdc2Δ519 mutation was first integrated by transformation and homologous recombination into the genome of the commercial EC1118 and the native Mab2C wine yeasts. Considering the ploidy of these strains, EC1118 has been reported to be a diploid (Novo et al. 2009) and according to a PCR test performed in our lab, Mab2C would be at least a diploid since both mating types were present. After the insertion of one pdc2Δ519 mutation copy, we performed a growth curve in aerobic conditions and lab-scale vinifications to compare the genetically modified EC1118Δ519 and Mab2CΔ519 with their respective wild type control strains. The experiments were performed with the same conditions used for the lab strains. With respect to the growth in aerobic conditions, both EC1118Δ519 and Mab2CΔ519 mutants grew normally in YPD medium showing no difference with their respective wild type controls (data not shown). As for the vinification experiments V3 and V4, Fig. 2 shows the curve for the accumulated CO2 weight loss (panels c, d) and Table 3 summarizes the value obtained for the main fermentative parameters. In contrast with the lab strains, the vinifications were in this case much faster and lasted around eight days, which is the expected time for well adapted wine yeasts. Interestingly, the mutants were not affected by the mutation and their kinetics was very similar to the wild type strains. The analysis of the fermentative parameters revealed a remarkable 15% total ethanol reduction for the mutant EC1118Δ519 and 10% for Mab2CΔ519 comparing with the wild type controls. Both EC1118Δ519 and Mab2CΔ519 mutants were also less efficient to produce ethanol showing statistically significant lower values of glucose yield. According to the values obtained for the glucose yield, EC1118Δ519 would reduce the ethanol almost 2 degrees (1.89) and Mab2CΔ519 almost 1.5 degrees (1.36) for a wine with a prediction of 15.5% (v/v). As it happened with the laboratory BY4743pdc2Δ519 mutant strain, the acetic acid concentrations of EC1118Δ519 and Mab2CΔ519 were also unaffected by the mutation. The ethanol reduction obtained with EC1118Δ519 was about two-fold higher than that of the laboratory BY4743pdc2Δ519 mutant strain. The Mab2CΔ519 native strain also displayed a higher ethanol reduction (about 1.5-fold) when compared with the laboratory BY4743pdc2Δ519 mutant strain. Thus, we were not only able to reproduce the phenotype observed in the lab strain, but also we improved it. In agreement with the ethanol reduction displayed, the carbon balance of the mutants are considerable lower than the wild type controls indicating that part of the carbon flux is being redirected away from the ethanol biosynthesis pathway.
Table 3.
Determination of fermentative parameters for mutant and wild type wine yeast strains EC1118 and Mab2C in two independent lab-scale vinifications
| Parameter | EC1118 | EC1118Δ519 | Mab2C | Mab2CΔ519 |
|---|---|---|---|---|
| V3 | V3 | V4 | V4 | |
| Main compounds (g/L) | ||||
| Consumed sugar | 210.38 ± 0.64A | 203.95 ± 2.51B | 212.03 ± 0.44A | 209.01 ± 0.63B |
| CO2 | 94.84 ± 2.05A | 91.56 ± 3.01A | 95.60 ± 3.50A | 93.51 ± 1.40A |
| Ethanol | 102.31 ± 2.99A | 87.05 ± 2.77B | 95.73 ± 4.05A | 86.00 ± 1.58B |
| Acetate | 1.13 ± 0.16A | 1.12 ± 0.19A | 0.83 ± 0.17A | 0.86 ± 0.19A |
| Balance (%) | ||||
| Carbon | 94.42 ± 1.81A | 88.81 ± 1.48B | 90.82 ± 3.19A | 86.78 ± 0.47A |
| Yield | ||||
| Ethanol production (% [v/v]) | 12.97 ± 0.38A | 11.03 ± 0.35B | 12.13 ± 0.51A | 10.90 ± 0.20B |
| EtOH (g/g glucose consumed) | 0.49 ± 0.013A | 0.43 ± 0.08B | 0.47 ± 0.021A | 0.42 ± 0.008B |
| Glucose (g) required for 1% (v/v) ethanol production | 16.23 ± 0.42A | 18.49 ± 0.36B | 17.50 ± 0.71B | 19.18 ± 0.32A |
| Residual sugar (g/L) | 6.22 ± 0.64A | 12.65 ± 2.51B | 4.57 ± 0.44B | 7.59 ± 0.63A |
Distinct letters correspond to statistical significant difference for a Fischer test with p < 0.05
Discussion
Before quantifying the fermentative parameters of each laboratory mutant strain we tested the growth capability of the pdc2Δ519 and pdc2Δ344 mutants under aerobic conditions with glucose as carbon source. All mutants were able to grow normally showing no difference with their respective controls. This was already a positive result considering the inability of the full Δpdc2 deletion to grow under such conditions (Hohmann 1993; Nevoigt and Stahl 1996). Nevertheless, it is quite surprising that none of the mutants showed a growth defect, especially BY4741pdc2Δ519 that carries only the mutant version of Pdc2p with a deletion of 519 amino acids which accounts for 56% of the wild type protein. Apparently, the activity of the binding site alone is sufficient to sustain a normal growth of the yeast.
With respect to the mutant’s fermentative parameters, they were determined by lab-scale vinifications. The phenotypic analysis showed that BY4743pdc2Δ519 is the most interesting mutant strain, displaying a consistent reduction of the ethanol concentration of up to 7.4%, as a result of a slight inefficiency for its production. It is important to remark that this ethanol reduction did not provoke an increment of the concentration of acetic acid, in contrast to previous GMO based strategies where the carbon flux was diverted to glycerol (Remize et al. 1999; Lopes et al. 2000; Varela et al. 2012). The moderate reduction observed in BY4743pdc2Δ519 was within the expectable, and it could be the result of a competence phenomenon between the wild type and the mutant Pdc2p protein for the DNA binding site and some activating protein. Although it is just a speculation, there is some indirect evidence which supports this proposition. On one hand, it has been shown that the Pdc2p DNA-binding site alone retains some DNA binding activity, and on the other hand, an activating protein has been proposed at least for the regulation by PDC2 of the THI genes (Nosaka et al. 2012). In any case, the molecular mechanism underlying the regulation of PDC1 by PDC2 is still unknown (Brion et al. 2014) and more investigation would be required to clarify this matter.
In view of the good results obtained with the BY4743pdc2Δ519 strain our next goal was to test this mutation in wine yeast strains. For this purpose the pdc2Δ519 mutation was transformed into the commercial EC1118 and native Mab2C wine yeast strains. EC1118 has been widely used in the wine industry and it is known for its reliability and excellent fermentation performance (Aceituno et al. 2012). Meanwhile, Mab2C is a native strain previously selected in our laboratory for its excellent oenological properties in the elaboration of Malbec wine. Both EC1118Δ519 and Mab2CΔ519 mutants grew normally in aerobic conditions with glucose as a carbon source and displayed typical fermentation kinetics for a wine yeast strain. As it happened with the laboratory BY4743pdc2Δ519 mutant strain, the acetic acid concentrations of EC1118Δ519 and Mab2CΔ519 were also unaffected by the mutation. The ethanol reduction obtained with EC1118Δ519 and Mab2CΔ519 was about two-fold higher than that of the laboratory BY4743pdc2Δ519 mutant strain. Thus, we were not only able to reproduce the phenotype observed in the lab strain, but also we improved it. These results demonstrate that the wine yeasts mutants EC1118Δ519 and Mab2CΔ519 are good candidates to develop a yeast starter for the elaboration of wines with reduced ethanol content. Still, it will be necessary to determine how the carbon flux is being redirected. In an exploratory experiment, we determined the glycerol concentration for the laboratory strain BY4743pdc2Δ519 and we found no significant increment compared to the control. Perhaps, a clue could come from the analysis of the CO2 weight loss data. Despite the ethanol reduction observed for BY4743pdc2Δ519, EC1118Δ519 and Mab2CΔ519 mutants, there was little or no difference in the total CO2 weight loss comparing with the wild type controls. It seems that another decarboxylation reaction is compensating the CO2 formed during the ethanol biosynthesis. Following this reasoning, two good candidates could be acetoin and 2,3-butanediol. Both compounds are derived from the secondary metabolism of yeast, and are produced from pyruvate in a series of chemical reactions where at least one decarboxylation reaction is involved (Romano and Suzzi 1996). In a recent work, an ethanol reduction of 1.3% (v/v) was achieved by combining adaptive laboratory evolution strategies with hybridization (Tilloy et al. 2014). Interestingly, the enhancement of glycerol production in the selected yeast was accompanied by an increment in 2,3-butanediol, which is consider a neutral organoleptic compound.
In this study we present an alternative microbiological strategy to reduce the ethanol content in wine, through a genetic modification of the S. cerevisiae Pdc2p transcription factor. Our results demonstrate that the insertion of the pdc2Δ519d mutation in a wine yeast strain can reduce the ethanol concentration up to 1.89% v/v without affecting the fermentation performance. In contrast to non-GMO based strategies, our approach permits the insertion of the selected mutation in any locally selected wine strain, making possible to produce quality wines with regional characteristics and lower alcohol content. This makes our work a valuable contribution to the problem of high ethanol concentration in wine. Nevertheless, pilot-scale trials complemented with sensorial analysis of the produced wines are required for a full evaluation of our strain’s potential for its application in the wine industry.
Authors’ contributions
RAC Participated in the design and coordination of the study, performed the experiments, interpreted the data, and drafted the manuscript. KJFM performed the experiments and the data analysis. LAM performed the experiments and the data analysis. MC participated in its design and coordination, interpreted the data, and helped to draft the manuscript. IFC conceived of the study, participated in its design and coordination, interpreted the data, and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This worked was supported by a PICT 2008-206 project of the Fondo para la investigación Científica y Tecnológica (FonCyT) Argentina. R.A.C. is a fellow of CONICET.
Abbreviations
- ANOVA
analysis of variance
- GMO
genetically modified organism
- LSD
least significant difference
- PDC
pyruvate decarboxylase
Contributor Information
Raúl Andrés Cuello, Email: cuello.raul@inta.gob.ar.
Karina Johana Flores Montero, Email: kfloresmontero@yahoo.com.ar.
Laura Analía Mercado, Email: mercado.laura@inta.gob.ar.
Mariana Combina, Email: combina.mariana@inta.gob.ar.
Iván Francisco Ciklic, Phone: +54-261-4963020, Email: ciklic.ivan@inta.gob.ar.
References
- Aceituno FF, Orellana M, Torres J, Mendoza S, Slater AW, Melo F, Agosin E. Oxygen response of the wine yeast Saccharomyces cerevisiae EC1118 grown under carbon-sufficient, nitrogen-limited enological conditions. Appl Environ Microbiol. 2012;78:8340–8352. doi: 10.1128/AEM.02305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito S, Morata A, Palomero F, González MC, Suárez-Lepe JA. Formation of vinylphenolic pyranoanthocyanins by Saccharomyces cerevisiae and Pichia guillermondii in red wines produced following different fermentation strategies. Food Chem. 2011;124:15–23. doi: 10.1016/j.foodchem.2010.05.096. [DOI] [Google Scholar]
- Bogianchini M, Cerezo AB, Gomis A, López F, García-Parrilla MC. Stability, antioxidant activity and phenolic composition of commercial and reverse osmosis obtained dealcoholised wines. LWT Food Sci Technol. 2011;44:1369–1375. doi: 10.1016/j.lwt.2011.01.030. [DOI] [Google Scholar]
- Brion C, Ambroset C, Delobel P, Sanchez I, Blondin B. Deciphering regulatory variation of THI genes in alcoholic fermentation indicate an impact of Thi3p on PDC1 expression. BMC Genom. 2014;15:1085. doi: 10.1186/1471-2164-15-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher WA, Siler CE, Morris JR, Threlfall RT, Main GL, Cone GC. High alcohol wine production from grape juice concentrates. Am J Enol Vitic. 2001;52:345–351. [Google Scholar]
- Cambon B, Monteil V, Remize F, Camarasa C, Dequin S. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl Environ Microbiol. 2006;72:4688–4694. doi: 10.1128/AEM.02975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico L, Comitini F, Oro L, Ciani M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front Microbiol. 2016;7:278. doi: 10.3389/fmicb.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani M, Ferraro L. Enhanced glycerol content in wines made with immobilized Candida stellata cells. Appl Environ Microbiol. 1996;62:128–132. doi: 10.1128/aem.62.1.128-132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani M, Beco L, Comitini F. Fermentation behavior and metabolic interactions of multistarter wine yeast fermentations. Int J Food Microbiol. 2006;108:239–245. doi: 10.1016/j.ijfoodmicro.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Ciani M, Morales P, Comitini F, Tronchoni J, Canonico L, Curiel JA, Oro L, Rodrigues AJ, Gonzalez R. Non-conventional yeast species for lowering ethanol content of wines. Front Microbiol. 2016;7:642. doi: 10.3389/fmicb.2016.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzud I, Ciani M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;5:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Contreras A, Hidalgo C, Schmidt S, Henschke PA, Curtin C, Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int J Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Di Maio S, Genna G, Gandolfo V, Amore G, Ciaccio M, Oliva D. Presence of Candida zemplinina in sicilian musts and selection of a strain for wine mixed fermentations. S Afr J Enol Vitic. 2012;33:80–87. [Google Scholar]
- Eglinton JM, Heinrich AJ, Pollnitz AP, Langridge P, Henschke PA, de Barros Lopes M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast. 2002;19:295–301. doi: 10.1002/yea.834. [DOI] [PubMed] [Google Scholar]
- Gawel R, Francis L, Waters EJ. Statistical correlations between the in-mouth textural characteristics and the chemical composition of Shiraz wines. J Agric Food Chem. 2007;55:2683–2687. doi: 10.1021/jf0633950. [DOI] [PubMed] [Google Scholar]
- Gawel R, Van Sluyter S, Waters EJ. The effects of ethanol and glycerol on the body and other sensory characteristics of Riesling wines. Aust J Grape Wine Res. 2007;13:38–45. doi: 10.1111/j.1755-0238.2007.tb00070.x. [DOI] [Google Scholar]
- Gietz DR, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/S0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Comitini F, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: a strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013;33:271–281. doi: 10.1016/j.fm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Güldener U, Heck S, Fiedler T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S. Characterization of PDC2, a gene necessary for high level expression of pyruvate decarboxylase structural genes in Saccharomyces cerevisiae. Mol Gen Genet. 1993;1:657–666. doi: 10.1007/BF00279908. [DOI] [PubMed] [Google Scholar]
- Kontoudakis N, Esteruelasa M, Forta F, Canalsa JM, De Freitas V, Zamora F. Influence of the heterogeneity of grape phenolic maturity on wine composition and quality. Food Chem. 2011;124:767–774. doi: 10.1016/j.foodchem.2010.06.093. [DOI] [Google Scholar]
- Kutyna DR, Varela C, Henschke PA, Chambers PJ, Stanley GA. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci Technol. 2010;21:293–302. doi: 10.1016/j.tifs.2010.03.004. [DOI] [Google Scholar]
- Lopes M, Ur-Rehman A, Gockowiak H, Heinrich AJ, Langridge P, Henschke PA. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae glycerol 3-phosphate dehydrogenase gene (GPD2) Aust J Grape Wine Res. 2000;6:208–215. doi: 10.1111/j.1755-0238.2000.tb00181.x. [DOI] [Google Scholar]
- Magyar I, Tóth T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011;28:94–100. doi: 10.1016/j.fm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Mojzita D, Hohmann S. Pdc2 coordinates expression of the THI regulon in the yeast Saccharomyces cerevisiae. Mol Genet Genomics. 2006;276(2):147–161. doi: 10.1007/s00438-006-0130-z. [DOI] [PubMed] [Google Scholar]
- Morales P, Rojas V, Quirós M, Gonzalez R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl Microbiol Biotechnol. 2015;9:3993–4003. doi: 10.1007/s00253-014-6321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevoigt E, Stahl U. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast. 1996;12:1331–1337. doi: 10.1002/(SICI)1097-0061(199610)12:13<1331::AID-YEA28>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Esaki H, Onozuka M, Konno H, Hattori Y, Akaji K. Facilitated recruitment of Pdc2p, a yeast transcriptional activator, in response to thiamin starvation. FEMS Microbiol Lett. 2012;330:140–147. doi: 10.1111/j.1574-6968.2012.02543.x. [DOI] [PubMed] [Google Scholar]
- Novo M, Bigey F, Beyne E, Galeote V, Gavory F, Mallet S, Cambon B, Legras JL, Wincker P, Casaregola S, Dequin S. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc Natl Acad Sci. 2009;38:16333–16338. doi: 10.1073/pnas.0904673106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIV International methods of analysis of wines and musts (2015) Compendium of international methods of analysis of wines and musts, vol 1, sections 3.1.1 Sugars, 3.1.2 Alcohols and 3.1.3 Acids
- Orduña RM. Climate change associated effects on grape and wine quality and production. Food Res Intern. 2010;43:1844–1855. doi: 10.1016/j.foodres.2010.05.001. [DOI] [Google Scholar]
- Remize F, Roustan JL, Sablayrolles JM, Barre P, Dequin S. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl Environ Microbiol. 1999;65:143–149. doi: 10.1128/aem.65.1.143-149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano P, Suzzi G. Origin and production of acetoin during wine yeast fermentation. Appl Environ Microbiol. 1996;62:309–315. doi: 10.1128/aem.62.2.309-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyerd D, Gallardo-Chacóna JJ, Ballesterc J, Vichie S, Guérin-Schneider R, Caixache J, Alexandre H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012;32:243–253. doi: 10.1016/j.fm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Tilloy V, Ortiz-Julien A, Dequin S. Reduction of ethanol yield and improvement of glycerol formation by adaptive evolution of the wine yeast Saccharomyces cerevisiae under hyperosmotic conditions. Appl Environ Microbiol. 2014;80:2623–2632. doi: 10.1128/AEM.03710-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Kutyna DR, Solomon MR, Black CA, Borneman A, Henschke PA, Pretorius IS, Chambers PJ. Evaluation of gene modification strategies for the development of low-alcohol-wine yeasts. Appl Environ Microbiol. 2012;78:6068–6077. doi: 10.1128/AEM.01279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Martini A, Martini A. Determination of ethanol production. In: Kurtzman CP, Fell JW, editors. The yeasts: a taxonomic study. Amsterdam: Elsevier; 1998. pp. 358–371. [Google Scholar]
- Vazquez F, Figueroa L, Toro ME. Enological characteristics of yeasts. Food Microbiol Protoc Methods Biotechnol. 2001;7:297–306. [Google Scholar]
- Zwietering M, Jongenburger I, Rombouts F, van Riet K. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


