Abstract
AIM
To evaluate the surgical treatment and visual outcomes of eyes with cataract and persistent hyperplastic primary vitreous (PHPV).
METHODS
This retrospective study included patients with cataract and PHPV treated with various strategies. Anterior PHPV was treated using phacoemulsification with underwater electric coagulation on posterior capsule neovascularization, posterior capsulotomy, anterior vitrectomy, and intraocular lens (IOL) implantation. Posterior PHPV was treated with lensectomy, posterior vitrectomy, retinal photocoagulation, and IOL implantation or silicone oil tamponade. Visual acuity (VA), pattern visual evoked potential (P-VEP), anatomic recovery, postoperative complications, and amblyopia outcome were examined. Subjects were followed-up for 3-48mo after surgery.
RESULTS
Of the 30 patients (33 eyes) with congenital cataract and PHPV included (average age, 39.30±35.47mo), 9 eyes had anterior PHPV and 24 had posterior PHPV. Thirty-two eyes were surgically treated. Eyes with anterior PHPV received an IOL during one-stage (6 eyes) and two-stage (3 eyes) implantation. Postoperative complications included retinal detachment (1 eye) and recurrent anterior chamber hemorrhage (1 eye). In eyes with posterior PHPV, 6 and 11 eyes received IOLs in one- and two-stage procedures, respectively. Silicone oil was retained in 2 eyes, and IOLs were not implanted in 4 eyes. VA significantly improved in 25 eyes following operations and 3-48mo of amblyopia treatment. P-VEP P100 was improved following surgery in both PHPV types.
CONCLUSION
Our surgical strategies are appropriate and effective for anterior and posterior PHPV. Early surgical intervention and amblyopia therapy result in positive treatment outcomes.
Keywords: congenital cataract, persistent hyperplastic primary vitreous, phacoemulsification, intraocular lens implantation, lensectomy, vitrectomy, visual acuity
INTRODUCTION
Persistent hyperplastic primary vitreous (PHPV), also referred to as persistent fetal vasculature (PFV), is a congenital anomaly of the eye that results from failure of embryological primary vitreous and hyaloid vasculature to regress. It is characterized by the persistence of various portions of the primary vitreous (embryonic hyaloid vascular system) with hyperplasia of the associated embryonic connective tissue, and associated with microphthalmia, cataract, and glaucoma. Approximately 90% of patients with the condition have unilateral disease and associated poor vision in the affected eye[1]–[3]. Though the exact prevalence remains unknown, PHPV is not considered to be a very rare disease. A study on childhood blindness and visual loss in the United States showed that PHPV accounts for about 5% of all cases of blindness[4]. A pilot study of ocular disease screening for neonates in China showed that it accounts for about 0.351% among 15 398 newborns[5].
Clinically, PHPV is classified into anterior and posterior subtypes[6]. Anterior PHPV occurs when the abnormality is located in front of the original vitreous artery and remains integrated with the posterior capsule. Clinical manifestations can occur together and include a retrolental vascular fibrous membrane, iris vascular remnants, microphthalmia, shallow anterior chamber, lens opacity, elongated ciliary processes, and glaucoma. In contrast, clinical manifestations of posterior PHPV can occur in combination with anterior and posterior abnormalities, e.g. vitreous stalk, preretinal membranes, optic hypoplasia, retinal folds, and retinal detachment. The remnant vascular stalk is seen arising off the optic nerve and radiating out in all directions. For example, it may be anteriorly integrated into the lens and elongate the ciliary processes, reach around to the peripheral retina, where the retinal folds are created, or posteriorly integrate into the optic nerve vascular bundle[7].
For posterior PHPV, some researchers do not recommend surgery and are more likely to choose conservative treatment[8], while others claim favorable results with new techniques, yielding good cosmetic results and even useful vision in recent literature[9]–[10]. However, we believe that, if left untreated, some patients with PHPV can become blind due to serious complications. Thus, we recommend early surgical intervention to salvage useful vision and achieve acceptable cosmetic outcomes. Moreover, we have achieved some favorable outcomes in a relatively large number of patients with cataract and PHPV through different choices of surgical methods and the introduction of sophisticated microsurgical techniques, combined with aggressive antiamblyopic therapy.
SUBJECTS AND METHODS
Study Subjects and Preoperative Examinations
The medical records of patients diagnosed with “cataract combined with PHPV” at the First Affiliated Hospital of Zhengzhou University between January 2010 and May 2015 were included in the retrospective analyses. All patients underwent comprehensive systemic and ophthalmological examinations, which included color Doppler imaging, computed tomography (CT) or magnetic resonance imaging (MRI), and slit-lamp biomicroscopy, ophthalmoscopy, fundus photography, visual activity, intraocular pressure (IOP), and corneal endothelial cell density. Imaging examination was used to visualize the posterior ocular structure.
A non-contact ophthalmic tonometer (CT-80A, TOPCON, Japan) was used to measure IOP when possible; however a hand-held resilience ophthalmic tonometer (TA03, Icare Finland Oy, Vanda, Finland) was used when patients were uncooperative. Intraocular lens (IOL) power was calculated with the SRK/T formula based on corneal curvature and axial length measurements (IOLMaster, CarlZeiss-Meditec, Jena, Germany).
Corneal endothelial cell densities were measured by a corneal endothelial cell counter (SP-3000P, TOPCON, Japan). Additionally, the latency and amplitude of the pattern visual evoked potential (P-VEP) P100 waveforms were used to assess visual electrophysiology (MonPack3 system, Metrovision, Parenchies, France). General patient information was also recorded, including age, sex, and initial symptoms reported.
Ethical Approval and Informed Consent
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University in January 2010 (Approval No.20100115), which waived the requirement of informed consent due to the study's retrospective nature. All study conduct adhered to the tenets of the Declaration of Helsinki. All subjects, when of age and capable, and their legal representatives provided informed consent to undergo standard-of-care procedures after the risks and benefits of surgery were fully explained.
Surgical Procedures
All surgical procedures were performed by one experienced surgeon (Zheng GY). After the successful induction of general anesthesia, cataract combined with anterior PHPV removal was performed through a 1.8-mm corneoscleral tunnel incision made at the 12 o'clock position. After making the incision, viscoelastic material (DisCoVisc, Alcon, Ft. Worth, TX, USA) was injected and a continuous 5.5-6.0 mm curvilinear capsulorhexis was made. Lens cortex extraction was then performed using phacoemulsification and aspiration (Stellaris, Bausch & Lomb, Rochester, NY, USA). Since most remnant fetal vessels are integrated with the posterior capsule and iris, a retrolental vascular fibrous membrane is usually present. Therefore, any neovascularization integrated with the posterior capsule or iris was cauterized using underwater electric coagulation. A 4.0-mm posterior capsulotomy, together with PFV fibrous tissue excision and anterior vitrectomy, was performed under irrigation using a 25-gauge vitreous cutter system and an infusion cannula inserted through a corneal limbal side port (Stellaris PC vitrectomy system, Bausch & Lomb, Rochester, NY, USA). A posterior chamber IOL was then implanted into the double capsulorhexis, capsular bag after injection of viscoelastic material into the anterior chamber. A balanced saline solution was then used to flush and exchange the viscoelastic material of the anterior chamber and capsular bag. The tunnel incision was sutured and the anterior chamber was reconstructed to be watertight. At the end of the surgical procedure, both to bramycin (20 mg) and dexamethasone (3 mg) were administered subconjunctivally. An antibiotic ointment was then applied to the ocular surface and the eye was packed and dressed.
During the operation to treat the cataract and posterior PHPV, most fetal vascular remnants that extended outward from the optic disk and integrated with the posterior capsule (some as far as retinal folds or the peripheral retina) required removal. Therefore, a routine three-port vitrectomy through the pars plicata/plana was also performed, along with lensectomy and a circular anterior capsulectomy and retrolental fibrous membrane removal using a 25-gauge vitreous cutter system. During removal of the vitreous abnormalities, the retina, which was pulled away from the back of the eye, was spread and reattached immediately. It was then illuminated using a xenon lamp light guide fiber and observed carefully through a panretinal mirror. If the retina avoided the optic disk and macular region, and there were no retinal tears, detachment, or obvious abnormalities observed during surgery, retinal photocoagulation was performed in areas with degeneration or dysplasia. A posterior chamber IOL (sulcus or in the top of the residual anterior capsule circular hole) was implanted through the tunnel incision after three vitrectomy incisions were sutured using 8-0 absorbable sutures. If any obvious and serious retinal breaks or folds were identified, endophotocoagulation was performed around the hole or folds and silicone oil was used for tamponade. Three or six months later, if the retina remained attached, the silicone oil was removed and an IOL was implanted. If a closed, funnel-shaped retinal detachment was identified, silicone oil was permanently kept in the vitreous cavity.
Postoperative Assessments
Corneal transparency, anterior chamber depth, aqueous flare, IOL location, and pupil size, shape, and reaction were examined using a slit-lamp to identify postoperative complications. Ophthalmoscopy was also performed to inspect the fundus and fundus photographs were obtained.
Distance visual acuity was tested at 5 m in children who could use an international standard vision chart before the operation, three months postoperatively, and at final follow up. Children who were younger than 3 years old had their distance visual acuity tested with a pediatric vision chart at 2.5 m. The measured visual acuity at a distance of 2.5 m was then converted to the 5 m equivalent. All visual acuity measurements were recorded in logarithm of the minimum angle of resolution (logMAR) units. The latency and amplitude of P-VEP P100, measured with both high (15′) and low (60′) frequency stimuli, were recorded and analyzed (MonPack3 system, Metrovision, Parenchies, France). In children who were uncooperative, Baby Vision (MonPack3 system) was used to obtain visual acuity and scanning stimuli were used to obtain visual evoked potentials (VEP).
Aphakic subjects over the age of 6mo who had no severe posterior lesions underwent correction with spectacles following retinoscopy. All patients who cooperated began amblyopia training as early as possible. Training methods included wearing corrective glasses, patching, using a composite amblyopia cure apparatus (BS, Boshi Medical and Health Research Institute, Guangzhou, China), and use of a 3 D or 4 D visual function training instrument (JCSX-01, Seein4D, Beijing, China). Subjects underwent amblyopia therapy for at least 1y. The effect of amblyopia treatment was evaluated by comparing patient visual function before therapy and at the end of follow-up. Parameters evaluated included uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) and the latency and amplitude of the P-VEP P100 waveform.
Statistical Analysis
All statistical analyses were performed using SPSS statistical software (ver. 19.0, SPSS, Inc., Chicago, IL, USA). Data normality was examined using the Shapiro-Wilk test. Normally distributed data were analyzed using paired Student's t-tests. Data that were not normally distributed were examined using Mann-Whitney U tests. Statistical significance was defined as P<0.05.
RESULTS
Subject Characteristics
A total of 33 eyes from 30 patients with congenital cataract and PHPV were included in this study. Subjects had an average age of 39.30±35.47mo (range: 3-108mo) and were followed up for 3-48mo postoperatively.
Nine of the 33 eyes (27.3%), eyes 1 to 9, had PHPV of the anterior type; the remaining 24 eyes (72.7%), eyes 10 to 33, had PHPV of the posterior type. One subject (No.5) had bilateral congenital cataract and anterior PHPV, two subjects (No.16 and No.17) had bilateral congenital cataract with posterior PHPV. Additionally, one subject (No.15) was diagnosed with morning glory syndrome associated with PHPV, two subjects (No.17 and No.30) were diagnosed with microphthalmia. None of the patients had other congenital abnormalities or a positive family history for related conditions.
Patients were divided into the anterior PHPV and posterior PHPV groups. There were no significant differences between groups in their gender distribution (P=0.50), age (P=0.84), IOP (P=0.84), axial length (P=0.07), or corneal endothelial cell density (P=0.43). Subject characteristics are summarized in Table 1 and representative cases are shown in Figures 1, 2 and 3.
Table 1. Subject characteristics of 30 cases with cataract and PHPV at clinical presentation.
| Case/No. | Eye | Gender | Age (mo) | Eye | Diagnosis | Axial length (mm) | Surgical treatment | Preop. UCVA (logMAR) | Postop. UCVA (logMAR) | BCVA at final follow up (logMAR) |
| 1 | 1 | F | 4 | OS | cata+aPHPV | 18.92 | Phaco+dia+aV+2°IOL | Unmeasurable | CF | CF |
| 2 | 2 | M | 36 | OS | cata+aPHPV | 22.01 | Phaco+dia+aV+IOL | 1.10 | 0.52 | 0.30 |
| 3 | 3 | M | 48 | OD | cata+aPHPV | 23.11 | Phaco+dia+aV+IOL | 1.0 | 0.30 | 0.22 |
| 4 | 4 | F | 36 | OD | cata+aPHPV | 21.91 | Phaco+dia+aV+IOL | 1.70 | 0.52 | 0.30 |
| 5 | 5 | M | 60 | OU | cata+aPHPV | 22.01 | Phaco+dia+aV+IOL | 0.92 | 0.40 | 0.30 |
| 5 | 6 | M | 60 | OU | cata+aPHPV | 21.01 | Phaco+dia+aV+IOL | 0.82 | 0.30 | 0.22 |
| 6 | 7 | M | 6 | OD | cata+aPHPV | 20.09 | Phaco+dia+aV+2° IOL | Unmeasurable | 0.52 | 0.30 |
| 7 | 8 | F | 48 | OD | cata+aPHPV | 22.80 | Phaco+dia+aV+IOL | 1.0 | 0.60 | 0.22 |
| 8 | 9 | F | 12 | OD | cata+aPHPV | 21.90 | Phaco+dia+aV+2° IOL | Unmeasurable | 0.40 | 0.30 |
| 9 | 10 | M | 36 | OD | cata+pPHPV | 21.06 | Phaco+pV+ RP+IOL | 1.30 | 0.52 | 0.40 |
| 10 | 11 | M | 96 | OD | cata+pPHPV | 22.09 | Phaco+pV+ RP+IOL | 1.70 | 0.00 | 0.00 |
| 11 | 12 | M | 96 | OS | cata+pPHPV | 22.30 | Phaco+pV+RP+IOL | HM | 0.60 | 0.40 |
| 12 | 13 | M | 120 | OS | cata+pPHPV | 19.07 | Phaco+pV+RP+IOL | HM | CF | 0.30 |
| 13 | 14 | F | 36 | OS | cata+pPHPV | 23.62 | Phaco+pV+RP+IOL | 1.70 | 0.82 | 0.52 |
| 14 | 15 | F | 60 | OS | cata+pPHPV | 21.98 | Phaco+pV+RP+IOL | 1.00 | 0.60 | 0.40 |
| 15 | 16 | M | 24 | OS | MGS+pPHPV | 22.00 | Phaco+pV+2° IOL | Unmeasurable | 0.92 | 0.52 |
| 16 | 17 | F | 5 | OU | cata+pPHPV | 20.87 | Phaco+pV+2° IOL | 1.00 | 0.52 | 0.30 |
| 16 | 18 | F | 5 | OU | cata+pPHPV | 20.01 | Phaco+pV+2° IOL | 0.92 | 0.40 | 0.40 |
| 17 | 19 | M | 5 | OU | cata+pPHPV | 20.10 | Phaco+pV+2° IOL | 1.10 | 0.92 | 0.52 |
| 17 | 20 | M | 5 | OU | pPHPV+microphthalmia | 16.76 | Phaco+pV+2° IOL | 1.00 | 0.82 | 0.40 |
| 18 | 21 | M | 6 | OD | cata+pPHPV | 20.90 | Phaco+pV+2° IOL | Unmeasurable | 0.40 | 0.30 |
| 19 | 22 | M | 3 | OD | cata+pPHPV | 21.80 | Phaco+pV+2° IOL | Unmeasurable | 0.60 | 0.40 |
| 20 | 23 | F | 96 | OS | cata+pPHPV | 22.30 | Phaco+pV+RP+oil+2° oil removal with IOL | HM | 1.10 | 0.40 |
| 21 | 24 | M | 108 | OS | cata+pPHPV | 21.54 | Phaco+pV+RP+oil+2° oil removal with IOL | 1.00 | 0.00 | 0.00 |
| 22 | 25 | M | 30 | OS | cata+pPHPV | 17.27 | Phaco+pV+RP+oil+2° oil removal with IOL | 1.00 | 0.60 | 0.40 |
| 23 | 26 | F | 48 | OD | cata+pPHPV | 21.09 | Phaco+pV+RP+oil+2° oil removal with IOL | 1.10 | 0.22 | 0.22 |
| 24 | 27 | M | 84 | OS | cata+pPHPV | 19.00 | Phaco+pV+RP+oil | LP | CF | CF |
| 25 | 28 | M | 48 | OS | cata+pPHPV | 21.45 | Phaco+pV+RP+oil | CF | CF | CF |
| 26 | 29 | M | 6 | OS | cata+pPHPV | 20.00 | Phaco+pV (no IOL) | Unmeasurable | Grabs objects | Grabs objects |
| 27 | 30 | F | 3 | OD | cata+pPHPV | 19.01 | Phaco+pV (no IOL) | Unmeasurable | Grabs objects | Grabs objects |
| 28 | 31 | F | 3 | OS | cata+pPHPV | 18.98 | Phaco+pV (no IOL) | Unmeasurable | Grabs objects | Grabs objects |
| 29 | 32 | F | 3 | OD | cata+pPHPV | 18.92 | Phaco+pV (no IOL) | Unmeasurable | Grabs objects | Grabs objects |
| 30 | 33 | M | 60 | OS | cata+pPHPV+microphthalmia | 14.76 | Untreated | - | - | - |
UCVA: Uncorrected visual acuity; BCVA: Best-corrected visual acuity; cata: Cataract; aPHPV: Anterior persistent hyperplastic primary vitreous; Phaco: Phacoemulsification; dia: Diathermy; aV: Anterior vitrectomy; 2° IOL: IOL implanted in a second procedure; CF: Count fingers; pPHPV: Posterior persistent hyperplastic primary vitreous; pV: Posterior vitrectomy; RP: Retinal photocoagulation; HM: Hand movement; MGS: Morning glory syndrome; oil: Silicone oil.
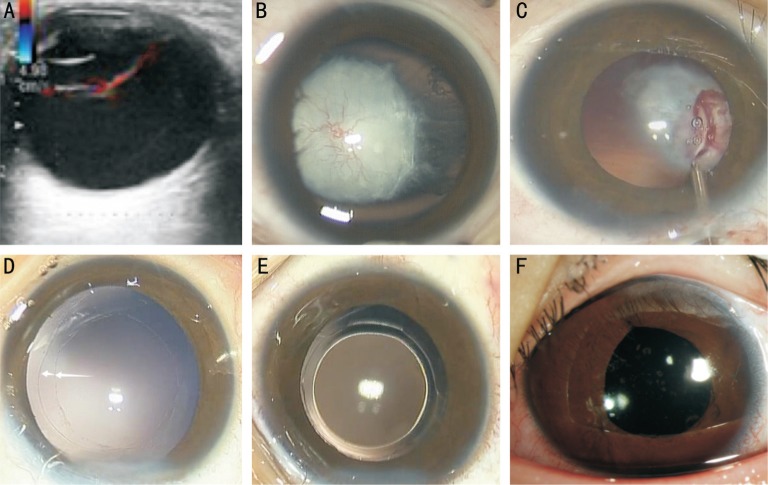
Figure 1. Color Doppler ultrasound and anterior segment photograph of a 36-month-old child with congenital cataract and anterior PHPV in Case 4.
A: A retrolental vascular fibrous membrane is found using color Doppler ultrasound; B: The remnant fetal vessels that integrate with the anterior and posterior capsule and iris have formed the retrolental vascular fibrous membrane; C: Some neovascularization integrated with the anterior and posterior capsule or iris is being cauterized using underwater electric coagulation; D: Continuous curvilinear capsulorhexis of 6.0 mm and 4.0 mm respectively, have been made in the anterior and posterior capsule; E: A posterior chamber IOL has been implanted into the double capsulorhexis capsular bag; F: The anterior segment photograph of the slip-lamp after implantation of the IOL, taken 90d after the surgery.
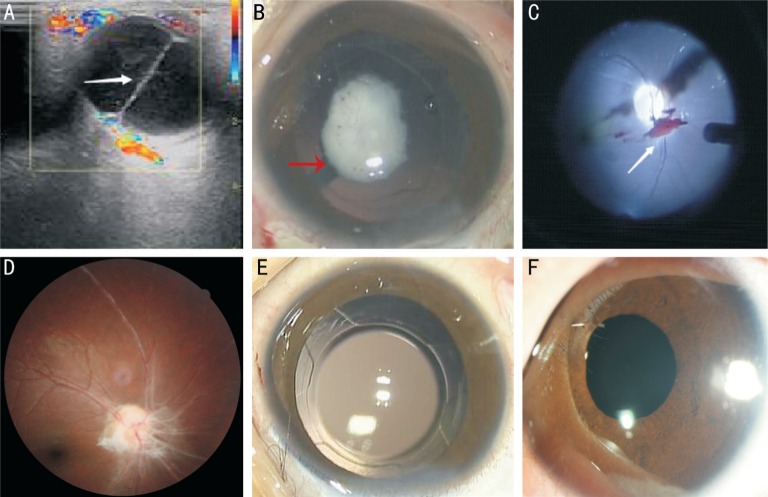
Figure 2. Imaging examination and fundus photographs of a 96-month-old child with congenital cataract and posterior PHPV in Case 10.
A: A persistent hyaloidal stalk that extends outward from the optic disk and anteriorly integrates with the posterior capsule is found in color Doppler ultrasound; B:The persistent hyaloidal stalk extends outward from the posterior segment of eye and anteriorly integrates into the posterior lens capsule under surgical microscope; C: The fetal vascular remnants above the optic nerve are being removed using a 25-gauge vitreous cutter system through a panretinal mirror; D: The remnant primary vitreous cord above the optic disc and a healthy fundus are observed; E: Because the tractional forces of the remnant primary vitreous cord avoid the optic disk and macular region, and there are no retinal tears, detachment, or obvious abnormalities observed during surgery, the posterior chamber IOL is implanted into the double capsulorhexis capsular bag; F: An anterior segment photograph of the slip-lamp after the IOL implantation, taken 90d after surgery.
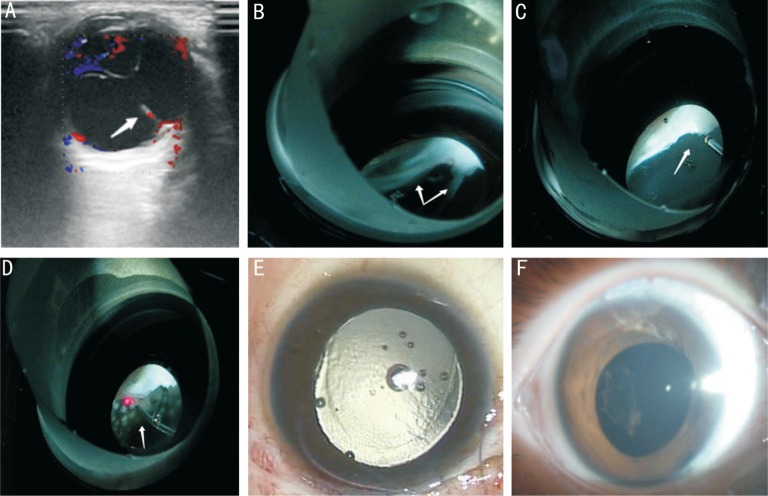
Figure 3. Imaging examination and fundus photographs of a 84-month-old child with congenital cataract and posterior PHPV in Case 24.
A: A persistent hyaloidal stalk that extends outward from the optic disk and integrats with the posterior capsule and elongates the ciliary processes is found via color Doppler ultrasound; B: The persistent hyaloidal stalk anteriorly integrates into the posterior lens capsule and elongates the ciliary processes, the abnormality reaches the peripheral retina, where retinal folds and retinal tears are created, and posteriorly integrates into the optic nerve vascular bundle, as viewed through a panretinal mirror; C: The retrolental fibrous membrane is being removed using a 25-gauge vitreous cutter system through a panretinal mirror; D: Retinal photocoagulation is being performed in cases of obvious and serious retinal breaks or folds through a panretinal mirror; E: Silicone oil is used for tamponade; F: An anterior segment photograph of the slip-lamp after silicon oil tamponade, taken 7d after surgery.
Selection of Surgical Procedures and Associated Complications
A total of 32 eyes (97%) from 29 subjects were surgically treated. Nine eyes (100%) from eight subjects with anterior PHPV underwent phacoemulsification with underwater electric coagulation (to treat neovascularization), posterior capsulotomy, anterior vitrectomy, and IOL implantation. Six eyes from five subjects (No.2, No.3, No.4, No.5 and No.7) underwent a single-stage operation and three eyes from three subjects (No.1, No.6 and No.8) received an IOL in a second procedure because the subject was younger than 3-year-old at the time of the initial procedure.
Very few intra- or post-operative complications were observed in patients treated for anterior PHPV. One 4-month-old subject with anterior PHPV (No.1) had repeated bleeding caused by neovascularization relapse in the retrolental fibrous membrane. One year after surgery, this subject unfortunately developed a retinal detachment caused by proliferative vitreoretinopathy. Therefore, a posterior vitrectomy with silicone oil tamponade was performed. It was difficult to keep the young patient in the recovery position required, and as a result band-shaped corneal degeneration developed after the vitrectomy procedure. Persistent anterior chamber bleeding also occurred in a 36-month old subject (No.2). This complication was resolved by IOL removal. The logMAR BCVA at the last follow-up visit was 0.30 (Snellen equivalent: 20/40).
A total of 23 eyes (95.8%) from 21 subjects with PHPV underwent phacoemulsification with posterior vitrectomy, retinal photocoagulation, and IOL implantation or silicone oil tamponade. Six eyes (26.1%) from six subjects (No.9-No.14) underwent retinal photocoagulation and IOL implant in a single procedure because no retinal abnormalities were identified. Seven eyes (30.4%) from five subjects (No.15-No.19) under 3 years of age underwent a second surgery to have an IOL implanted. Additionally, obvious peripheral retinal breaks and retinal detachment were found in four eyes (17.4%) from four subjects (No.20-No.23). These patients were treated with retinal photocoagulation and silicone oil tamponade. When retinal function had recovered (at least 3-6mo later), the silicone oil was removed and a posterior chamber IOL was implanted. Finally, six eyes (26.1%) from six subjects (No.24-No.29) did not receive an IOL during the observation period. Two of these eyes (two subjects, No.24 and No.25) permanently required silicone oil in the vitreous cavity because a closed, funnel-shaped retinal detachment developed. One month after surgery, these eyes had developed proliferative membranes in the pupil. Fortunately, IOP and the anterior segment were stable at subsequent follow-up visits. The other four patients (four eyes, No.26-No.29) were not implanted with an IOL because they were under 3 years of age during the entire observation period. These four subjects were given spectacles to correct visual acuity and underwent amblyopia training with patching. Surgical procedures and their associated complications and outcomes are summarized in Table 1.
Anatomical and Physiological Recovery of the Anterior Segment
No inflammation or other complications occurred after IOL implantation. All eyes had a transparent cornea, normal anterior chamber depth, normal pupillary light reaction, normal IOP, no aqueous flare, and a round pupil. Additionally, the IOL was in the proper location and no pigment was present on the IOL surface. Complete recovery of the anterior and posterior anatomic structures was achieved, with the exception of one eye that developed a band-shaped corneal degeneration and two eyes that developed a pupillary proliferative membrane. These representative cases are respectively shown in Figures 1, 2 and 3.
Postoperative Visual Acuity
The visual acuity of each individual patient pre- and post-operatively is included in Table 1. UCVA for the anterior and posterior PHPV groups after the operation (0.40±0.26) and best corrected vision at the final follow-up visit (0.53±0.20) was significantly better compared with uncorrected vision (0.09±0.04) before surgery (P=0.00).
Pattern Visual Evoked Potential
Examination of the P-VEP before and after surgery showed that the P100 latency in anterior PHPV was significantly shorter in response to both low (P<0.00) and high (P<0.00) frequency stimuli after surgery. The P100 amplitude in eyes with anterior PHPV also increased for both low (P=0.01) and high (P=0.04) frequency stimuli. In eyes with posterior PHPV, P100 latency (low P<0.00, high P<0.00) and P100 amplitude (low P<0.00, high P<0.00) were significantly better after surgery compared with before surgery. The results are summarized in Table 2.
Table 2. Latency and amplitude of P-VEP P100 waveform.
| Parameters | Low frequency |
High frequency |
||
| Latency (ms) | Amplitude (µV) | Latency (ms) | Amplitude (µV) | |
| Anterior PHPV (n=9 eyes) | ||||
| Preoperative | 128.00±7.47 | 13.44±4.86 | 132.78±8.09 | 13.44±4.95 |
| Postoperative | 105.48±13.79 | 19.74±3.62 | 108.59±13.27 | 20.19±7.46 |
| P | 0.00 | 0.01 | 0.00 | 0.04 |
| Posterior PHPV (n=23 eyes) | ||||
| Preoperative | 133.46±7.16 | 12.31±3.86 | 128.92±8.89 | 10.63±2.87 |
| Postoperative | 113.29±13.60 | 17.04±5.66 | 117.08±10.79 | 14.15±4.80 |
| P | 0.00 | 0.00 | 0.00 | 0.00 |
PHPV: Persistent hyperplastic primary vitreous. Low frequency stimuli had a spatial frequency of 60′, high frequency stimuli had a spatial frequency of 15′. All P values were calculated using paired Student's t-tests.
Amblyopia Treatment Outcomes
A total of 25 eyes underwent amblyopia treatment. Three eyes with silicone oil (No.1, No.24 and No.25) were excluded from these analyses, along with four aphakic eyes of children (No.26-No.29) who were not cooperative during visual acuity testing. After 1y or more of amblyopia treatment, BCVA improved in all 25 eyes included in the amblyopia treatment analyses. Both uncorrected (P=0.04) and corrected (P=0.03) visual acuity were significantly better after amblyopia therapy than before amblyopia treatment. Additionally, P100 latency was significantly shorter and P100 amplitude was significantly higher for both low- and high-frequency stimuli after therapy (all P<0.00). The results are summarized in Table 3.
Table 3. Effect of amblyopia treatment on visual acuity and P-VEP P100 waveform.
| Parameters | Before therapy | After therapy | P |
| P100 latency (ms) | |||
| High frequency | 128.38±8.30 | 112.93±11.14 | 0.00a |
| Low frequency | 131.66±7.26 | 112.05±12.77 | 0.00 |
| P100 amplitude (µV) | |||
| High frequency | 12.98±3.84 | 19.09±4.96 | 0.00a |
| Low frequency | 11.61±3.63 | 17.45±5.94 | 0.00 |
| UCVA (logMAR) | 0.46±0.62 | 0.35±0.68 | 0.04a |
| BCVA (logMAR) | 0.39±0.66 | 0.31±0.74 | 0.03a |
UCVA: Uncorrected visual acuity; BCVA: Best-corrected visual acuity; logMAR: Logarithm of the minimum angle of resolution. aA Mann-Whitney U test was used to determine statistical significance. Other P values were calculated using paired Student's t-tests.
n=25 eyes
DISCUSSION
We believe our study compares favorably with other contemporary studies in terms of its innovation, and interesting results: we performed surgery in eyes with the latest minimally invasive technology, and with aggressive amblyopia therapy, and achieved good visual results in a relatively “big” patient cohort.
It is generally known that PHPV is difficult to find and diagnose early because it is always hidden behind the leukocoria. Some relevant studies (e.g. color Doppler imaging, CT, and MRI)[1],[11]–[12] have reported that radiological examinations are important for its diagnosis, especially in patients in whom the posterior segment of the eye is not visible. Depending on which part of the primary vitreous remains (e.g. vitreous artery) and which part of the eye is affected (e.g. posterior capsule, ciliary body, or retina via vitreous traction), the condition is classified as either anterior or posterior PHPV. Each PHPV type is treated using different surgical procedures.
In our study, the outcome of the surgery on PHPV appears to depend on the extent and traction of the original vitreous artery involvement. For anterior PHPV, the presence and extension of a retrolental fibrovascular membrane, in particular neovascularization integrated into the lens, iris, and ciliary processes should be removed first. Thus, cauterizing neovascularization by underwater electric coagulation to prevent hemorrhaging is critical to the success of surgery in the initial treatment. Secondly, extension of the fibrous membrane integrated into the lens and ciliary body should be removed as much as possible. Thirdly, vitrectomy is performed on only part of the anterior vitreous and the posterior vitreous should not be interfered with if the visual axis vitreous is clear. Finally, an IOL should be implanted in the ring-shaped double capsular bag, in which both the anterior and posterior capsule are formed by continuous circular capsulorhexis to facilitate and maintain correct positioning of the IOL. Using these minimally traumatic surgical approaches, we have achieved a good result, and satisfactory visual rehabilitation. Our study's outcomes is in agreement with Pollard[13], Anteby et al[10], and Vasavada et al[14] who found that anterior PHPV is associated with a good visual outcomes when adequate treatment is provided.
Posterior PHPV is generally thought to result in poor visual outcomes, even in the absence of complications[15]–[16]. Sisk et al[2] insisted that conservative treatment of posterior PHPV was preferential. However, recent studies[17]–[18] indicate that early surgical intervention with vitrectomy is beneficial, resulting in improved intraocular anatomy, acceptable ocular cosmetic appearance, and visual prognosis (with vision rehabilitation). Our study showed that approximately 50% of patients had good visual acuity [0.30-1.00 (20/40 to 20/200)] following surgical treatment in agreement with Morrison et al[19], however, our outcomes were dependent upon the severity of traction between the proliferative vitreous and the retina. When tractional forces were created by the primary vitreous cord being pulled away from the macular region and optic disc, the performance of a combined vitrectomy and lensectomy procedure resulted in a healthy fundus and good vision implementation in six eyes (six patients) after fundus retinal photocoagulation surgery. Another 11 aphakic eyes (nine patients) also had improved vision when an IOL was implanted in a second procedure. LogMAR BCVA improved to 0.00 (20/20) in all patients following IOL implantation. In contrast, if the primary vitreous cord was causing traction between the macular region and the optic disc, patients underwent a combined vitrectomy and lensectomy procedure, resulting in the rehabilitation of the posterior segment, but with poor visual outcomes after surgery.
Although visual acuity measurement remains the preferred assessment for postoperative evaluation when patients are cooperative, visual acuity measurements in very young children can vary with patient mood and other factors. This inaccuracy is especially evident in infants and young children who cannot correctly identify vision chart items and have a limited cognitive ability. However, the VEP can be used as a more objective method for evaluating visual function in infants. In 1976, many scholars, including Banks[20] used the P-VEP as a measure of visual function. The test is still used and is currently the only non-invasive, objective, method for evaluating neural function in the pathway between retinal ganglion cells and the visual cortex[21]. Latency of the P100 waveform reflects photochemical signal conduction velocity from central retinal (20°) inner ganglion cell axonal fibers to the visual cortex. The P100 amplitude is also an important indicator of macular and visual pathway status[22]. Therefore, our patients underwent electrophysiological testing both before and after surgery to objectively observe the effects of surgery on vision. Baby vision and scanning VEP based on visual tracking are the two most effective methods of vision testing when using the visual acuity chart is not an option, especially in children less than 3 years of age. Our baby vision testing and P100 latency and amplitude analyses showed significant improvements in vision, P100 latency, and P100 amplitude following surgery (all P<0.05).
Positive visual results following surgery in our study are attributed to not only the choice of surgical treatment but also refractive correction, and permanent and intensive amblyopia therapy. In particular, parental motivation, patient interest and compliance, aggressive amblyopia management, and frequent hospital visits over the long-term also play a significant role in the excellent visual outcomes. Many studies have shown that amblyopia therapy should be recommended and obviously improve visual acuity following PHPV removal[6],[10]. In agreement, our study has achieved excellent visual outcome that 25 of 25 eyes (100%) improve in visual function after at least 1y of amblyopia therapy. BCVA and UCVA significantly improved, P100 latency significantly decreased, and P100 amplitude significantly increased.
The limitations of this study include the concern that vision measurement is not accurate because the age of some children is too small. Secondly, we do not measure endothelial cell density again postoperatively, and do not revisit the changes in refractive status and myopic shift after implantation of the lens accompanying the growth of axial length. In a future study, we should perform longer follow-ups to observe both vision and complications.
In summary, although PHPV is always hidden behind leukocoria and other congenital abnormalities in infants, once diagnosis has been established, satisfactory visual rehabilitation can be achieved when PHPV eyes are managed surgically through a personalized surgical procedure. Other successful visual outcomes after surgery have been associated with early surgical intervention, implantation of an IOL for optical correction, and aggressive antiamblyopic therapy. Therefore, when surgery of a PHPV eye is considered, an individualized early surgical intervention treatment plan and an intensive management approach (implantation of an IOL and aggressive antiamblyopic therapy) can result in positive outcomes.
Acknowledgments
Author Contributions: Li L: conception and design, analysis and interpretation of data, and drafting the article; Fan DB: table drawing and language modification; Zhao YT: clinical data collection and patient visits; Li Y: clinical data collection and patient visits; Cai FF: picture collection; Zheng GY: manuscript edit and revision.
Foundation: Supported by the Science and Technology Research Projects of Henan Province, China (No.201202010).
Conflicts of Interest: Li L, None; Fan DB, None; Zhao YT, None; Li Y, None; Cai FF, None; Zheng GY, None.
REFERENCES
- 1.Jain TP. Bilateral persistent hyperplastic primary vitreous. Indian J Ophthalmol. 2009;57(1):53–54. doi: 10.4103/0301-4738.44487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sisk RA, Berrocal AM, Feuer WJ, Murray TG. Visual and anatomic outcomes with or without surgery in persistent fetal vasculature. Ophthalmology. 2010;117(11):2178–2183.e1-2. doi: 10.1016/j.ophtha.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 3.Makino S, Ohkubo Y, Tampo H. Prepapillary vascular loop associated with persistent hyperplastic primary vitreous. Case Rep Ophthalmol Med. 2013;2013:259797. doi: 10.1155/2013/259797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khaliq S, Hameed A, Ismail M, Anwar K, Leroy B, Payne AM, Bhattacharya SS, Mehdi SQ. Locus for autosomal recessive nonsyndromic persistent hyperplastic primary vitreous. Invest Ophthalmol Vis Sci. 2001;42(10):2225–2228. [PubMed] [Google Scholar]
- 5.Nie WY, Wu HR, Qi YS, Zhang M, Hou Q, Yang HX, Gong LX, Dong YR, Guo YL, Shi JN, Yin SY, Li PY. A pilot study of ocular diseases screening for neonates in China. Zhonghua Yan Ke Za Zhi. 2008;44(6):497–502. [PubMed] [Google Scholar]
- 6.Khokhar S, Tejwani LK, Kumar G, Kushmesh R. Approach to cataract with persistent hyperplastic primary vitreous. J Cataract Refract Surg. 2011;37(8):1382–1385. doi: 10.1016/j.jcrs.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Fei P, Zhang Q, Li J, Zhao P. Clinical characteristics and treatment of 22 eyes of morning glory syndrome associated with persistent hyperplastic primary vitreous. Br J Ophthalmol. 2013;97(10):1262–1267. doi: 10.1136/bjophthalmol-2013-303565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard ZF. Results of treatment of persistent hyperplastic primary vitreous. Ophthalmic Surg. 1991;22(1):48–52. [PubMed] [Google Scholar]
- 9.Schulz E, Griffiths B. Long-term visual function and relative amblyopia in posterior persistent hyperplastic primary vitreous (PHPV) Strabismus. 2006;14(2):121–125. doi: 10.1080/09273970600701242. [DOI] [PubMed] [Google Scholar]
- 10.Anteby I, Cohen E, Karshai I, BenEzra D. Unilateral persistent hyperplastic primary vitreous: course and outcome. J AAPOS. 2002;6(2):92–99. doi: 10.1067/mpa.2002.121324. [DOI] [PubMed] [Google Scholar]
- 11.Sanghvi DA, Sanghvi CA, Purandare NC. Bilateral persistent hyperplastic primary vitreous. Australas Radiol. 2005;49(1):72–74. doi: 10.1111/j.1440-1673.2005.01402.x. [DOI] [PubMed] [Google Scholar]
- 12.Hu A, Yuan M, Liu F, Yang Y, Ding X, Pei X. Ultrasonographic feature of persistent hyperplastic primary vitreous. Eye Sci. 2014;29(2):100–103. [PubMed] [Google Scholar]
- 13.Pollard ZF. Persistent hyperplastic primary vitreous: diagnosis, treatment and results. Trans Am Ophthalmol Soc. 1997;95:487–549. [PMC free article] [PubMed] [Google Scholar]
- 14.Vasavada AR, Vasavada SA, Bobrova N, Praveen MR, Shah SK, Vasavada VA, Pardo A JV, Raj SM, Trivedi RH. Outcomes of pediatric cataract surgery in anterior persistent fetal vasculature. J Cataract Refract Surg. 2012;38(5):849–857. doi: 10.1016/j.jcrs.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Pollard ZF. Treatment of persistent hyperplastic primary vitreous. J Ophthalmic Nurs Technol. 1991;10(4):155–159. [PubMed] [Google Scholar]
- 16.Hunt A, Rowe N, Lam A, Martin F. Outcomes in persistent hyperplastic primary vitreous. Br J Ophthalmol. 2005;89(7):859–863. doi: 10.1136/bjo.2004.053595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrakis G, Scott IU, Flynn HW, Jr, Murray TG, Feuer WJ. Visual acuity outcomes with and without surgery in patients with persistent fetal vasculature. Ophthalmology. 2000;107(6):1068–1072. doi: 10.1016/s0161-6420(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo T. Intraocular lens implantation in unilateral congenital cataract with minimal levels of persistent fetal vasculature in the first 18 months of life. Springerplus. 2014;3:361. doi: 10.1186/2193-1801-3-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison DG, Wilson ME, Trivedi RH, Lambert SR, Lynn MJ, Infant Aphakia Treatment Study Group Infant Aphakia Treatment Study: effects of persistent fetal vasculature on outcome at 1 year of age. J AAPOS. 2011;15(5):427–431. doi: 10.1016/j.jaapos.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banks MS. Visual acuity development in human infants: a re-evaluation. Invest Ophthalmol Vis Sci. 1977;16(2):191–193. [PubMed] [Google Scholar]
- 21.McBain VA, Robson AG, Hogg CR, Holder GE. Assessment of patients with suspected non-organic visual loss using pattern appearance visual evoked potentials. Graefes Arch Clin Exp Ophthalmol. 2007;245(4):502–510. doi: 10.1007/s00417-006-0431-2. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZM, Yin ZQ. How to use of visual evoked potential testing technology reasonable. Zhonghua Yan Ke Za Zhi. 2013;49(12):1061–1063. [PubMed] [Google Scholar]