Abstract
AIM
To report the epidemiology and incidence of vitreous hemorrhage and to evaluate risk factors for patients with vitreous hemorrhage (VH) in Taiwan.
METHODS
A retrospective population-based study. Analyzing a sample of one million subjects from all enrollees of the Taiwan Health Insurance programme. All data were obtained from the Taiwan Health Insurance Research Database, which contained patient sex, date of birth, all records of clinical visits and hospitalizations, and diagnosis codes as included in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The main outcome measures were the incidence and risk factors of VH.
RESULT
From 2001 to 2010, the database claim 4379 newly diagnosed cases were identified with VH. The average incidence of VH in Taiwan was 4.8 cases per ten-thousand person-years generally and increased with time especially in subjects who aged between 40 and 59y and when the VH was associated with proliferative diabetic retinopathies or retinal vein occlusions. A definitely upward trends in the incidence of VH from 2001 to 2010 were noted (P-value for increasing trend <0.001). Univariate Cox's regression analysis pointed out that older age (for 40-59, P<0.001, HR=9.39; for ≥60, P<0.001, HR=11.39), male gender (P=0.03, HR=1.07) and subjects who had been prescribed anti-coagulation drug included aspirin, warfarin and clopidogrel (P<0.001, HR=2.20) were significant risk factors for suffering from VH.
CONCLUSION
The incidence of VH is estimated being 4.8 cases per 10 000 person-years in Taiwan. Age, male gender and having been prescribed anti-coagulation drugs are associated with the incidence of VH.
Keywords: vitreous hemorrhage, incidence, risk factor
INTRODUCTION
Vitreous hemorrhage (VH) is the extravasation of blood into the vitreous body[1]. The vitreous gel is avascular. VH occurs when blood leaks from ruptured vessels into the vitreous cavity. It may occur suddenly, without pain.
VH usually happens in adult patients with proliferative diabetic retinopathy, retinal break, retinal vein occlusion, posterior vitreous detachment, or ocular trauma[2]–[5]. Furthermore, retinal arterial macroaneurysms, choroidal neovascularization, intraocular tumors, and other diseases may also lead to VH. The most common cause of VH in children is blunt or penetrating trauma[3],[6]–[7].
Patients with VH may suffer from acute blurring of the affected eye. The influence on visual acuity caused by VH itself may persist from days to months until the blood is removed or absorbed. Nevertheless, the underlying diseases which lead to VH might cause permanent visual impairment. Traditionally, vitrectomy is recommended if the blood in the vitreous has not been absorbed after 2 to 3mo. However, some underlying diseases, for example, retinal break and detachment, might progress rapidly during this period. Recent studies have suggested that an early vitrectomy reduces the overall retinal detachment rate and results in a better visual prognosis[8]–[10].
Owing to the potentially severe impact of VH and its underlying diseases on vision, affected patients may be unable to return to work following treatment, which could affect the socioeconomic status of patients and their families. However, studies on the epidemiology of VH are scanty. The incidence of dense VH was estimated to be 7 cases per 100 000 person-years in Europe and was even lower in children[1],[4],[11]. The Taiwan Health Insurance program was implemented in 1995. This single-payer medical healthcare system covers more than 99% of residents in Taiwan. The aim of this population-based study was to analyze the incidence of VH in Taiwan using data obtained from the Health Insurance Research Database. We believe this is the first long-term population-based epidemiological study of VH in a Chinese population.
SUBJECTS AND METHODS
Database and Study Sample
This population-based study analyzed one million subjects from all enrollees in the Taiwan Health Insurance program. Subjects were randomly selected from the registry data in 2000. All data were obtained from the Taiwan Health Insurance Research Database, which contains subjects' sex, date of birth, all records of clinical visits and hospitalizations, and diagnosis codes included in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The validity, representativeness, and clinical consistency of this database have been previously reported[12]. No informed consent was necessary because this study analyzed the secondary data.
Inclusion and Exclusion Criteria
All patients who sought medical care with a new diagnosis of VH (ICD-9-CM code 379.23) between January 1, 2001 and December 31, 2010 were identified. We excluded subjects with incorrect age (n=28) and missing gender (n=383). In addition, patients diagnosed as having VH before January 1, 2001 (n=985) were excluded.
Study Variables
Factors including age, gender, urbanization level of living or working area, and prescription of anti-coagulation drugs were analyzed in this study. Age was stratified into the following 3 groups: younger than 40 years (children and younger group), 40 to 59 years of age (middle-aged group), and 60 years or older (elderly group). The urbanization level of the living or working areas were categorized as urban, suburban, and rural, based on population density and the availability of medical resources. Subjects with diabetes or hypertension were identified using ICD-9-CM codes. Subjects who had ever been prescribed anti-coagulation drugs such as aspirin, warfarin and clopidogrel were identified from the prescription data.
Regarding the possible causes of VH, the first qualified diagnosis made by an ophthalmologist after the occurrence of VH was obtained. The possible causes of VH were categorized as retinal detachment (ICD-9-CM: 361), proliferative diabetic retinopathy (ICD-9-CM: 362.0), retinal vein occlusion (ICD-9-CM: 362.3) and age-related macular degeneration (ICD-9-CM: 362.5). If there was more than one diagnosis made simultaneously or no qualified diagnosis, the patient was categorized as “others or undefined”.
Statistical Analysis
Age group-stratified cumulative incidence rates were calculated as persons with newly developed VH divided by the total population at risk on the first day of that year annually from 2001 to 2010 (number of incident cases/total years of follow-up in total population). To determine the association between each factor and VH, a univariate analysis was performed by Cox regression analysis. A significance level of P=0.05 was selected. The SAS statistical package version 9.2 was used for all statistical calculations.
This study was approved by the Ethical Review Board of the Taichung Veterans General Hospital (CE14320A#1).
RESULTS
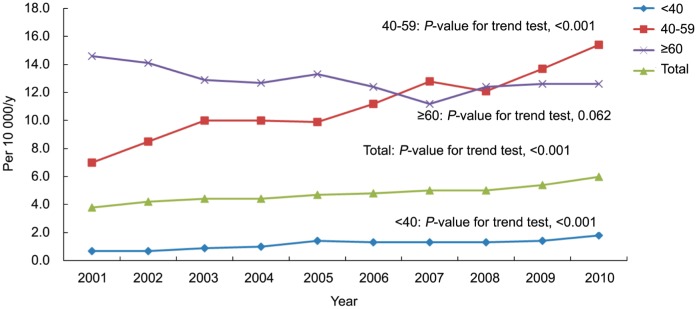
Interestingly, upward trends in the incidence of VH were noted from 2001 to 2010 (Table 1, P-value for increasing trend <0.001). After stratifying by age groups, the constant increasing trend was most significant in patients aged 40 to 59y (from 7.0 to 15.4 cases per 10 000 person-years, P-value for increasing trend <0.001) and in patients younger than 40y (from 0.7 to 1.8 cases per 10 000 person-years, P-value for increasing trend <0.001). However, the incidence of VH remained stable with time in patients who were older than 60 years old (P-value for decreasing trend =0.062) (Figure 1).
Table 1. Incidence of VH in Taiwan, 2001-2010.
| Year | VH |
|||||||||||
| Total population |
Incidence case |
Incidence per 104/year |
||||||||||
| <40 | 40-59 | ≥60 | Total | <40 | 40-59 | ≥60 | Total | <40 | 40-59 | ≥60 | Total | |
| 2001 | 613 116 | 225 894 | 114 026 | 953 036 | 40 | 159 | 167 | 366 | 0.7 | 7.0 | 14.6 | 3.8 |
| 2002 | 607 507 | 224 413 | 110 071 | 941 991 | 45 | 191 | 155 | 391 | 0.7 | 8.5 | 14.1 | 4.2 |
| 2003 | 603 525 | 223 023 | 106 182 | 932 730 | 53 | 222 | 137 | 412 | 0.9 | 10.0 | 12.9 | 4.4 |
| 2004 | 602 095 | 221 710 | 102 424 | 926 229 | 59 | 222 | 130 | 411 | 1.0 | 10.0 | 12.7 | 4.4 |
| 2005 | 601 349 | 220 436 | 98 566 | 920 351 | 83 | 218 | 131 | 432 | 1.4 | 9.9 | 13.3 | 4.7 |
| 2006 | 600 544 | 219 085 | 94 642 | 914 271 | 76 | 245 | 117 | 438 | 1.3 | 11.2 | 12.4 | 4.8 |
| 2007 | 599 616 | 217 547 | 90 985 | 908 148 | 78 | 278 | 102 | 458 | 1.3 | 12.8 | 11.2 | 5.0 |
| 2008 | 598 642 | 216 003 | 87 166 | 901 811 | 79 | 262 | 108 | 449 | 1.3 | 12.1 | 12.4 | 5.0 |
| 2009 | 597 355 | 214 335 | 83 179 | 894 869 | 86 | 294 | 105 | 485 | 1.4 | 13.7 | 12.6 | 5.4 |
| 2010 | 596 022 | 212 521 | 79 419 | 887 962 | 110 | 327 | 100 | 537 | 1.8 | 15.4 | 12.6 | 6.1 |
Figure 1. Incidence of VH in Taiwan during 2001-2010.
Incidence cases included in this population-based study are summarized in Table 2. In total, 4379 newly diagnosed cases of VH were identified in the database from 2001 to 2010. The average incidence of VH in Taiwan was 4.8 cases per ten thousand person-years, 13.2 cases per ten thousand person-years in elders (60 years or older), 11.1 cases per ten thousand person-years in middle-aged persons (40 to 59 years old) and 1.2 cases per ten thousand person-years in younger people (younger than 40 years old).
Table 2. Age-specific incidence of VH in Taiwan during 2001-2010.
| Age group (a) | Incidence case |
Population (person-years)1 |
Incidence rate per 104/year |
||||||
| Female | Male | Total | Female | Male | Total | Female | Male | Total | |
| <40 | 292 | 417 | 709 | 2 944 762 | 3 063 450 | 6 008 212 | 1.0 | 1.4 | 1.2 |
| 40-59 | 1166 | 1252 | 2418 | 1 103 006 | 1 083 983 | 2 186 989 | 10.6 | 11.5 | 11.1 |
| ≥60 | 636 | 616 | 1252 | 472 917 | 474 939 | 947 856 | 13.4 | 13.0 | 13.2 |
| Total | 2094 | 2285 | 4379 | 4 520 684 | 4 622 372 | 9 143 057 | 4.6 | 4.9 | 4.8 |
1Person-years means the total years of follow-up in population who were at risk.
Overall the mean age of the patients was 52.0 years old, with a standard deviation of 14.3y, and most patients were aged between 40 and 59y. The incidence rate of VH was higher in males (52.18%) than in females (Table 3). Among all incident cases, 56.77% were also diagnosed as having diabetes and 61.57% were diagnosed as having hypertension. Regarding the possible causes of VH, 36.83% of VH cases were possibly due to proliferative diabetic retinopathy, 12.42% were possibly due to retinal detachment, 4.34% were possibly due to age-related macular degeneration, and 3.68% were possibly due to retinal vein occlusion. Regrettably, about 42.73% of VH were classified into the “others or undefined” group. Furthermore, we also estimated the incidence of VH due to each possible cause separately and the results showed that the incidence of VH associated with proliferative diabetic retinopathy and retinal vein occlusion among adults also increased with time.
Table 3. Characteristics of study subjects with VH.
| Variables | VH |
| Age (a, x±s) | 52.0±14.3 |
| <40 | 709 (16.19) |
| 40-59 | 2418 (55.22) |
| ≥60 | 1252 (28.59) |
| Gender | |
| F | 2094 (47.82) |
| M | 2285 (52.18) |
| Urbanization1 | |
| 1 | 1250 (29.10) |
| 2 | 1227 (28.56) |
| 3 | 1819 (42.34) |
| Diabetes | |
| No | 1893 (43.23) |
| Yes | 2486 (56.77) |
| Hypertension | |
| No | 1683 (38.43) |
| Yes | 2696 (61.57) |
| Anti-coagulation drug (aspirin warfar in clopidogrel) | |
| No | 2359 (53.87) |
| Yes | 2020 (46.13) |
| Possible reason of VH | |
| RD | 544 (12.42) |
| PDR | 1613 (36.83) |
| RVO | 161 (3.68) |
| AMD | 190 (4.34) |
| Others or undefined | 1871 (42.73) |
Urbanization: 1 indicates the highest level of urbanization and 3 the lowest; RD: Retinal detachment; PDR: Proliferative diabetic retinopathy; RVO: Retinal vein occlusion; AMD: Age-related macular degeneration.
n=4379 (%)
The risk factors found to be associated with incidence of VH are shown in Table 4. Univariate Cox regression analysis revealed that (for 40-59, P<0.001, HR=9.39; for ≥60, P<0.001, HR=11.39) male gender (P=0.03, HR=1.07) and use of an anti-coagulation drug, including aspirin, warfarin, or clopidogrel (P<0.001, HR=2.20) were significant risk factors for VH. The incidence of VH increased as the urbanization level of the subjects' residential area decreased, but the result was not statistically significant.
Table 4. Hazard ratios of risk associated with baseline characteristics in univariate Cox's regression analysis.
| Variables | VH |
||
| HR | 95%CI | P | |
| Age (a) | |||
| <40 | 1.00 | ||
| 40-59 | 9.39 | (8.64, 10.21) | <0.001 |
| ≥60 | 11.39 | (10.39, 12.49) | <0.001 |
| Sex | |||
| F | 1.00 | ||
| M | 1.07 | (1.01, 1.13) | 0.03 |
| Urbanization | |||
| 1 | 1.00 | ||
| 2 | 1.01 | (0.94, 1.09) | 0.41 |
| 3 | 1.06 | (0.99, 1.14) | 0.11 |
| Anti-coagulation drug | |||
| No | 1.00 | ||
| Yes | 2.20 | (2.07, 2.33) | <0.001 |
Urbanization level: 1 indicates the highest level of urbanization and 3 the lowest.
DISCUSSION
In this study, we found that the crude incidence of VH in a Chinese population was 4.8 cases per 10 000 person-years. We also showed that the incidence of VH in elders and middle-aged adults was much higher than that in younger people. Our results also showed that the annual cumulative incidence of VH increased from 3.8 cases to 6.1 cases per 10 000 person-years within 10y. In a study by Lindgren et al[4], there were approximately 7 cases of spontaneous VH per 100 000 population each year in Sweden, which was much lower than the rate found in the present study. The main reason for this discrepancy is that our study included patients with any cause of VH and also included VH patients with all levels of severity. Moreover, the difference in incidence rates might also support one of our findings that the incidence of VH did increase with time.
Our results showed that the incidence of VH increased significantly in subjects aged between 40 and 59y. Hence we further analyzed the causes of VH in this age group and the results showed that 43.3% were possibly due to proliferative diabetic retinopathy, 11.4% were possibly due to retinal detachment, 3.06% were possibly due to retinal vein occlusion, and 2.44% were possibly due to age-related macular degeneration (data not shown). Furthermore, we also estimated the incidence of VH due to each possible cause separately and the results showed that the incidence of VH associated with proliferative diabetic retinopathy and retinal vein occlusion among adults also increased with time (P-value for increasing trend <0.01 and =0.02, respectively, data not shown). Meanwhile, the incidence of VH associated with retinal detachment and age-related macular degeneration did not show any significant trend.
Another consideration is the universality of small incision sutureless vitrectomy, which greatly reduces the surgery time and complication rates[9]. It is unclear whether ophthalmologists make the diagnosis of VH more frequently with a view to applying for reimbursement for vitrectomy. Therefore, we performed a sensitivity analysis which only estimated the incidence of VH in cases that required vitrectomy. The result of this analysis showed that the incidence of VH in cases requiring vitrectomy also increased from 1.0 case per 10 000 person-years in 2001 to 1.3 cases per 10 000 person-years in 2010. The percentages of cases that received vitrectomy among all VH patients ranges from 0.20 to 0.27 during 2001 to 2010.
The incidence of VH was shown to increase significantly with age. Many diseases such as retinal vein occlusion and age-related macular degenerations mostly tend to occur in older people. The prevalence and severity of diabetic retinopathy also increase with the duration of diabetes[13]. Furthermore, the Beaver Dam Eye Study showed the prevalence of vein occlusion was associated with hypertension and diabetes[14]. As demonstrated in other studies, systemic hypertension was frequently found among patients with macroaneurysm and branch retinal vein occlusion[15]–[16]. It is therefore not surprising that more than half of the patients with VH in our study had hypertension and diabetes.
The causes of VH may depend on the study population and tend to vary with the mean age of the patients as well as the region in which previous studies were performed[3]–[4],[17]. Proliferative diabetic retinopathy (19.1%-54.0%) and retinal break (27%-37.3%) were the most common causes of VH in previous studies. Vitreous detachment with or without a tear was only reported as the most common cause of VH in a case series by Lindgren et al[4]. In our study, proliferative diabetic retinopathy accounted for 36.8% of VH and retinal detachment accounted for 12.4% of VH. This finding is compatible with the results of previous reports. Up to 42.7% of VH cases were classified as “others or undefined” causes, which was much higher than in previous reports. A major reason for this result is that some causes cannot be clearly identified using ICD-9-CM codes, such as ocular trauma and posterior vitreous detachment. Another explanation is that patients with multiple diagnoses simultaneously made after VH were categorized as “VH due to others or undefined cause”. For example, proliferative diabetic retinopathy might coexist with retinal vein occlusion or age-related macular degeneration. It may therefore not be possible to define the possible causes of VH in such conditions without access to a patient's medical records. However, this discrepancy implicated that diabetes or hypertension might be the underlying risk factor.
In our study, males had a higher incidence of VH than females. The finding was consistent with the results of previous studies. Many reports have shown that male sex was independently associated with causes of VH but not directly with VH. Novak and Welch[18] suggested that men have a four-fold greater risk of developing retinal tear after vitreous detachment than women. A Meta-estimate of previous studies also reported that the sex distribution in retinal detachment indicates a male proportionality of between 52% and 59%[19]. Furthermore, male sex was independently associated with the presence of diabetic retinopathy, as well as poorer blood sugar and blood pressure control[20].
Our results showed that subjects who have been prescribed anti-coagulation drugs for any reason had a higher incidence of VH than those who had not been prescribed these medications. The findings are not consistent with the results of previous studies on patients with diabetes and retinal vein occlusion[21]. However, the design and purpose of our study differ from those of previous studies and so such comparisons may not be meaningful. Our study did not adjust for underlying diseases of VH which require a prescription for these medications. This finding suggests that regardless of the type of disease requiring anti-coagulation medication or the disease cause, the patient would have a higher risk for VH. Furthermore, a recent study found that taking aspirin, clopidogrel, or warfarin was more likely to result in VH as a result of an acute posterior vitreous detachment[22].
The present study was limited by lack of clinical variables in the Taiwan Health Insurance Research Database. It is difficult to establish a definite cause of VH without chart review, especially when the disease cannot be clarified by ICD-9-CM codes or when many diagnoses were made simultaneously. Second, detection bias is a notable problem in retrospective studies, especially when the data are derived from a database. Patients who suffered from minor VH or had a poor general condition, such as patients who are bed-ridden or have dementia, might not have sought medical help and could have been missed by our study. Although this study was highly representative to the total population, it is a crucial limitation that we could only use the ICD codes for diagnoses. Without detail medical data, the validity of diagnoses, the severity of diseases, the exact cause of VH might be biased. Besides, we cannot obtain the visual acuity in this study, which is an indicator for severity of the VH. Although the healthcare utilization in Taiwan is very high due to the National Health Insurance program, the incidence of VH may have been underestimated in our study.
In conclusion, the incidence of VH was estimated to be 4.8 cases per 10 000 person-years in Taiwan and increased with time, especially in subjects between 40 and 59 years old and in VH patients with proliferative diabetic retinopathy or retinal vein occlusion. Age, male gender, and use of anti-coagulation drugs were associated with incidence of VH. We recommend that clinicians and policymakers should pay more attention to the increasing trend of incidence of VH in Taiwan. Further clinical studies are needed to confirm the findings of our study.
Acknowledgments
The authors would like to thank the Healthcare Service Research Center (HSRC) of Taichung Veterans General Hospital for statistical support. This study is based in part on data from the Taiwan Health Insurance Research Database provided by the Ministry of Health and Welfare (Registered number 101095, 102148).
Foundations: Supported in part by Grants from Taichung Veterans General Hospital, Taiwan (TCVGH-NHRI10405, TCVGH-1047324D, TCVGH-1047312C, TCVGH-104G211).
Conflicts of Interest: Wang CY, None; Cheang WM, None; Hwang DK, None; Lin CH, None.
REFERENCES
- 1.Spraul CW, Grossniklaus HE. Vitreous hemorrhage. Surv Ophthalmol. 1997;42(1):3–39. doi: 10.1016/s0039-6257(97)84041-6. [DOI] [PubMed] [Google Scholar]
- 2.Butner RW, McPherson AR. Spontaneous vitreous hemorrhage. Surv Ophthalmol. 1982;14(3):268–270. [PubMed] [Google Scholar]
- 3.Dana MR, Werner MS, Viana MA, Shapiro MJ. Spontaneous and traumatic vitreous hemorrhage. Ophthalmology. 1993;100(9):1377–1383. doi: 10.1016/s0161-6420(93)31472-7. [DOI] [PubMed] [Google Scholar]
- 4.Lindgren G, Sjodell L, Lindblom B. A prospective study of dense spontaneous vitreous hemorrhage. Am J Ophthalmol. 1995;119(4):458–465. doi: 10.1016/s0002-9394(14)71232-2. [DOI] [PubMed] [Google Scholar]
- 5.Morse PH, Aminlari A, Scheie HG. Spontaneous vitreous hemorrhage. Arch Ophthalmol. 1974;92(4):297–298. doi: 10.1001/archopht.1974.01010010307006. [DOI] [PubMed] [Google Scholar]
- 6.Cekic O, Totan Y, Batman C. Traumatic vitreous hemorrhage from a persistent hyaloid artery. J Pediatr Ophthalmol Strabismus. 2000;37(2):117–118. doi: 10.3928/0191-3913-20000301-13. [DOI] [PubMed] [Google Scholar]
- 7.Spirn MJ, Lynn MJ, Hubbard GB., 3rd Vitreous hemorrhage in children. Ophthalmology. 2006;113(5):848–852. doi: 10.1016/j.ophtha.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Dhingra N, Pearce I, Wong D. Early vitrectomy for fundus-obscuring dense vitreous haemorrhage from presumptive retinal tears. Graefes Arch Clin Exp Ophthalmol. 2007;245(2):301–304. doi: 10.1007/s00417-006-0278-6. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Morita S, Bando H, Sato S, Ikeda T, Emi K. Early vitreous hemorrhage after vitrectomy with preoperative intravitreal bevacizumab for proliferative diabetic retinopathy. Middle East Afr J Ophthalmol. 2013;20(1):51–55. doi: 10.4103/0974-9233.106387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan HS, Mura M, Bijl HM. Early vitrectomy for vitreous hemorrhage associated with retinal tears. Middle East Afr J Ophthalmol. 2010;150(4):529–533. doi: 10.1016/j.ajo.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Nie WY, Wu HR, Qi YS, Zhang M, Hou Q, Yang HX, Gong LX, Dong YR, Guo YL, Shi JN, Yin SY, Li PY. A pilot study of ocular diseases screening for neonates in China. Zhonghua Yan Ke Za Zhi. 2008;44(6):497–502. [PubMed] [Google Scholar]
- 12.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Takemori M, Kitano S, Hori S, Fukushima H, Numaga J, Yamashita H. Retinopathy in older patients with diabetes mellitus. Diabetes Res Clin Pract. 2002;58(3):187–192. doi: 10.1016/s0168-8227(02)00155-9. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98 [PMC free article] [PubMed] [Google Scholar]
- 15.Panton RW, Goldberg MF, Farber MD. Retinal arterial macroaneurysms: risk factors and natural history. Br J Ophthalmol. 1990;74(10):595–600. doi: 10.1136/bjo.74.10.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeshaya A, Treister G. Pars plana vitrectomy for vitreous hemorrhage and retinal vein occlusion. Ann Ophthalmol. 1983;15(7):615–617. [PubMed] [Google Scholar]
- 17.Sudhalkar A, Chhablani J, Jalali S, Mathai A, Pathengay A. Spontaneous vitreous hemorrhage in children. Am J Ophthalmol. 2013;1561267(6):1271.e1262. doi: 10.1016/j.ajo.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Novak MA, Welch RB. Complications of acute symptomatic posterior vitreous detachment. Am J Ophthalmol. 1984;97(3):308–314. doi: 10.1016/0002-9394(84)90628-7. [DOI] [PubMed] [Google Scholar]
- 19.Mitry D, Charteris DG, Yorston D, Siddiqui MA, Campbell H, Murphy AL, Fleck BW, Wright AF, Singh J. The epidemiology and socioeconomic associations of retinal detachment in Scotland: a two-year prospective population-based study. Invest Ophthalmol Vis Sci. 2010;51(10):4963–4968. doi: 10.1167/iovs.10-5400. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong DS, Barton FB, Bresnick GH. Impaired color vision associated with diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report No. 15. Am J Ophthalmol. 1999;128(5):612–617. doi: 10.1016/s0002-9394(99)00227-5. [DOI] [PubMed] [Google Scholar]
- 22.Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina. 2013;33(3):621–626. doi: 10.1097/IAE.0b013e3182671006. [DOI] [PubMed] [Google Scholar]