Abstract
AIM
To strengthen the biomechanics of collagen by crosslinking rabbit scleral collagen with genipin to develop a new therapy for preventing myopic progression.
METHODS
Ten New Zealand rabbits were treated with 0.5 mmol/L genipin injected into the sub-Tenon's capsule in the right eyes. Untreated contralateral eyes served as the control. The treated area was cut into scleral strips measuring 4.0 mm×10.0 mm for stress-strain measurements (n=5). The remaining five treated eyes were prepared for histological examination.
RESULTS
Compared to the untreated scleral strips, the genipin-crosslinked scleral strips showed that the ultimate stress and Young's modulus at 10% strain were increased by the amplitude of 130% and 303% respectively, ultimate strain was decreased by 24%. There had no α-smooth muscle actin (α-SMA) positive cells in control and treated sclera. Histologically, there was no sign of apoptosis in the sclera, choroid, and retina; and no side effects were found in the peripheral cornea and optic nerve adjacent to the treatment area.
CONCLUSION
Genipin induced crosslinking of collagen can increase its biomechanical behavior by direct strengthening of the extracellular matrix in rabbit sclera, with no α-SMA expression seen in the myofibroblasts. As there is no evidence of cytotoxicity in the scleral, choroidal, and retinal cells, genipin is likely a promising agent to strengthen the weakened sclera to prevent myopic progression.
Keywords: genipin, crosslinking, biomechanics, sclera, myopia
INTRODUCTION
Myopia is one of the most prevalent eye diseases, in which the refractive power of the eye is incompatible with its axial length. Epidemiological studies have shown that its prevalence was about 20%-30% of the general population in the USA and Europe. Overall, the prevalence was 52.6% in school-aged children, with numbers as high as 80%-90% among school-leavers in East Asia[1]–[3]. For most patients, myopia develops mainly during the school years and stabilizes in the teenage years[4]. However, in some adult patients with high myopia, the myopia continues to progress. Saka et al[5] followed up with 184 high myopic eyes in adults for about 8.2y; they noted that the axial length increased by more than 1 mm in 31% of the eyes. Axial length is the most important determinant factor for myopic progression.
The elongation of the eye was associated with the biomechanical properties of sclera[6]. During myopia development, the sclera undergoes an active remodeling process, which causes progressive thinning and weakening of the tissue. A reduction in the size of individual collagen fibers as well as the number of collagen fibers of the sclera are known to be the main pathogenic factors in progressive myopia; the sclera thus becomes weaker and more extensible[7]. Therefore, even under normal intraocular pressure, the sclera could expand, leading to axis elongation. Despite extensive research, there is still no effective method to prevent the progression of myopia. Recently, riboflavin/UVA-induced collagen crosslinking was successfully used to prevent the progression of keratoconus and keratectasia after photorefractive keratectomy (PRK) and laser in situ keratomileusis (LASIK)[8]–[9]. Therefore, for typically collagenous tissues like the cornea, it can be logically hypothesized that scleral collagen crosslinking may strengthen the sclera to prevent myopic progression.
Genipin is a natural crosslinking reagent that may be obtained from gardenia fruits. As a part of traditional Chinese medicine or herbal medicine, genipin has been used to treat jaundice (to increase bile secretion) and various other inflammatory and hepatic diseases[10]. A previous study showed that genipin could also significantly strengthen the stiffness of porcine cornea and sclera in vitro[11]–[12]. Further, it also showed promising results as a therapeutic agent for strengthening the weakened sclera in progressive myopia[13]. With this background, we designed the present study to explore in vivo the biomechanical efficiency of genipin-induced scleral crosslinking and its possible cytotoxicity in rabbits.
MATERIALS AND METHODS
Animals
Ten New Zealand rabbits (weight, 2.5-3.5 kg) were included for treatment. The crosslinked right eyes were used for biomechanical measurements (n=5) and histological examination (n=5). The untreated contralateral eyes were used as control.
Treatment Procedure
Alcaine eye drops were instilled for induction of local anesthesia. Our previous study showed that 0.5 mmol/L genipin (Wako, Japan) could improve the stiffness of porcine sclera, and resulted in no significant discoloration[13]. Therefore, this in vivo study, we chose the same concentration, i.e. 0.5 mmol/L genipin (0.5 mL) to be injected into the sub-Tenon's capsule at 3.0 mm behind the limbus in the superonasal quadrant (1 o'clock position in the right eyes) by using a 1.0 mL syringe with a sharp 25-gauge injection needle. The injections were repeated 4 times within two weeks with 2-3d intervals between injections. After the injections, tobramycin eye drops and ointment were used. Two weeks after the first injection (i.e. 2d after the last injection), the animals were humanely killed under general anesthesia using an overdose of intramuscular ketamine hydrochloride and chlorpromazine. All animal procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Institutional Animal Care and Use Committee of the Sun Yet-sen University (2012-087).
Biomechanical Measurement
After sacrificing, a scleral strip measuring 4.0 mm×10.0 mm was cut from an area 2.0 mm-12.0 mm behind the limbus at the 1 o'clock position (11 o'clock position in the left eyes) in the direction of the optic nerve, by using a self-constructed double-blade scalpel. To preserve the hydration, the strips were submerged in hypromellose eye drops. The scleral thickness was measured using a digimatic caliper [Sanling group (HK, China) Ltd.] before undergoing the stress-strain tests.
The biomechanical measurement was performed at room temperature in air. The stress-strain test of the scleral strips was measured using an Instron 3343 microtester (Illinois Tool Works Inc., Glenview, IL, USA) in air. During the examination, the strips were submerged in hypromellose eye drops. To perform the stress-strain test on the scleral strips, each strip was subjected to 5 cycles of deformation-load testing at a stress level of 0.003 MPa. Strain was increased linearly at a velocity of 1.5 mm/min until tissue rupture. The ultimate stress, ultimate strain, and Young's modulus at 10% strain were measured.
Histology
After sacrificing, the treated area was positioned with 10-0 silk thread. Subsequently, enucleation was performed and the eyeballs were immersed in neutral buffered formalin (obtained from Zhongshan Ophthalmic Center) for 2d. Subsequently, the following sections were cut for histological analysis: 2 mm×4 mm strips including the sclera, choroid, and retina at the equator; the adjacent corneal tissue measuring about 2 mm×4 mm (at the 1 o'clock position in the right eyes and 11 o'clock position in the left eyes); and the optical nerve measuring about 2 mm in length, adjacent to the eye ball. All the sections were subsequently embedded in paraffin.
To compare the histological change, 4 µm thick paraffin sections of the cornea; wall of the eyeball (including sclera, choroid, and retina); and optic nerve were prepared for staining with hematoxylin and eosin. Terminal deoxynucleotidyl transferase (TdT) mediated biotin dUTP nick-end labeling (TUNEL) assay was performed using the TUNEL assay kit (Promega, USA), and was carried out according to the manufacturer's instructions.
α-smooth muscle actin (α-SMA) expression is a reliable marker of myofibroblast proliferation. To evaluate this, mouse monoclonal anti-α-SMA antibody (1:100 dilution in PBS, Merck KGaA, Darmstadt, Germany) was used along with biotinylated goat anti-mouse IgG (code B-6398; Sigma-Aldrich) as the secondary antibody, according to the manufacturer's instructions.
All sections were examined with light microscopy (Axioplan 2 imaging; Zeiss, Oberkochen, Germany). Five fields were randomly selected from each section at a magnification of 400× for histological evaluation. Data are reported as the number of TUNEL and α-SMA positive cells per field counted manually.
Statistical Analysis
The ultimate stress, ultimate strain, and Young's modulus data at 10% strain between untreated and treated groups were analyzed using the independent sample's t-test. A P value of <0.05 was considered statistically significant.
RESULTS
Observation with Slit Lamp
In the treated area, a bubble-like elevation appeared immediately after injection, which gradually vanished within the next 1-2d. During the follow-up, the conjunctiva showed slight congestion, but there was no discoloration of the conjunctiva and sclera in the treated eyes (Figure 1). There were no cloudy and edematous regions, and no loss of corneal epithelium in the area adjacent to the treated area.
Figure 1. Observation after genipin injection in the sub-Tenon's capsule.
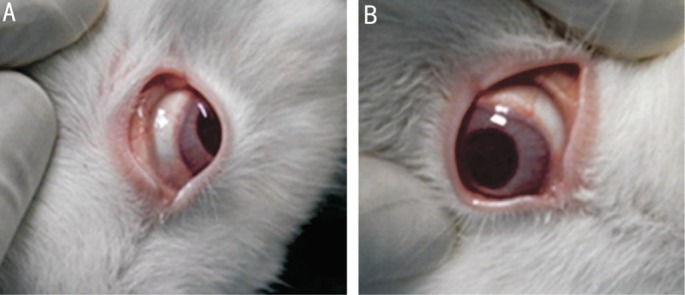
No discoloration in untreated (A) and treated (B) eyes. Slight conjunctival congestion (B).
Biomechanical Properties
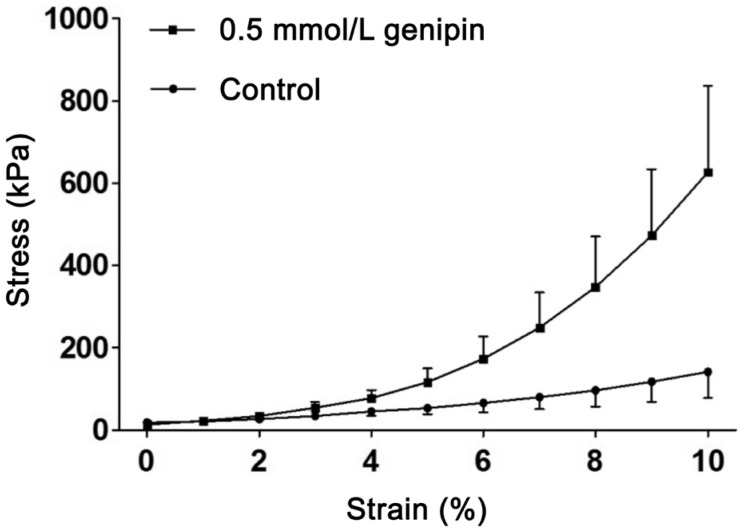
The biomechanical properties of genipin-treated sclera were improved (Table 1). Compared with the untreated scleral strips, the ultimate stress, and Young's modulus at 10% strain of genipin-crosslinked sclera was increased by amplitude of 130% and 303%, respectively. The ultimate strain was decreased by 24%. The stress-strain curves of strips treated with genipin were steeper than those of untreated strips (Figure 2).
Table 1. The biomechanical parameters of rabbit scleral strips.
| Groups | Ultimate stress (kPa) | Ultimate strain (%) | Young's modulus (MPa) |
| Control | 4.36±1.64 | 46.66±5.42 | 5.56±1.95 |
| Genipin 0.5 mmol/L | 10.03±1.36 | 35.44±5.80 | 22.42±12.61 |
| aP | 0.000 | 0.013 | 0.018 |
at-test.
x±s
Figure 2. Stress-strain curves in rabbit scleral strips treated with genipin.
Histology Analysis
The rabbit scleral thickness was 389.40±4.95 µm (SE) and 377.00±5.73 µm (SE) in the controls and treated eyes, respectively (P=0.140).
There was no evidence of stromal edema and loss of keratocytes and corneal epithelium in the peripheral cornea immediately adjacent to the injection site in all treated eyes. The endothelium was integrated (Figure 3A, 3B). The sclera appeared normal, specifically, without inflammatory infiltrates or scarring (Figure 3C, 3D). The choroid and retina in all samples were without abnormalities. Additionally, there was no nerve fiber swelling and rupture in the optic nerve (Figure 3E, 3F).
Figure 3. Photomicrographs of tissues in rabbit eye hematoxylin & eosin.
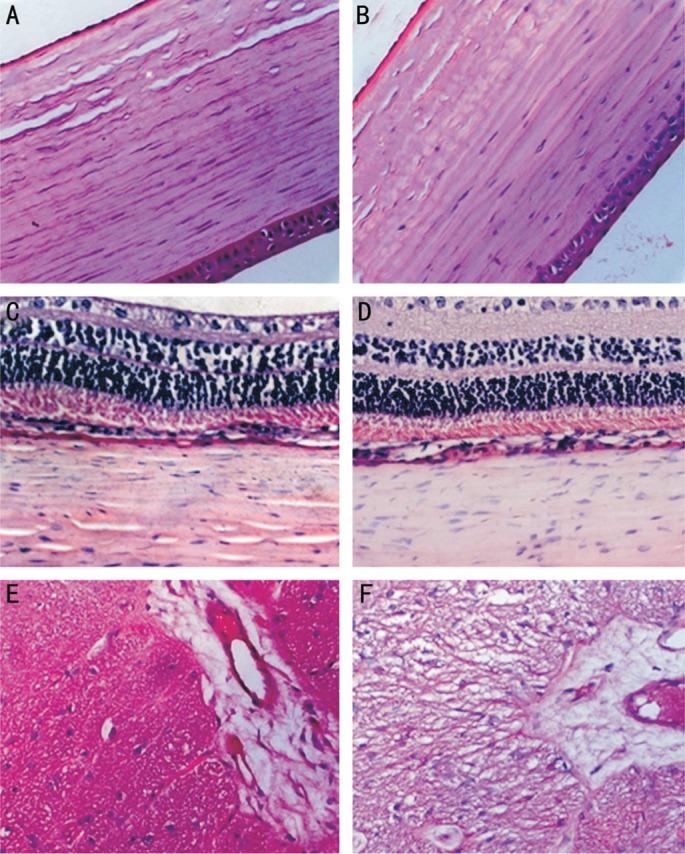
Panels (A, C, and E) are sections of untreated eyes, while panels (B, D, and F) are those of treated eyes. A, B: The cornea immediately adjacent to the scleral treatment quadrant with integrated endothelium and without stromal edema and loss of keratocytes; C, D: Intact retina, choroid, and sclera; E, F: The adjacent optic nerve without fiber swelling and rupture (400×).
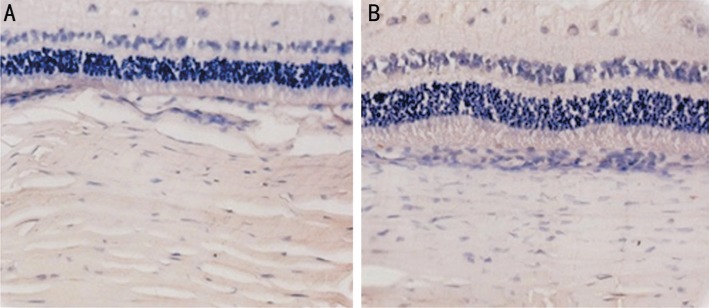
To detect the cytotoxicity of 0.5 mmol/L genipin in rabbit tissues, we used the TUNEL assay to check for cellular apoptosis (Figure 4). However, there were no TUNEL-positive cells in the inner and outer nuclear retinal layer, choroid, or sclera in both treated and untreated eyes.
Figure 4. Photomicrograph of the TUNEL assay.
No TUNEL-positive cells in the sclera, choroid, and retina in both untreated (A) and treated (B) eyes (400×).
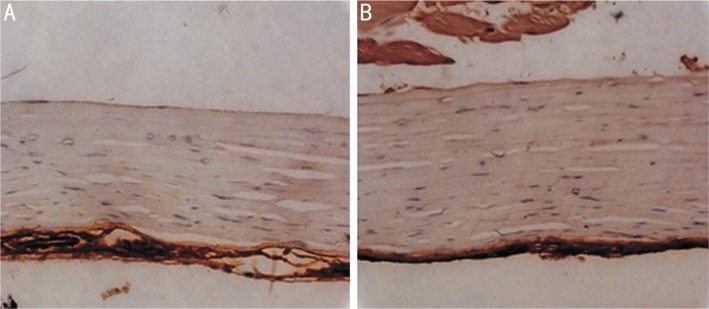
Figure 5 showed no staining for α-SMA in either treated or untreated rabbit sclera.
Figure 5. Immunohistochemical staining of α-SMA.
No α-SMA-positive cells seen in the sclera of untreated (A) and treated (B) eyes (400×).
DISCUSSION
In this study, we examined the in vivo efficacy, safety, and biomechanics of genipin crosslinking in rabbit scleral collagen. It has been shown that macromolecules as large as 150 000 Da could be transclerally delivered to the choroid and retina in rabbit sclera[14]. Hence, we hypothesized that it was possible for genipin (MW 226) to infiltrate the retina across the sclera or diffuse into adjacent tissues.
Axis elongation is the main feature of myopic progression, and has thus understandably been the focus of myopia research. It has been related to the weakened biomechanical properties of the sclera[15]. The biomechanical properties of myopic sclera including the ultimate stress, ultimate load, and the Young's modulus were lower than that of the normal sclera, but ultimate strain was higher[16]. This was because of the insufficient formation of stabilizing intra- and inter-molecular crosslinks in the myopic scleral collagen[17]. Therefore, modulation of axial length might be the key factor to halt myopic progression through scleral collagen crosslinking to control scleral resistance[18].
Collagen crosslinking is a commonly understood phenomenon, which can strengthen the collagenous structure by induction of intra- and inter-molecular covalent bonds. In diabetic patients, the collagenous tissues were strengthened through glucose-induced crosslinking[19]. Further, the biomechanics of collagenous tissues has been reported to increase with age[20]. This is probably why axial elongation of myopia among diabetic patients and elderly people is rare.
There are several methods to strengthen collagenous tissues by collagen crosslinking. Ultraviolet A (UVA) radiation could lead to retinal damage[1], and it is difficult to use UVA radiation in the posterior pole sclera. Glyceraldehyde is nontoxic and is a natural product of metabolism; however, it requires several sub-Tenon injections[2]. As a natural crosslinking reagent obtained from gardenia fruits, genipin has been found to crosslink gelatin by nucleophilic attack through primary amine groups on lysine and arginine residues on the C3 atom of genipin[1], and then embedding a tertiary nitrogen in the six-membered ring in place of an oxygen atom[2]. Moreover, genipin crosslinking has been shown to improve the mechanical properties of biological tissues[21]. Our present experiment showed that the application of genipin in vivo had an obvious stiffening effect on crosslinked rabbit sclera. The ultimate stress and Young's modulus at 10% strain of genipin-crosslinked sclera were increased by an amplitude of 130% and 303%, respectively; the ultimate strain was decreased by 24%. The stress-strain curve, which reflected the scleral capacity of deformation under different stress, was steeper than that of untreated strips. This implied that sclera crosslinked with genipin could resist higher stress and deform less than non-crosslinked sclera under the same stress. However, the correct degree of scleral crosslinking to halt myopic progression is still unknown. Further studies in animal models of myopia are required in order to explore the correct degree of scleral collagen crosslinking to prevent progression of myopia.
Some recent studies have shown that, same as biochemical structure, myofibroblast was also the contributor of biomechanical properties in sclera[22]–[23]. The sclera is mostly composed of extracellular matrix, particularly collagen and few cells. Scleral fibroblasts are the characteristic cells types of the sclera and can differentiate into myofibroblasts upon undergoing any insult such as physical or chemotherapeutic-agent stimulation, especially stress and β-TGF[23]. Myofibroblasts have potential contractile capacities that can be identified owing to the main marker protein known- α-SMA. Once the stress was applied, these cells could rapidly contract to limit the expansion of the surrounding matrix[24]. Hinz et al[25] showed that α-SMA played an important role in fibroblast contractility, as increased α-SMA expression could enhance fibroblast contractile activity.
In monkeys, humans, and tree shrews, scleral fibroblasts displayed a myofibroblastic phenotype[26]–[28]. Thus, we wanted to explore whether myofibroblasts contribute to the biomechanics of rabbit sclera. Interestingly, we did not detect any α-SMA positive cells in either untreated or treated sclera. The absence of myofibroblasts in rabbit sclera implied that these cell types did not contribute to the biomechanical properties of rabbit sclera. Further, genipin did not improve the biomechanics through modulating the α-SMA expression. However, little is still known about the characterization and functions of scleral fibroblasts and myofibroblasts and the influence of genipin on them, thereby requiring further investigation.
The cytotoxicity of genipin is dose dependent, which was well tolerated by the scleral cells[29]–[30], and the threshold of genipin cytotoxicity varied among different tissues or cells. Previous data showed that even when exposed to 10 mmol/L genipin, crosslinked chitosan was still compatible with human RPE cells[31]. L929 fibroblasts treated with 1 mmol/L genipin for 24h experienced mild toxicity[29]. In the present study, while the genipin concentration was highest in the vicinity of the injection area, it did not cause the loss and apoptosis of scleral cells. Additionally, we examined the effect of genipin on adjacent tissues. Our study showed there were no adverse effects to the peripheral cornea, optical nerve, retina, or choroid. Meanwhile, negligible discoloration was exhibited in the sclera, sub-Tenon's capsule, and conjunctiva. These results suggest that 0.5 mmol/L genipin might be safe for use in rabbit eyes in vivo.
However, strip extensometry is a simple technique for measuring biomechanics, which could not accurately simulate true conditions of myopic onset and progression. Moreover, the effects of the treatment on myopic sclera might be different from that of normal sclera. Therefore, further studies should be performed in myopic animal models to verify the effect and safety of genipin.
In conclusion, our study showed that genipin-induced crosslinking could increase the biomechanical properties by direct strengthening of the extracellular matrix in rabbit sclera; further, α-SMA expression of myofibroblasts was not detected. Moreover, 0.5 mmol/L genipin caused no cytotoxicity in the scleral, choroidal, or retinal cells. Negligible discoloration was exhibited in the treated area. Genipin is likely a promising natural, crosslinking agent to increase scleral capacity by increasing its stiffness and preventing myopic progression.
Acknowledgments
Foundation: Supported by Guangdong Province Science and Technology Projects (No.2007B031002001).
Conflicts of Interest: Liu TX, None; Wang Z, None.
REFERENCES
- 1.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthal. 2009;127(12):1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 3.Atta Z, Arif AS, Ahmed I, Farooq U. Prevalence of refractive errors in madrassa students of haripur district. J Ayub Med Coll Abbottabad. 2015;27(4):850–852. [PubMed] [Google Scholar]
- 4.Goss DA, Winkler RL. Progression of myopia in youth: age of cessation. Am J Optometry Physiol Optics. 1983;60(8):651–658. doi: 10.1097/00006324-198308000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Saka N, Ohno-Matsui K, Shimada N, Sueyoshi S, Nagaoka N, Hayashi W, Hayashi K, Moriyama M, Kojima A, Yasuzumi K, Yoshida T, Tokoro T, Mochizuki M. Long-term changes in axial length in adult eyes with pathologic myopia. Am J Ophthalmol. 2010;150(4):562–568.e1. doi: 10.1016/j.ajo.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Harper AR, Summers JA. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res. 2015;133(4):100–111. doi: 10.1016/j.exer.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35(9):1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 8.Goldich Y, Barkana Y, Wussuku LO, Marcovich AL, Hirsh A, Avni I, Zadok D. Corneal collagen cross-linking for the treatment of progressive keratoconus: 3-year prospective outcome. Can J Ophthalmol. 2014;49(1):54–59. doi: 10.1016/j.jcjo.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Richoz O, Mavrakanas N, Pajic B, Hafezi F. Corneal collagen cross-linking for ectasia after LASIK and photorefractive keratectomy: long-term results. Ophthalmol. 2013;120(7):1354–1359. doi: 10.1016/j.ophtha.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Akao T, Kobashi K, Aburada M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol Pharm Bull. 1994;17(12):1573–1576. doi: 10.1248/bpb.17.1573. [DOI] [PubMed] [Google Scholar]
- 11.Avila MY, Navia JL. Effect of genipin collagen crosslinking on porcine corneas. J Cataract Refract Surg. 2010;36(4):659–664. doi: 10.1016/j.jcrs.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Wong FF, Lari DR, Schultz DS, Stewart JM. Whole globe inflation testing of exogenously crosslinked sclera using genipin and methylglyoxal. Exp Eye Res. 2012;103(10):17–21. doi: 10.1016/j.exer.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu TX, Wang Z. Collagen crosslinking of porcine sclera using genipin. Acta Ophthalmol. 2013;91(4):e253–257. doi: 10.1111/aos.12172. [DOI] [PubMed] [Google Scholar]
- 14.Ambati J, Canakis CS, Miller JW, Gragoudas ES, Edwards A, Weissgold DJ, Kim I, Delori FC, Adamis AP. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci. 2000;41(5):1181–1185. [PubMed] [Google Scholar]
- 15.Sergienko NM, Shargorogska I. The scleral rigidity of eyes with different refractions. Graefe's Arch Clin Exp Ophthalmol. 2012;250(7):1009–1012. doi: 10.1007/s00417-012-1973-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang CY, Chen WY, He L, Wu HP, Liu YQ, Tan SE. Scleral biomechanical properties in high myopia. Chinese Ophthalmic Research. 2003;21(2):113–115. [Google Scholar]
- 17.Funata M, Tokoro T. Scleral change in experimentally myopic monkeys. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):174–179. doi: 10.1007/BF00935729. [DOI] [PubMed] [Google Scholar]
- 18.Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg. 2004;30(3):689–695. doi: 10.1016/j.jcrs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Pierro L, Brancato R, Robino X, Lattanzio R, Jansen A, Calori G. Axial length in patients with diabetes. Retina. 1999;19(5):401–404. doi: 10.1097/00006982-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Bailey AJ. Structure, function and ageing of the collagens of the eye. Eye (Lond) 1987;1(Pt 2):175–183. doi: 10.1038/eye.1987.34. [DOI] [PubMed] [Google Scholar]
- 21.Lima EG, Tan AR, Tai T, Marra KG, DeFail A, Ateshian GA, Hung CT. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009;91(3):692–700. doi: 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22(3):307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 23.Phillips JR, McBrien NA. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest Ophthalmol Vis Sci. 2004;45(3):758–763. doi: 10.1167/iovs.03-0732. [DOI] [PubMed] [Google Scholar]
- 24.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backhouse S, Phillips JR. Effect of induced myopia on scleral myofibroblasts and in vivo ocular biomechanical compliance in the guinea pig. Invest Ophthalmol Vis Sci. 2010;51(12):6162–6171. doi: 10.1167/iovs.10-5387. [DOI] [PubMed] [Google Scholar]
- 27.Watson PG, Young RD. Scleral structure, organisation and disease. A review. Exp Eye Res. 2004;78(3):609–623. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 28.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250(2):273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 29.Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI. Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J Biomed Mater Res A. 2008;87(2):308–320. doi: 10.1002/jbm.a.31715. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Lau TT, Loh WL, Su K, Wang DA. Cytocompatibility study of a natural biomaterial crosslinker--Genipin with therapeutic model cells. J Biomed Mater Res B Appl Biomater. 2011;97(1):58–65. doi: 10.1002/jbm.b.31786. [DOI] [PubMed] [Google Scholar]
- 31.Lai JY, Li YT, Wang TP. In vitro response of retinal pigment epithelial cells exposed to chitosan materials prepared with different cross-linkers. Int J Mol Sci. 2010;11(12):5256–5272. doi: 10.3390/ijms11125256. [DOI] [PMC free article] [PubMed] [Google Scholar]