Abstract
AIM
To compare visual, surgical and topographic outcomes of deep anterior lamellar keratoplasty (DALK) and penetrating keratoplasty (PK) for keratoconus (KC).
METHODS
In this multicenter, prospective, randomized clinical trial 76 eyes of 71 KC patients operated between January 2011 and July 2014 in 2 tertiary referral hospitals were included. Consecutive patients were alternately selected to receive one of the two surgical methods. Thirty eight eyes underwent DALK with the big-bubble technique and 38 eyes underwent PK.
RESULTS
Mean best spectacle corrected visual acuity (BSCVA) at the first postoperative week (P=0.012) and the first postoperative month (P<0.001) was statistically significantly higher in DALK group. The mean BSCVA at 12mo was not significantly different for DALK (0.30±1.99 logMAR) versus PK (0.40±0.33 logMAR) (P=0.104). The 76.3% of the eyes had a BSCVA over 0.5 in DALK and 47.4% in PK group (P=0.009). The 7.9% of the eyes had a BSCVA of 1.0 in DALK and 5.3% in PK group (P=0.644). Mean spherical equivalent was -2.94 D in DALK and -3.09 D in PK group. Mean topographic astigmatism was 4.62 D and 4.18 D respectively. Regular topographic patterns were observed in 31 (81.6%) of DALK and 29 (76.3%) of PK (P=0.574). The most frequent topographic pattern was oblate asymmetric bow tie, seen in 39.5% in DALK and 23.7% in PK.
CONCLUSION
Big bubble DALK provides an earlier visual improvement compare to PK. However, visual and topographic outcomes are similar to those in PK at 1y. Postoperative complications including rejection and intraocular pressure elevation are more frequent in PK. DALK is a safer alternative to PK for KC. However, intraoperative perforation of the Descemet's membrane is a significant complication.
Keywords: deep anterior lamellar keratoplasty, keratoconus, penetrating keratoplasty
INTRODUCTION
Keratoconus (KC) is a progressive ectatic disorder of the cornea. Keratoplasty is indicated in its advanced stage involving contact lens intolerance and significant scarring. Penetrating keratoplasty (PK) has been the mainstay of surgical treatment for decades[1]. It remains the most commonly performed procedure world wide since it is relatively simple and standardized technique with good results[2]–[3]. However, after the introduction of Anwar's big bubble technique, deep anterior lamellar keratoplasty (DALK) has become increasingly popular since it provides better graft survival that is especially important for young KC patients[4]. DALK leaves the healthy endothelium of the recipient cornea intact that eliminates endothelial rejection, prevents postoperative endothelial loss and subsequent graft failure[5]–[6]. As an extraocular procedure, it is theoretically safer as well[7]. It offers an increased donor availibility, since endothelial quality is irrelevant. It is even possible to avoid of stromal graft rejection by grafting cryopreserved donor corneas without live cells[8].
However, lamellar surgery is difficult and time-consuming. The major technical difficulty lies in judging the depth of the corneal dissection as close as possible to Descemet's membrane (DM) without perforation, unless this is achieved visual outcome is likely to be compromised[3],[7],[9]. Even in experienced hands, surgery can be completed as planned in approximately 80% of the cases[10]–[11]. Up to now DALK has not been technically standardized, in 10%-20% a conversion to PK is required due to perforation of DM[3],[12]. Prior hydrops with DM discontinuity is a contraindication to DALK[8]. Although these disadvantages, currently DALK is considered to be the first line of treatment of KC by many surgeons[13]–[15].
There are several studies comparing DALK and PK in the literature with similar visual outcomes[2],[7],[13]. However, to our knowledge, there is no study comparing the two techniques in terms of postoperative topographic patterns and surface geometry of the corneal graft. This study was conducted to compare the visual outcomes and topographic features of DALK with PK in the surgical management of KC.
SUBJECTS AND METHODS
In this multi center, prospective, randomized clinical trial, 76 eyes of 71 consecutive patients with moderate to severe KC operated between January 2011 and July 2014 in two tertiary referral hospitals were included. The study followed the tenets of the Declaration of Helsinki and had local Ethical Committee approval. An informed consent was obtained from all participants. Consecutive patients were alternately selected to receive one of the two surgical methods. By using the same randomization method, 12 eyes of 12 patients received DALK, and 12 eyes of 12 patients received PK in Istanbul Kartal Hospital. Twenty six eyes of 22 patients receieved DALK and 26 eyes of 25 patients received PK in Izmir Bozyaka Hospital. Inclusion criteria were moderate (mean keratometric values between 47 and 52 D) to advanced KC (mean keratometric values >52 D or immeasurable on topography) patients who had a poor spectacle corrected visual acuity and rigid gas permeable contact lens intolerance and minimum postoperative follow-up of 12mo. Exclusion criteria included co-existing ocular pathologies, such as cataract, retinal disorders or glaucoma. Drop outs from follow-up were also excluded from the analysis. Since visual acuity and topography could not be measured, patients with Down syndrome were not included. Preoperatively most of the eyes in both groups had anterior stromal scar or Vogt striae. In all DALK operated eyes, scars were pre-descemetic and DM was intact. Few patients with previous corneal hydrops and a discontinuity in DM were selected for PK.
Thirty eight eyes underwent DALK with the big-bubble technique and 38 eyes underwent PK. Four patients received bilateral DALK, 1 patient received bilateral PK. Patients underwent an ophthalmic examination preoperatively and 1wk, 1 and 12mo postoperatively. Ocular examinations included evaluation of Snellen best spectacle corrected visual acuity (BSCVA), biomicroscopy of the anterior segment, intraocular pressure (IOP) measurement with Goldmann applanation tonometer or Tonopen, and fundus examination. Corneal topographies were acquired with Keratograph III (Oculus, Wetzlar, Germany) 1mo after suture removal. Visual acuity was recorded using decimal charts and was converted to the logarithm of minimum angle of resolution (logMAR) for analysis.
Minimum postoperative follow-up was 12mo. Drop outs from follow-up were excluded. Main outcome measures were BSCVA at three different time points (week 1, month 1 and year 1) as well as spherical equivalent (SE) refraction, topographic corneal power (K), astigmatism, eccentricity (ECC), Q value and topographic patterns at year 1. Secondary outcome measures were immune rejection, graft clarity, IOP, intraoperative and postoperative complications.
Surgical Technique
All patients were operated by two corneal surgeons (Yüksel B and Kandemir B). DALK surgeries were performed by using Anwar's big bubble technique. A partial thickness trephination of the recipient cornea was performed with a Hessburg-Barron vacuum trephine 7.0 to 8.0 mm in diameter. Air was injected with a 30 G needle to separate the DM. When the big bubble was achieved, a partial thickness keratectomy was performed with a crescent knife. Stromal cap was cut and predescemetic space filled with 1% Na hyaluronate. Stromal layers were cut into four parts and removed. Donor cornea was cut from the endothelial side. The endothelium was stained with Trypan blue and removed. Donor cornea was sutured in place with a single running 10-0 nylon suture. In PK cases, full thickness recipient cut was followed by suturing of the graft by using the same suture technique.
Postoperative Management
Topical prednisolone acetate 1% was used to prevent rejection. After initial administration of 2-hourly and then reducing four times daily, the dose was tapered over 3-6mo depending on the condition of the eye and switched to low intensity steroids (fluorometholone or loteprednol). Steroid eye drops were applied to the eyes after DALK for at least six months and after PK for at least twelve months. Sutures were removed at 1y following PK. In DALK cases, postoperative management was similar to PK except that lower intensity steroids were started after 2-3mo and sutures were removed at 8mo. Loose sutures were removed upon diagnosis.
Statistical Analysis
Statistical analysis was performed using SPSS software for Windows (version 21.0, SPSS, Inc.). Comparison among groups was performed using Chi-square test and independent samples test. P values <0.05 were considered to be significant.
RESULTS
There were no statistically significant difference between PK and DALK groups in terms of age, gender, graft size, and donor-host trephine disparity (Table 1). No intraoperative complications occurred in PK group. Whereas, 2 micro and 4 macro perforations of DM occurred in DALK group (15.8%). Three of these eyes (7.9%) converted to PK and included in PK group. Postoperative double anterior chamber occurred in 1 eye (2.6%) with micro perforation. It was succesfully managed with air injection into the anterior chamber.
Table 1. Comparison of the demographic features of PK and DALK groups.
| Parameters | PK | DALK | P |
| Age (x±s, a) | 35.3±10.9 | 34.9±12.5 | 0.876a |
| Gender (F:M) | 23:15 | 28:10 | 0.222b |
| Eye (R:L) | 18:20 | 22:16 | 0.358b |
| Recipient trephine (%) | 0.231b | ||
| 7.00 mm | 1 (2.6) | 1 (2.6) | |
| 7.50 mm | 25 (65.8) | 29 (76.3) | |
| 7.75 mm | 0 | 2 (5.3) | |
| 8.00 mm | 12 (31.6) | 6 (15.8) | |
| Host-donor disparity (%) | 0.301b | ||
| 0 mm | 2 (5.3) | 0 | |
| 0.25 mm | 32 (84.2) | 32 (84.2) | |
| 0.50 mm | 4 (10.5) | 6 (15.8) |
PK: Penetrating keratoplasty; DALK: Deep anterior lamellar keratoplasty; SD: Standard deviation. a Independent sample test; bChi-square test.
n=38
Postoperative complications were significantly more frequent after PK (Table 2). IOP elevations occurred at mean 4.7 (1-10)mo and were controlled with brimonidine or its fixed combination with timolol. A fixed dilated pupil occurred in 1 eye due to prolonged use of cyclopentolate after the operation. Endothelial rejection episodes occurred in two eyes at 2 and 4mo. They were treated with intravenous pulsed methyl prednisolone 500 mg for three days followed by oral route. Grafts cleared after the treatment. No rejection episode was observed in 38 DALK-operated eyes. Transient wrinkles of DM occurred in 36.8% of the DALK eyes and disappeared within a mean of 7.1 (3-11)mo.
Table 2. Postoperative complications.
| Complications | PK | DALK | Pa |
| IOP elevation | 10 (26.3) | 1 (2.6) | 0.003b |
| Rejection episode | 4 (10.5) | 0 | 0.040b |
| Suture loosening | 2 (5.3) | 2 (5.3) | 1.000 |
| Dilated pupil | 1 (2.6) | 0 | 0.314 |
| Double anterior chamber | 0 | 1 (2.6) | 0.314 |
PK: Penetrating keratoplasty; DALK: Deep anterior lamellar keratoplasty; IOP: Intraocular pressure. aChi-square test; bStatistically significant P values.
n=38 (%)
There was no significant difference between PK and DALK groups in the mean preoperative BSCVA (Table 3). It was 0.05 in decimal score for both groups. Mean postoperative BSCVA, at the first week and the first month, was statistically significantly higher in DALK group than PK group. However, that difference no longer existed at 12mo. It was mean 0.49 decimal score in PK and 0.55 in DALK-operated eyes. At the last visit, the percentage of the eyes with a BSCVA ≥0.5 was 76.3% in DALK and 47.4% in PK group (P=0.009). The percentage of the eyes with a BSCVA of 1.0 was 7.9% and 5.3% respectively (P=0.644).
Table 3. Comparison of postoperative visual acuity between PK and DALK groups in three different time points.
| BSCVA (logMAR) | PK (n=38) | DALK (n=38) | Pa |
| Preoperative | 1.79±0.95 (3.00-0.54) | 1.81±0.87 (3.00-0.30) | 0.938 |
| Postoperative week 1 | 0.94±0.76 (2.00-0.30) | 0.76±0.28 (1.30-0.18) | 0.012b |
| Postoperative month 1 | 0.80±0.35 (1.30-0.00) | 0.53±0.23 (1.30-0.00) | < 0.001b |
| Postoperative year 1 | 0.40±0.33 (1.30-0.00) | 0.30±1.99 (0.80-0.00) | 0.104 |
PK: Penetrating keratoplasty; DALK: Deep anterior lamellar keratoplasty; BSCVA: Best spectacle corrected visual acuity. aIndependent sample test; bStatistically significant P values.
x±s (range)
Preoperatively, in patients with readable topographic maps, preoperative mean keratometry was calculated as 62.80±6.30 D in PK group and 57.07±6.91 D in DALK group (P=0.025, independent sample test). In patients with measurable refraction, mean spherical spectacle correction was -5.5 D and myopic cylinder was 3.8 D in PK group. These values were -5.2 D and 3.3 D in DALK group (P=0.762 for spherical spectacle correction, P=0.739 for myopic cylinder, independent sample test).
Mean postoperative myopic SE, K, ECC and astigmatism did not show any statistically significant difference between two groups at year one. The only statistically significant difference was found in Q value between DALK and PK-operated eyes (Table 4). Regular topographic patterns were observed in 31 (81.6%) of DALK and 29 (76.3%) of PK eyes (P=0.574). The most frequent topographic pattern was oblate asymmetric bow tie that was seen in 39.5% of DALK and 23.7% of PK-operated eyes (Figure 1).
Table 4. Mean values of SE refraction and topographic values measured at postoperative year one for PK and DALK groups.
| Parameters | PK (n=38) | DALK (n=38) | Pa |
| Spheric equivalent | -3.09±4.34 (-17.00-4.50) | -2.94±4.16 (-20.00-5.25) | 0.880 |
| K | 44.24±5.11 (31.12-63.60) | 44.11±3.59 (37.90-51.05) | 0.901 |
| ECC | 0.17±0.95 (-1.23-4.20) | -0.21±0.68 (-1.35-0.96) | 0.220 |
| Q | 11.81±0.38 (10.80-12.60) | 11.46±0.53 (9.80-12.20) | 0.002b |
| Topo astigmatism | 4.18±2.37 (0.90-11.80) | 4.62±2.25 (1.20-9.80) | 0.409 |
SD: Standard deviation; PK: Penetrating keratoplasty; DALK: Deep anterior lamellar keratoplasty; K: Topographic corneal power; ECC: Eccentricity; Q: Topographic Q value; Topo astigmatism: Topographic astigmatism. aIndependent sample test; bStatistically significant P values.
x±s (range)
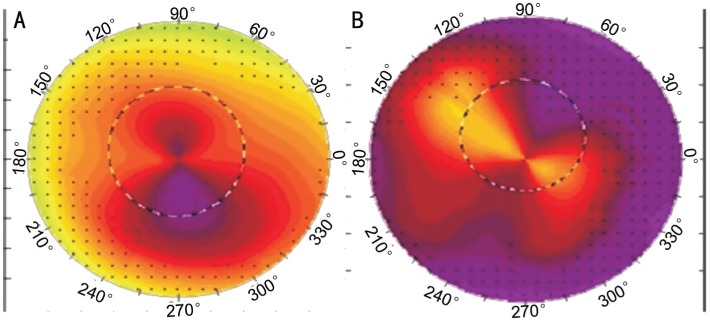
Figure 1. Topographic maps showing Prolate Asymmetric Bow Tie pattern in a 42-year-old male with PK (A) and Oblate Asymmetric Bow Tie pattern in 49-year-old female with DALK (B) are seen.
At postoperative 1y the primary study end-point, spectacle correction was achieved in 24/38 (63.2%) of the eyes in PK group and 36/38 (94.7%) in DALK group (P=0.001, Chi-square test). Mean postoperative spherical correction was -0.98±2.19 D (-11.0 to +4.0) in PK group and -1.66±3.16 (-16.0 to +4.0) in DALK group (P=0.465, independent sample test). Mean refractive cylinder was 4.14±2.18 D in PK and 4.27±2.10 D in DALK (P=0.817, independent sample test).
DISCUSSION
In the current study, mean BSCVA at the first postoperative week (P=0.012) and the first postoperative month (P<0.001) was found statistically significantly higher in DALK group. This outcome indicates that an earlier visual rehabilitation occurs after DALK surgery compare to PK. However, mean BSCVA was similar at 12mo as described by previous reports[2],[7],[16]–[17]. Akdemir et al[18] reported mean BSCVA at 1y 0.15 logMAR for DALK and 0.21 for PK without a statistical significance. Donoso et al [13] reported 0.17 for both surgeries. These values were 0.30 and 0.40 respectively in our study (P=0.104). Smadja et al[19] reported a logMAR BCVA of 0.88 for DALK at 12mo, which is considerably lower than our results, with a rate of BSCVA≥0.5 in 100%. In our study, this rate was 76.3% in DALK group which was significantly higher than PK group (47.4%). Söğütlü et al[20] reported these rates 83% and 85% respectively without any statistical difference. Although 20/20 vision reported more frequent in PK cases[2], that rate was similar in both groups of our study.
Mean postoperative BSCVA, SE, K, ECC and astigmatism values were found similar in DALK and PK at one year. Although, a significantly higher postoperative myopia was reported in DALK compare to PK at 12mo (-4.22 D vs -2.73 D) as well as in long term (-6.1 D vs -5.0 D)[14],[17], mean SE was found approximately -3.0 D in either groups of our study, in accordance with Söğütlü et al's[20] report. Mean K was 44.1 D in DALK and 44.2 D in PK. It was reported 46.9 and 47.8 D by Kim et al[17]. As a result of progressive steepening, it may reach 48.8 D and 47.3 D respectively at 9y[14]. Different outcomes may be related to the differences in patient populations. We found mean topographic astigmatism 4.2 D in PK and 4.6 D in DALK without statistical significance. Zhang et al[8] also reported similar astigmatism results ranging 3 to 4 D, consistent with other studies[7],[14],[17].
In our DALK surgeries, intraoperative DM perforation occurred in 15.8% and conversion to PK in 7.9%. DM perforation rate has been reported between 4.0%-39.2% and large DM tears needing conversion to PK between 2.3%-27.3% in the literature[8],[11],[19]. Our rates are consistent with these reports. These complications usually decrease after the first 10 cases[19]. Formation of type 2 bubble during air injection is associated with high perforation rate[21]. Postoperative complications are reported more frequent after PK (57.1%) compared to DALK (26.5%) and 31.8% vs 4.5% in another study[2],[19]. Overall incidence of complications was 44.7% in PK and 10.5% in DALK group of our study. IOP elevation occurred in 26.3% of PK and 2.6% of DALK eyes. This finding is consistent with previous report of 46.2% vs 1.3%[8]. It is likely to be a consequence of the use of steroid drops for shorter period and lower intensity after DALK.
Endothelial rejection episodes were observed in 10.5% of our PK cases. Cohen et al[16] reported this rate as 13.3% for PK and Donoso et al[13] as 8%. Zhang et al[8] reported 7.7% allograft rejection not causing graft failure. Although stromal rejection episodes have been reported in 1.7% to 11.3 % within 4y, no rejection episode was observed in our DALK cases[11],[22]. No significant difference in graft survival between DALK and PK has been found in a series of 4521 cases[23]. The median predicted survival was reported as 49.0y in DALK and 17.3y in PK[24]. By contrast, MacIntyre et al[2] reported a higher graft survival in PK: 100% vs 93.5%. No cases of late endothelial failure occurred in our cases as reported earlier[16]. An endothelial loss of 8.6% and 14.0% has been reported at 1 and 2y after DALK, stabilizing around 14.0% in following three years. Whereas in PK, a steady loss occurs, reaching at 34.6% in 5y[8]. Reported annual loss rates of 14.1% in PK and 5.8% in DALK are much higher than physiologic loss of 0.6% per year[15],[25]. It suggests that the removal of stroma and exposure of DM may lead to endothelial cell loss due to indirect trauma.
ECC designates the amount by which the cornea diverges from a perfect sphere. It is 0 for a sphere, 0.1-0.9 for an ellipsoid and 0.3-0.6 for an average cornea[26]. Negative ECC may be seen after keratoplasty[27]. Although ECC was closer to normal in PK (0.17) compare to DALK (-0.21), the difference was not statistically significant (P=0.220). Q value is an indicator of asphericity of the cornea. Prolate means (negative Q) the radius in the periphery is larger than the center, oblate (positive Q) is the opposite. Average Q for a normal cornea is -0.26 to -0.42 and 0 for a sphere[28]. Almost all postkeratoplasty corneas have been reported to be oblate[29]. Likewise, in our study, mean Q value was found 11.81 in PK and 11.46 in DALK indicating highly oblate corneas. However, corneal shape was closer to normal in DALK compare to PK (P=0.002).
The limitations of this study include small sample size and relatively short follow-up. Although, optical and visual outcomes at one year including BSCVA, astigmatism, SE and K values are similar in both groups. Results of our study suggest that DALK offers an earlier visual rehabilitation and a more natural corneal shape with fewer postoperative complications. Given the advantages including no risk of endothelial rejection, long-term preservation of endothelial cells and structurally stronger globe, DALK should be the first choice for the surgical treatment of KC except three conditions: regrafting in eyes with PK, prior hydrops with discontinuity in DM and deep scars[3],[6]–[8],[14],[18]. However, intraoperative perforation of the DM remains as a significant complication.
Acknowledgments
Conflicts of Interest: Yüksel B, None; Kandemir B, None; Uzunel UD, None; Çelik O, None; Ceylan S, None; Küsbeci T, None.
REFERENCES
- 1.Parker JS, van Dijk K, Melles GR. Treatment options for advanced keratoconus: a review. Surv Ophthalmol. 2015;60(5):459–480. doi: 10.1016/j.survophthal.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 2.MacIntyre R, Chow SP, Chan E, Poon A. Long-term outcomes of deep anterior lamellar keratoplasty versus penetrating keratoplasty in Australian keratoconus patients. Cornea. 2014;33(1):6–9. doi: 10.1097/ICO.0b013e3182a9fbfd. [DOI] [PubMed] [Google Scholar]
- 3.Seitz B, Cursiefen C, El-Husseiny M, Viestenz A, Langenbucher A, Szentmáry N. DALK and penetrating laser keratoplasty for advanced keratoconus. Ophthalmologe. 2013;110(9):839–848. doi: 10.1007/s00347-013-2822-1. [DOI] [PubMed] [Google Scholar]
- 4.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28(3):398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 5.Keane M, Coster D, Ziaei M, Williams K. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus. Cochrane Database Syst Rev. 2014;22(7):CD009700. doi: 10.1002/14651858.CD009700.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Hu DN, Xia Y, Yang L, Xue C, Huang Z. Comparison of femtosecond laser-assisted deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus. BMC Ophthalmol. 2015;15:144. doi: 10.1186/s12886-015-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhart WJ, Musch DC, Jacobs DS, Lee WB, Kaufman SC, Shtein RM. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplastya report by the American academy of ophthalmology. Ophthalmology. 2011;118(1):209–218. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YM, Wu SQ, Yao YF. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14(5):438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana L, Parente G, Sincich A, Tassinari G. Influence of graft-host interface on the quality of vision after deep anteriorlamellar keratoplasty in patients with keratoconus. Cornea. 2011;30(5):497–502. doi: 10.1097/ico.0b013e3181d25e4d. [DOI] [PubMed] [Google Scholar]
- 10.Feizi S, Javadi MA, Daryabari SH. Factors influencing big-bubble formation during deep anterior lamellar keratoplasty in keratoconus. Br J Ophthalmol. 2015;100(5):622–625. doi: 10.1136/bjophthalmol-2015-307111. [DOI] [PubMed] [Google Scholar]
- 11.Kubaloglu A, Sari ES, Unal M, Koytak A, Kurnaz E, Cinar Y, Ozertürk Y. Long-term results of deep anterior lamellar keratoplasty for the treatment ofkeratoconus. Am J Ophthalmol. 2011;151(5):760–767. doi: 10.1016/j.ajo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Schiano-Lomoriello D, Colabelli-Gisoldi RA, Nubile M, Oddone F, Ducoli G, Villani CM, Mastropasqua L, Pocobelli A. Descemetic and predescemetic DALK in keratoconus patients: a clinical and confocal perspective study. Biomed Res Int. 2014;2014:123156. doi: 10.1155/2014/123156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donoso R, Díaz C, Villavicencio P. Comparative study of keratoconus between Anwar's deep anterior lamellar keratoplasty versus converted penetrating keratoplasty. Arch Soc Esp Oftalmol. 2015;90(6):257–263. doi: 10.1016/j.oftal.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Huang T, Hu Y, Gui M, Hou C, Zhang H. Comparison of refractive outcomes in three corneal transplantation techniques for keratoconus. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1947–1953. doi: 10.1007/s00417-015-3091-2. [DOI] [PubMed] [Google Scholar]
- 15.Kubaloglu A, Koytak A, Sari ES, Akyol S, Kurnaz E, Ozerturk Y. Corneal endothelium after deep anterior lamellar keratoplasty and penetratingkeratoplasty for keratoconus: a four-year comparative study. Indian J Ophthalmol. 2012;60(1):35–40. doi: 10.4103/0301-4738.90490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen AW, Goins KM, Sutphin JE, Wandling GR, Wagoner MD. Penetrating keratoplasty versus deep anterior lamellar keratoplasty for the treatment of keratoconus. Int Ophthalmol. 2010;30(6):675–681. doi: 10.1007/s10792-010-9393-9. [DOI] [PubMed] [Google Scholar]
- 17.Kim KH, Choi SH, Ahn K, Chung ES, Chung TY. Comparison of refractive changes after deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus. Jpn J Ophthalmol. 2011;55(2):93–97. doi: 10.1007/s10384-010-0914-x. [DOI] [PubMed] [Google Scholar]
- 18.Akdemir MO, Kandemir B, Sayman IB, Selvi C, Kamil Dogan O. Comparison of contrast sensitivity and visual acuity between deep anterior lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Int J Ophthalmol. 2012;5(6):737–741. doi: 10.3980/j.issn.2222-3959.2012.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smadja D, Colin J, Krueger RR, Mello GR, Gallois A, Mortemousque B, Touboul D. Outcomes of deep anterior lamellar keratoplasty for keratoconus: learning curve and advantages of the big bubble technique. Cornea. 2012;31(8):859–863. doi: 10.1097/ICO.0b013e318242fdae. [DOI] [PubMed] [Google Scholar]
- 20.Söğütlü ES, Kubaloğlu A, Ünal M, Piñero Llorens D, Koytak A, Ofluoglu AN, Özertürk Y. Penetrating keratoplasty versus deep anterior lamellar keratoplasty: comparison of optical and visual quality outcomes. Br J Ophthalmol. 2012;96(8):1063–1067. doi: 10.1136/bjophthalmol-2011-301349. [DOI] [PubMed] [Google Scholar]
- 21.Goweida MB. Intraoperative review of different bubble types formed during pneumodissection (big-bubble) deep anterior lamellar keratoplasty. Cornea. 2015;34(6):621–624. doi: 10.1097/ICO.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 22.Romano V, Iovieno A, Parente G, Soldani AM, Fontana L. Long-term clinical outcomes of deep anterior lamellar keratoplasty in patients with keratoconus. Am J Ophthalmol. 2015;159(3):505–511. doi: 10.1016/j.ajo.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Kasbekar SA, Jones MN, Ahmad S, Larkin DF, Kaye SB. Corneal transplant surgery for keratoconus and the effect of surgeon experienceon deep anterior lamellar keratoplasty outcomes. Am J Ophthalmol. 2014;158(6):1239–1246. doi: 10.1016/j.ajo.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Borderie VM, Sandali O, Bullet J, Gaujoux T, Touzeau O, Laroche L. Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology. 2012;119(2):249–255. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 25.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38(3):779–782. [PubMed] [Google Scholar]
- 26.Courville CB, Kleyce SD. Corneal topography. In: Smolin G, Foster CS, Azar Dt, Dohlman CH, editors. Smolin and Thoft's The Cornea. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 181. [Google Scholar]
- 27.Gruenauer-Kloevekorn C, Fischer U, Kloevekorn-Norgall K, Duncker GI. Pellucid marginal corneal degeneration: evaluation of the corneal surface and contact lens fitting. Br J Ophthalmol. 2006;90(3):318–323. doi: 10.1136/bjo.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal A, Agarwal A, Jacop S, editors. Dr.Agarwal's textbook on corneal topography. New Delhi: Jaypee Bros Medical P Ltd; 2010. [Google Scholar]
- 29.Sarhan AR, Dua HS, Beach M. Effect of disagreement between refractive, keratometric, and topographic determination of astigmatic axis on suture removal after penetrating keratoplasty. Br J Ophthalmol. 2000;84(8):837–841. doi: 10.1136/bjo.84.8.837. [DOI] [PMC free article] [PubMed] [Google Scholar]