Abstract
Background
The management of acne in adult females is problematic, with many having a history of treatment failure and some having a predisposition to androgen excess. Alternatives to oral antibiotics and combined oral contraceptives (COCs) are required.
Objective
Our aim was to conduct a hybrid systematic review of the evidence for benefits and potential harms of oral spironolactone in the management of acne in adult females.
Methods
The review was conducted according to a previously published protocol. Three reviewers independently selected relevant studies from the search results, extracted data, assessed the risk of bias, and rated the quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Results
Ten randomized controlled trials (RCTs) and 21 case series were retrieved. All trials were assessed as being at a ‘high risk’ of bias, and the quality of evidence was rated as low or very low for all outcomes. Apart from one crossover trial that demonstrated statistical superiority of a 200 mg daily dose versus inflamed lesions compared with placebo, data from the remaining trials were unhelpful in establishing the degree of efficacy of lower doses versus active comparators or placebo. Menstrual side effects were significantly more common with the 200 mg dose; frequency could be significantly reduced by concomitant use of a COC. Pooling of results for serum potassium supported the recent recommendation that routine monitoring is not required in this patient population.
Conclusion
This systematic review of RCTs and case series identified evidence of limited quality to underpin the expert endorsement of spironolactone at the doses typically used (≤100 mg/day) in everyday clinical practice.
Electronic supplementary material
The online version of this article (doi:10.1007/s40257-016-0245-x) contains supplementary material, which is available to authorized users.
Key Points
| Oral spironolactone is used off-label to treat persistent and late-onset acne in adult females. |
| There is low-quality evidence for benefits and side effects from randomized controlled trials and case series; superiority over placebo has not been established for doses <200 mg/day. |
| Prescribing recommendations must continue to rely on consensus and expert opinion until high-quality evidence becomes available. |
Introduction
Acne is the eighth most prevalent disease globally [1]. While this chronic inflammatory skin condition affects mostly adolescents, adult females represent a significant and increasing proportion of cases in which quality of life is severely affected [2–5].
A number of variants of acne in adult women are recognized, based on age of onset, distribution and type of lesions, recalcitrance to conventional drug-based remedies, predisposing factors (e.g. smoking, ethnicity), and endocrine disposition, most commonly polycystic ovarian syndrome (PCOS) [2, 3, 6–9]. However, many patients have no signs of peripheral hyperandrogenism other than acne. Serum profiles of androgens and gonadotrophins are often normal [10, 11].
In both teenagers and adults, acne is, de facto, a disease of sebogenesis [12]. Beginning during adrenarche, rising levels of androgens and insulin-like growth factor (IGF)-1 mediate the onset of sebum production in both sexes [13]. Anaerobic bacteria, particularly Propionibacterium acnes proliferate within acne-prone pilosebaceous follicles, which are blocked as a result of abnormal keratinocyte proliferation in response to signals from sebum components. This triggers leukocyte infiltration via both innate and adaptive immune mechanisms. Characteristically, a cell-mediated inflammatory response ensues, in which macrophages and T helper (Th)-1 and Th-17 cells predominate [13, 14].
Spironolactone, a synthetic 17-lactone steroid, acts as a non-selective mineralocorticoid receptor antagonist with moderate affinity for both progesterone and androgen receptors [15]. Spironolactone is predominantly utilized in clinical practice as a potassium-sparing diuretic, however it has been used off-label for acne since the 1980s. A reduction in sebum may be achieved by blocking dihydrotestosterone binding to the androgen receptor within sebocytes and inhibiting androgen-induced sebocyte proliferation [16, 17]. The systemic effects of spironolactone on adrenal synthesis of androgen precursors may also contribute to clinical efficacy, although at therapeutic doses this may be unlikely [18]. The diuretic effect of spironolactone may benefit women who experience a premenstrual acne flare associated with fluid retention [19].
Successful long-term management of acne in adult women presents a considerable therapeutic challenge. As an anti-androgen and potential inhibitor of sebogenesis, spironolactone represents a possible alternative to oral isotretinoin and combined oral contraceptives (COCs), the only licensed anti-acne medications that significantly reduce sebum secretion, but which may be associated with serious adverse effects in some patients [20, 21]. Antibiotics are often over-prescribed in acne, drive antimicrobial resistance in targeted and non-targeted bacteria, and have no effect on sebum synthesis [22].
A Cochrane review focusing primarily on hirsutism included only one randomized controlled trial (RCT) of oral spironolactone for acne in its analyses and concluded there was insufficient evidence for effectiveness in treating acne [23]. In contrast, a narrative review, based largely on clinical experience, highlighted the potential therapeutic usefulness of oral spironolactone in the management of acne in adult females, and detailed recommendations about appropriate use and monitoring during therapy [24].
Take-home messages from these different reviews are contradictory. In view of this clinical uncertainty, we conducted a hybrid systematic review of all studies that had assessed the clinical efficacy of oral spironolactone for acne in women. The primary aim was to determine whether oral spironolactone monotherapy produces a degree of improvement in acne that is clinically important and comparable to conventional drug-based remedies. Secondary aims were to identify evidence that could better inform clinicians on the selection of patients likely to benefit, and/or reveal the most appropriate dosing regimen.
Methods
The protocol for this review was published in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/) with the accession number 42016038496.
Search Strategies
Electronic searches were conducted between 10 and 15 May 2016 and included the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, Science Citation Index and LILACS. The search strategy for MEDLINE and EMBASE is shown in Appendix 1 (electronic supplementary material). The following trials registers were searched using the search terms spironolactone AND acne or ‘polycystic ovarian syndrome’.
metaRegister of Controlled Trials (www.controlled-trials.com);
US National Institutes of Health Ongoing Trials Register (http://www.clinicaltrials.gov);
Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch);
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/).
The reference lists of all identified RCTs and key review articles were checked for citations to potentially relevant studies. No language or date restrictions were applied.
Outputs of searches were imported into Rayyan to facilitate sorting [25], and full-text copies of all potentially eligible studies were obtained. Two authors (AE and ZF) independently assessed the full-text papers and resolved any disagreements on the eligibility of included studies through discussion and consensus, or through a third party (EvZ).
Inclusion Criteria
We included RCTs in females of any ethnicity over 18 years of age with acne vulgaris of the face and/or trunk, or PCOS if acne status or severity was measured as an outcome. Case series were included if they provided supplementary evidence on the benefits or side effects.
Outcome Measures
The following primary outcomes were prespecified: (1) physician-assessed change in total lesion count; and (2) physician-assessed change in global acne severity using a recognized or validated scale. Prespecified secondary outcomes were (1) participant-reported improvement in global acne severity (e.g. Likert scale); (2) change in health-related quality of life (HRQOL) assessed using any validated instrument (generic, dermatology-specific, or acne-specific); (3) number and proportion of participants reporting each type of adverse event; (4) duration of remission post-treatment; and (5) time to improvement (as assessed by either primary outcome or patient-reported outcome at time points within the first 8 weeks).
Data Extraction
Data extraction using piloted forms, risk of bias assessments and analyses were carried out independently by three authors (AE, ZF and EvZ) and any disagreements were resolved by consensus. Risk of bias assessments for the RCTs were made using the Cochrane domain-based risk of bias tool and were used to support conclusions regarding the overall quality of evidence in the review [26]. Data were analyzed using RevMan version 5.3 [27]. Dichotomous outcomes were expressed as risk ratios (RRs) and were reported with their associated 95% confidence interval (CI), while continuous outcomes were reported as mean differences (MDs) with 95% CI. Attempts, although only partially successful, were made to obtain missing trial details by contacting the lead investigators of the studies. The protocol specified that data would be reanalyzed according to the intention-to-treat (ITT) principle; however, for the majority of trials this was not possible due to inadequate or incomplete reporting. Therefore, in general, the per-protocol (PP) population was used. Analyses of side effect rates were conducted using the ITT population with and without acne. For numbers included in each type of analysis, see Electronic Supplementary Table 1. When available, and unless otherwise stated, assessments at month 3 were used as the basis of comparison between studies. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) software (GRADEpro GDT) was used to rate the quality of evidence from RCTs for the individual outcomes and to produce summary of findings tables [28]. Following recommendations in the GRADE handbook, case series are considered to provide low quality evidence and are often further downgraded to very-low-quality evidence [29]. Data from case series were not reanalyzed but were pooled if the studies were clinically similar.
Results
Study Characteristics
Full details of the study selection process are reported in Fig. 1. We identified 10 RCTs [30–39], 18 case series in which acne status or severity was an outcome [40–52, 54, 55, 57, 59, 60], and three [53, 56, 58] articles reporting on the side effects of spironolactone in female patients with acne, but which contained no data on clinical outcomes. Eleven studies [61–71] were out of scope and five were unobtainable [72–76] (see Electronic Supplementary Table 2).
Fig. 1.
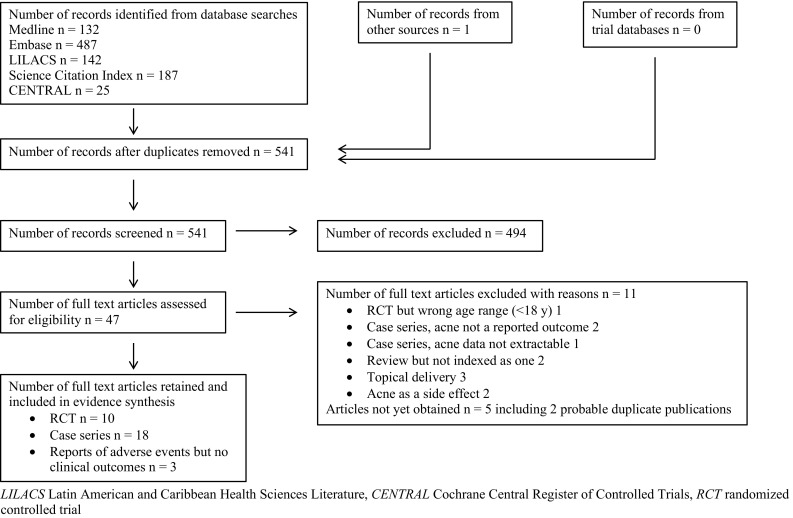
Study flow diagram
Randomized Controlled Trials (RCTs)
All 10 RCTs were single-center studies that had been conducted in Canada (1), UK (2), India (3), Bangladesh (1), Thailand (1), Israel (1) and China (1). Baseline acne severity ranged from mild to severe and was not reported in four trials [30, 32, 34, 39]. The sites affected by acne were reported for seven trials and always included the face [31–33, 35, 36, 38, 39]. Of the four trials that included males, two reported results for females separately [33, 39] and two did not [31, 36]. Six trials did not mention any sources of funding [31, 33, 36–39], one was funded by a manufacturer of spironolactone [30], two were funded by non-industrial sponsors [34, 35] and one received no support from a pharmaceutical company [32]. A spironolactone manufacturer supplied the active and placebo treatment in two trials [31, 37]. Declarations stating no conflicts of interest were provided for two trials [32, 35]. For further details, see Table 1.
Table 1.
Characteristics of included RCTs
| Study ID | Age range, years | Comparator | Concomitant medications | Dose of spironolactone | Duration of therapy, months | Primary diagnosis | Diagnoses excluded | Main clinical outcome measure (acne) | Blinding |
|---|---|---|---|---|---|---|---|---|---|
| Cusan et al. [30] | 19–40 | Flutamide 250 mg bid + levonorgestrel/EE | Levonorgestrel/EE | 50 mg bid on days 5–25 of menstrual cycle | 9 | Hirsutism, idiopathic or associated with polycystic ovaries | No ovarian/adrenal tumors, no other identifiable medical problem | Combined score for acne, seborrhea and alopecia [77] | Blinded assessment of clinical outcomes |
| Goodfellow et al. [31] | 18–38 | Placebo | None | 50, 100, 150 or 200 mg/day | 3 | Acne | NR | Subjective improvement in acne severity (Likert scale) | Double-blind |
| Hagag et al. [32] | 18–30 | (a) Norgestimate/EE; (b) cyprotetone acetate/EE + 10 mg cyproterone acetate | Norgestimate/EE | 100 mg/day for 21/28 days | 12 | Hirsutism associated with PCOS | Endocrine disorders predisposing to acne | Acne score using the Burke and Cunliffe grading method [81] | No details provided |
| Hatwal et al. [33] | 14–25 | Cimetidine 1.6 g/day in divided doses | None | 100 mg/day | 3 | Recalcitrant acne | NR | Acne score using the Michaëlsson method [78] | Trial described as ‘open’ by the authors |
| Kriplani et al. [34] | 16–40 | Finasteride 5 mg/day + cyproterone acetate/EE | Cyproterone acetate/EE | 100 mg/day | 12 | Hirsutism, idiopathic or associated with PCOS | Endocrine disorders predisposing to acne | Acne score using the Indian grading system [79] | Blinded assessment of clinical outcomes |
| Leelaphiwat et al. [35] | 20–35 | Cyproterone acetate/EE | Desogestrel/EE | 25 mg/day for 21/28 days | 3 | PCOS | Endocrine disorders predisposing to acne | Acne score using the global acne grading system [80] | No details provided |
| Mansurul and Islama [36] | 10–40 | Placebo (vitamin B1) | None | 25 mg bid | 3 | Acne | Cushing’s syndrome, steroid acne | Inflamed lesion count (NR) | Double-blind |
| Muhlemann et al. [37] | NR | Placebo | Six women taking an unspecified oral contraceptive | 200 mg/day | 3 | Acne | NR | Inflamed lesion count | Double-blind |
| Vaswani and Pandhi [38] | 12–25 | Cimetidine 1.4 mg/day | None | 100 mg/day for 21/28 days | 3 | Acne | Hirsutism | Inflamed and non-inflamed lesion counts | No details provided |
| Wang et al. [39] | 16–33 | (a) ketoconazole 200 mg/day initially; (b) cimetidine 400 mg tid initially; (c) tetracycline 250 mg qid initially |
Topical treatment with unspecified active | 20 mg tid | 2 | Acne | Endocrine disorders, gynecological problems | Acne lesion count (unclear if non-inflamed and inflamed were included) | No details provided |
RCTs randomized controlled trials, bid twice daily, EE ethinyl estradiol, PCOS polycystic ovarian syndrome, tid three times daily, qid four times daily, NR not reported
aIn this trial, treatment allocation was quasi-random (odd versus even numbers)
Risk of Bias in Included RCTs
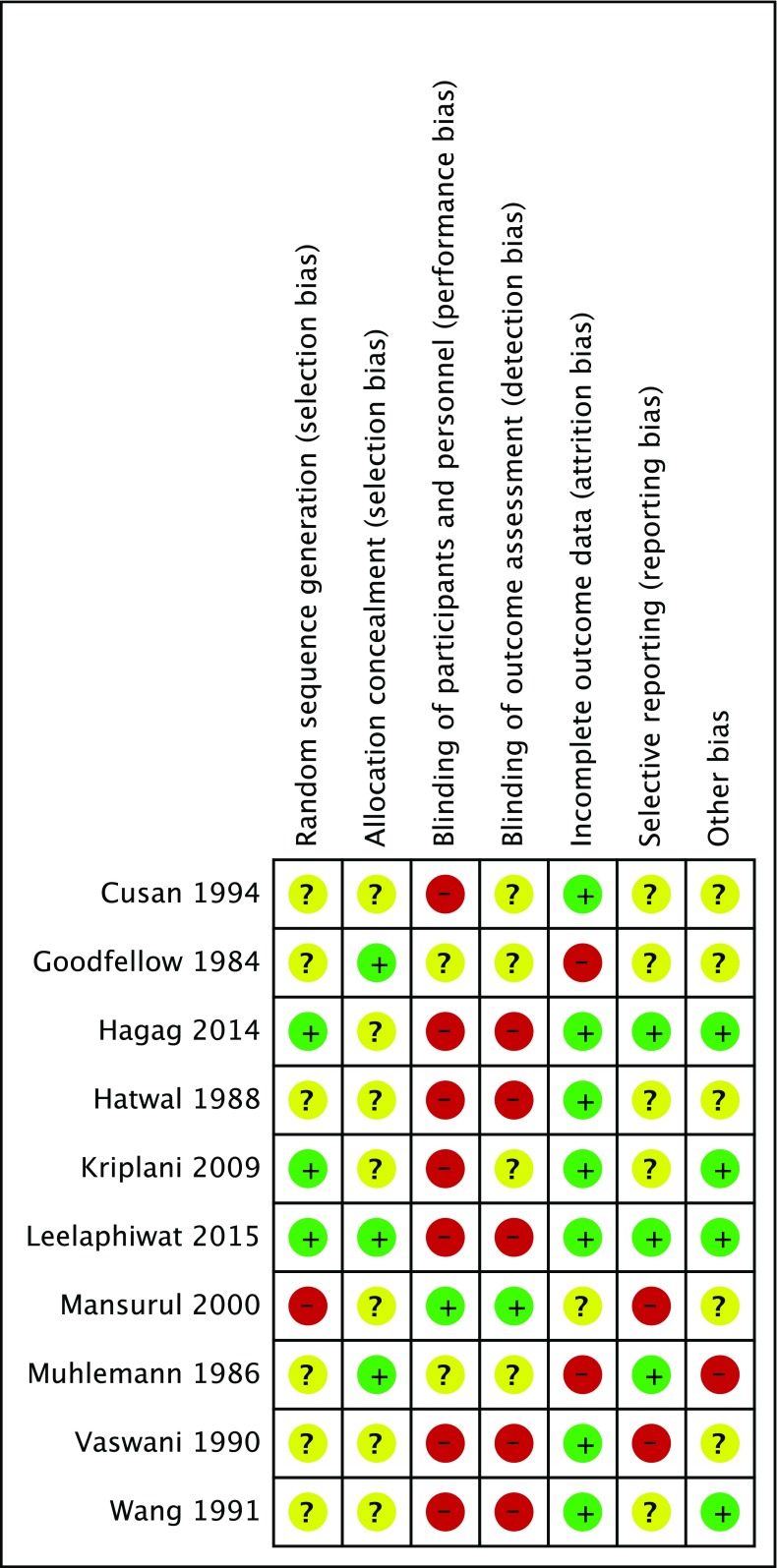
All of the trials were considered to be at ‘high risk of bias’ (plausible bias that seriously weakens confidence in the results) because one or more domains, most commonly lack of blinding, received a judgment of high risk (see Fig. 2).
Fig. 2.
Risk of bias summary: authors’ judgments about each risk of bias item for every randomized controlled trial included. + indicates low risk, − indicates high risk, ? indicates unclear risk of bias
Use of Outcome Measures Within the RCTs
Heterogeneity of outcome measures and methods of reporting meant that pooling of data from different trials versus the same comparator was not feasible. Only two studies [31, 37] reported the same outcome in the same way for a similar patient population. Of our primary outcomes, four trials included a lesion count [36–39] and five included an investigator-assessed change in global acne severity, all but one of which used recognized but different severity scales [32–35]. Of our secondary outcomes, three trials included participant-assessed global improvement [31, 32, 37], none measured changes in HRQOL and only two included a post-treatment follow-up [30, 34]. One trial included time points prior to month 3, theoretically enabling calculation of time to improvement [30].
All but one trial reported side effects in full; one reported side effects associated with discontinuation only [34]. Only two trials reported the number of women experiencing any side effect [32, 38]. Seven trials monitored changes in serum potassium (risk of hyperkalemia), but none reported the number of women with raised levels as a result of treatment [31–33, 35, 36, 38, 39].
Effect of the Interventions
The ten RCTs included 16 comparisons of spironolactone versus placebo or active treatment. The quality of the evidence was assessed for all comparisons and was downgraded by several levels, principally due to limitations in study design and implementation, and imprecision due to low sample sizes, and was consequently rated low or very low for our predefined outcomes. Inadequate data reporting did not permit calculation of RRs or MDs for any of the outcomes in four trials [30, 31, 34, 36].
Spironolactone versus Placebo
Three trials evaluated this comparison [31, 36, 37]. One provided useful data for a 200 mg daily dose in a crossover trial that examined 29 women [37]. For the inflamed lesion count, the MD in favor of spironolactone was 26.1 lesions fewer, despite baseline imbalance in favor of the placebo (p < 0.00001; PP population of 21, both phases combined). Data for the first phase, which would be free of any potential carryover effects, were not reported. Combined data from both phases showed that 18/21 women taking spironolactone, compared with 5/21 taking placebo, reported improvement (RR 3.6, 95% CI 1.64–7.89; p = 0.001), and 15/20 versus 4/20 had at least a 50% reduction in inflamed lesion count (RR 3.75, 95% CI 1.51–9.34; p = 0.005). In a mixed gender RCT [36], 24/30 participants receiving spironolactone (50 mg/day, including 27 women) improved, compared with 2/25 receiving placebo (PP population); separate data were unavailable for females. A similar problem arose in the third trial, which also included males [31]. Data could not be extracted for three women receiving the 50 mg dose, but 6/9 women receiving doses of 100–200 mg/day improved, irrespective of which outcome measures were used (PP population). In neither mixed gender trial was the extent of improvement quantified. For details and quality of evidence, see Table 2.
Table 2.
Summary of findings for spironolactone versus placebo
| Spironolactone (50, 100, 150, 200 mg/day and 25 mg bid) compared with placebo for adult females with acne vulgaris | |||||
|---|---|---|---|---|---|
| Patient or population: females over 18 years of age with acne vulgaris. Mansurul and Islam [36] included an unknown number of women under 18 years of age and who could not be excluded from the analyses. Ages were not reported by Muhlemann et al. [37] but participants were described as women implying they were over 18 years of age Intervention: Spironolactone (50, 100, 150, 200 mg/day and 25 mg bid) Comparison: Placebo | |||||
| Outcomes | Anticipated absolute effectsa (95% CI) | No. of participants (no. of studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with spironolactone (50, 100, 150, 200 mg/day and 25 mg bid) | ||||
| Physician-assessed change in total lesion count (inflamed lesions) Follow-up: mean 12 weeks |
The mean physician-assessed change in total lesion count (inflamed lesions) was 1.2 (10.75) inflamed lesions | The mean physician-assessed change in total lesion count (inflamed lesions) in the intervention group was 26.1 inflamed lesions fewer (34.74 fewer to 17.46 fewer) | 21 (1 RCT) | ⊕◯◯◯ Very lowb,c,d |
Usable data were only obtained from the study by Muhlemann et al. [37] for a dose of 200 mg/day. Treatment with spironolactone was more effective than placebo for inflamed lesions (p < 0.00001). Mansurul and Islam [36] failed to report data for the inflamed lesion count |
| Physician-assessed change in global acne severity Follow-up: mean 12 weeks |
In the study by Goodfellow et al. [31], 6/9 females receiving spironolactone (≥100 mg/day) had improved (extent not reported). No data were available for females receiving placebo. In the study by Mansurul and Islam [36], 24/30 patients receiving spironolactone (27 women, 3 men) versus 2/25 receiving placebo (including up to six men) had less acne (extent unclear) In the study by Muhlemann et al. [37], data from both phases combined, expressed as the number with at least 50% reduction in lesion count, showed 15/20 patients in the spironolactone group versus 4/20 in the placebo group, with a RR of 3.75 (95% CI 1.51–9.34; p = 0.005). This outcome was not assessed for one woman in the PP population |
34 + an unknown number of females in the study by Mansurul and Islam [36] (3 RCTs) | ⊕◯◯◯ Very lowd,e,f |
Data reporting was limited and incomplete in all three studies. Goodfellow et al. [31] did not report how many females were randomized to each of five arms, and only one woman receiving placebo completed. Mansurul and Islam [36] did not report how many women were included in the PP population for the placebo group | |
| Participant-reported improvement in global acne severity Follow-up: mean 12 weeks |
In the study by Goodfellow et al. [31], 6/9 women receiving spironolactone ≥ 100 mg reported improvement (extent not specified) In the study by Muhlemann et al. [37], data from both phases combined, and assessed on a 5-point Likert scale (improved = better or much better), showed 18/21 considered themselves improved while receiving spironolactone versus 5/21 in the placebo group, with an RR of 3.6 (95% CI 1.64–7.89; p = 0.001) |
34 (2 RCTs) | ⊕◯◯◯ Very lowd,g |
In the study by Goodfellow et al. [31], it was not possible to determine how many women receiving the 50 mg dose improved | |
| Change in health-related quality of life–not measured | – | – | – | – | None of the studies assessed this outcome |
| Number and proportion of participants reporting each type of adverse event throughout the study period Follow-up: mean 12 weeks |
In the study by Goodfellow et al. [31], 7/12 subjects reported metrorrhagia, 2 reported polyuria, and 1 reported depression. The text is silent as to which dose(s) these side effects were associated with. In the study by Mansurul and Islam [36], 13 women reported polymenorrhea, and 1 or 2 reported diarrhea. No side effects were reported for the placebo group in either trial. In the study by Muhlemann et al. [37], 11/15 spironolactone-treated patients not using an oral contraceptive reported menstrual irregularity, 3/21 reported nausea, 1 reported dizziness, 2 reported breast enlargement, and 9 reported less greasy skin and hair. No side effects were reported for the placebo phase | 34 + an unknown number of females in the study by Mansurul and Islam [36] (3 RCTs) |
⊕◯◯◯ Very lowd,f |
The reported effects of spironolactone on the menstrual cycle are well known. No unexpected side effects were reported. RRs could not be calculated as the number of women experiencing any event was not reported in any of the three RCTs | |
| Duration of remission post- treatment – not measured | – | – | – | – | None of the studies assessed this outcome |
| Time to improvement within the first 8 weeks – not measured | – | – | – | – | None of the studies assessed this outcome |
GRADE Working Group grades of evidence: High quality (⊕⊕⊕⊕): We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality (⊕⊕⊕◯): We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect but there is a possibility that it is substantially different. Low quality (⊕⊕◯◯): Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality (⊕◯◯◯): We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
bid twice daily, RCT randomized controlled trial, CI confidence interval, PP per-protocol, GRADE Grading of Recommendations Assessment, Development and Evaluation, RR risk ratio
aThe risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
bDowngraded two levels for very serious risk of bias as there was attrition bias (27.6%) and baseline imbalance. Combining data from both phases may have reduced the magnitude of the difference in lesion count reduction, which was already large, if there had been carryover of the spironolactone effect after the washout period had ended
cAlthough only inflamed lesions were counted, and therefore not exactly meeting our outcome, we chose not to downgrade as we had already downgraded for risk of bias and imprecision
dDowngraded one level for serious imprecision, small sample size
eDowngraded two levels for very serious risk of bias. The studies by Goodfellow et al. [31] and Muhlemann et al. [37] were at high risk for attrition bias, while the study by Mansurul and Islam [36] was at high risk for selection bias and selective reporting
fAlthough the data reported did not exactly meet our prespecified outcome, we have already downgraded for risk of bias and imprecision
gDowngraded two levels for very serious risk of bias. There was attrition bias in both studies (27.8% and 27.6%) and baseline imbalance in the study by Muhlemann et al. [37]
Spironolactone versus Cimetidine
Three trials compared spironolactone with cimetidine using different daily doses of one or both drugs and different outcome measures [33, 38, 39]. One also used a shorter treatment duration (2 months) and an unspecified topical therapy in both arms [39]. Despite these inconsistencies, none detected a difference in efficacy between the two drugs. The earliest trial [33] found no difference in acne severity score between spironolactone (100 mg/day) and cimetidine (1.6 g/day) [MD −4.20, 95% CI −17.48 to 9.08; p = 0.54] using the Michaëlsson scale [78]. Similarly, the second study [38] found no difference between the same dose of spironolactone and cimetidine 1.4 g/day, using reduction in inflamed and non-inflamed lesions as the outcome measure. MDs were −3.3 inflamed lesions (95% CI −8.14 to 1.54; p = 0.18) and −10.70 non-inflamed lesions (95% CI −24.08 to 2.68; p = 0.12). Lesion counts were also converted to a categorical improvement in 11/15 women receiving spironolactone and 6/14 women receiving cimetidine, showing at least a 50% reduction in lesions (RR 1.71, 95% CI 0.87–3.37; p = 0.12). In the final trial [39], a lower dose of spironolactone (60 mg/day) was not significantly different to cimetidine for the number of participants with at least 50% reduction improvement (physician-assessed) at an initial dose of 1.2 g/day (RR 0.96, 95% CI 0.89–1.03; p = 0.25). For details and quality of evidence, see Electronic Supplementary Tables 3 and 4.
Spironolactone Plus a Combined Oral Contraceptive (COC) versus the Same or a Different COC Alone or Combined with an Anti-Androgen
Among the remaining comparisons, four compared spironolactone in combination with a COC versus a COC alone [35] or combined with an anti-androgen: flutamide [30], finasteride [34] or additional cyproterone acetate [32]. One trial compared 25 mg/day spironolactone plus desogestrel/ethinyl estradiol (EE) versus cyproterone acetate/EE [35]. Assessed using the global acne grading system [80], both treatments were similarly effective in reducing the acne severity score from baseline, with an MD of −2.0 (95% CI −4.59 to 0.59; p = 0.13). For details and quality of evidence, see Electronic Supplementary Table 5.
A second trial compared spironolactone (100 mg/day) plus norgestimate/EE with (1) norgestimate/EE alone and (2) cyproterone acetate/EE plus 10 mg/day additional cyproterone acetate [32]. Randomization was 3:3:1, with fewer women in the norgestimate/EE arm. After 12 months of treatment, no significant difference was reported in the proportion of women with at least 50% improvement, using the Burke and Cunliffe acne grading system [81], between the three arms. Participants’ self-assessed improvement confirmed these data. For details and quality of evidence, see Electronic Supplementary Tables 6 and 7.
The remaining two trials employed multiple assessment time points, which, in one case, included the first and second month of treatment [30], and included post-treatment follow-up for 6 months (Table 4). Neither conducted any intergroup tests of significance and both presented data graphically, with no measures of dispersion of mean values, therefore MDs could not be calculated. Hence, the studies provided limited usable data. Using the Indian grading system [79], one study [34] found no difference in the rate of improvement or magnitude of the reduction in severity score for spironolactone plus cyproterone acetate/EE compared with finasteride plus the same COC (p > 0.05 for all time points, investigators’ calculation). The reduced acne severity score (10% of baseline value at month 12) was maintained almost unchanged during the 6-month post-treatment follow-up period. The other trial [30] compared spironolactone plus levonorgestrel/EE versus flutamide plus the same COC using the Cremoncini score [77], which included seborrhea and alopecia as well as acne. The score fell more rapidly in the flutamide plus COC arm and the reduction in score, which was maximal by month 3 in both groups, was also greater for the comparator (50% vs. 85% reduction; statistical significance not reported by the investigators. At month 9 (end of the treatment phase), 5/10 or 7/10 in the spironolactone arm (investigators’ text unclear) versus 11/12 in the flutamide arm had a lower severity score [30]. Relapse occurred over 6 months in both arms. For further details and quality of evidence, see Electronic Supplementary Tables 8 and 9.
Table 4.
Summary of common and very common adverse side effects of spironolactone (≥1% of the ITT population for RCTs and/or case series)
| Side effect | RCTs (eligible ITT population = 326 unless stated) | Case series (eligible ITT population = 663 unless stated) | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Menstrual irregularities | 38 | 13.4 (of 283) | 216 | 33.4 (of 646) |
| Breast tenderness | 8 | 2.5 | 30 | 4.5 |
| Breast enlargement | 7 | 2.1 | 13 | 2.0 |
| Dizziness/vertigo/lightheadedness | 11 | 3.4 | At least 19a | ≥2.9 |
| Headache | 5 | 1.5 | At least 10a | ≥1.5 |
| Nausea with/without vomiting | 6 | 1.8 | 24 | 3.6 |
| Weight gainb | 5 | 1.5 | 1 | 0.2 |
| Abdominal pain | 0 | 0 | At least 11a | ≥1.7 |
| Polyuria | 2 | 0.6 | 8 | 1.2 |
| Fatigue/lethargy | 1 | 0.3 | At least 12a | ≥1.8 |
ITT intention to treat, RCTs randomized controlled trials
aPrecise figures not available due to inadequate reporting
bNot monitored in most studies
Spironolactone versus Ketoconazole and Tetracycline
Spironolactone (60 mg/day) was compared with (1) 200 mg/day ketoconazole and (2) oral tetracycline at an initial de-escalating dose of 1 g/day [39]. All three treatments were similarly effective using the proportion of women with at least 50% improvement in lesion count as the outcome measure (Table 4). However, as the numbers of participants in each treatment arm were not equal, the study may not have been sufficiently powered to detect a difference between spironolactone (n = 63) and tetracycline (n = 14). For details and quality of evidence, see Electronic Supplementary Tables 10 and 11.
Supplementary Efficacy Data from Case Series and Comparison with RCTs
Of the 18 case series, 13 were in English, two in Spanish, and one each in French, Czech and Turkish. As well as these, three additional articles included some data on side effects in women with acne [53, 56, 58]; they did not address clinical effectiveness. One definite case series [74] (in Portuguese) and two possible case series (in Czech) including acne patients treated with spironolactone [72, 75] were unobtainable and had either no abstract or an uninformative abstract. Two further unobtainable articles, one in Spanish [73] and one in Turkish [76], had abstracts containing sufficient information to identify them as duplicate publications of two included case series [44, 60].
Acne severity ranged from mild to severe and was not reported in nine of the case series [41, 43, 45, 46, 50–52, 54, 55]. The location of lesions was reported in only three case series [48, 54, 57]. Seven studies did not make a clear statement about concomitant medications, making attribution of any clinical effect to spironolactone uncertain [41, 43–45, 49, 51, 55]. Further details are reported in Table 3. Insufficient data were provided to conduct any subgroup analyses on the effect of dose or duration of spironolactone therapy on efficacy or side effects. Within the case series, between 216 and 259 of 728 women (29.7–35.6%) were receiving daily doses of ≥150 mg, compared with only 35/343 women (10.2%) in the RCTs.
Table 3.
Characteristics of the included case series
| Study ID | Prospective? | What was reported | Age range, years | Primary diagnosis | Secondary diagnoses | Diagnoses excluded | Dose of spironolactone | Duration of therapy, months | Concomitant medications |
|---|---|---|---|---|---|---|---|---|---|
| Azizlerli et al. [40] | Yes (clarified by email) | Efficacy and safety | 18–33 | Acne | Hirsutism 9, menstrual irregularities 7 | NR | Starting at 200 mg and reducing to 100 or 50 mg/day over 5 months | 1–18 (mean 9) | Levonorgestrel/EE 12 |
| Beksac et al. [41] | Unclear | Efficacy and safety | 18–27 | Idiopathic hirsutism | Therapy resistant acne 7, menstrual irregularities 6 | Obesity, major abnormalities in serum androgens | 50 mg bid (n = 16); 50 mg/day (n = 6) from days 5 to 26 of the menstrual cycle | 9 | NR |
| Bravo Garcia et al. [42] | Unclear | Efficacy and safety | 12–37 | Acne | Polycystic ovaries 35, idiopathic hyperandrogenemia 18, hirsutism and/or irregular menses 23 | Hyperthyroidism, hyperprolactinemia, congenital adrenal hyperplasia, Cushing’s syndrome, ovarian and adrenal tumors |
50 mg bid from days 5 to 21 of the menstrual cycle | 6 | None permitted |
| Burke and Cunliffe [43] | Unclear (probably retrospective) | Efficacy | NR | Acne 8, hirsutism 12, alopecia 7 | Primary diagnoses were mutually exclusive | NR | 200 mg/day | 6 | NR |
| Cortez de Castro et al. [44] | Unclear | Efficacy and safety | 18–44 | Persistent recalcitrant acne | None reported Patients were otherwise healthy | No evidence of endocrinopathies, no menstrual irregularities | 2 × 25 mg bid (12 h apart) from start of menstruation for 15 days | 1–11 | NR |
| Hana et al. [45] | Unclear | Efficacy and safety | 24–34 | Hirsutism | Seborrhea 6, Abnormal menses 5, PCOS 4, adrenal carcinoma 1, acromegaly 1 |
NR | 75 mg/day | 24–34 | NR |
| Hughes and Cunliffe [46] | No | Efficacy (not properly reported) and safety | 21–51 | Hirsutism 24, acne 21, acne and hirsutism 8, seborrhea 1 | NR | NR | 200 mg/day initially, reduced in 8 patients as not tolerated | 1–45 | Unspecified oral contraceptive in 23 patients |
| Krunic et al. [47] | Yes | Efficacy and safety (latter not properly reported) | 18–43 | Severe papulo-pustular or nodulo-cystic acne that had failed to respond to at least one standard treatment | None reported | Pre-existing hyperkalemia, liver or kidney disease, diabetes mellitus | 100 mg od in the morning | Up to 6 | Drospirenone/EE. Previously prescribed topical acne treatments continued |
| Lessner et al. [48] | No | Efficacy and safety | 19–57 | Cyclical late-onset acne vulgaris (i.e. acne worsening premenstrually, with lesions predominantly on the lower face and neck) |
NR | Receiving oral or topical antibiotics or treated with PDT | Initial dose 50 mg/day, escalated to 200 mg/day as and when necessary in 25 mg increments every 3 months. 11 patients were increased to 75 mg, and 4 were increased from 75 to 100 mg | 2–102 | Majority also treated with topical tretinoin Or Adapalene™ at bedtime. Most had been receiving the topical retinoid prior to starting spironolactone |
| Lubbos et al. [49] | Unclear | Efficacy and safety | 14–38 | Idiopathic acne | Oligomenorrhea 13 | NR | 50 mg bid on days 5–21 of the menstrual cycle | 2–49 Therapy continued unless ‘significant’ adverse effects occurred |
NR |
| Masahashi et al. [50] | Unclear | Efficacy and safety | 21–36 | Hyperandrogenism | Oligomenorrhea 8, amenorrhea 9, acne and oily skin 5, oily skin 1, hirsutism 1, hirsutism 9, oily skin 1 | NR | 100–150 mg/day from day 5 of the menstrual cycle, then continuously | 2–11 (mean 4.4) | None 7, clomiphene (100 mg/day for 5 days from week 4) 10, plus bromocriptine (1.5–2.5 mg/day from week 8) in 3 patients |
| Messina et al. [51] | No | Efficacy (not properly reported) and safety | 19–33 | Hirsutism without any other sign of virilization | Acne (unknown number), seborrhea (unknown number), PCOS 1 All but the PCOS case had regular menses |
Hirsutism associated with congenital adrenal hyperplasia or androgen-secreting tumor | Group A (n = 10): 400 mg/day for first 10 days then 200–300 mg. Group B (n = 8): 200 mg/day continuously Therapy started on the fifth day of the menstrual cycle in both groups |
Up to 8.3 | NR |
| Pugeat et al. [52] | Unclear | Efficacy and safety | 16–42 | Hirsutism | Amenorrhea 8 | ‘Associated pathologies’ | 75 mg/day from the start of menstruation Demegestone 0.5 mg on days 14–24 of the cycle added from month 7 | 12 | None permitted |
| Plovanich et al. [53] | No | Safety (serum electrolyte data only) | 18–45 | Acne | Endocrine disorders including PCOS, hirsutism, alopecia, and hyperandrogenism 298 | Heart failure, renal failure, renal disease | 50–200 mg/day (personal communication) | NR | NR |
| Saint-Jean et al. [54] | Unclear | Efficacy and safety | >20 | Recalcitrant acne | One patient had PCOS, 5 had irregular menses, 4 reported a premenstrual flare | NR | 75–150 mg/day | Mean 17 months | A topical acne treatment (not specified) |
| Sato et al. [55] | Yes | Efficacy and safety | 15–46, both genders | Acne | NR | NR | 200 mg/day for first 8 weeks, then reduced by 50 mg every 4 weeks | 5 months | NR |
| Shaw [56] | No | Safety (serum electrolyte data only) | NR | Acne | NR | NR | 50–150 mg/day | NR | NR |
| Shaw [57] | No | Efficacy and safety | 18 –52 | Inflammatory papular or nodular acne in 51/85 cases of adult onset; recalcitrant in 76 cases | Hormonal influence 68, hirsutism 16, menstrual flare 23, history of ovarian cysts 4 | NR | 50–100 mg/day | 2–24 (mean 10) | Oral antibiotics, COC (norethindrone/EE), or both. 17 patients (20%) were treated with spironolactone alone; 46 (54%) were treated with a combination of spironolactone and systemic antibiotics; 10 (12%) received spironolactone plus oral contraceptives; 12 (14%) received spironolactone plus antibiotics and oral contraceptives |
| Shaw and White [58] | No | Safety | 18–52 | Adult acne | NR | NR | 50–100 mg/day | 0.5–122 (mean 28.5) | Topical therapies, systemic antibiotics or oral contraceptives |
| Turowksi and James [59] | No | Efficacy and safety | 18–59 | Recalcitrant acne | NR | NR | 50–100 mg, decreasing according to response. One patient receiving 200 mg/day | Mean 19.5 | Trimethoprim 2, cotrimoxazole 3 or amoxicillin 2 initiated at the same time as spironolactone. Concomitant topical medications (started earlier) included topical retinoid 22, azelaic acid 4 and benzoyl peroxide 11. 14 or 15 women were already taking a COC, 2 were using a depot hormonal contraceptive, and 4 were started on a COC |
| Yemisci et al. [60] | Yes | Efficacy and safety | 18–31 | Acne in adult females | NR | Pregnant women, women using oral contraceptives or other drugs with possible effects on hormone levels, and women with irregular menstruation or hirsutism were excluded | 50 mg bid on days 5–21 of the cycle | 3 | Not permitted |
od once daily, bid twice daily, COC combined oral contraceptive, EE ethinyl estradiol, PCOS polycystic ovarian syndrome, PDT photodynamic therapy, NR not reported
The most commonly used outcome measure was physician-assessed global improvement in acne severity, which was reported in all but three case series [46, 49, 54]. Some used a 4- or 5-point Likert-like improvement scale, whereas others simply recorded improved/not improved without further categorization. Dichotomizing the pooled data shows that acne improved (to any extent) in 427/550 women (77.6%, ITT population) receiving spironolactone at any dose. Using the PP population, the proportion who improved was 427/454 (94.1%). These improvement rates are significantly higher than in the RCTs that also assessed this outcome (164/213, 76.1%; PP population, not calculable for ITT population due to incomplete reporting; RR 1.22, 95% CI 1.13–1.32; p < 0.00001).
The only other clinical outcomes reported in the case series were change in acne grade [43, 59], change in lesion count [54, 60] and post-treatment relapse rate [40, 60]. Using their own grading method [81], Burke and Cunliffe [43] observed a 35% reduction in acne severity at month 3, and an average 52% reduction at month 6, in eight women receiving a spironolactone dose of 200 mg/day (no measures of dispersion or p values were reported). Turowski and James [59] recorded a dramatic improvement in acne score in 39 women, from a median of two pretreatment to eight post-treatment (no measure of dispersion) on a scale of 1–10 (lower is worse). Most of the women in this study, which used spironolactone doses of 50–100 mg/day, were receiving concomitant medications and were treated for an average of 19.5 months. In the study by Yemisci et al. [60], the mean lesion count in 28 women (PP population) had decreased by month 3, from 32.86 (standard deviation [SD] 16.15) to 6.92 (SD 4.99), a reduction of 78.9% with spironolactone monotherapy at 100 mg/day (p < 0.001, investigators’ calculation). Furthermore, in the study by Saint-Jean et al. [54], the mean inflamed lesion count fell from 9.0 to 4.6, and the non-inflamed lesion count fell from 15.0 to 8.5 (no measures of dispersion and no p-values) in 14 women treated with a spironolactone dose of 75–150 mg/day for an average of 17 months. Two studies [46, 51] did not report any quantifiable outcomes data and two followed-up patients after treatment ended [40, 60]. In the study by Azizlerli et al. [40], 5 of 14 women (35.7%) followed for 3–18 months relapsed, while in the study by Yemisci et al. [60], acne returned in 5/28 women (17.9%) during the 6-month follow-up period. Interestingly, Bravo Garcia et al. [42] observed that residual acne post-treatment in 23/30 women was purely comedonal, whereas only 10/53 had exclusively comedonal acne prior to treatment (p < 0.00001, Chi-squared).
Side Effects Reported in RCTs and Case Series
None of the studies carried a clear statement as to how information on side effects had been elicited, e.g. spontaneous reporting versus an open-ended question at each visit. Within the RCTs, there was no difference in the proportion of participants who dropped out due to side effects: 14/303 (4.6%) receiving spironolactone at any dose versus 10/343 (2.9%) receiving the comparators (RR 1.58, 95% CI 0.71–3.52; p = 0.26). Dropout rates due to side effects could not be calculated for two trials [31, 36]. In the case series, 49/729 (6.7%) women dropped out due to the side effects of spironolactone (ITT population). This was not significantly different to the rate in the RCTs (RR 0.69, 95% CI 0.39–1.23; p = 0.20).
Due to inadequate reporting in 8/10 RCTs, the proportion of women experiencing any side effect(s) could not be calculated [30, 31, 33–37, 39]. In the case series, at least 241 women experienced side effects equivalent to 48.0% of the PP and 43.9% of the ITT population. Reported rates varied from 0 to 90.7%, with the highest rates associated with the 200 mg/day dose [46, 55]. The most common side effect in both the RCTs and case series was menstrual irregularities: 38/264 (14.4%) in the RCTs and 216/543 (39.8%) in the case series (RR 0.36, 95% CI 0.26–0.49; p < 0.00001). From the case series, it was apparent these were dose-related: 137/176 women receiving 200 mg/day experienced menstrual disturbances, compared with 66/349 on lower daily doses (RR 4.12, 95% CI 3.27–5.19; p < 0.00001).
Within the RCTs, it was possible to compare the incidence of menstrual disturbances in women receiving spironolactone with and without concomitant use of a COC. The incidence appeared significantly lower when spironolactone was combined with a COC: 32/146 without a COC versus 6/112 with a COC (RR 0.24, 95% CI 0.11–0.56; p = 0.001). This was also observed by Hughes and Cunliffe [46], who studied the incidence of side effects in a series of 53 women receiving a dose of 200 mg/day. The number of women with menstrual irregularities was 12/23 receiving a COC versus 21/24 receiving spironolactone monotherapy (RR 0.60, 95% CI 0.39–0.91; p = 0.02). In a minority of women with abnormal menses, spironolactone therapy was reported to normalize the menstrual cycle [41, 52, 57].
Other commonly reported side effects in the RCTs and case series are shown in Table 4. No side effect, apart from menstrual disturbances, had an incidence above 5% in the RCTs or case series. Uncommon side effects (0.1–1.0%) were postural hypotension, depression, diarrhea, muscle pain, increased appetite, drowsiness, rashes/drug eruptions, chloasma-like skin pigmentation, polydipsia, weakness, edema of the legs, change in libido, and palpitations. Some investigators mentioned that certain side effects were considered beneficial: breast enlargement, reduced symptoms of premenstrual syndrome, and less greasy skin and hair [31, 46, 57]. Due to the use of concomitant medications, especially COCs, in many of the studies, side effects could not be unambiguously attributed to spironolactone. Among the 10 case series of spironolactone monotherapy in 370 women in which concomitant therapies were not mentioned [41, 43–45, 49, 51, 55] or were not permitted [42, 52, 60], the only common side effects reported were nausea (11), abdominal pain (≥10), polyuria (4) and breast tenderness (4).
Risk of Hyperkalemia
Serum electrolytes were measured in 7/10 RCTs [31–33, 35, 36, 38, 39] involving 157 women (PP population) and 10/18 case series [41, 45, 47, 49–52, 55, 57, 60] involving 312 women (PP population) as a means of detecting possible adverse effects of spironolactone on fluid balance and kidney function. Serum electrolyte data reporting was inadequate in almost all cases and no results were presented for two RCTs that monitored this [36, 38]. No women in the remaining five RCTs were reported to have elevated levels. Fourteen women (4.5%) in two case series [51, 57], one that used doses ≥200 mg/day (4/18) and the other 50–100 mg/day (10/73), were reported to have slightly raised levels post-treatment. In an earlier study (not one of the 18 case series), serum potassium was measured in otherwise healthy women with acne treated with 50–150 mg/day of spironolactone [56]. Mild hyperkalemia was detected in 6/60 (10%) women. It is impossible to determine whether the women in the later study were a separate group to those in the earlier study, and thus whether these were independent samples. Most recently, Plovanich et al. [53] conducted a retrospective analysis of serum potassium levels in 974 women aged 18–45 years taking 50–200 mg/day of spironolactone for acne, seen in two US hospitals between December 2000 and March 2014. Among 1802 measurements, 13 were slightly elevated, yielding a mild hyperkalemia rate of 0.72%, similar to the expected baseline rate of 0.76% derived from all available serum potassium measurements for females of the same age (32/4209). Repeat testing in 6/13 women yielded values within the normal range, suggesting initial measurements could have been erroneous.
Discussion
Two authors of this review (JDR, AML) confirm that, based on their extensive experience of successful use of the drug to treat large numbers of women with persistent or late-onset acne, spironolactone is pivotal to their clinical practice. Such experience suggests that the important role of spironolactone in this hard-to-manage patient population has been largely underrecognized and provided the rationale for this systematic review. What the review has highlighted is a paucity of high-quality evidence for the effectiveness of oral spironolactone in the management of acne in adult females. The results should not be misinterpreted to mean that the drug is ineffective at safe dosages, but rather that there is a lack of robust evidence in support of expert opinion, which currently drives treatment recommendations either for or against more widespread use.
Every one of the 10 RCTs was at high risk of bias, with the most common reason being lack of blinding. Even in the more recent trials, sample sizes were not justified by reporting of power calculations or the assumptions on which they had been based. This lack of difference in efficacy between treatments might have been due, at least in part, to insufficient power. Moreover, as no single outcome measure had been used consistently across the trials, pooling of data from similar comparisons was not possible.
The trials fell into two categories: (1) comparisons of spironolactone monotherapy versus placebo; and (2) comparisons of mono or combination therapy versus active treatment. GRADE assessments of the quality of the evidence showed that all of the comparisons versus placebo were rated ‘very low quality’ and all of the comparisons versus active therapy were of low or very low quality. With one exception [39], the choices of active comparator did not include standard oral therapies such as antibiotics or isotretinoin, nor was there any direct head-to-head comparison of spironolactone monotherapy with a COC of proven anti-acne efficacy, such as cyproterone acetate plus ethinyl estradiol.
Despite the somewhat limited evidence available, some tentative conclusions can still be drawn. First, at a daily dose of 200 mg, spironolactone was found to be significantly superior to placebo versus inflamed lesions in a crossover trial in which 21 of 29 women completed 3 months of treatment. Baseline imbalance and a possible carryover effect of spironolactone into the second phase would have reduced the magnitude of the difference in efficacy between spironolactone and placebo, which was already large. This statistically significant result was not reported by the authors of a frequently cited Cochrane review, which evaluated the effects of spironolactone for hirsutism and acne [23]. Equally, the Cochrane review may have also inadvertently misled authors of subsequent reports by stating that “there was no evidence for effectiveness [of spironolactone] for the treatment of acne vulgaris”. In contrast to the crossover trial, no conclusions can be drawn from the two parallel group comparisons versus placebo regarding the absolute efficacy of lower doses of spironolactone. Spironolactone appeared to be more effective than placebo, but by how much was not quantified and indeed the difference may not have been clinically significant. The very-low-quality evidence provided by the case series consistently shows that lower doses do have a measure of anti-acne activity, but they contributed no information on relative efficacy.
The trials that assessed comparative efficacy versus the anti-androgens flutamide, finasteride, cimetidine, ketoconazole and various COCs consistently found no difference between the spironolactone arm and the anti-androgen arm. With the exception of the COCs for which anti-acne efficacy has been established beyond doubt [83], independent verification of the efficacy of the comparators is scarce and/or contradictory [84–92]. What these anti-androgens have in common with spironolactone and COCs is the expectation that they will reduce sebum secretion at the doses used via their effects on androgen synthesis, action or sequestration (see Table 5). Demonstrating no difference in efficacy versus these agents is unhelpful in terms of quantifying the effect size for spironolactone, especially as some of the trials most likely had too few participants to detect a significant difference, if in fact such a difference existed.
Table 5.
Summary of the modes of action in acne of spironolactone and anti-androgens used as comparators or concomitant medications within the RCTs
| Drug | Proposed mode of action | Main mechanism | Other relevant actions | Androgenicity of progestin component |
|---|---|---|---|---|
| Spironolactone | Suppression of sebum excretion at ≥100 mg/day [31, 33] | AR antagonist/very weak partial agonist | Steroidogenesis inhibitora (specifically 17α-hydroxylase and 17, 20-lyase), possibly only at supraphysiological doses in man. Data from over 50 articles reporting effects on serum androgens are equivocal. One report showed inhibition of 5α-reductase type I in genital skin—relevance to acne at therapeutic doses unclear | NA |
| Cimetidine | Suppression of sebum excretion at doses ≥1 g/day [82, 84, 93] | AR antagonist | Inhibits 2-hydroxylation of estradiol, thereby reducing testosterone production | NA |
| Cyproterone acetate/EE (Diane-35™) | Suppression of sebum excretion [94–99] | AR antagonist/partial agonist | Steroidogenesis inhibitor (mainly 3β-hydroxysteroid dehydrogenase and 17, 20-lyase); suppresses gonadotrophin release; increases hepatic SHBG production but does not bind to it | None |
| Desogestrel/EE (Marvelon™) | Suppression of sebum excretion [94, 99–103] | Steroidogenesis inhibitor | Suppresses gonadotrophin release, thereby decreasing ovarian androgen output; increases hepatic SHBG production (significantly more than levonorgestrel). Active metabolite binds to SHBG | Low-affinity agonist |
| Levonorgestrel/EE (Triphasil™) | Suppression of sebum excretion (no in vivo evidence in humans) | Steroidogenesis inhibitor | Suppresses gonadotrophin release, thereby decreasing ovarian androgen output; increases hepatic SHBG production and binds to it with high affinity | Agonist (more potent than desogestrel and much more potent than norgestimate) |
| Norgestimate/EE (Ortho-Cyclen™) | Suppression of sebum excretion [100, 104] | Steroidogenesis inhibitor | Suppresses gonadotrophin release, thereby decreasing ovarian androgen output; increases hepatic SHBG production; possible 5α-reductase inhibitor. Almost no binding to SHBG | Very-low-affinity agonist |
| Finasteride | Suppression of sebum excretion (no in vivo evidence in humans) | Competitive inhibitor of 5α-reductase types II and III | None. Does not bind to AR. Evidence for inhibition of sebaceous gland 5α-reductase (types I and III) in human skin not yet obtained | NA |
| Flutamide | Suppression of sebum excretion (no in vivo evidence in humans) | AR antagonist: first-pass metabolite (2-OH-flutamide) responsible for activity | Pure anti-androgen. No effect on other hormone receptors Steroidogenesis inhibitor (specifically 17α-hydroxylase and 17, 20-lyase) | NA |
| Ketoconazole | Suppression of sebum excretion at ≥200 mg/day [105, 106] | Steroidogenesis inhibitor (primarily of 17α-hydroxylase and 17, 20-lyase) | Low-affinity AR antagonist at supraphysiological doses. Reduces serum androgen levels at doses of 400 mg/day and over | NA |
AR androgen receptor, SHBG sex hormone binding globulin, NA not applicable, EE ethinyl estradiol, RCTs randomized controlled trials
Information on modes of action and androgenicity was complied from Schmidt [107], Carr [108], del Marmol et al. [109] and Schindler et al. [110]
aInhibition of steroidogenesis in the adrenals, ovaries and/or peripheral tissues, including skin, reduces serum levels of androgens and androgen precursors
Four of the RCTs evaluated spironolactone in combination with a COC. Hirsutism or PCOS was the primary diagnosis and acne was the secondary diagnosis. Since COCs are potent anti-androgens, and are effective as monotherapy for acne, superiority of the combination needs to be demonstrated over the COC alone. Despite this, only one trial included a COC monotherapy arm and found no benefit of adding spironolactone to norgestimate/EE [32], although, at trend, the combination was more effective. In this three-arm trial, treatment allocation was unbalanced, with three times as many women in the combination arm as those receiving COC monotherapy. Any added benefit of spironolactone might have been revealed had treatment arms been of equal size.
One potentially more useful trial put spironolactone head-to-head against oral tetracycline and found no difference in efficacy over 8 weeks of treatment [39]; however, caution is necessary in interpreting the results as this trial was also unbalanced, with 63 women receiving spironolactone but only 14 receiving tetracycline. Interestingly, the duration of therapy was consistently short (2–3 months) for those RCTs in which acne was the primary diagnosis, and much longer (9–12 months) for the trials in which hirsutism was the primary diagnosis. After only 3 months of treatment, the response of acne to spironolactone may not be optimal. The case series show that clinicians often use longer courses to manage acne in the real world. Using the PP population, improvement rates were significantly higher in the case series compared with those RCTs that had also used an outcome measure that could be dichotomized. This difference in efficacy rates may be an indication that longer treatment is likely to be more successful.
Only two RCTs conducted time courses with post-treatment follow-up [30, 34]. One found [30] that improvement was maximal at month 3 (approximately 50% reduction vs. baseline), whereas the other showed that improvement continued until month 12, when the reduction was 89% [34]. Both used 100 mg/day of spironolactone in combination with a COC—the former in combination with triphasic levonorgestrel/EE and the latter in combination with cyproterone acetate/EE. In the follow-up phase, acne returned to baseline levels over 6 months in those treated with spironolactone plus levonorgestrel/EE, but there was no relapse in those treated with cyproterone acetate/EE. The data for the spironolactone plus levonorgestrel/EE combination may be somewhat misleading as a non-validated scoring method which combined acne with seborrhea and alopecia was used [77]. Neither study included a COC-only or spironolactone-only treatment arm, making it impossible to determine the contribution of spironolactone to the efficacy of the combination.
Two studies made apparently contradictory observations in respect of the efficacy of spironolactone against comedonal (non-inflamed) lesions. One case series found that residual acne was more likely to be comedonal [42], whereas an RCT showed what appeared to be a large reduction in comedonal acne compared with baseline and the comparator [38]; however, the lack of reporting of baseline data for the number of lesions in the RCT did not permit fair comparisons to be made. As only these two studies evaluated comedones, it is not possible to know whether spironolactone monotherapy is effective versus non-inflamed lesions. Expert reviews [24, 56], commentaries [111], and acne treatment guidelines that include spironolactone, such as the recent US guidelines [112], are silent on this important point. ‘Hormonal acne’ is widely perceived as predominantly inflammatory with a paucity of comedones; however, such cases represent a minority of women with acne and, in most instances, comedones will be present [113].
Dropout rates due to side effects in spironolactone-treated participants were low in the RCTs and case series, suggesting that most women who begin the drug will continue to take it. However, side effect rates were significantly higher for the 200 mg/day dose than for lower doses. This was especially true for menstrual irregularities, the most common side effect reported in the RCTs and case series. Pooling of data has shown that the rate of menstrual irregularities can be significantly reduced by concomitant use of a COC, a practice that is widely recommended by experts [24, 56, 111]. However, experts also recommend dose escalation [24, 56, 111, 114, 115], which, paradoxically, was rarely used in the included trials or case series as a potential, but as yet untested, means of improving tolerance and adherence.
A recent multicenter study in 974 women [53] concluded that routine monitoring of serum potassium in healthy women taking spironolactone for acne is not necessary. The findings from this systematic review support that conclusion. Occasional testing may be justified on a case-by-case basis when risk factors are present. Although 14 women with raised potassium levels were identified among 469 women in the RCTs and case series, hyperkalemia was invariably mild and clinically insignificant. Some of the side effects of spironolactone, notably nausea, fatigue, and especially muscle weakness, can be indicative of hyperkalemia and, if persistent, could be used to indicate patients in whom testing may be justified. Crucially, the non-requirement for routine testing would reduce the overall direct costs of spironolactone treatment.
While endorsing key aspects of expert opinion with hard data and providing some new insights, this systematic review has highlighted existing important gaps in the evidence about how best to utilize oral spironolactone in managing acne in women, including clarifying the optimum dose and dosing regimen to maximize benefits and minimize the risk of side effects, the lowest effective dose, the possible requirement for concomitant therapies and what these should be, which types of acne are likely to be responsive, and how effective spironolactone is compared with standard therapies. It is interesting to note that several current acne guidelines and treatment recommendations include spironolactone on the basis of consensus and/or expert opinion [112, 116, 117]. Others either do not mention spironolactone [118–121], or specifically say it is regarded as ineffective, not recommended [122, 123] or there is insufficient evidence to support its use [124]. All, including those that purport to be evidence-based, have failed to identify the majority of studies that were included in this systematic review. Although there were five relevant studies that, to date, have proved unobtainable (two of which were almost certainly duplicate publications of included case series), we are confident that all the RCTs evaluating spironolactone for acne in women have been retrieved and no clinical trial evidence has been overlooked. Until such time as higher quality evidence becomes available, guideline developers will have to continue to rely on recommendations largely based on expert experience or reached via consensus of expert panels. While this review has identified some very-low-quality evidence which showed that the 200 mg daily dose was statistically significantly more effective than placebo versus inflamed lesions, it has also confirmed that this dose is associated with a significantly greater risk of adverse side effects than lower doses. Hence, there would appear to be no merit in using these higher doses for managing acne, except in exceptional circumstances (e.g. in obese women with PCOS). Data from the multiple case series suggest that any future RCT examining lower doses is likely to generate results that confirm the effectiveness and better safety profile of doses ≤100 mg/day.
The findings of this systematic review have several key implications for future research. First, there is an urgent need for a well-designed, adequately powered RCT versus placebo, preferably of monotherapy, so that it is possible to establish whether spironolactone is effective against inflamed and non-inflamed lesions without concomitant use of a topical agent that would inhibit comedogenesis (see Table 6). Women who are stably maintained on an oral contraceptive can be considered for inclusion in such a trial as long as they remain on the oral contraceptive throughout and have been taking it sufficiently long enough for any anti-acne effect to be maximalized. If such a study confirms the utility of spironolactone, then head-to-head comparisons versus widely used oral therapies (antibiotics, COCs, isotretinoin) could follow. The need for, and utility of, combinations could also be explored. Within these trials, validated outcome measures should be carefully selected and any dose-related effect on sebum secretion should be explored early on. Monitoring serum androgens within such RCTs is unnecessary unless the trial is intended to identify subgroups of women less/more likely to benefit based, at least in part, on a combination of hormone profiles and clinical presentation.
Table 6.
Summary of findings from this review, and recommendations for future research to fill the evidence gap: EPICOTa
| Element | Issues to consider | Status of research for this review and recommendations |
|---|---|---|
| Disease burden | Acne is the eighth most common disease globally, with peak prevalence in late adolescence. Acne in adult women is often recalcitrant to conventional medications, associated with a high degree of emotional distress and sometimes accompanied by hyperandrogenemia and/or other signs of peripheral hyperandrogenism, such as hirsutism and alopecia | |
| Evidence (E) | What is the current evidence? | This systematic review identified 10 RCTs and 21 case series that provided some evidence of the benefit and potential harms of oral spironolactone for acne in adult females. The most frequently reported outcome measure was physician-reported improvement in acne severity. Lesion counts were reported for two RCTs and none of the case series. Patient-assessed outcomes were reported for three RCTs and none of the case series Results from one RCT of crossover design at high risk of bias showed that spironolactone at a daily dose of 200 mg was significantly more effective than placebo against inflamed lesions (low-quality evidence, GRADE). Evidence for lower doses with respect to comparative efficacy versus placebo or active comparators was equivocal and of low or very low quality. There was some very-low-quality evidence that menstrual irregularities, the most common side effect observed in RCTs and case series, are dose-related and can be minimized by concomitant use of a combined oral contraceptive. Although serum potassium levels were measured in 7 RCTs and 12 case series, inadequate reporting meant that it was not possible to draw robust conclusions regarding the need for routine monitoring of hyperkalemia at any dose up to 200 mg/day in this patient population |
| Study type | What is the most appropriate study design to address the proposed question? | RCT |
| Population (P) | Diagnosis, disease stage, comorbidity, risk factors, gender, age, ethnic group, specific inclusion or exclusion criteria, clinical setting | Inclusion criteria: Premenopausal females aged 18 years and over with persistent or late-onset acne vulgaris Acne severity defined at baseline, e.g. at least moderate severity (IGA score of 3 on a 0–5 scale), with a minimum of 15 inflamed and 15 non-inflamed lesions on the face Women with PCOS can be included. In addition, women with additional signs of peripheral hyperandrogenism can also be included as long as the endocrinopathies listed below have been excluded Exclusion criteria: Pregnant or intending to become pregnant Androgen-secreting adrenal or ovarian tumor Cushing’s syndrome, late-onset congenital adrenal hyperplasia Unwilling to stop oral and topical anti-acne medications prior to the baseline visit Unwilling to use a barrier method of contraception for the duration of the study |
| Intervention (I) | Type, frequency, dose, duration, prognostic factor | Oral spironolactone at an initial dose of 25 or 50 mg/day, escalating as and if necessary to 100 mg/day after 6–8 weeks depending on response. Total treatment duration not <3 months, and preferably 6 months. It is recommended that concomitant topical therapy is not permitted for any study versus placebo (comparison one below) |
| Comparison (C) | Type, frequency, dose, duration, prognostic factor | In order of priority: 1. Matching placebo 2. An oral antibiotic of known magnitude of effect on acne 3. A combined oral contraceptive with low androgenicity of known magnitude of effect on acne |
| Outcome (O) | Which clinical or patient-related outcomes will the researcher need to measure, improve, influence, or accomplish? Which methods of measurement should be used? | 1. Change in the number of acne lesions (inflamed and non-inflamed) 2. Participant-assessed global improvement in acne severity using a Likert-like scale with photographic anchor at baseline 3. Change in HRQOL assessed using any validated or recognized quality-of-life instrument or as part of a validated patient-reported outcome measure 4. Proportion of participants who reported an adverse effect (putative drug-related adverse event) throughout the study period; number and type of adverse effects 5. Change in sebum excretion rate on the face using a validated method Note clinical outcomes and measures (1–3) may be subject to change as a result of ongoing work by the Acne Core Outcomes Research Network |
| Timelines | Time aspects of core elements: | |
| Age of population | 18 years and over; premenopausal | |
| Duration of intervention | At least 3 months, preferably 6 months | |
| Length of follow-up | At least 3 months, preferably 6 months (ideally with topical maintenance therapy) | |
| Time stamp (T) | Date of literature search or recommendation | November 2016 |
GRADE Grading of Recommendations Assessment, Development and Evaluation, RCTs randomized controlled trials, IGA Investigator’s Global Assessment, HRQOL health-related quality of life
aBrown et al. [125]
Conclusions
This systematic review has revealed a lack of high-quality evidence on the benefits and potential harms of oral spironolactone for managing acne in women. However, it has shown that (1) there is low-quality, but statistically highly significant, evidence that 200 mg/day effectively reduces inflamed lesion counts; (2) side effects, in particular menstrual irregularities, are dose-related; and (3) concomitant use of a COC significantly reduces the incidence of menstrual disturbances. It has also confirmed the recommendation of Plovanich et al. [53] that routine potassium monitoring is largely unnecessary unless risk factors are present.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Helen Weir, Library Manager at Harrogate District Hospital, for her help with literature searches and the retrieval of articles; Kwan Bevan for translation of the article by Wang et al. [39] from Mandarin to English; and Mohammed Delowar for making it possible to obtain the publication by Mansurul and Islam [36].
Compliance with Ethical Standards
Funding
No funding was received for the preparation of this review.
Conflict of interest
None of the authors of this article have received funding or payments of any kind from the manufacturers of any of the products included in this review.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s40257-016-0245-x) contains supplementary material, which is available to authorized users.
References
- 1.Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- 2.Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(Suppl 1):3–8. doi: 10.1159/000354887. [DOI] [PubMed] [Google Scholar]
- 3.Kim GK, Michaels BD. Post-adolescent acne in women: more common and more clinical considerations. Drugs Dermatol. 2012;11:708–713. [PubMed] [Google Scholar]
- 4.Perkins AC, Maglione J, Hillebrand GG, Miyamoto K, Kimball AB. Acne vulgaris in women: prevalence across the life span. J Women’s Health (Larchmt). 2012;21:223–230. doi: 10.1089/jwh.2010.2722. [DOI] [PubMed] [Google Scholar]
- 5.Tan JK, Li Y, Fung K, Gupta AK, Thomas DR, Sapra S, et al. Divergence of demographic factors associated with clinical severity compared with quality of life impact in acne. J Cutan Med Surg. 2008;12:235–242. doi: 10.2310/7750.2008.07053. [DOI] [PubMed] [Google Scholar]
- 6.Dréno B, Layton A, Zouboulis CC, Lopez-Estebaranz JL, Zalewska-Janowska A, Bagatin E. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063–1070. doi: 10.1111/jdv.12061. [DOI] [PubMed] [Google Scholar]
- 7.Di Landro A, Cazzaniga S, Cusano F, Bonci A, Carla C, Musumeci ML, et al. Adult female acne and associated risk factors: results of a multicenter case-control study in Italy. J Am Acad Dermatol. 2016;75(6):1134–1141.e1. [DOI] [PubMed]
- 8.Callender VD, Alexis AF, Daniels SR, Kawata AK, Burk CT, Wilcox TK, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7(7):19–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672–690. doi: 10.1016/j.jaad.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 10.da Cunha MG, Fonseca FL, Machado CD. Androgenic hormone profile of adult women with acne. Dermatology. 2013;226:167–171. doi: 10.1159/000347196. [DOI] [PubMed] [Google Scholar]
- 11.Falsetti L, Gambera A, Andrico S, Sartori E. Acne and hirsutism in polycystic ovarian syndrome: clinical, endocrine-metabolic and ultrasonographic differences. Gynecol Endocrinol. 2002;16:275–284. doi: 10.1080/gye.16.4.275.284. [DOI] [PubMed] [Google Scholar]
- 12.Khondker L, Khan SI. Acne vulgaris related to androgens: a review. Mymensingh Med J. 2014;23:181–185. [PubMed] [Google Scholar]
- 13.Bergfeld WF. The pathophysiology of acne vulgaris in children and adolescents. Part 1. Cutis. 2004;74:92–97. [PubMed] [Google Scholar]
- 14.Kistowska M, Meier B, Proust T, Feldmeyer L, Cozzio A, Kuendig T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110–118. doi: 10.1038/jid.2014.290. [DOI] [PubMed] [Google Scholar]
- 15.Garthwaite SM, McMahon EG. The evolution of aldosterone antagonists. Mol Cell Endocrinol. 2004;217:27–31. doi: 10.1016/j.mce.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Berardesca E, Gabba P, Ucci G, Borroni G, Rabbiosi G. Topical spironolactone inhibits dihydrotestosterone receptors in human sebaceous glands: an autoradiographic study in subjects with acne vulgaris. Int J Tissue React. 1988;10:115–119. [PubMed] [Google Scholar]
- 17.Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5-alpha dihydrotestosterone in vitro. J Invest Dermatol. 1993;100:660–662. doi: 10.1111/1523-1747.ep12472325. [DOI] [PubMed] [Google Scholar]
- 18.Menard RH, Guenthner TM, Kon H, Gillette JR. Studies on the destruction of adrenal and testicular cytochrome P-450 by spironolactone. Requirement for the 7α-thio group and evidence for the loss of the heme and apoproteins of cytochrome P-450. J Biol Chem. 1979;254:1726–1733. [PubMed] [Google Scholar]
- 19.Williams M, Cunliffe WJ. Explanation for premenstrual acne. Lancet. 1973;2:1055–1057. doi: 10.1016/S0140-6736(73)92661-5. [DOI] [PubMed] [Google Scholar]
- 20.Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502–515. doi: 10.1016/j.clindermatol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ganceviciene R, Zouboulis CC. Isotretinoin: state of the art treatment for acne vulgaris. J Dtsch Dermatol Ges. 2010;8(Suppl 1):47–59. doi: 10.1111/j.1610-0387.2009.07238.x. [DOI] [PubMed] [Google Scholar]
- 22.Del Rosso JQ, Webster GF, Rosen T, Thiboutot D, Leyden JJ, Gallo R, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: Part 1. Antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18–24. [PMC free article] [PubMed]
- 23.Brown J, Farquhar C, Lee O, Toomath R, Jepson RG. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;(2):CD000194.
- 24.Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37–50. [PMC free article] [PubMed] [Google Scholar]
- 25.Rayyan, the systematic reviews web app. https://rayyan.qcri.org/.Accessed 3 Nov 2106.
- 26.The Cochrane Collaboration’s tool for assessing risk of bias. In: Higgins JPT, Green S (eds). The Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011)]. The Cochrane Collaboration, 2011. www.handbook.cochrane.org. Accessed 3 Nov 2106.
- 27.Review Manager (RevMan) (computer program). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- 28.GRADE profiler version 3.6. http://tech.cochrane.org/revman/other-resources/gradepro/download. Accessed 3 Nov 2106.
- 29.Quality of evidence: In: Schünemann H, Brożek J, Oxman A (eds). GRADE: Handbook for grading quality of evidence and strength of recommendation. Updated October 2013. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. Accessed 3 Nov 2106.
- 30.Cusan L, Dupont A, Gomez JL, Tremblay RR, Labrie F. Comparison of flutamide and spironolactone in the treatment of hirsutism: a randomized controlled trial. Fertil Steril. 1994;61:281–287. doi: 10.1016/S0015-0282(16)56518-2. [DOI] [PubMed] [Google Scholar]
- 31.Goodfellow A, Alaghband-Zadeh J, Carter G, Cream JJ, Holland S, Scully J, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111:209–214. doi: 10.1111/j.1365-2133.1984.tb04045.x. [DOI] [PubMed] [Google Scholar]
- 32.Hagag P, Steinschneider M, Weiss M. Role of the combination spironolactone-norgestimate-estrogen in hirsute women with polycystic ovary syndrome. J Reprod Med. 2014;59:455–463. [PubMed] [Google Scholar]
- 33.Hatwal A, Bhatt RP, Agrawal JK, Singh G, Bajpai HS. Spironolactone and cimetidine in treatment of acne. Acta Derm Venereol. 1988;68:84–87. [PubMed] [Google Scholar]
- 34.Kriplani A, Thulkar J, Agrawal N, Kulshrestha V, Ammini AC, Kumar G. A comparative study of Diane-35 plus spironolactone and Diane-35 plus finasteride in cases of hirsutism and acne. Int J Endocrinol Metab. 2009;7:235–241. [Google Scholar]
- 35.Leelaphiwat S, Jongwutiwes T, Lertvikool S, Tabcharoen C, Sukprasert M, Rattanasiri S, et al. Comparison of desogestrel/ethinylestradiol plus spironolactone versus cyproterone acetate/ethinylestradiol in the treatment of polycystic ovary syndrome: a randomized controlled trial. J Obstet Gynaecol Res. 2015;41:401–410. doi: 10.1111/jog.12543. [DOI] [PubMed] [Google Scholar]
- 36.Mansurul A, Islam AZM. Effect of spironolactone on acne vulgaris: a double blind study. Bangladesh J Dermatol Venereol Leprol. 2000;17:1–4. [Google Scholar]
- 37.Muhlemann MF, Carter GD, Cream JJ, Wise P. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227–232. doi: 10.1111/j.1365-2133.1986.tb05722.x. [DOI] [PubMed] [Google Scholar]
- 38.Vaswani N, Pandhi RK. Treatment of acne vulgaris with anti-androgens. Indian J Dermatol Venereol Leprol. 1990;56:34–36. [Google Scholar]
- 39.Wang XM, Zhang XP, Li CH. Comparative observation on the therapeutic effect of acne vulgaris with spironolactone, ketoconazole and cimetidine. J Clin Dermatol. 1991;20:303–305. [Google Scholar]
- 40.Azizlerli G, Özarmagan G, Taklifi B, Südogan S, Azizlerli H. Spironolactone therapy in acne patients [Aknede spironolatone ile tedavi] Deri Hastalıkları ve Frengi Arşivi. 1988;22:125–129. [Google Scholar]
- 41.Beksac MS, Önderglu LS, Atac B. Idiopathic hirsutism with spironolactone. Adv Contracept Deliv Syst. 1990;6:265–269. [Google Scholar]
- 42.Bravo Garcia M, Barroeta S. Mejia de Alejos M, Franco de Arias M, Yustiz MA, Mascia S. Use of spironoactone in patients with acne [Uso de spironolactona en pacientes con acne] Arch Argent Dermatol. 1987;37:231–238. [Google Scholar]
- 43.Burke BM, Cunliffe WJ. Oral spironolactone therapy for female patients with acne, hirsutism or androgenic alopecia. Br J Dermatol. 1985;112:124–125. doi: 10.1111/j.1365-2133.1985.tb02305.x. [DOI] [PubMed] [Google Scholar]
- 44.Cortez de Castro G, Pacheco A, Naranjo H, Palacios A. Spironolactone in thetreatment of acne [Espironolactona en el tratamiento del acne]. DermatologiaVenezolana. 1986;24:103–5.
- 45.Hana V, Marek J, Gregorova I. Long-term treatment of hirsutism with spironolactone [Dlouhodoba lecba hirsutismu spirolaktonem] Casopis Lekaru Ceskych. 1984;123:1273–1275. [PubMed] [Google Scholar]
- 46.Hughes BR, Cunliffe WJ. Tolerance of spironolactone. Br J Dermatol. 1988;118:687–691. doi: 10.1111/j.1365-2133.1988.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 47.Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60–62. doi: 10.1016/j.jaad.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Lessner E, Fisher S, Kobraei K, Osleber M, Lessner R, Elliot L, et al. Spironolactone and topical retinoids in adult female cyclical acne. J Drugs Dermatol. 2014;13:126–129. [PubMed] [Google Scholar]
- 49.Lubbos HG, Hasinski S, Rose LI, Pollock J. Adverse effects of spironolactone therapy in women with acne. Arch Dermatol. 1998;134:1162–1163. doi: 10.1001/archderm.134.9.1162-a. [DOI] [PubMed] [Google Scholar]
- 50.Masahashi T, Wu MC, Ohsawa M. Spironolactone therapy for hyperandrogenic anovulatory women. Clinical and endocrinological study. Acta Obstet Gynaecol Jpn. 1986;38:95–101. [PubMed]
- 51.Messina M, Manieri C, Biffignandi P, Massucchetti C, Novi RF, Molinatti GM. Antiandrogenic properties of spironolactone. Clinical trial in the management of female hirsutism. J Endocrinol Invest. 1983;6:23–27. doi: 10.1007/BF03350556. [DOI] [PubMed] [Google Scholar]
- 52.Pugeat M, Elmidani M, Dechaud H, Garoscio-Cholet M, Lejeune H, Tourniaire A. Treatment of hirsutism with spironolactone and progestagen combination [Traitement de l’hirsutisme par l’association de spironolactone et d’un progestatif] Presse Med. 1990;19:1529–1532. [PubMed] [Google Scholar]
- 53.Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941–944. doi: 10.1001/jamadermatol.2015.34. [DOI] [PubMed] [Google Scholar]
- 54.Saint-Jean M, Ballanger F, Nguyen JM, Khammari A, Dréno B. Importance of spironolactone in the treatment of acne in adult women. J Eur Acad Dermatol Venereol. 2011;25:1480–1481. doi: 10.1111/j.1468-3083.2010.03926.x. [DOI] [PubMed] [Google Scholar]
- 55.Sato K, Matsumoto D, Iizuka F, Aiba-Kojima E, Watanabe-Ono A, Suga H, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesthet Plast Surg. 2006;30:689–694. doi: 10.1007/s00266-006-0081-0. [DOI] [PubMed] [Google Scholar]
- 56.Shaw JC. Spironolactone in dermatologic therapy. J Am Acad Dermatol. 1991;24:236–243. doi: 10.1016/0190-9622(91)70034-Y. [DOI] [PubMed] [Google Scholar]
- 57.Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498–502. doi: 10.1067/mjd.2000.105557. [DOI] [PubMed] [Google Scholar]
- 58.Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow up study. J Cutan Med Surg. 2002;6:541–545. doi: 10.1177/120347540200600604. [DOI] [PubMed] [Google Scholar]
- 59.Turowski CB, James WD. The efficacy and safety of amoxicillin, trimethoprim-sulphamethoxazole and spironolactone for treatment resistant acne vulgaris. Adv Dermatol. 2007;23:155–163. doi: 10.1016/j.yadr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad DermatolVenereol. 2005;19:163–166. doi: 10.1111/j.1468-3083.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- 61.Anonymous. Different concentrations of spironolactone in the treatment of acne. Chinese J Dermatol. 1989;22:258-9.
- 62.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 63.Devoto E, Aravena L, Rios R. Treatment of hirsutism with spironolactone and with spironolactone plus dexamethasone. Rev Med Chil. 2000;128:868–875. [PubMed] [Google Scholar]
- 64.Ibanez L, Diaz M, Sebastiani G, Lopez-Bermejo A, De F. Oral contraception vs low-dose pioglitazone-spironolactone-metformin for adolescent girls with hyperinsulinaemic androgen excess: on-treatment divergences. Horm Res Paediatr. 2015;84(Suppl 1):251–252. [Google Scholar]
- 65.Jurzyk RS, Spielvogel RL, Rose LI. Antiandrogens in the treatment of acne and hirsutism. Am Fam Phys. 1992;45:1803–1806. [PubMed] [Google Scholar]
- 66.Karrer-Voegeli S, Rey F, Reymond MJ, Meuwly JY, Gaillard RC, Gomez F. Androgen dependence of hirsutism, acne, and alopecia in women. Retrospective analysis of 228 patients investigated for hyperandrogenism. Medicine. 2009;88:32–45. [DOI] [PubMed]
- 67.Messina M, Manieri C, Musso MC, Pastorino R. Oral and topical spironolactone therapies in skin androgenization. Panminerva Med. 1990;32:49–55. [PubMed] [Google Scholar]
- 68.Molinatti GM, Messina M, Biffignandi P, Manieri C. Therapeutic possibilities in women with skin androgenic manifestations [Possibilita terapeutichein donne affette da manifestazionidi androgenizzazione a livello cutaneo] G Ital Dermatol Venereol. 1980;115:579–584. [Google Scholar]
- 69.Pizzino D, Bettoli V, Varotti C. Treatment of acne with antiandrogens: validity of treatment with spironolactone [Trattamento antiandrogeno dell’acne: validata di unaterapia con spirolattone] G Ital DermatolVenereol. 1987;122:599–604. [PubMed] [Google Scholar]
- 70.Shilin DE, Dedov II, Grigor’eva EA. Thetherapeuticeffect of spironolactone in the hyperandrogenism syndrome [Lechebnyi effekt spironolaktona pri sindrome giperandrogenii]. Probl Endokrinol (Mosk).1993;39:55–60. [PubMed]
- 71.Ylöstalo P, Heikkinen J, Kauppila A, Pakarinen A, Järvinen PA. Low-dose spironolactone in the treatment of female hirsutism. Int J Fertil. 1987;32:41–45. [PubMed] [Google Scholar]
- 72.Arenberger P, Vohradnikova O, Zaruba F. Treatment of acne vulgaris with spironolactone. Cesk Dermatol. 1988;63:224–227. [Google Scholar]
- 73.Cortez de Castro G, Pacheco A, Naranjo H, Palacios A. Acnevulgaris: treatment with spironolactone [Acne vulgar: tratamiento con espironolactona]. Salus Militia. 1987;12:34–7.
- 74.Havrenne JED, Halbe HW. Treatment of idiopathic hirsutism with spironolactone [Tratamento do hirsutismo idiopático com espironolactona] Ginecol Obstet Bras. 1985;8:73–74. [Google Scholar]
- 75.Vohradnikova O, Arenberger P, Kratochvilova M, Zaruba F. Treatment of acne vulgaris with spironolactone and Androcur. Cesk Dermatol. 1988;63:308–310. [Google Scholar]
- 76.Yemisci A, Gorgulu A, Piskin S. Spironolactone therapy in acne vulgaris [Acne vulgaris tedavisinde spironolakton] Türkderm Deri Hastaliklari ve Frengi Arivi. 1994;28:209–212. [Google Scholar]
- 77.Cremoncini C, Vignati E, Libroia A. Treatment of hirsutism and acne in women with two combinations of cyproterone acetate and ethinylestradiol. Acta Eur Fertil. 1976;7:299–314. [PubMed] [Google Scholar]
- 78.Michaëlsson G, Juhlin L, Vahlquist A. Effects of oral zinc and vitamin A in acne. Arch Dermatol. 1977;113:31–36. doi: 10.1001/archderm.1977.01640010033003. [DOI] [PubMed] [Google Scholar]
- 79.Adityan B, Thappa DM. Profile of acne vulgaris: a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272–278. doi: 10.4103/0378-6323.51244. [DOI] [PubMed] [Google Scholar]
- 80.Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416–418. doi: 10.1046/j.1365-4362.1997.00099.x. [DOI] [PubMed] [Google Scholar]
- 81.Burke BM, Cunliffe WJ. The assessment of acne vulgaris: the Leeds technique. Br J Dermatol. 1984;111:83–92. doi: 10.1111/j.1365-2133.1984.tb04020.x. [DOI] [PubMed] [Google Scholar]
- 82.Caron P, Chateauneuf C, Bazex J, Louvet JP. Anti-seborrhea effect of cimetidine [Effet anti-séborrhée de cimétidine] Bio Med Pharmacother. 1987;41:253–254. [PubMed] [Google Scholar]
- 83.Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425. [DOI] [PMC free article] [PubMed]
- 84.Jones H, Simpson NB, Blanc D, Forster RA, Cunliffe WJ. Lack of effect of cimetidine in acne. Lancet. 1980;2(8205):1201–1202. doi: 10.1016/S0140-6736(80)92640-9. [DOI] [PubMed] [Google Scholar]
- 85.Wang HS, Wang TH, Soong YK. Low dose flutamide in the treatment of acne vulgaris in women with or without oligomenorrhea or amenorrhea. Changgeng Yi Xue Za Zhi. 1999;22:423–432. [PubMed] [Google Scholar]
- 86.Calaf J, Lopez E, Millet A, Alcañiz J, Fortuny A, Vidal O, et al. Long term efficacy and tolerability of flutamide compared with oral contraception in moderate to severe hirsutism: a 12-month, double-blind, parallel clinical trial. J Clin Endocrinol Metab. 2007;92:3446–3450. doi: 10.1210/jc.2006-2798. [DOI] [PubMed] [Google Scholar]
- 87.Adalatkhah H, Pourfarzi F, Sadeghi-Bazargani H. Flutamide versus a cyproterone acetate-ethinyl estradiol combination in moderate acne: a pilot randomized clinical trial. Clin Cosmet Investig Dermatol. 2011;4:117–121. doi: 10.2147/CCID.S20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carmina E, Lobo RA. A comparison of the relative efficacy of antiandrogens for the treatment of acne in hyperandrogenic women. Clin Endocrinol (Oxf). 2002;57:231–234. doi: 10.1046/j.1365-2265.2002.01594.x. [DOI] [PubMed] [Google Scholar]
- 89.Kohler C, Tschumi K, Bodmer C, et al. Effect of finasteride 5 mg (Proscar) on acne and alopecia in female patients with normal serum levels of free testosterone. Gynecol Endocrinol. 2007;23:142–145. doi: 10.1080/09513590701214463. [DOI] [PubMed] [Google Scholar]
- 90.Venturoli S, Fabbri R, Dal Prato L, Mantovani B, Capelli M, Magrini O, et al. Ketoconazole therapy for women with acne and/or hirsutism. J Clin Endocrinol Metab. 1990;71:335–339. doi: 10.1210/jcem-71-2-335. [DOI] [PubMed] [Google Scholar]
- 91.Vidal-Puig AJ, Muñoz-Torres M, Jódar-Gimeno E, García-Calvente CJ, Lardelli P, Ruiz-Requena ME, et al. Ketoconazole therapy: hormonal and clinical effects in non-tumoral hyperandrogenism. Eur J Endocrinol. 1994;130:333–338. doi: 10.1530/eje.0.1300333. [DOI] [PubMed] [Google Scholar]
- 92.Ghetti P, Patrone P, Tosti A. Ketoconazole in the treatment of acne in women. Arch Dermatol. 1986;122(6):629. doi: 10.1001/archderm.1986.01660180029008. [DOI] [PubMed] [Google Scholar]
- 93.Lyons F, Cook J, Shuster S. Inhibition of sebum secretion by an H2 blocker. Lancet. 1979;1(8131):1376. doi: 10.1016/S0140-6736(79)92011-7. [DOI] [PubMed] [Google Scholar]
- 94.Erkkola R, Hirvonen E, Luikku J, Lumme R, Mannikko H, Aydinlik S. Ovulation inhibitors containing cyproterone acetate or desogestrel in the treatment of hyperandrogenic symptoms. Acta Obstet Gynecol Scand. 1990;69:61–65. doi: 10.3109/00016349009021041. [DOI] [PubMed] [Google Scholar]
- 95.Falsetti L, Galbignani E. Long-term treatment with the combination ethinylestradiol and cyproterone acetate in polycystic ovary syndrome. Contraception. 1990;42:611–619. doi: 10.1016/0010-7824(90)90002-D. [DOI] [PubMed] [Google Scholar]
- 96.Greenwood R, Brummitt L, Burke B, Cunliffe WJ. Acne: double blind clinical and laboratory trial of tetracycline, oestrogen-cyproterone acetate, and combined treatment. Br Med J (Clin Res Ed). 1985;291:1231–1235. doi: 10.1136/bmj.291.6504.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller JA, Wojnarowska FT, Dowd PM. Anti-androgen treatment in women with acne: a controlled trial. Br J Dermatol. 1986;114:705–716. doi: 10.1111/j.1365-2133.1986.tb04880.x. [DOI] [PubMed] [Google Scholar]
- 98.vanVloten WA, van Haselen CW, van Zuuren EJ, Gerlinger C, Heithecker R. The effect of 2 combined oral contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis. 2002;69(4 Suppl):2–15. [PubMed]
- 99.Huber J, Foidart JM, Wuttke W, Merki-Feld GS, The HS, Gerlinger C, et al. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur J Contracept Reprod Health Care. 2000;5:25–34. doi: 10.1080/13625180008500375. [DOI] [PubMed] [Google Scholar]
- 100.Jaisamrarn U, Chaovisitsaree S, Angsuwathana S, Nerapusee O. A comparison of multiphasic oral contraceptives containing norgestimate or desogestrel in acne treatment: a randomized trial. Contraception. 2014;90:535–541. doi: 10.1016/j.contraception.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 101.Katz HI, Kempers S, Akin MD, Dunlap F, Whiting D, Norbart TC. Effect of a desogestrel-containing oral contraceptive on the skin. Eur J Contracept Reprod Health Care. 2000;5:248–255. doi: 10.1080/13625180008500411. [DOI] [PubMed] [Google Scholar]
- 102.Kränzlin HT, Nap MA. The effect of a phasic oral contraceptive containing desogestrel on seborrhea and acne. Eur J Contracept Reprod Health Care. 2006;11:6–13. doi: 10.1080/13625180500252638. [DOI] [PubMed] [Google Scholar]
- 103.Prilepskaya VN, Serov VN, Zharov EV, Golousenko IJ, Mejevitinova EA, Gogaeva EV, et al. Effects of a phasic oral contraceptive containing desogestrel on facial seborrhea and acne. Contraception. 2003;68:239–245. doi: 10.1016/S0010-7824(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 104.Thorneycroft IH, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis. 2004;74:123–130. [PubMed] [Google Scholar]
- 105.De Pedrini P, Rapisarda R, Spanò G. The effect of ketoconazole on sebum secretion in patients suffering from acne and seborrhea. Int J Tissue React. 1988;10:111–113. [PubMed] [Google Scholar]
- 106.Norris JF, Cunliffe WJ. Effect of ketoconazole on sebum excretion rate. Arch Dermatol. 1987;123:301. doi: 10.1001/archderm.1987.01660270033010. [DOI] [PubMed] [Google Scholar]
- 107.Schmidt JB. Other antiandrogens. Dermatology. 1998;196:153–157. doi: 10.1159/000017850. [DOI] [PubMed] [Google Scholar]
- 108.Carr BR. Uniqueness of contraceptive progestins. Contraception. 1998;58(3 Suppl):23–27. doi: 10.1016/S0010-7824(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 109.del Marmol V, Teichmann A, Gertsen K. The role of combined oral contraceptives in the management of acne and seborrhea. Eur J Contracept Reprod Health Care. 2004;9:107–124. doi: 10.1080/1362518042000221508. [DOI] [PubMed] [Google Scholar]
- 110.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. Classification and pharmacology of progestins. Maturitas. 2008;61:171–180. doi: 10.1016/j.maturitas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 111.Friedman. Spironolactone for adult female acne. Cutis. 2015;96:216–7. [PubMed]
- 112.Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945–973. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 113.Dréno B, Thiboutot D, Layton AM, Berson D, Perez M, Kang S, Global Alliance to Improve Outcomes in Acne Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096–1106. doi: 10.1111/jdv.12757. [DOI] [PubMed] [Google Scholar]
- 114.Danby FW. Spironolactone. J Am Acad Dermatol. 1992;26:137. doi: 10.1016/S0190-9622(08)80534-8. [DOI] [PubMed] [Google Scholar]
- 115.Bettoli V, Zauli S, Virgili A. Is hormonal treatment still an option in acne today. Br J Dermatol. 2015;172(Suppl 1):37–46. doi: 10.1111/bjd.13681. [DOI] [PubMed] [Google Scholar]
- 116.Del Rosso JQ, Harper JC, Graber EM, Thiboutot D, Silverberg NB, Eichenfield DZ, et al. Status report from the American Acne & Rosacea Society on medical management of acne in adult women, part 1: overview, clinical characteristics, and laboratory evaluation. Cutis. 2015;96:236–241. [PubMed] [Google Scholar]
- 117.Gollnick HP, Bettoli V, Lambert J, Araviiskaia E, Binic I, Dessinioti C, et al. A consensus-based practical and daily guide for the treatment of acne patients. J Eur Acad Dermatol Venereol. 2016;30:1480–1490. doi: 10.1111/jdv.13675. [DOI] [PubMed] [Google Scholar]
- 118.Nast A, Dreno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al. European evidence-based (S3) guideline for the treatment of acne—update 2016—short version. J Eur Acad Dermatol Venereol. 2016;30:1261–1268. doi: 10.1111/jdv.13776. [DOI] [PubMed] [Google Scholar]
- 119.NICE Clinical Knowledge Summary. Acne 2014. cks.nice.org.uk/acne-vulgaris. Accessed 3 Nov 2016 (not available outside the UK).
- 120.Abad-Casintahan F, Chow SK, Goh CL, Kubba R, Miyachi Y, Noppakun N, et al. Toward evidence-based practice in acne: consensus of an Asian Working Group. J Dermatol. 2011;38:1041–1048. doi: 10.1111/j.1346-8138.2011.01266.x. [DOI] [PubMed] [Google Scholar]
- 121.Goh CL, Abad-Casintahan F, Aw DC, Baba R, Chan LC, Hung NT, et al. South-East Asia study alliance guidelines on the management of acne vulgaris in South-East Asian patients. J Dermatol. 2015;42:945–953. doi: 10.1111/1346-8138.12993. [DOI] [PubMed] [Google Scholar]
- 122.CPG Secretariat. Management of acne. Malaysian clinical practice guideline 2012. http://www.moh.gov.my/attachments/7190.pdf. Accessed 3 Nov 2016.
- 123.Nast A, Bayerl C, Borelli C, et al. S2 k-guideline for therapy of acne [in German] J Dtsch Dermatol Ges. 2010;8(Suppl 2):1–59. doi: 10.1111/j.1610-0387.2010.07466.x. [DOI] [PubMed] [Google Scholar]
- 124.Asai Y, Baibergenova A, Dutil M, et al. Management of acne: Canadian clinical practice guideline. Can Med Assoc J. 2016;188:118–126. doi: 10.1503/cmaj.140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M. How to formulate research recommendations. BMJ. 2006;333:804–806. doi: 10.1136/bmj.38987.492014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.