Abstract
Aim
The aim of this study is to evaluate the protective effect of the salivary proteins on the demineralization of enamel.
Materials and methods
Twenty freshly extracted human molar teeth were used in this study. Enamel samples (2 mm thickness) were prepared from the buccal and lingual surfaces of the teeth selected. An acid-resistant nail varnish was used to cover every aspect of the sample, except an area of 5 * 5 mm limited by an adhesive tape. After drying, the adhesive tape was removed, exhibiting a rectangular area on the enamel surface. Samples were divided into two groups:
Group I (10 samples): Each sample was coated by 100 μg of albumin for 2 hours at 37°C.
Group II (10samples): Each sample was exposed to 100 μL of deionized water.
Samples were washed by dipping once in deionized water. They were then disposed into individual tubes containing demineralization solution for 1, 2, 3, and 4 minutes at 37°C with gentle agitation. The demineralization solution was utilized to determine the calcium loss from specimens at 1, 2, 3, 4 minutes using an ultraviolet-visible spectrophotometer.
Results
Calcium loss was less from the albumin-coated samples than control group at all times and was statistically significant (p < 0.05). Also, calcium loss was maximum at the end of 1 minute, and it decreased as time interval increased and was statistically significant (p < 0.001).
Conclusion
Albumin has provided a strong protection against enamel demineralization at all times compared to the one without it.
How to cite this article
Hegde MN, Sajnani AR. Salivary Proteins—A Barrier on Enamel Demineralization: An in vitro Study. Int J Clin Pediatr Dent 2017;10(1):10-13.
Keywords: Albumin, Demineralization, Enamel, Salivary protein.
INTRODUCTION
In the oral cavity, saliva surrounds the tooth and creates the milieu that bathes the tooth surface. It serves as the main vehicle for solubilizing and transporting potential harmful substances as well as protective factors to the biofilm covered tooth surface. Saliva’s protective role is mediated by its ability to clear cariogenic food substances and dilute, neutralize, and buffer organic acids formed by biofilm microorganisms. Thus, it reduces the demineral ization rate and enhances remineralization by providing calcium, phosphate, and fluoride in the fluid phase of the biofilm in close association with the tooth surface.1
The selective adsorption of salivary proteins leads to formation of an organic film on the tooth surface, i.e., known as the acquired enamel pellicle (AEP).2,3 It forms a protective interface between the tooth surface and the oral environment and acts as a selective permeability barrier that regulates demineralization/rem-ineralization processes.4 Also, it acts as a reservoir of remineralizing electrolytes, i.e., calcium, phosphate, and fluorides.1
Organic phase of saliva consist of proteins that may regulate the demineralization and remineralization processes. Similar to saliva, AEP also consist of albumin-, mucin-, and proline-rich proteins (PRPs), histatins and cystatins which have shown high affinity to enamel surfaces and are also involved with the maintenance of tooth integrity by favoring a suitable calcium phosphate environment. Recently, by using the proteomic technology, more than 130 different proteins have been identified in the dental pellicle.1
Despite the variety of proteins, information regarding the importance of salivary proteins on demineralization/ remineralization processes is scarce.5
Hence, the study aimed to evaluate the protective effect of the salivary proteins on the demineralization of enamel.
MATERIALS AND METHODS
Infection Control Protocol
The teeth were cleansed of visible blood and gross debris and were maintained in a hydrated state during storage. Then they were placed in sodium hypochlorite solution diluted with saline in a ratio of 1:10 in the container with a secure lid to prevent leakage.6
Inclusion Criteria
Freshly extracted human maxillary and mandibular molar teeth extracted for periodontal problems. Teeth were selected based on randomized sampling method.
Exclusion Criteria
Teeth with caries, hypoplastic lesions, white spots, cracks, erosion, developmental anomaly, or any other deformity.
Enamel Sample Preparation
Twenty freshly extracted human molar teeth were used in this study. Enamel samples (2 mm thickness) were prepared from the buccal and lingual surfaces of the teeth selected, using a double-faced diamond disk mounted on a contra-angle handpiece. An acid-resistant nail varnish was used to cover every aspect of the specimen, except an area of 5 × 5 mm limited by an adhesive tape. After drying, the adhesive tape was removed from the enamel using a sharp-tipped instrument exhibiting a rectangular area on the enamel surface.7
Groups
A total of 20 enamel samples were divided into two groups of 10 samples in each.
Group 1: Each sample was coated by 100 ug of albumin (bovine serum albumin was used as a standard model protein) for 2 hours at 37°C.
Group 2: Each sample will be exposed to 100 uL of deionized water.
Samples were washed by dipping once in deionized water. They were then disposed in demineralization solution: Calcium chloride (CaCl2) - 2.2 mM, sodium dihydrogen phosphate (NaH2PO4) - 2.2 mM, acetic acid - 0.005M, pH 4.5 (Siqueira et al) for 1, 2, 3, and 4 minutes at 37°C with gentle agitation.
The demineralization solution was utilized to determine the calcium loss from samples at 1, 2, 3, and 4 minutes using an ultraviolet-visible light spectro-photometer.
Assessment of the Extent of Demineralization
Amount of Calcium released
The calcium content of the samples was analyzed using a quantitative colorimetric calcium determination assay (Agappe Calcium Assay Kit, Ernakulum, Kerala, India), employing an ultraviolet-visible spectrophotometer (Systronics, Ahmedabad, Gujarat, India) determining the optical density at a wavelength of 612 nm.
Formula to calculate Calcium Loss
Amount of calcium loss(mg/dl) = (Optical density of test sample / Optical density of standard sample) × 10
Optical density of standard sample = 0.645
Statistical Analysis
Results were analyzed using Independent Sample t-test and Repeated Measure ANOVA Test.
RESULTS
Graph 1 shows that calcium loss was less from the albumin coated enamel samples than control group at all times. It was statistically significant (p < 0.05) except at the end of 1 minute.
Graph 1:
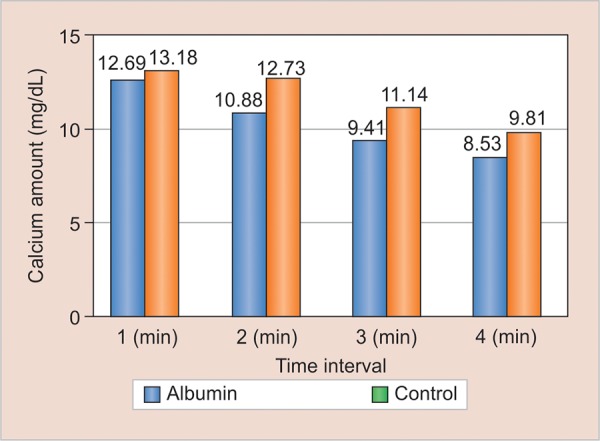
Comparison of calcium loss between study groups
Graph 2 shows that calcium loss was maximum at the end of 1 minute, and it decreased as time interval increased, i.e., at the end of 2, 3, and 4 minutes. Also, the amount of calcium loss at the end of 1 minute was more and statistically significant (p < 0.001) than at the end of 4 minutes.
Graph 2:
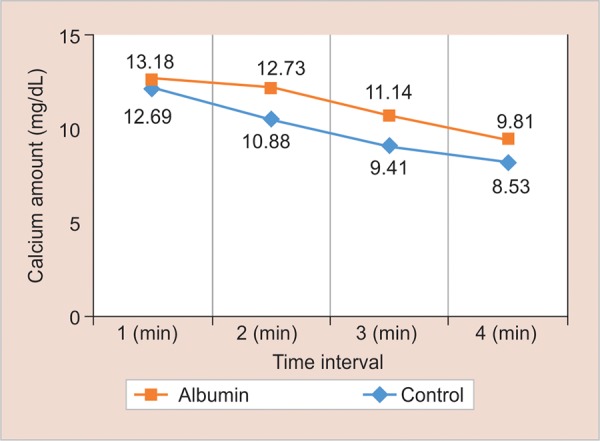
Comparison of calcium loss at different time intervals from the study groups
DISCUSSION
Saliva, a body fluid basically formed by proteins and ions, has multiple physiological functions, such as digestion, swallowing, lubrication, tooth integrity, and antimicrobial protection.4 A group of salivary proteins, namely, statherin, the acidic PRPs, albumin, histatins, and cystatins are also said to be multifunctional as they are partly responsible for the remineralization capacity of saliva.8
Albumin is the most abundant serum protein, accounting for more than 50% of all plasma proteins. In the oral cavity, albumin is considered as a serum ultrafiltrate to the mouth and is present in saliva due to contamination by either traces of blood or gingival fluid.9 It may also diffuse into the mucosal secretions. Concentration of salivary albumin varies considerably from person to person.10 As such there are no reference limits for salivary albumin, but it has shown to be increased in medically compromised patients, such as in immunosuppression, radiotherapy, and diabetes.11
In the present study enamel samples were coated with albumin for a period of 2 hours as there are several studies showing that AEP reaches a plateau within 2 hours on enamel.12,13 The loss of calcium from albumin coated enamel samples was less than the control group at all the time intervals, i.e., 1, 2, 3, and 4 minutes (Graph 1). This could be because enamel is mainly protected from demineralization by the inhibitory effects of salivary albumin which penetrates into the pores and binds to hydroxyapatite (HA) crystals in addition to the protective effects of AEP.14,15 These results were in accordance with the studies on other salivary proteins conducted by Kielbassa et al,15 Featherstone et al,16 Kosoric et al17 and Martins et al.4 Studies conducted by Hemingway et al18 has also shown that egg protein ovalbumin has potential antierosive properties and can be used as additive to drinks. Also, a study conducted by Hegde et al concluded that with the decrease in the levels of albumin there is increase in the levels of dental caries.19
In the present study, maximum calcium loss was seen from the enamel samples at the end of 1 minute from both albumin-coated enamel samples and control group, and it decreased as the time interval increased, i.e., 2, 3, and 4 minutes (Graph 2).
As stated earlier, saliva is a rich reservoir of calcium, phosphate, and fluoride ions. According to the law of saturation, a dynamic equilibrium exists between the mineral contents of the tooth and saliva. At “neutral pH,” the HA crystal dissolves minimally and releases the calcium, phosphate, and hydroxyl ions into saliva, but since it already contains the same minerals, saliva becomes supersaturated which results in the precipitation of these minerals back on to the tooth surface.20
Critical pH is a point at which a solution is just saturated with a mineral. It is inversely proportional to the amount of calcium and phosphate ions in the saliva of an individual, since the concentration of these ions vary from each individual, critical pH also changes. Research now suggests that in individuals with low calcium and phosphate ion concentration levels, the critical pH is 6.5, and those with higher concentration of these ions, critical pH is 5.5.21
At “acidic pH” (which in our study is 4.5), the phosphate ions and the hydroxyl ions react with the hydrogen ions in the tooth-biofilm interface by forming complexes, such as hydrogen phosphate and water. Thus saliva becomes undersaturated with respect to phosphate ions, which leads to the dissolution of hydroxyapettite crystals in an attempt to resaturate saliva.20
After a period of time, saliva saturates and mineral loss stops. When enough minerals (mainly calcium, phosphate, and hydroxyl ions) are available in the saliva surrounding the HA crystals, increasing its saturation level, balance returns.22 Thus, in our study, as the time interval increased the amount of calcium loss decreased.
Therefore, although pH is the strongest determinant for saturation level leading to demineralization or rem-ineralization under clinical conditions, but it is not the only important factor. Saturation is significantly affected by the calcium and phosphate ions concentration and the total ionic strength of plaque fluid.22
CONCLUSION
Under the conditions of this study, following conclusions can be derived:
Albumin has provided a strong protection against enamel demineralization at all times compared to the one without it.
Since it is an in vitro study, further research should be carried out in in vitro environment for clinical application.
Thus, albumin could be considered as an additive to oral hygiene products for caries-prone individuals with reduced salivary flow or altered saliva composition.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Hara TA, Zero TD. The caries environment: saliva, pellicle, diet and hard tissue ultrastructure. Dent Clin North Am. 2010 Jul;54(3):455–467. doi: 10.1016/j.cden.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Hannig M, Joiner A. The structure, function and properties of the acquired pellicle. Monogr Oral Sci. 2006;19:29–64. doi: 10.1159/000090585. [DOI] [PubMed] [Google Scholar]
- 3.Siqueira WL, Helmerhorst EJ, Zhang W, Salih E, Oppenheim FG. Acquired enamel pellicle and its potential role in oral diagnostics. Ann N Y Acad Sci. 2007 Mar;1098:504–509. doi: 10.1196/annals.1384.023. [DOI] [PubMed] [Google Scholar]
- 4.Martins C, Castro GF, Siqueira MF, Xiao Y, Yamaguti PM, Siqueira WL. Effect of dialyzed saliva on human enamel demineralization. Caries Res. 2013;47(1):56–62. doi: 10.1159/000343574. [DOI] [PubMed] [Google Scholar]
- 5.Siqueira WL, Margolis HC, Helmerhorst EJ, Mendes FM. Oppenheim FG: Evidence of intact histatins in the in vivo acquired enamel pellicle. J Dent Res. 2010;89:626–630. doi: 10.1177/0022034510363384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantera EA, Jr, Schuster GS. Sterlization of extracted human teeth. J Dent Educ. 1990 May;54(5):283–285. [PubMed] [Google Scholar]
- 7.Shetty S, Hegde MN, Bopanna TP. Enamel remineralization assessment after treatment with three different remineral-izing agents using surface microhardness: an in vitro study. J Conserv Dent. 2014 Jan;17(1):49–52. doi: 10.4103/0972-0707.124136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlén A, Börjesson A, Nikdel K, Olsson J. Composition of pellicles formed in vivo on tooth surfaces in different parts of the dentition and in vitro on hydroxyapatite. Caries Res. 1998;32(6):447–455. doi: 10.1159/000016486. [DOI] [PubMed] [Google Scholar]
- 9.Selby C, Lobb PA, Jeffcoate WJ. Sex hormone binding globulin in saliva. Clin Endocrinol. 1988;28:19–24. doi: 10.1111/j.1365-2265.1988.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 10.Niswander JD, Shreffler DC, Neel JV. Genetic studies of quantitative variation in a component of human saliva. Ann Hum Genet. 1963;27:319–328. doi: 10.1111/j.1469-1809.1963.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 11.Meurman JH, Rantonen P, Pajukoski H, Sulkava R. Salivary albumin and other constituents and their relation to oral and general health in the elderly. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002 Oct;94(4):432–438. doi: 10.1067/moe.2002.122345. [DOI] [PubMed] [Google Scholar]
- 12.Eggen KH, Rolla G. Further studies on the composition of the acquired enamel pellicle. Scand J Dent Res. 1983;91:439–446. doi: 10.1111/j.1600-0722.1983.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JL, Lamkin MS, Oppenheim FG. Adsorption of human salivary proteins to hydroxyapatite: a comparison between whole saliva and glandular salivary secretions. J Dent Res. 1992;71:1569–1576. doi: 10.1177/00220345920710090501. [DOI] [PubMed] [Google Scholar]
- 14.Van Der Linden AH, Booij M, Ten Bosch JJ, Arends J. Albumin interaction with caries-like lesions in bovine enamel. Caries Res. 1989;23(6):393–398. doi: 10.1159/000261216. [DOI] [PubMed] [Google Scholar]
- 15.Kielbassa AM, Oeschger U, Schulte-Monting J. Meyer-lueckel H. Microradiographic study on the effects of salivary proteins on in vitro demineralization of bovine enamel. J Oral Rehabil. 2005 Feb;32(2):90–96. doi: 10.1111/j.1365-2842.2004.01392.x. [DOI] [PubMed] [Google Scholar]
- 16.Featherstone JD, Behrman JM, Bell JE. Effect of whole saliva components on enamel demineralization in vitro. . Crit Rev Oral Biol Med. 1993;4(3-4):357–362. doi: 10.1177/10454411930040031401. [DOI] [PubMed] [Google Scholar]
- 17.Kosoric J, Hector MP, Anderson P. The influence of proteins on demineralization kinetics of hydroxyapatite aggregates. J Biomed Mater Res A. 2010 Sep;94(3):972–977. doi: 10.1002/jbm.a.32759. [DOI] [PubMed] [Google Scholar]
- 18.Hemingway CA, Shellis RP, Parker DM, Addy M, Barbour ME. Inhibition of hydroxyapatite dissolution by ovalbumin as a function of pH, calcium concentration, protein concentration and acid type. Caries Res. 2008;42(5):348–353. doi: 10.1159/000151440. [DOI] [PubMed] [Google Scholar]
- 19.Hegde MN, Bhat R, Punja A, Shetty C. Correlation between dental caries and salivary albumin in adult Indian population— An in vivo study. Br J Med Med Res. 2014 Sep;4(25):4238–4244. [Google Scholar]
- 20.Carounanidy U, Sathyanarayanan R. Dental caries - A complete changeover (Part I). J Conserv Dent. 2009 Apr;12(2):46–54. doi: 10.4103/0972-0707.57631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ericson Y. Enamel apetite solubility. Investigation into the calcium phosphate equilibrium between enamel and saliva and its relation to dental caries. Actra Odont Scand. 1949;8(Suppl 3):1–139. [Google Scholar]
- 22.Gonzalez-Cabezas C. The chemistry of caries: remineralization and demineralization events with direct clinical relevance. Dent Clin North Am. 2010 Jul;54(3):469–478. doi: 10.1016/j.cden.2010.03.004. [DOI] [PubMed] [Google Scholar]


