Abstract
AIM
To analyze the relationship between the score obtained in the Risk Score System (RSS) proposed by Hicks et al with penetrating keratoplasty (PKP) graft failure at 1y postoperatively and among each factor in the RSS with the risk of PKP graft failure using univariate and multivariate analysis.
METHODS
The retrospective cohort study had 152 PKPs from 152 patients. Eighteen cases were excluded from our study due to primary failure (10 cases), incomplete medical notes (5 cases) and follow-up less than 1y (3 cases). We included 134 PKPs from 134 patients stratified by preoperative risk score. Spearman coefficient was calculated for the relationship between the score obtained and risk of failure at 1y. Univariate and multivariate analysis were calculated for the impact of every single risk factor included in the RSS over graft failure at 1y.
RESULTS
Spearman coefficient showed statistically significant correlation between the score in the RSS and graft failure (P<0.05). Multivariate logistic regression analysis showed no statistically significant relationship (P>0.05) between diagnosis and lens status with graft failure. The relationship between the other risk factors studied and graft failure was significant (P<0.05), although the results for previous grafts and graft failure was unreliable. None of our patients had previous blood transfusion, thus, it had no impact.
CONCLUSION
After the application of multivariate analysis techniques, some risk factors do not show the expected impact over graft failure at 1y.
Keywords: penetrating keratoplasty, graft failure, score
INTRODUCTION
There are several risk factors that can compromise the successful outcome of a penetrating keratoplasty (PKP) graft. In addition, the increased severity of a certain factor also increases the risk of graft failure[1].
The assessment of an accurate preoperative risk of failure for a penetrating keratoplasty graft is, in many cases, hazardous and difficult. Although risk factors for PKP graft failure have been well recognized[1], there is a need for a useful tool to integrate all these factors and establish a preoperative risk of failure, in order to offer the optimum management for an individual patient.
A Risk Score System (RSS) has been proposed by Hicks et al[2] based on factors proved to reduce the graft survival at 1y postoperatively. Seven risk factors for graft failure were analyzed: diagnosis (disease leading to a need for a corneal graft), lens status, ocular inflammation, ocular hypertension, quadrants of corneal neovascularization, number of previous grafts and blood transfusion. For a certain level of a risk factor a score was given, according to probability of 1y graft survival, obtained from the Australian Corneal Graft Registry (ACGR), and the total risk score before corneal replacement was calculated using a worksheet (Table 1).
Table 1. Worksheet for calculating a patient's total risk score[2].
| Factors present before graft | Points | Patient's score | |
| Original condition | |||
| Keratoconus | 0 | ||
| Bullous keratopathy | 1 | ||
| Corneal dystrophy | 0 | ||
| HSV | 1 | ||
| Non HSV scar | 1 | ||
| Non HSV ulcer | 4 | ||
| Trauma | 2 | ||
| Non HSV infection | 5 | ||
| Quadrants of vascularization | |||
| 0 | 1 | ||
| 1 | 2 | ||
| 2 | 2 | ||
| 3 | 3 | ||
| 4 | 4 | ||
| Lens status | |||
| Aphakic, no intention to implant IOL | 3 | ||
| Phakic pre and post | 1 | ||
| Intend to place/replace IOL at graft | 1 | ||
| No IOL change | 2 | ||
| Number of previous corneal grafts | |||
| 0 | 1 | ||
| 1 | 3 | ||
| 2 | 4 | ||
| 3 | 5 | ||
| 4 or more | 6 | ||
| Glaucoma before planned procedure | |||
| No history of raised IOP | 1 | ||
| History of raised IOP, controlled | 3 | ||
| Persistent raised IOP | 3 | ||
| Uveitis | |||
| None, ever | 0 | ||
| Previous, ever | 1 | ||
| Currently inflamed | 3 | ||
| Previous and currently inflamed | 3 | ||
| Blood transfusion | |||
| None | 1 | ||
| Previous transfusion | 2 | ||
| Total score |
|||
| Date of surgery | Date of latest follow up | ||
| Graft failure? | Date of failure if occurred | ||
HSV: Herpes simplex virus; IOP: Intraocular pressure.
Patients in that study were stratified depending on the total score obtained, and were classified into 4 groups: PKP without immunosuppression, PKP with immunosuppression, lamellar keratoplasty, and keratoprostheses.
In the new era of lamellar keratoplasty, PKP remains as the gold standard in the treatment of corneal disorders which require corneal replacement. In a recent study[3] of 13 920 PKPs, 858 deep anterior lamellar keratoplasties (DALK) and 2287 endothelial keratoplasties (EK) survival of DALK or EK grafts was poorer than that of PKP for the same indications over the same timeframe, although these newer techniques require a learning curve. However, many studies show that the rejection rate is lower, and graft survival is higher, following lamellar keratoplasty compared with PKP[4]–[5]. Thus, it is not possible to assess whether the risk of failure given by a certain factor following PKP is the same than following lamellar keratoplasty, or even keratoprostheses.
It is also of interest to highlight that some of the risk factors traditionally considered to be of great impact over graft failure may not show the expected relationship after adjustment for other risk factors[6].
Application of the RSS can therefore be useful for cases treated with PKP. We applied this RSS to a sample of 134 eyes of 134 patients who underwent PKP for different reasons. Distribution of diagnoses is shown in Table 2.
Table 2. Distribution of diagnosis and rate of graft failure related to each condition at 1y postoperative.
| Diagnosis | No. of eyes | Failure |
| Keratoconus | 63 | 3 (4.76) |
| Pseudophakic bullous keratopathy | 31 | 14 (45.16) |
| Trauma | 6 | 2 (33.33) |
| Herpes simplex virus | 10 | 3 (30.00) |
| Non herpetic corneal ulcer | 9 | 6 (66.67) |
| Non herpetic corneal scar | 4 | 4 (100) |
| Corneal dystrophy | 11 | 4 (36.36) |
In the RSS both keratoconus and corneal dystrophy have the same score, according to the probability of graft failure shown by the ACGR[1]. However, the rate of failure in our sample for each condition is very different. Among the 4 cases having corneal dystrophy (Fuchs endothelial dystrophy) as primary diagnosis showing graft failure at 1y, 1 case had 4 quadrants of corneal neovascularization, and another case had a previous failed graft with chronic uveitis and high intraocular pressure, making reasonable to consider that it may be the features of a certain condition what is related to graft failure, rather than the diagnosis itself.
n (%)
None of our patients had systemic immunosuppression previous to, or following, PKP. We use our very homogeneous sample in order to get more reliable results for this risk score and a better assessment of the relationship between score obtained and graft survival. We also analyzed the impact of every single risk factor with graft failure using univariate and multivariate techniques.
SUBJECTS AND METHODS
The project was deemed to meet criteria for a retrospective cohort study by the Asociación Para Evitar la Ceguera-Hospital Luis Sánchez Bulnes Institutional Review Board (Mexico City, Mexico). Information collected from patient charts included preoperative, operative and postoperative data. The study and data accumulation were in conformity with all state laws, and were in adherence to the tenets of the Declaration of Helsinki. Using the same RSS, the factors involved in this study were diagnosis, lens status, ocular hypertension, inflammation, quadrants of neovascularization, previous grafts and blood transfusion. The status of each factor previous to corneal replacement was recorded after being validated by an expert corneal surgeon. All corneas were evaluated pre operatively with specular microscopy, however, RSS evaluates the above mentioned host risk factors and not those from the donor. Therefore, this has not been included in the study.
According to the RSS proposed by Hicks, a number of points was given for a certain level of each factor, based on the probability of 1y survival for a PKP graft: a probability of 1y survival of 95% accounts for 0 points, a probability of 90%-94% accounts for 1 point, a probability of 85%-89% accounts for 2 points, a probability of 80%-84% accounts for 3 points, a probability of 70%-79% accounts for 4 points, a probability of 60%-69% accounts for 5 points, and less than 60% accounts for 6 points. Points are additive for all the risk factors included in the RSS. Table 3 shows an example of the points collected in relation to the degree of a risk factor (in this table, corneal neovascularization)[2].
Table 3. Point allocation for quadrants of deep vascularization (from Hicks et al[2]).
| Quadrants of vascularization | 1a survival (%) | Contribution to risk score |
| 0 | 93.8 | 1 |
| 1 | 87.8 | 2 |
| 2 | 87.8 | 2 |
| 3 | 83.8 | 3 |
| 4 | 73.8 | 4 |
Points given for a single factor increase in relation to decreasing probability of 1y survival of a PKP graft. The sum of the points obtained for every factor compose the final score for a certain individual. A worksheet (Table 1) was used to calculate the total risk score for every individual patient.
This single centre retrospective cohort study involved data of patients receiving PKP between the 1st of January 2011 and the 31st of December 2011, with a minimum follow up of 12mo. None of the patients had systemic immunosuppression before PKP. After PKP no patient had mid term or long term systemic immunosuppression. It is difficult to assess from retrospective data why none of the patients had systemic immunosuppression as not enough details were recorded in the notes, however social or economic factors may have played a role on this. Nevertheless, it is interesting to highlight that it would help to better stablish the impact of host risk factors for graft failure as our sample of patients was very homogeneus with regards of post operative management.
Graft failure was defined as hazy, edematous cornea with no possibility for any medical treatment to re-establish corneal deturgescence and transparency, and another corneal graft is required to restore vision, or the decision of no further grafts has been made. The date of graft failure recorded, in case it occurred, was the first visit where the corneal graft was considered to have failed.
Patients with incomplete medical records, follow up less of 1y, or having primary graft failure were excluded from our study. Anonymous data were recorded according to the worksheet. Correlation coefficient was calculated to establish the relationship between final score and failure of the graft at 1y. The relationship between factors included in the RSS and graft failure was also calculated by univariate and multivariate logistic regression analysis techniques. Provided that in the RSS there is a certain number of points for each level of every risk factor, the variables are analyzed as quantitative ones. Therefore, the odds ratio (OR) value represents the increased risk for every point increase in the score. Receiver operating characteristics (ROC) curve were calculated for RSS and for a modified RSS. These curves address the capability for each model to predict graft failure and serve for comparison between both the RSS and a modified RSS.
SPSS Statistics 17.0 (Chicago, SPSS Inc.) was used for this purpose.
RESULTS
We retrospectively studied 152 PKPs from 152 patients, from which 18 cases were excluded from our study due to primary failure (10 cases), incomplete medical notes (5 cases) or follow up less than 1y (3 cases). According to the mentioned criteria, 134 eyes of 134 patients were included, of whom 57 (42.5%) were women, and 62 (46.3%) were right eyes. Age of patients ranged from 2 to 85y. Graft failure within 12mo was observed in 36 eyes (26.9%), 18 from males and 18 from females.
Points in the risk score can range from a minimum of 5 points, to a maximum of 26 points. In our sample, minimum score obtained was 5 points (55 eyes), whereas maximum score obtained was 18 points (2 eyes). Table 4 shows all cases in our sample stratified by points obtained in the risk score, and the number of cases in each stratus showing graft failure at 1y post PKP.
Table 4. Patients stratified by points obtained in the risk score and number of cases with graft failure in each stratus.
| Score | No. of eyes (% of total) | Failure (% of stratus) |
| 5 | 55 (41.04) | 3 (5.45) |
| 6 | 3 (2.23) | 1 (33.3) |
| 7 | 16 (11.94) | 1 (6.25) |
| 8 | 10 (7.46) | 3 (30) |
| 9 | 9 (6.71) | 4 (44.45) |
| 10 | 10 (7.46) | 4 (40) |
| 11 | 7 (5.22) | 2 (28.55) |
| 12 | 5 (3.73) | 3 (60) |
| 13 | 5 (3.73) | 1 (20) |
| 14 | 2 (1.49) | 2 (100) |
| 15 | 2 (1.49) | 2 (100) |
| 16 | 4 (2.98) | 4 (100) |
| 17 | 4 (2.98) | 4 (100) |
| 18 | 2 (1.49) | 2 (100) |
| Total | 134 | 36 |
We suggest that a score from 5 to 8 points could be considered of low risk (8 failures among 84 cases, 9.52%), a score form 9 to 13 of moderate risk (14 failures among 36 cases; 38.89%), and above 13 points of high risk (14 failures among 14 cases, 100%).
Results for univariate analysis for the relationship between each risk factor and graft failure are shown in Table 5.
Table 5. Results of univariate analysis.
| Risk factors | Odds ratio | Interval confidence | P |
| Age | 1.017 | 1.001-1.034 | 0.0365 |
| Diagnosis | 2.307 | 1.560-3.718 | 0.0001 |
| Lens status | 2.356 | 1.263-4.509 | 0.0073 |
| Neovascularization | 2.648 | 1.800-4.155 | 0.0001 |
| Previous grafts | 0.930 | 0.590-1.383 | 0.7295 |
| Ocular hypertension | 4.317 | 2.739-7.098 | 0.0001 |
| Inflammation | 5.331 | 2.827-12.04 | 0.0001 |
According to these results all the risk factors studied show a significant correlation with graft failure, with the exception of “number of previous grafts”. Given the small number of cases having 4 or more previous grafts, we consider that this result could be unreliable. Age showed a significant correlation in the univariate analysis, however, its inclusion in the multivariate analysis did not represent any remarkable change to our proposal for a modified RSS. Diagnosis and lens status showed a significant correlation with graft failure in the univariate analysis, unlike the multivariate analysis.
Multivariate logistic regression analysis showed a significant relationship between ocular hypertension (P=0.0004), ocular inflammation (P=0.0055), and corneal neovascularization (P=0.0019) with graft failure at 1y. Table 6 shows corresponding OR values for these factors, highlighting their impact over graft failure at 1y postoperative.
Table 6. Results of multivariate analysis between risk factors and graft failure at 1y.
| Risk factors | OR | 95%CI | P |
| Lens status | 1.434 | 0.584-3.477 | 0.4248 |
| Ocular hypertension | 2.849 | 1.594-5.267 | 0.0004 |
| Previous inflammation | 2.911 | 1.351-7.239 | 0.0055 |
| Previous graft | 0.349 | 0.126-0.767 | 0.0063 |
| Neovascularization | 2.368 | 1.355-4.561 | 0.0019 |
| Diagnosis | 1.037 | 0.543-1.802 | 0.9032 |
The difference in the results obtained in the univariate and multivariate analysis for the risk factors “lens status” and “diagnosis”.
The rate of graft failure increased with higher scores obtained. Spearman coefficient showed a significant relationship between total score and graft failure at 1y (r=0.544, P<0.001).
Interestingly, surprising results were obtained for the risk factor “number of previous grafts”. Although statistically significant (P=0.0063), the OR obtained for this factor was lower than 1. Further details are discussed later in this paper.
Unlike the univariate analysis, lens status and diagnosis didn't show a statistically significant relationship with graft failure at 1y in the multivariate analysis (P=0.4248 and P=0.9032 respectively). Blood transfusion had no effect in the final outcome, as none of our patients had previous records of blood transfusion. Table 5 shows the 95% confidence interval, OR and P value for every risk factor involved in the study.
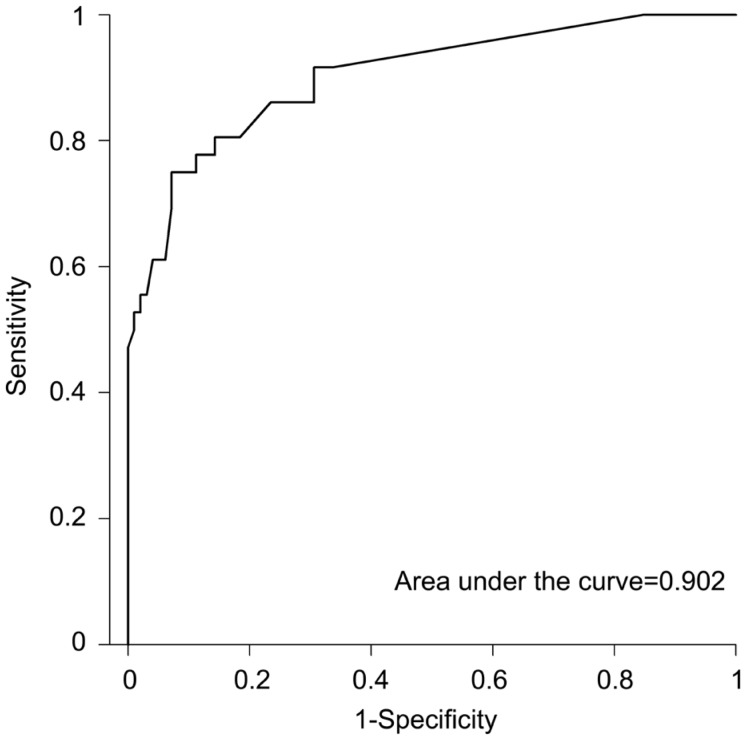
Area under the ROC curve including all the risk factors proposed by Hicks et al[2] (with exception of blood transfusion, as none of our patients had records of it) is 0.902 (Figure 1).
Figure 1. ROC curve for RSS model.
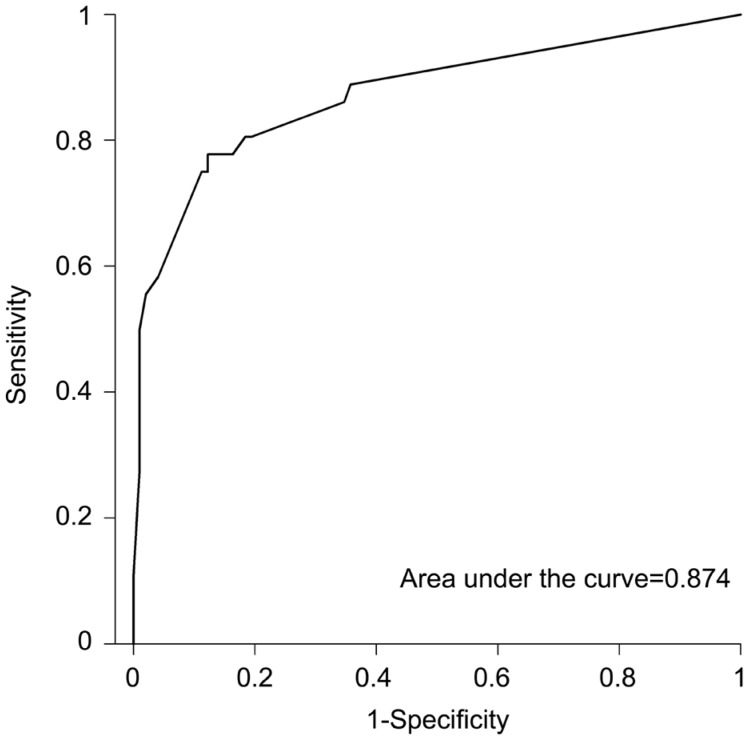
Interestingly, taking into account only the factors showing a statistically significant relationship with graft failure, area under ROC curve remains as high as 0.895. Moreover, when considering only the factors with a statistically significant OR>1 in the multivariate logistic regression analysis, area under ROC curve is 0.874 (Figure 2).
Figure 2. ROC curve for modified RSS model.
DISCUSSION
PKP remains as the most common procedure to restore vision for patients who developed a decrease in visual acuity related to corneal opacity or irregularity. Although in last years there is a trend towards lamellar keratoplasty based on studies which show advantages of these procedures over PKP (such as lower rate of graft rejection, better corneal biomechanics or endothelial cell survival)[4]–[5],[7]–[13], other studies support PKP as the gold standard for visual restoration in corneal diseases[3].
Our knowledge about how a combination of certain factors in a same case can affect the final outcome of a PKP is relatively low. Hicks et al[2] developed a model, based on data obtained from the ACGR[1], in which seven factors related to poor prognosis at 1y postoperative were considered in order to establish a risk score (Table 1). Although donor quality is of paramount importance with regards of graft survival, the RSS only takes into account seven host risk factors. This is the reason why we do not provide a detailed explanation of donor cornea evaluation in this paper. The results from the ACGR on which the RSS is based are mainly obtained from a huge sample of patients operated of PKP, however, Hicks et al[2] make the assumption that this probability of graft survival at 1y could be also applied for lamellar grafts, and even keratoprostheses.
Based on published data, it is difficult to assure whether the risk of graft failure related to a certain factor is the same for a PKP or a lamellar keratoplasty, as the rate of graft rejection can be much lower when only the diseased layer of the cornea is replaced[10],[14]–[15]. For this reason, we applied the RSS described by Hicks et al[2] to a sample of PKP grafts only.
In our sample, the relationship between a PKP graft failure and the score obtained in the RSS is significant, that is, the higher the score, the more likely is graft failure within 1y. This result is consistent with that described by Hicks, where in both groups of PKP patients, higher scores were related to higher rates of graft failure, especially in the group of PKP with immunosuppression. Table 4 shows our sample stratified by points obtained in the risk score, and the number of cases in each stratus showing graft failure at 1y post PKP. According to our results, a score between 5 to 8 points could be considered a low risk graft, from 9 to 13 points it could be considered a moderate risk graft, and above 13 points a high risk graft. Table 4 also shows the rate of graft failure for the mentioned scoring, making reasonable to suggest that for those considered of high risk, systemic immunosuppression should be advisable.
Results of univariate analysis are shown in Table 5. With the exception of “previous grafts”, all the other risk factors proposed by Hicks, as well as age of patients, showed a statistically significant relationship with graft failure. Nevertheless, the inclusion of “age” in the multivariate analysis did not add significant information.
Interestingly, multivariate analysis showed a significant relationship between graft survival at 1y postoperative with ocular hypertension, ocular inflammation, and corneal neovascularisation, but not with diagnosis or lens status. Special attention needs to be paid for the analysis of an interesting factor such as number of previous grafts. Although statistically significant (P=0.0063), the OR obtained for this factor was <1. This result can be explained by the low number of cases having 2 or more previous grafts, and by the fact that the only case having 4 previous grafts didn't develop graft failure during follow up of 1y, yielding unreliable results for this particular variable in our sample.
Diagnosis (the disease leading to the need for a PKP) and lens status had a non statistically significant relationship with the 1y outcome in our sample after the multivariate analysis. Furthermore, the results obtained regarding diagnosis support those from Maguire et al[6]. In this study, multivariate survival analyses techniques were used to estimate the magnitude of risk factors. With the exception of chemical burn, the rate of graft failure varied little among the primary diagnostic groups. Moreover, we have to take into account that an important feature in a chemical burn is the degree of injury to limbal stem cells. Provided that this factor has not been analyzed in Maguire's report, this result could be biased, as stem cell deficiency is by itself an important factor when analyzing the rate of graft failure. However, the inclusion in the RSS of limbal stem cell deficiency is very difficult to assess, due to the number of confounding factors which interfere, as it is not only the condition, but the treatment offered for it, what could influence the final outcome of the graft; whether the patient had previous limbal stem cell auto or allografting (from first degree living relative or cadaveric donor), with or without amniotic membrane grafting, and with or without immunosuppression following the procedure. Therefore, as there is not a unique standardized technique for its treatment, it is not possible to obtain a reliable score for this risk factor[16]–[17].
An explanation to our results could be the lack of independence of these two variables, that is, diagnosis and lens status, meaning that the likelihood of graft failure related to both factors is dependent on the presence or absence of ocular hypertension, ocular inflammation or corneal neovascularization related to each condition. According to the RSS, points given for “keratoconus” and “corneal dystrophy” as primary diagnosis are equivalent. However, in our sample, the rate of failure related to each condition is markedly different (Table 2), making reasonable to consider that the features of a certain condition are more related to graft failure, rather than the diagnosis itself. Although there is an imbalance in our study towards those cases with a diagnosis considered to be of low risk, we believe that this can also be representative of the routine practice of a corneal surgeon, as the incidence of pathologies such as keratoconus or Fuchs endothelial dystrophy are more frequent than others[18]–[19]. For sure, another study with a higher number of cases, particularly those considered to be of high risk, could yield to more reliable results.
We obtained the ROC curve for the RSS (taking into account all the risk factors with exception of blood transfusion, as none of our patients had records of it) to assess the accuracy of this model and to compare it with a modified RSS model, obviating the factors not showing a statistically significant relationship with graft failure at 1y.
For the RSS model, area under the curve was 0.902 (Figure 1). Interestingly, taking into account the factors showing a statistically significant relationship with graft failure (ocular hypertension, ocular inflammation, corneal neovascularization, number of previous grafts), area under ROC curve remains as high as 0.895. Moreover, when considering only the factors with a statistically significant OR>1 in the multivariate logistic regression analysis (ocular hypertension, ocular inflammation, corneal neovascularization), area under ROC curve is 0.874 (Figure 2).
We are aware of the limitations of a retrospective study. Despite of these disadvantages, we conclude that our results support those of Hicks et al[2] showing the RSS as a useful tool to predict the success of PKP surgery or whether other modalities of treatment should be better recommended for certain cases, such as lamellar grafts, keratoprostheses or even biosynthetic implants[20]. We have also suggested score cut points where systemic immunosuppression would be advisable, and showed that our results could be supporting that diagnosis and lens status are very important risk factors for graft failure when studied as an independent variable; however, when a certain disease is split into its different features, and studied in a multivariate analysis, the diagnosis may lose impact over the final outcome.
Further applications (with certain adaptations) for the RSS can be obtained. We suggest a modified RSS eliminating the factors which showed no impact over graft survival at 1y in the multivariate analysis, which is easier to calculate as it includes a lower number of variables, and based on the results of ROC curves shown in this paper, with comparable results to that of the RSS on predicting graft failure.
Acknowledgments
The authors would like to thank Prof. Dr. J. L. Alio for his contribution to this paper.
Conflicts of Interest: Tourkmani AK, None; Sánchez-Huerta V, None; De Wit G, None; Martínez JD, None; Mingo D, None; Mahíllo-Fernández I, None; Jiménez-Alfaro I, None.
REFERENCES
- 1.Williams KA, Muehlberg SM, Bartlett CM, et al. Adelaide: Snap Printing; 2000. The Australian Corneal Graft Registry 1999 Report. Dept. Ophthalmology, Flinders Medical Centre, Australia. [Google Scholar]
- 2.Hicks CR, Macvie O, Crawford GJ, Constable IJ. A risk score as part of an evidence-based approach to the selection of corneal replacement surgery. Cornea. 2005;24(5):523–530. doi: 10.1097/01.ico.0000153103.27399.e6. [DOI] [PubMed] [Google Scholar]
- 3.Coster DJ, Lowe MT, Keane MC, Williams KA, Australian Corneal Graft Contributors. A comparison of lamellar and penetrating keratoplasty outcomes: a registry study. Ophthalmology. 2014;121(5):979–987. doi: 10.1016/j.ophtha.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YM, Wu SQ, Yao YF. Long term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14(5):438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price FW, Jr, Price MO. Evolution of endothelial keratoplasty. Cornea. 2013;32:S28–S32. doi: 10.1097/ICO.0b013e3182a0a307. [DOI] [PubMed] [Google Scholar]
- 6.Maguire MG, Stark WJ, Gottsh JD, Stulting RD, Sugar A, Fink NE, Schwartz A. Risk factors for corneal graft failure and rejection in the Collaborative Corneal Transplantation Studies. Ophthalmology. 1994;101(9):1536–1547. doi: 10.1016/s0161-6420(94)31138-9. [DOI] [PubMed] [Google Scholar]
- 7.Melles GRJ, Eggink FAGJ, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998;17(6):618–626. doi: 10.1097/00003226-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Melles GRJ, Ong TS, Ververs B, van der Wees J. Preliminary clinical results of Descemet membrane endothelial keratoplasty (DMEK) Am J Ophthalmol. 2008;145(2):222–227. doi: 10.1016/j.ajo.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Baydoun L, Ham L, Borderie V, Dapena I, Hou J, Frank LE, Oellerich S, Melles GRJ. Endothelial survival after Descemet's membrane endothelial keratoplasty: effect of surgical indication and graft adherence status. JAMA Ophthalmol. 2015;133(11):1277–1285. doi: 10.1001/jamaophthalmol.2015.3064. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Deep anterior lamellar keratoplasty versus penetrating keratoplasty: a meta-analysis of randomized control trials. Cornea. 2016;35(2):169–174. doi: 10.1097/ICO.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 11.Feizi S, Daryabari SH, Najdi D, Javadi MA, Karimian F. Big bubble deep anterior lamellar keratoplasty using central vs peripheral air injection: a clinical trial. Eur J Ophthalmol. 2016;26(4):297–302. doi: 10.5301/ejo.5000702. [DOI] [PubMed] [Google Scholar]
- 12.Busin M, Scorcia V, Leon P, Nahum Y. Outcomes of air injection within 2 mm inside a deep trephination for deep anterior lamellar keratoplasty in eyes with keratoconus. Am J Ophthalmol. 2016;164:6–13. doi: 10.1016/j.ajo.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28(3):398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 14.Xu JJ, Le QH, Sun XH, et al. Comparative study on deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Zhonghua Yan Ke Za Zhi. 2007;43(7):583–588. [PubMed] [Google Scholar]
- 15.Terry MA, Chen ES, Shamie N, Hoar KL, Friend DF. Endothelial cell loss after Descemet's stripping endothelial keratoplasty in a large prospective series. Ophthalmology. 2008;115(3):488–496. doi: 10.1016/j.ophtha.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Daya SM, Ilari FA. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology. 2001;108(1):126–134. doi: 10.1016/s0161-6420(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 17.Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116(4):431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 19.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38(2):149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 20.Fagerholm P, Lagali NS, Ong JA, et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35(8):2420–2427. doi: 10.1016/j.biomaterials.2013.11.079. [DOI] [PubMed] [Google Scholar]