Abstract
AIM
To evaluate the efficacy and safety of intravitreal ranibizumab (IVR) with panretinal photocoagulation (PRP) followed by trabeculectomy compared with Ahmed glaucoma valve (AGV) implantation in neovascular glaucoma (NVG).
METHODS
This was a retrospective comparative study. We reviewed the cases of a total of 45 eyes from 45 NVG patients among which 23 eyes underwent AGV implantation and the other 22 underwent trabeculectomy. The causes of neovascular glaucoma included: diabetic retinopathy (25 eyes), and retinal vein occlusion (20 eyes). All patients received preoperative IVR combined with postoperative PRP. The mean best-corrected visual acuities (BCVA) were converted to the logarithms of the minimum angle of resolution (logMAR) for the statisitical analyses. Intraocular pressure (IOP), the logMAR BCVA and surgical complications were evaluated before and after surgery. The follow-up period was 12mo.
RESULTS
A total of 39 cases showed complete regression of iris neovascularization at 7d after injection, and 6 cases showed a small amount of residual iris neovascularization. The success rates were 81.8% and 82.6% at 12mo after trabeculectomy and AGV implantation, respectively. In the trabeculectomy group, the logMAR BCVA improved at the last follow-up in 14 eyes, remained stable in 6 eyes and decreased in 2 eyes. In 4 cases, slight hyphemas developed after trabeculectomy. A shallow anterior chamber developed in 2 cases and 2 vitreous hemorrhages. In the AGV group, the logMAR BCVA improved in 14 eyes, remained stable in 5 eyes and decreased in 4 eyes. Slight hyphemas developed in 3 cases, and a shallow anterior chamber in 3 cases. The mean postoperative IOP was significantly lower in both groups after surgery (F=545.468, P<0.05), and the mean postoperative logMAR BCVA was also significantly improved (F=10.964, P<0.05) with no significant difference between two groups.
CONCLUSION
It is safe and effective to treat NVG with this combined procedure, and we found similar results after IVR+AGV implantation+PRP and IVR+trabeculectomy+PRP in eyes with NVG.
Keywords: neovascular glaucoma, trabeculectomy, Ahmed drainage valve implantation, ranibizumab, panretinal photocoagulation
INTRODUCTION
Neovascular glaucoma (NVG) is a type of refractory glaucoma characterized by neovascularization of the iris and anterior chamber angle. Contraction of this neovascular tissue results in progressive angle closure and increased intraocular pressure (IOP). It is difficult to control elevated IOP with medications, and the visual prognosis is very poor[1]. NVG is closely related to retinal ischemic diseases such as proliferative diabetic retinopathy (PDR) and ischemic retinal vein occlusion[2]. Conventional trabeculectomy and drainage valve implantation show a very high failure rate, as the procedure cannot fundamentally solve the problem of retinal ischemia and hypoxia. Furthermore, ciliary body photocoagulation, condensation and other destructive procedures often cause intractable low IOP, which can result in eye atrophy. Lucentis (ranibizumab) is a vascular endothelial growth factor (VEGF) inhibitor that can effectively inhibit neovascularization of the iris and anterior chamber angle, reduce complications of filtration surgery and facilitate long-term control of IOP. A large number of studies have confirmed that panretinal photocoagulation (PRP) can significantly improve the ischemic condition of the retina and promote the regression of neovascularization[3]–[5]. We have achieved a good therapeutic effect with two operation strategies to treat NVG. Here, we provide evidence for the clinically reasonable choice of surgical approach by retrospectively analyzing and comparing the efficacy and safety of these two surgical methods.
SUBJECTS AND METHODS
In this retrospective comparative study, we reviewed the cases of 45 NVG patients from the Affiliated Hospital of Qingdao University between May 2014 and July 2015. The patients (23 males, 22 females) were aged from 43-70y, with a mean age of 52.42±12.78y. The logMAR BCVA was 1.32±0.49, and the mean IOP was 44.76±10.32 mm Hg. Primary diseases included PDR in 25 eyes and retinal vein occlusion in 20 eyes. Ocular examination included visual acuity assessment, IOP measurement, slit-lamp examination, gonioscopy, ultrasound biomicroscopy (UBM), ocular ultrasonography and indirect ophthalmoscopy. The inclusion criteria were as follows: 1) no primary glaucoma history; 2) IOP>30 mm Hg with the maximum use of more than two types of anti-glaucoma medications; 3) neovascularization of the iris surface and/or an anterior chamber angle closed more than halfway with goniosynechia; 4) no apparent opacity of the refractive medium and/or retinal detachment; 5) good condition of the body overall and no surgical contraindications. The exclusion criteria were as follows: 1) patients with other serious uncontrolled diseases who could not tolerate surgery; 2) patients with other types of glaucoma or other serious eye diseases; 3) patients who were unwilling or unable to return for follow-up. In total, 23 patients underwent intravitreal ranibizumab (IVR)+Ahmed glaucoma valve (AGV)+PRP, while the other 22 patients underwent IVR+trabeculectomy+PRP. The IVR was administered 1wk before the trabeculectomy or AGV implantation. We tried to perform the PRP postoperatively when possible. The study was approved by the Ethics Committee of the Review Board of the Affiliated Hospital of Qingdao University. All patients signed an informed consent form before participation in this study, and all study procedures adhered to the Declaration of Helsinki.
Preoperative information (before IVR administration) included gender, age, history of laser and surgical treatments, glaucoma diagnosis, lens status, anti-glaucoma medications, IOP and the logMAR BCVA. Postoperative data included IOP; the logMAR BCVA; and the incidence of complications at months 1, 2, 3, 6 and 12. There was no significant difference in age, sex, preoperative visual acuity, IOP or the primary cause of disease between the two groups (P>0.05).
Success was categorized as either complete or qualified. Complete success was defined by the following criterion: postoperative IOP between 6 and 21 mm Hg without anti-glaucoma medications and without further anti-glaucoma surgery. The qualified success criterion was IOP between 6 and 21 mm Hg with one or more medications. Failure was defined as IOP>21 mm Hg despite the use of maximum medications. If a patient maintained stable IOP after an additional surgery because of a complication, this was not considered failure of the procedure; however, the additional surgery could not be an anti-glaucoma surgery. The total success rate was calculated as the sum of the complete success rate and the qualified success rate. Potential postoperative complications included hyphema, an early postoperative shallow anterior chamber, choroidal detachment, AGV exposure, persistent hypotony, bleb leak, vitreous hemorrhage and corneal loss of compensation.
Surgical Techniques
All of the surgical procedures were carried out by Dr. Da-Bo Wang.
For intravitreal ranibizumab
The patients were given levofloxacin eye drops for 3d before the injection. After topical anesthesia and disinfection with iodine in the operating room, ranibizumab (Lucentis, 10 mg/mL; Novartis, Basel, Switzerland) at a dose of 0.5 mg/0.05 mL was injected into the vitreous cavity via the pars plana (4 mm away from the corneal limbus) using a tuberculin syringe with a 30-G needle. The puncture site was then pressed with a cotton swab for 1min. IOP and light perception were examined after the injection. The patients subsequently received an anti-glaucoma medication for 7d, as intravitreal injections may be associated with a risk of further IOP elevation.
For trabeculectomy
Seven days after the IVR administration, when the neovascularization of the iris had regressed significantly and the anterior chamber angle had improved, we created a limbus-based conjunctival flap for trabeculectomy. After creation of a 3×4 mm half-thickness scleral flap, small pieces of surgical sponge soaked in 0.02% mitomycin C (MMC) were inserted under the conjunctival flap for 5min. The eye was then rinsed thoroughly with 200 mL saline. Afterward, we punctured the anterior chamber and slowly released a small amount of aqueous humor to reduce the IOP, and then we partly resected the trabecular tissue and peripheral iris. The scleral and conjunctival flaps were subsequently sutured with 10-0 nylon sutures. Postoperatively, we massaged the eyeball routinely. Topical antibiotic and steroid eye drops were used for 2wk.
For Ahmed glaucoma valve implantation
AGV implantation was conducted 7d after IVR injection. After anesthesia, a fornix-based conjunctival Tenon's flap was created in the superotemporal quadrant. A piece of cotton containing 0.02% MMC was placed 9-12 mm posterior to the limbus, between the conjunctival flap and sclera, for 3-5min and was then washed thoroughly with balanced salt solution (BSS). The tube of the valve was also irrigated with BSS to open the valve mechanism. The anterior edge of the plate was fixed with 8-0 sutures on the sclera at 8-9 mm from the limbus. The tube tip was also cut obliquely to protect the tube lumen from the iris. A half-thickness limbus-based closed scleral flap was then created. A 23-G needle tract was used to enter the anterior chamber under the scleral flap, after which the AGV tube was placed into the anterior chamber, parallel to the iris plane, through the needle tract. The tube in the anterior chamber was positioned anterior to the iris and away from the corneal endothelium. Finally, the conjunctiva and Tenon's capsule were sutured with 8-0 absorption sutures. The anterior chamber was reformed with BSS through a paracentesis tract. Topical antibiotic and steroid eye drops were used for 2wk.
For panretinal photocoagulation
Seven days after surgery, PRP was applied in 2-3 sessions. The settings were as follows: pulse duration: 200-300ms; spot size: 200-500 µm; power: 200-400 mW; shots: 800-1000 points each time; and interval: 7d. We aim to further perfect the method for retinal photocoagulation in accordance with follow-up and to strive to achieve full retinal coverage.
Statistical Analysis
Statistical analysis was performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Normally distributed data are shown as the mean±standard deviation (SD). Comparisons of preoperative and follow-up data, including IOP and logMAR BCVA data, were performed using repeated-measures analysis of variance (ANOVA). The long-term efficacy was calculated using covariance analysis. P<0.05 was considered statistically significant.
RESULTS
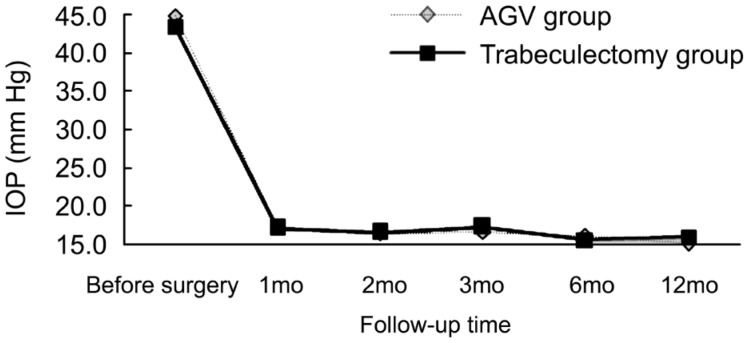
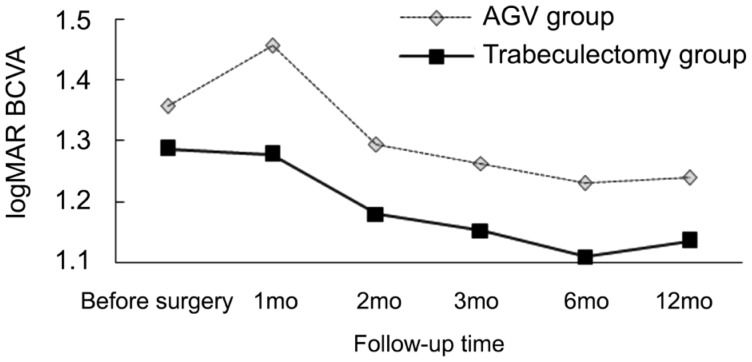
The total success rate in the AGV group (82.6%) was better than that in the trabeculectomy group (81.8%), but the difference was not statistically significant. The surgical success rates in both groups at 1y are presented in Table 1. In the AGV group, visual acuity improved in 14 cases, remained unchanged in 5 cases and decreased in 4 cases. Additionally, 4 cases showed poor IOP control, and 4 cases achieved IOP control with one medication; 2 cases, with two medications; and 1 case, with three medications. In the trabeculectomy group, visual acuity improved in 14 cases, remained unchanged in 6 cases and decreased in 2 cases. Four cases showed poor IOP control, and 3 cases achieved IOP control with one medication; 3 cases, with two medications; and 2 cases, with three medications. Postoperative IOP was significantly decreased in both groups (F=545.468, P<0.05) and was relatively stable throughout long-term follow-up. The outcomes are presented in Table 2. There was no significant difference in postoperative IOP control between the two groups (F=0.726, P>0.05). There was also no interaction between time and the surgical procedure (F=0.002, P>0.05) (Figure 1). The mean logMAR BCVA values before and after surgery in both groups are shown in Table 3. The mean postoperative logMAR BCVA values were improved in both groups, but there was no significant difference between the groups (F=10.964, P<0.05). There was no significant difference in postoperative logMAR BCVA values between the two groups (F=0.543, P=0.634). There was also no interaction between time and the surgical procedure (F=0.601, P>0.05) (Figure 2).
Table 1. The surgical success rates in both groups at 1y.
| Surgical success rate | AGV (n=23) | Trabeculectomy (n=22) |
| Complete success | 12 (52.2) | 10 (45.5) |
| Qualified success | 7 (30.4) | 8 (36.4) |
| Failure | 4 (17.4) | 4 (18.2) |
n (%)
Table 2. Mean IOP in both groups at follow-up time intervals.
| Duration | AGV | Trabeculectomy |
| Preoperative | 44.89±4.67 (28.5-57.6) | 43.45±3.9 (30.2-58.4) |
| 1mo | 17.23±5.13 (6.4-32.8) | 17.15±6.14 (7.2-33.4) |
| 2mo | 16.49±4.54 (7.8-37.6) | 16.61±6.3 (8.5-38.2) |
| 3mo | 16.64±5.2 (9.5-39.4) | 17.31±5.94 (8.4-47.6) |
| 6mo | 16.05±4.25 (10.5-46.6) | 15.59±4.54 (9.5-44.2) |
| 12mo | 15.09±4.94 (9.2-44.2) | 15.94±5.49 (8.8-46.6) |
x±s, mm Hg
Figure 1. Distribution of IOP measurements at follow-up visits.
Table 3. Mean logMAR BCVA in both groups at follow-up time intervals.
| Groups | Preoperative | 1mo | 2mo | 3mo | 6mo | 12mo |
| AGV | 1.36±0.55 | 1.46±0.52 | 1.29±0.54 | 1.26±0.56 | 1.23±0.62 | 1.24±0.61 |
| Trabeculectomy | 1.29±0.45 | 1.28±0.47 | 1.18±0.49 | 1.15±0.46 | 1.11±0.51 | 1.14±0.48 |
x±s
Figure 2. Distribution of logMAR BCVA measurements at follow-up visits.
After 1y of follow-up, we found no significant difference in efficacy between the two groups (F=0.35, P>0.05). A total of 39 patients demonstrated complete regression of iris neovascularization at 7d after IVR administration, and the remaining 7 patients displayed a small amount of residual iris neovascularization. After 1y of follow-up, certain patients experienced partial recurrence of new blood vessel formation. Complications in the trabeculectomy group included non-functional filtering blebs in 5 patients, 4 cases of slight hyphemas after surgery and a shallow anterior chamber in 2 cases in the early postoperative period. Vitreous hemorrhage also occurred in 2 cases between 3 and 6mo after surgery. We achieved good IOP control in these cases by means of vitrectomy. Complications in the AGV group included 3 cases of slight hyphemas after surgery and a shallow anterior chamber in 3 cases in the early postoperative period. Additionally, fiber-wrapped drainage disks occurred in 3 cases. There were no complications such as obstruction or exposure of the drainage valve.
DISCUSSION
NVG is a refractory glaucoma that has long been recognized as very difficult to manage. Patients often experience intolerable pain and loss of vision unless they receive timely and effective treatment. Currently, the consensus view is that any treatment must include treatment of the primary disease, improvement of retinal ischemia and effective control of IOP[6]. Previous treatments, such as cyclocryotherapy and cyclophotocoagulation, have led to loss of vision and even atrophy of the eyeball. Conventional trabeculectomy alone has a low success rate, probably due to postoperative recurrence of neovascularization, scarring and fibrosis of the filtering channel[7]–[8]. Shen et al[9] compared the effect of trabeculectomy and AGV implantation for the treatment of NVG in a retrospective study and found long-term success rates at 1y of 65% and 70%, respectively. The authors stated that the reason for the low success rate might have been that the primary factor inducing neovascularization was not removed. Additionally, because of a large amount of neovascularization in the iris and the angle, postoperative complications such as hyphema are the major risk factors for primary treatment failure[10]. In recent years, a large number of studies have confirmed that VEGF gene overexpression induced by retinal ischemia and hypoxia results in higher VEGF content in the aqueous humor and vitreous humor, eventually leading to the formation of new vessels. When neovascular fibrous tissues occupy the trabecular meshwork, aqueous outflow is inhibited, and IOP rises. This elevated IOP leads to further retinal ischemia, which in turn induces more neovascularization, eventually causing irreversible damage to visual function[11]. To date, anti-VEGF drugs have been widely used in the treatment of various neovascular diseases, including NVG, with satisfactory clinical effects[12]–[13]. As a next-generation anti-VEGF drug, ranibizumab is a fully humanized murine monoclonal antibody fragment that can provide stronger binding to and inhibition of a number of isoforms of VEGF-A. Indeed, studies have confirmed that ranibizumab is more effective than bevacizumab[14]. Ranibizumab can remarkably suppress neovascularization and avoid hemorrhagic complications[15], and this drug can also create better conditions for further surgery and improve surgical success rates[16]–[18]. However, studies have reported that although anti-VEGF agents can cause regression of neovascularization in the iris, the effect dissipates over time[19]. Kobayashi et al[20] specifically found that preoperative IVR injection could increase the surgical success rate of trabeculectomy but that multiple injections were needed for a long-term effect. This need for repeated treatment undoubtedly places a huge economic burden on patients. According to previous studies[3]–[5], PRP can effectively improve retinal ischemic conditions and reduce the release of VEGF, thus preventing the development of NVG. PRP also facilitates control of IOP and improves the long-term effect of surgery[21]. All of our patients received IVR injection before the subsequent surgery; according to this design, we achieved a better clinical result than in previous studies[22]–[23]. Our treatment success rate specifically reached 81.8% in the trabeculectomy group and 82.6% in the AGV group.
Saito et al[24] reported an incidence rate of hyphema after trabeculectomy of 34.3%-63.0%, while Lai et al[25] reported that the incidence rate of fiber-wrapped drainage disks was 24.6% in Chinese patients. In recent years, certain studies[26] have confirmed that preoperative IVR injection can effectively reduce hyphema. In our study, we observed that neovascularization of the iris and anterior chamber angle appeared to regress in 39 eyes 7d after IVR injection, while only 6 cases demonstrated a small amount of bulky new vessels. Postoperative complications such as non-functional filtering blebs, fiber-wrapped drainage disks, slight hyphema and a shallow anterior chamber were significantly decreased, which we believe may have been due to the preoperative IVR injection and postoperative PRP.
Postoperative BCVA was improved to varying degrees in 28 patients. We believe that one reason for this result was that the surgeries reduced IOP and restored corneal transparency. Another possible reason is that retinal and macular edema was reduced after the preoperative IVR injection and postoperative PRP. We also found that the postoperative BCVA and IOP values were stable in both groups during follow-up, with no significant difference between groups. Therefore, we have reason to believe that there are no significant differences in the safety and efficacy of these two types of surgery under the premise of combined surgery.
In summary, IVR injection combined with trabeculectomy or AGV implantation for the treatment of NVG is safe and effective. The preoperative application of ranibizumab can significantly reduce postoperative complications, and surgery combined with timely and adequate PRP can effectively inhibit the recurrence of neovascularization, improve retinal ischemia and hypoxia and increase the success rate of anti-glaucoma surgery. In the current study, with skilled operative technique, there was no significant difference in postoperative visual acuity, IOP control or the success rate between these two surgical approaches. However, compared with AGV implantation, trabeculectomy is more affordable and should be used as the first clinical option. Due to the limited follow-up period of our study, however, the long-term safety and effectiveness of these methods require further observation.
Acknowledgments
Conflicts of Interest: Sun JT, None; Liang HJ, None; An M, None; Wang DB, None.
REFERENCES
- 1.Lüke J, Nassar K, Lüke M, Grisanti S. Ranibizumab as adjuvant in the treatment of rubeosis iridis and neovascular glaucoma-results from a prospective interventional case series. Graefes Arch Clin Exp Ophthalmol. 2013;251(10):2403–2413. doi: 10.1007/s00417-013-2428-y. [DOI] [PubMed] [Google Scholar]
- 2.Yildirim N, Yalvac IS, Sahin A, Ozer A, Bozca T. A comparative study between diode laser cyclophotocoagulation and the Ahmed glaucoma valve implant in neovascular glaucoma: a long-term follow-up. J Glaucoma. 2009;18(3):192–196. doi: 10.1097/IJG.0b013e31817d235c. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Society of Ophthalmic Diabetology, Subcommittee on the Study of Diabetic Retinopathy Treatment. Sato Y, Kojimahara N, Kitano S, Kato S, Ando N, Yamaguchi N, Hori S. Multicenter randomized clinical trial of retinal photocoagulation for preproliferative diabetic retinopathy. Jpn J Ophthalmol. 2012;56(1):52–59. doi: 10.1007/s10384-011-0095-2. [DOI] [PubMed] [Google Scholar]
- 4.Alkawas AA, Shahien EA, Hussein AM. Management of neovascular glaucoma with panretinal photocoagulation, intravitreal bevacizumab, and subsequent trabeculectomy with mitomycin C. J Glaucoma. 2010;19(9):622–626. doi: 10.1097/IJG.0b013e3181ccb794. [DOI] [PubMed] [Google Scholar]
- 5.Tatsumi T, Yamamoto S, Uehara J, Sugawara T, Baba T, Inoue M, Hata H, Mitamura Y. Panretinal photocoagulation with simultaneous cryoretinopexy or intravitreal bevacizumab for neovascular glaucoma. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1355–1360. doi: 10.1007/s00417-012-2236-9. [DOI] [PubMed] [Google Scholar]
- 6.Sivak-Callcott JA, O'Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108(10) doi: 10.1016/s0161-6420(01)00775-8. [DOI] [PubMed] [Google Scholar]
- 7.SooHoo JR, Seibold LK, Kahook MY. Recent advances in the management of neovascular glaucoma. Semin Ophthalmol. 2013;28(3):165–172. doi: 10.3109/08820538.2012.730103. [DOI] [PubMed] [Google Scholar]
- 8.Takihara Y, Inatani M, Fukushima M, Iwao K, Iwao M, Tanihara H. Trabeculectomy with mitomycin C for neovascular glaucoma: prognostic factors for surgical failure. Am J Ophthalmol. 2009;147(5) doi: 10.1016/j.ajo.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Shen CC, Salim S, Du H, Netland PA. Trabeculectomy versus Ahmed glaucoma valve implantation in neovascular glaucoma. Clin Ophthalmol. 2011;5:281–286. doi: 10.2147/OPTH.S16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakatake S, Yoshida S, Nakao S, Arita R, Yasuda M, Kita T, Enaida H, Ohshima Y, Ishibashi T. Hyphema is a risk factor for failure of trabeculectomy in neovascular glaucoma: a retrospective analysis. BMC Ophthalmol. 2014;14:55. doi: 10.1186/1471-2415-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010;21(2):112–117. doi: 10.1097/ICU.0b013e3283360aad. [DOI] [PubMed] [Google Scholar]
- 12.Writing Committee for the UK Age-Related Macular Degeneration EMR User group The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121(5):1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Waisbourd M, Shemesh G, Kurtz S, Rachmiel R, Moisseiev E, Zayit-Soudri S, Loewenstein A, Barequet I. Topical bevacizumab for neovascular glaucoma: a pilot study. Pharmacology. 2014;93(3-4):108–112. doi: 10.1159/000358600. [DOI] [PubMed] [Google Scholar]
- 14.Klettner A, Roider J. Comparison of bevacizumab, ranibizumab, and pegaptanib in vitro: efficiency and possible additional pathways. Invest Ophthalmol Vis Sci. 2008;49(10):4523–4527. doi: 10.1167/iovs.08-2055. [DOI] [PubMed] [Google Scholar]
- 15.Kang JY, Nam KY, Lee SJ, Lee SU. The effect of intravitreal bevacizumab injection before Ahmed valve implantation in patients with neovascular glaucoma. Int Ophthalmol. 2014;34(4):793–799. doi: 10.1007/s10792-013-9875-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhou MW, Wang W, Huang WB, Chen SD, Li XY, Gao XB, Zhang XL. Adjunctive with versus without intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in the treatment of neovascular glaucoma. Chin Med J (Engl) 2013;126(8):1412–1417. [PubMed] [Google Scholar]
- 17.Zhang HT, Yang YX, Xu YY, Yang RM, Wang BJ, Hu JX. Intravitreal bevacizumab and Ahmed glaucoma valve implantation in patients with neovascular glaucoma. Int J Ophthalmol. 2014;7(5):837–842. doi: 10.3980/j.issn.2222-3959.2014.05.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevim MS, Buttanri IB, Kugu S, Serin D, Sevim S. Effect of intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in neovascular glaucoma. Ophthalmologica. 2013;229(2):94–100. doi: 10.1159/000345490. [DOI] [PubMed] [Google Scholar]
- 19.Park UC, Shin JY, McCarthy LC, Kim SJ, Park JH, Chung H, Yu HG. Pharmacogenetic associations with long-term response to anti-vascular endothelial growth factor treatment in neovascular AMD patients. Mol Vis. 2014;20:1680–1694. [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi S, Inoue M, Yamane S, Sakamaki K, Arakawa A, Kadonosono K. Long-term outcomes after preoperative intravitreal injection of bevacizumab before trabeculectomy for neovascular glaucoma. J Glaucoma. 2016;25(3):281–284. doi: 10.1097/IJG.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 21.Kiuchi Y, Nakae K, Saito Y, Ito S, Ito N. Pars plana vitrectomy and panretinal photocoagulation combined with trabeculectomy for successful treatment of neovascular glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244(12):1627–1632. doi: 10.1007/s00417-006-0321-7. [DOI] [PubMed] [Google Scholar]
- 22.Canut MI, Alvarez A, Nadal J, Abreu R, Abreu JA, Pulido JS. Anterior segment changes following intravitreal bevacizumab injection for treatment of neovascular glaucoma. Clin Ophthalmol. 2011;5:715–719. doi: 10.2147/OPTH.S17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miki A, Oshima Y, Otori Y, Matsushita K, Nishida K. One-year results of intravitreal bevacizumab as an adjunct to trabeculectomy for neovascular glaucoma in eyes with previous vitrectomy. Eye (Lond) 2011;25(5):658–659. doi: 10.1038/eye.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito Y, Higashide T, Takeda H, Ohkubo S, Sugiyama K. Beneficial effects of preperative intravitreal bevacizumab on trabeculectomy outcomes in neovascular glaucoma. Acta Ophthalmol. 2010;88(1):96–102. doi: 10.1111/j.1755-3768.2009.01648.x. [DOI] [PubMed] [Google Scholar]
- 25.Lai JS, Poon AS, Chua JK, Tham CC, Leung AT, Lam DS. Efficacy and safety of the Ahmed glaucoma valve implant in Chinese eyes with complicated glaucoma. Br J Ophthalmol. 2000;84(7):718–721. doi: 10.1136/bjo.84.7.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang HB, Han JW, Yim HB, Lee NY. Beneficial effects of adjuvant intravitreal bevacizumab injection on outcomes of Ahmed glaucoma valve implantation in patients with neovascular glaucoma: systematic literature review. J Ocul Pharmacol Ther. 2015;31(4):198–203. doi: 10.1089/jop.2014.0108. [DOI] [PubMed] [Google Scholar]