Abstract
The neozoan species raccoon dog (Nyctereutes procyonoides) and raccoon (Procyon lotor) are widespread in Europe and potential vectors of many diseases that can threaten human and domestic animal health. Facing a further spread of these species, it is important to know about (i) pathogens imported and/or (ii) pathogens acquired in the new habitat. Thus, we investigated the parasite fauna of wild raccoon dogs and raccoons from Austria, at the edge of their new distribution range. The eight examined raccoons were nearly free of pathogens including Baylisascaris procyonis, and thus assumed to have a low epidemiological impact, so far. Out of ten raccoon dog specimens, we found one from western Austria to be infected with Echinococcus multilocularis and another three from the eastern wetland regions to harbour adults of Alaria alata. Furthermore, we detected Babesia cf. microti in five of eight raccoon dogs all over Austria but none of our samples were tested positive for Trichinella spp. Nevertheless, the raccoon dog seems to be a relevant host, at least for the zoonotic pathogens E. multilocularis and A. alata, and we suggest to further monitor the raccoon dogs parasite fauna.
Keywords: Alaria alata, Echinococcus multilocularis, Babesia cf. microti, Trichinella spp., Baylisascaris procyonis, Neozoa
Introduction
Two of the most widespread non-indigenous wildlife species in Europe are the raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor). Both species have been introduced to Europe in the twentieth century but their origin is completely the opposite, namely the USA for the raccoon and the Far East for the raccoon dog (Lutz 1984; Nowak 1973; Stubbe 1975). In the former decades, these new carnivore species expanded their range, increased in abundance, and even became the most common carnivore species in some parts of Europe (Kowalczyk 2014; Laurimaa et al. 2015). Consequently, there is a need to spend attention to these neozoa as additional wildlife reservoir of zoonotic diseases in Europe.
The most serious problem concerning the raccoon dog invasion seems to be the transmission of zoonotic diseases (Kauhala and Kowalczyk 2011). In Europe, the raccoon dog can be infected with a minimum of 32 helminth species of which 19 are zoonotic (Laurimaa et al. 2016). Its parasite fauna is similar to that of the indigenous red fox (Vulpes vulpes) (Al-Sabi et al. 2013; Bružinskaitė-Schmidhalter et al. 2012; Laurimaa et al. 2015; Thiess et al. 2001). The raccoon dog is assumed to be an important additional host species of Echinococcus multilocularis (Kauhala and Kowalczyk 2011; Schwarz et al. 2011; Tackmann et al. 2003) and moreover is highly susceptible for Alaria alata (Al-Sabi et al. 2013; Bružinskaitė-Schmidhalter et al. 2012; Laurimaa et al. 2016). The raccoon dog is also known to be an important reservoir host of Trichinella spp. (Bružinskaitė-Schmidhalter et al. 2012; Kauhala and Kowalczyk 2011; Thiess et al. 2001) and has been identified as important rabies vector in northeastern Europe (Holmala and Kauhala 2006; Singer et al. 2009). Due to broad oral vaccination campaigns, rabies is of less relevance in central Europe and is known to be eradicated in Austria since 2008 (Hirk et al. 2014). However, spill-over from neighbouring countries may still occur (Duscher et al. 2015). As the raccoon dog population is still growing and spreading, its role as a vector of this dangerous virus as well as of other zoonotic diseases may further increase in Europe (Kauhala and Kowalczyk 2011; Sutor et al. 2014).
While the raccoon is known to be the host of many helminth parasite species in its native range (e.g. Harkema and Miller 1964), previous studies from Germany, the country with the highest raccoon density in Europe, showed a remarkably low endoparasitic burden (e.g. Schwarz et al. 2015). The most prevalent parasites of raccoons found in recent studies were Mesocestoides spp. (Karamon et al. 2014; Schwarz et al. 2015). But the most serious zoonotic disease transmitted by raccoons in Europe seems to be the Larva migrans syndrome caused by Baylisascaris procyonis. The estimated prevalence of this parasite in European raccoon populations showed high geographical variations, ranging from 0 up to 71% (Al-Sabi et al. 2016; Gey 1998; Karamon et al. 2014; Schwarz et al. 2015; Winter et al. 2005). Concerning rabies, the raccoon is not considered to be a reservoir host in Europe, probably because of a low susceptibility for the relevant rabies virus variants (Vos et al. 2012). Among the piroplasmids, Babesia cf. microti was found in raccoons and raccoon dogs so far (Alvarado-Rybak et al. 2016; Han et al. 2010). This pathogen is frequently confirmed in foxes in Austria (Duscher et al. 2014), as well as in other regions of Europe sometimes infecting dogs (Camacho et al. 2004). This group comprises different clades and is also known as Babesia microti-like, Babesia annae, Babesia “Spanish dog isolate”, Theileria annae and Babesia vulpes (Baneth et al. 2015), but due to the lack of a valid description, it only can be informally named as the “microti group” (Harris 2016). Additionally, it has been stated that raccoons might get infected with at least three different Babesia species (Alvarado-Rybak et al. 2016).
The distribution and transmission of parasites depend on several environmental factors and thus differ across geographical regions (Dybing et al. 2013; Mackenstedt et al. 2015; Monello and Gompper 2011). So far, all studies concerning the parasite fauna of the raccoon dog as well as of the raccoon in Europe were conducted in the northeastern countries of its introduced range. Facing a range expansion of these neozoan species towards South Europe (Kauhala and Winter 2006; Winter 2006), further studies are needed to understand their role and impact of maintaining and transmitting zoonotic diseases in Europe’s diverse landscapes and climatic regions. Thus, we investigated the parasite fauna of wild raccoon dogs and raccoons from Austria, on the edge of both species’ geographic ranges. So far, the population densities in Austria do not reach high numbers but hunting bags are increasing (Duscher 2016). Therefore, sampling of statistical sufficient numbers to calculate parameters hardly seems possible. Nevertheless, any data of those species in the newly spread area is of great interest. It may give hints about (i) pathogens imported and/or (ii) pathogens acquired in the new habitat.
Material and methods
Raccoons and raccoon dogs were obtained via regular hunting and catching events between February 2010 and January 2016 all over Austria under the restrictions of the Austrian game laws. Samples of in total eight raccoons and 13 raccoon dogs were obtained. Due to the small number of samples collected over 6 years, this is neither a longitudinal nor a cross-sectional study design. In some cases, only parts (e.g. intestines or tissue samples) were provided by the hunters. Either the whole animal or the intestines only, which were excised under high safety standards, were frozen at −80 °C for a minimum of 14 days to inactivate E. multilocularis oncosphaera. The intestines of seven raccoons and ten raccoon dogs were analysed by using the “shaking in a vessel” technique which was previously described (Duscher et al. 2005) (Table 1). Muscle tissues, at least 5 g if available (diaphragm or limb muscle), were analysed for Trichinella spp. by artificial digestion method at the National Reference Laboratory (Institute for Veterinary Disease Control, Austrian Agency for Health and Food Safety, Innsbruck, Austria). Spleens of four raccoons and eight raccoon dogs were analysed for piroplasmids (Babesia/Theileria spp.) and Anaplasmataceae (Anaplasma spp./Ehrlichia canis and Candidatus Neoehrlichia spp.) by using a nested and a single PCR, respectively, according to a protocol previously published (Duscher et al. 2013; Hodžić et al. 2015).
Table 1.
Numbers of raccoons and raccoon dogs with the available material and the method used for analysis
| Species/no. | Material available/method used | Collection year | Province | ||
|---|---|---|---|---|---|
| Intestines | Muscle | Spleen | |||
| Raccoon 189 | SVT | n.a. | n.a. | 2010 | Salzburg |
| Raccoon 229 | SVT | n.a. | n.a. | 2010 | Lower Austria |
| Raccoon 251 | SVT | n.a. | n.a. | 2011 | Styria |
| Raccoon 253 | SVT | n.a. | n.a. | 2010 | Styria |
| Raccoon 311 | n.a. | n.a. | PCR | 2013 | Lower Austria |
| Raccoon 328 | SVT | n.a. | PCR | 2015 | Carinthia |
| Raccoon 400 | SVT | Diaphragm (<5 g) | PCR | 2015 | Lower Austria |
| Raccoon 401 | SVT | Diaphragm | PCR | 2016 | Lower Austria |
| Raccoon dog 211 | SVT | n.a. | n.a. | 2010 | Burgenland |
| Raccoon dog 247 | SVT | n.a. | n.a. | 2011 | Burgenland |
| Raccoon dog 255 | SVT | Limb | n.a. | 2011 | Burgenland |
| Raccoon dog 256 | SVT | Limb (<5 g) | n.a. | 2011 | Lower Austria |
| Raccoon dog 266 | SVT | Limb | n.a. | 2011 | Lower Austria |
| Raccoon dog 293 | SVT | n.a. | PCR | 2012 | Burgenland |
| Raccoon dog 314 | SVT | n.a. | PCR | 2014 | Vorarlberg |
| Raccoon dog 321 | n.a. | n.a. | PCR | 2014 | Lower Austria |
| Raccoon dog 322 | n.a. | n.a. | PCR | 2014 | Lower Austria |
| Raccoon dog 324 | n.a. | n.a. | PCR | 2014 | Lower Austria |
| Raccoon dog 389 | SVT | Diaphragm | PCR | 2015 | Lower Austria |
| Raccoon dog 394 | SVT | Diaphragm | PCR | 2015 | Styria |
| Raccoon dog 397 | SVT | Limb/Diaphragm | PCR | 2015 | Lower Austria |
PCR polymerase chain reaction used for piroplasmids and Anaplasmatacae, SVT shaking in a vessel technique, n.a. not available
Results
Concerning the human relevant parasites, we detected A. alata in the intestines of three raccoon dogs (30%) as well as E. multilocularis in one raccoon dog intestine (10%) (Table 2). The examined raccoon dogs were also infected with Uncinaria stenocephala (40%), Mesocestoides spp. (40%), Molineus spp. (30%), Toxocara canis (20%), Taenia spp. (20%), Isthmiophora melis (20%), Dipylidium caninum (10%) and Toxascaris leonina (10%). Seven examined raccoons were free of gastrointestinal parasites, and one specimen was infected with Molineus spp.
Table 2.
Parasite species detected in the examined raccoon (left) and raccoon dog (right) specimens and infestation intensity (middle intense for Echinococcus multilocularis: 101–1000 specimens, low intense for Mesocestoides sp.: below 30 specimens)
| Species | Raccoon | Raccoon dog | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | 189 | 229 | 251 | 253 | 311 | 328 | 400 | 401 | 211 | 247 | 255 | 256 | 266 | 293 | 314 | 321 | 322 | 324 | 389 | 394 | 397 |
| Alaria alata | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 6 | 0 | 23 | 0 | 37 | 0 | 0 | – | – | – | 0 | 0 | 0 |
| Isthmiophora melis | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 9 | 1 | 0 | 0 | 0 | – | – | – | 0 | 0 | 0 |
| Dipylidium caninum | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | 0 | 0 | 1 |
| Echinococcus multilocularis | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Middle intense | – | – | – | 0 | 0 | 0 |
| Mesocestoides sp. | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | Low intense | 5 | – | – | – | 0 | 0 | 0 |
| Taenia sp. | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | 1 | 0 | 3 |
| Molineus sp. | 1 | 0 | 0 | 0 | – | 0 | 0 | 0 | 1 | 12 | 1 | 0 | 0 | 0 | 0 | – | – | – | 0 | 0 | 0 |
| Toxascaris leonina | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | – | – | – | 0 | 0 | 0 |
| Toxocara canis | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | – | – | – | 0 | 0 | 5 |
| Trichinella sp. | – | – | – | – | – | – | 0a | 0 | – | – | 0 | 0a | 0 | – | – | – | – | – | 0 | 0 | 0 |
| Uncinaria stenocephala | |||||||||||||||||||||
| Babesia cf. microti | – | – | – | – | 0 | 0 | 0 | 0 | – | – | – | – | – | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Anaplasmatacae sp. | – | – | – | – | 0 | 0 | 0 | 0 | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
aLess than 5 g of muscle available
The results of the PCR showed no Anaplasmataceae infections of the four raccoons as well as of the eight raccoon dogs examined. However, none of the raccoons but five of eight raccoon dogs were tested positive for B. cf. microti (Genbank® accession number [KY246306]). The sequence (partial 18S rRNA) were 100% identical to each other and to the sequences found in foxes from Austria (e.g. [KM115972]). The Trichinella analysis showed all of the investigated raccoon and raccoon dog samples were free of Trichinella spp.—although, two of the samples consisted of less than 5 g and thus were probably not representative.
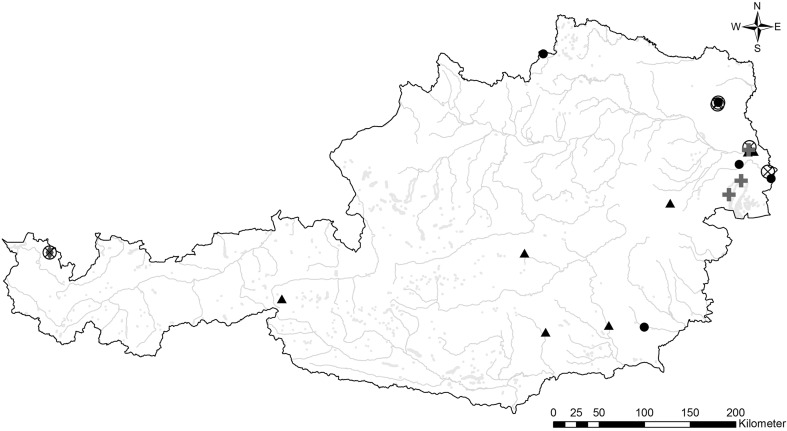
The A. alata infected raccoon dogs originated from eastern Austria, precisely from the Lake Neusiedl and the Danube floodplain areas (Fig. 1), while the E. multilocularis positive raccoon dog was shot in the northwestern edge of the country. Tissues of the racoon dogs infested with B. cf. microti were sampled in the east as well as in the west.
Fig. 1.
Geographical distribution of raccoon dogs infected with Alaria alata ( ), Babesia cf. microti (
), Babesia cf. microti ( ) and Echinococcus multilocularis (
) and Echinococcus multilocularis ( ) as well as of raccoons (▲) and raccoon dogs (•) not infected with the relevant parasites (see above)
) as well as of raccoons (▲) and raccoon dogs (•) not infected with the relevant parasites (see above)
Discussion
Interestingly, the E. multilocularis appeared in the western areas of Austria, those areas of known high endemicity of this cestode in foxes (Duscher et al. 2006). The host competence of the raccoon dog for E. multilocularis is in accordance with studies from other countries (e.g. Al-Sabi et al. 2013; Laurimaa et al. 2015; Schwarz et al. 2011; Thiess et al. 2001). But, due to its feeding habits, the raccoon dog seems to play a minor role as a reservoir of E. multilocularis than the red fox does (Al-Sabi et al. 2013; Bružinskaitė-Schmidhalter et al. 2012).
In contrast, the trematode A. alata was found in the eastern parts surrounding the Lake Neusiedl, and in the Danube floodplains where this parasite was also found in foxes (Duscher 2011), and where the densities of paratenic hosts are high. These wetlands are also the areas with a high probability of raccoon dog presence (Duscher and Nopp-Mayr under review), and where future population densities are expected to be high. Our results confirm previous European studies that showed a high abundance of A. alata in raccoon dogs and thus a probable high susceptibility of the raccoon dog for this gastrointestinal parasite with high zoonotic relevance (Al-Sabi et al. 2013).
Furthermore, B. cf. microti was found in five out of eight investigated raccoon dogs. To our knowledge, this is the first evidence of a B. cf. microti infection of raccoon dogs in Europe and is in accordance with the idea of sharing the fox parasites, as seen for the gastrointestinal parasites. In this context, the raccoon dog may be seen as additional reservoir of B. cf. microti as already suggested by Han et al. (2010). Contrary to that, the raccoons were negative for this pathogen, although the B. cf. microti species found in Austrian foxes is closely related to those found in raccoons in Japan (Baneth et al. 2015). So, in this case, the same type of pathogen also could circulate among the host species. Unfortunately, the sample size is too low to either confirm or reject this assumption.
No Anaplasmataceae were found, which might have occurred in the raccoons, e.g. the raccoon-associated bacteria Candidatus Neoehrlichia lotoris (CNL) as stated in previous works (Hodžić et al. 2015, 2016). These bacteria were found and described in raccoons from the USA (Yabsley et al. 2008), but recently were accidentally found in foxes from Austria and the Czech Republic (Hodžić et al. 2015, 2016), far away from the US distribution. Likewise, all examined raccoon and raccoon dog samples were free of Trichinella spp., but due to the small sample size, no clear declaration can be made.
Generally, our examined raccoons were almost pathogen free, which also was observed in other studies within their introduced range (e.g. Schwarz et al. 2015). The idea is that in some regions, introduced raccoons were originating from fur farms and dewormed in regular manner. Thus, geographical differences in the worm prevalence, e.g. B. procyonis, within their introduced range are supposed to depend on the source population (Schwarz et al. 2015; Winter et al. 2005). In opposite to the raccoon dog, the raccoon is not that suitable for fox parasites (Schwarz et al. 2015; Thiess et al. 2001). This might be the explanation for the overall lower prevalence in the investigated raccoons in this study.
Conclusion
Due to the low population densities of the raccoon and the raccoon dog in Austria (Duscher 2016), our sample size is quite low and varying. Nevertheless, our results support the thesis of Michler and Michler (2012) that the epidemiological meaning of the raccoon in Europe is still low. However, a further spread and population increase of this non-indigenous species will obviously lead to an admixture of the different founder populations (Biedrzycka et al. 2014; Fischer et al. 2015), and therewith to a further spread of B. procyonoides as well. In the case of the raccoon dog, our results confirm it as a host of E. multilocularis as well as a probable indicator species of A. alata. As the origins of A. alata positive samples are also the regions with expected high raccoon dog population densities (Duscher and Nopp-Mayr under review), A. alata infections is an increasing risk. Moreover, our study confirms the raccoon dog as an additional host of B. cf. microti with a relevant impact as a reservoir. Concerning its epidemiological impact and facing a further spread in Europe, the raccoon dog should be monitored more intensively in the matters of vector-borne diseases (Sutor et al. 2014).
Acknowledgement
Open access funding is provided by the University of Veterinary Medicine Vienna, Austria. We thank all hunters, preparators and museums staff who provided specimen, gastrointestinal tracts and/or tissue sample for our examination. Furthermore, we thank the team of the pathology lab at the Research Institute of Wildlife Ecology for their help.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
References
- Al-Sabi MNS, Chriél M, Jensen TH, Enemark HL. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012—a comparative study. In J Parasitol: Parasite Wildlife. 2013;2:144–151. doi: 10.1016/j.ijppaw.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sabi MNS, Chriél M, Hansen MS, Enemark HL. Baylisascaris procyonis in wild raccoons (Procyon lotor) in Denmark. Veterinary Parasitology: Regional Studies and Reports. 2016;1-2:55–58. doi: 10.1016/j.vprsr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Alvarado-Rybak M, Solano-Gallego L, Millan J. A review of piroplasmid infections in wild carnivores worldwide: importance for domestic animal health and wildlife conservation. Parasit Vectors. 2016;9:538. doi: 10.1186/s13071-016-1808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneth G, Florin-Christensen M, Cardoso L, Schnittger L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit Vectors. 2015;8:207. doi: 10.1186/s13071-015-0830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedrzycka A, Zalewski A, Bartoszewicz M, Okarma H, Jędrzejewska E. The genetic structure of raccoon introduced in Central Europe reflects multiple invasion pathways. Biol Invasions. 2014;16:1611–1625. doi: 10.1007/s10530-013-0595-8. [DOI] [Google Scholar]
- Bružinskaitė-Schmidhalter R, Šarkūnas M, Malakauskas A, Mathis A, Torgerson PR, Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:120–127. doi: 10.1017/S0031182011001715. [DOI] [PubMed] [Google Scholar]
- Camacho AT, et al. Azotemia and mortality among Babesia microti-like infected dogs. J Vet Intern Med. 2004;18:141–146. doi: 10.1892/0891-6640(2004)18<141:aamabm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Duscher GG. Der Dunckersche Muskelegel—Alaria alata beim Rotfuchs in Österreich in Relation zum Vorkommen von Wildschweinen. Wiener Tierärztliche Monatsschrift. 2011;98:251–254. [Google Scholar]
- Duscher T (2016) The current status of the raccoon (Procyon lotor) and the raccoon dog (Nyctereutes procyonoides) in Austria. Beitr Jagd Wildforsch 41:285–293
- Duscher T, Nopp-Mayr U (under review) Species distribution modelling for the invasive raccoon dog (Nyctereutes procyonoides) in Austria and first range predictions for alpine environments. Arch Biol Sci
- Duscher GG, Prosl H, Joachim A. Scraping or shaking—a comparison of methods for the quantitative determination of Echinococcus multilocularis in fox intestines. Parasitol Res. 2005;95:40–42. doi: 10.1007/s00436-004-1260-z. [DOI] [PubMed] [Google Scholar]
- Duscher GG, Pleydell D, Prosl H, Joachim A. Echinococcus multilocularis in Austrian foxes from 1991 until 2004. J Vet Med B Infect Dis Vet Public Health. 2006;53:138–144. doi: 10.1111/j.1439-0450.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- Duscher GG, Kubber-Heiss A, Richter B, Suchentrunk F. A golden jackal (Canis aureus) from Austria bearing Hepatozoon canis—import due to immigration into a non-endemic area? Ticks and tick-borne diseases. 2013;4:133–137. doi: 10.1016/j.ttbdis.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Duscher GG, Fuehrer H-P, Kübber-Heiss A. Fox on the run—molecular surveillance of fox blood and tissue for the occurrence of tick-borne pathogens in Austria. Parasite Vectors. 2014;7:521. doi: 10.1186/s13071-014-0521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duscher GG, Leschnik M, Fuehrer HP, Joachim A. Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int J Parasitol: Parasite Wildlife. 2015;4:88–96. doi: 10.1016/j.ijppaw.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybing NA, Fleming PA, Adams PJ. Environmental conditions predict helminth prevalence in red foxes in Western Australia. Int J Parasitol: Parasite Wildlife. 2013;2:165–172. doi: 10.1016/j.ijppaw.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ML, et al. Historical invasion records can be misleading: genetic evidence for multiple introductions of invasive raccoons (Procyon lotor) in Germany. PLoS One. 2015;10:e0125441. doi: 10.1371/journal.pone.0125441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gey AB (1998) Synopsis der Parasitenfauna des Waschbären (Procyon lotor) unter Berücksichtigung von Befunden aus Hessen. Dissertation, Justus Liebig-Universität Gießen
- Han J-I, Lee S-J, Jang H-J, Na K-J. Asymptomatic Babesia microti–like parasite infection in wild raccoon dogs (Nyctereutes procyonoides) in South Korea. J Wildl Diseases. 2010;46:632–635. doi: 10.7589/0090-3558-46.2.632. [DOI] [PubMed] [Google Scholar]
- Harkema R, Miller GC. Helminth parasites of the raccoon, Procyon lotor in the southeastern United States. J Parasitol. 1964;50:60–66. doi: 10.2307/3276029. [DOI] [PubMed] [Google Scholar]
- Harris DJ. Naming no names: comments on the taxonomy of small piroplasmids in canids. Parasit Vectors. 2016;9:289. doi: 10.1186/s13071-016-1567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirk S, Allerberger F, Huhulescu S, Indra A, Kallab V, Lachner P, Schmid D, Wewalka G (2014) Tollwut. Bundesministerium für Gesundheit and AGES - Österreichische Agentur für Gesundheit und Ernährungssicherheit GmbH, Wien
- Hodžić A, Cézanne R, Duscher GG, Harl J, Glawischnig W, Fuehrer H-P. Candidatus Neoehrlichia sp. in an Austrian fox is distinct from Candidatus Neoehrlichia mikurensis, but closer related to Candidatus Neoehrlichia lotoris. Parasit Vectors. 2015;8:539. doi: 10.1186/s13071-015-1163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A, Mitkovà B, Modrý D, Juránková J, Frgelecová L, Forejtek P, Steinbauer V, Duscher GG. A new case of the enigmatic Candidatus Neoehrlichia sp. (FU98) in a fox from the Czech Republic. Mol Cell Probe. 2016 doi: 10.1016/j.mcp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Holmala K, Kauhala K. Ecology of wildlife rabies in Europe. Mammal Rev. 2006;36:17–36. doi: 10.1111/j.1365-2907.2006.00078.x. [DOI] [Google Scholar]
- Karamon J, Kochanowski M, Cencek T, Bartoszewicz M, Kusyk P. Gastrointestinal helminths of raccoons (Procyon lotor) in western Poland (Lubuskie province) - with particular regard to Baylisascaris procyonis. Bull Vet Inst Pulawy. 2014;58:547–552. doi: 10.2478/bvip-2014-0084. [DOI] [Google Scholar]
- Kauhala K, Kowalczyk R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: history of colonization, features behind its success, and threats to native fauna. Curr Zool. 2011;57:584–598. doi: 10.1093/czoolo/57.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauhala K, Winter M (2006) Nyctereutes procyonoides. Species factsheet. DAISIE - Delivering Alien Invasive Species Inventories for Europe. http://www.europe-aliens.org/pdf/Nyctereutes_procyonoides.pdf. Accessed 15 Nov 2016
- Kowalczyk R (2014) Nyctereutes procyonoides. NOBANIS – Invasive Alien Species Fact Sheet. https://www.nobanis.org/globalassets/speciesinfo/n/nyctereutes-procyonoides/nyctereutes_procyonoides-final.pdf. Accessed 05 Feb 2016
- Laurimaa L, Suld K, Moks E, Valdmann H, Umhang G, Knapp J, Saarma U. First report of the zoonotic tapeworm Echinococcus multilocularis in raccoon dogs in Estonia, and comparisons with other countries in Europe. Vet Parasitol. 2015;212:200–205. doi: 10.1016/j.vetpar.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Laurimaa L, Suld K, Davison J, Moks E, Valdmann H, Saarma U. Alien species and their zoonotic parasites in native and introduced ranges: the raccoon dog example. Vet Parasitol. 2016;219:24–33. doi: 10.1016/j.vetpar.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Lutz W. Die Verbreitung des Waschbären (Procyon lotor, Linné 1758) im mitteleuropäischen Raum. Z Jagdwiss. 1984;30:218–228. [Google Scholar]
- Mackenstedt U, Jenkins D, Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas international. J Parasitol: Parasite Wildlife. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler F, Michler B. Ökologische, ökonomische und epidemiologische Bedeutung des Waschbären (Procyon lotor) in Deutschland–eine aktuelle Übersicht. Beitr Jagd Wildforsch. 2012;37:389–397. [Google Scholar]
- Monello RJ, Gompper ME. Effects of resource availability and social aggregation on the species richness of raccoon endoparasite infracommunities. Oikos. 2011;120:1427–1433. doi: 10.1111/j.1600-0706.2011.19260.x. [DOI] [Google Scholar]
- Nowak E. Ansiedlung und Ausbreitung des Marderhundes (Nyctereutes procyonoides GRAY) in Europa. Beitr Jagd Wildforsch. 1973;8:351–383. [Google Scholar]
- Schwarz S, Sutor A, Staubach C, Mattis R, Tackmann K, Conraths FJ. Estimated prevalence of Echinococcus multilocularis in raccoon dogs Nyctereutes procyonoides in northern Brandenburg, Germany. Curr Zool. 2011;57:655–661. doi: 10.1093/czoolo/57.5.655. [DOI] [Google Scholar]
- Schwarz S, Sutor A, Mattis R, Conraths FJ. Der Waschbärspulwurm (Baylisascaris procyonis)-kein Zoonoserisiko für Brandenburg? Berl Munch Tierarztl Wochenschr. 2015;128:34–38. [PubMed] [Google Scholar]
- Singer A, Kauhala K, Holmala K, Smith GC. Rabies in northeastern Europe—the threat from invasive raccoon dogs. J Wildl Diseases. 2009;45:1121–1137. doi: 10.7589/0090-3558-45.4.1121. [DOI] [PubMed] [Google Scholar]
- Stubbe M. Der Waschbär Procyon lotor (L., 1758) in der DDR. Hercynia. 1975;12:80–91. [Google Scholar]
- Sutor A, Schwarz S, Conraths FJ. The biological potential of the raccoon dog (Nyctereutes procyonoides, Gray 1834) as an invasive species in Europe-new risks for disease spread? Acta Theriol. 2014;59:49–59. doi: 10.1007/s13364-013-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackmann K, Goretzki J, Conraths FJ. Das Neozoon Marderhund als neue Endwirtpopulation für Echinococcus multilocularis in Ostdeutschland - ein Risiko? Angewandte Wissenschaft - Schriftreihe des BMVEL. 2003;498:176–181. [Google Scholar]
- Thiess A, Schuster R, Nöckler K, Mix H. Helminthenfunde beim einheimischen Marderhund Nyctereutes procyonoides (Gray, 1834) Berl Munch Tierarztl Wochenschr. 2001;114:273–276. [PubMed] [Google Scholar]
- Vos A, Ortmann S, Kretzschmar AS, Köhnemann B, Michler F. The raccoon (Procyon lotor) as potential rabies reservoir species in Germany: a risk assessment. Berl Munch Tierarztl Wochenschr. 2012;125:228–235. [PubMed] [Google Scholar]
- Winter M (2006) Procyon lotor. Species factsheet. DAISIE - Delivering Alien Invasive Species Inventories for Europe. http://www.europe-aliens.org/pdf/Procyon_lotor.pdf. Accessed 15 Nov 2016
- Winter M, Stubbe M, Heidecke D. Zur Ökologie des Waschbären (Procyon lotor L., 1758) in Sachsen-Anhalt. Beitr Jagd Wildforsch. 2005;30:303–322. [Google Scholar]
- Yabsley MJ, Murphy SM, Luttrell MP, et al. Characterization of “Candidatus Neoehrlichia lotoris” (family Anaplasmataceae) from raccoons (Procyon lotor) Int J Syst Evol Microbiol. 2008;58:2794–2798. doi: 10.1099/ijs.0.65836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]