Abstract
Local healthcare providers often question the possible steroidal activity of traditional Chinese medicine (TCM) herbs or herbal products and implicate them as a cause for adrenal insufficiency or Cushing’s syndrome in patients with a history of TCM intake. We conducted a comprehensive database search for evidence of potential glucocorticoid, mineralocorticoid, androgenic or oestrogenic activity of herbs or herbal products. Overall, there are not many herbs whose steroidal activity is well established; among these, most cases were based on preclinical studies. Liquorice root may cause pseudoaldosteronism through interference with the steroidogenesis pathway. Although ginseng and cordyceps have some in vitro glucocorticoid activities, the corroborating clinical data is lacking. Deer musk and deer antler contain androgenic steroids, while epimedium has oestrogenic activity. On the other hand, adulteration of herbal products with exogenous glucocorticoids is a recurrent problem encountered locally in illegal products masquerading as TCM. Healthcare providers should stay vigilant and report any suspicion to the relevant authorities for further investigations.
Keywords: adulteration, androgen, corticosteroids, oestrogen, traditional Chinese medicine
INTRODUCTION
Endogenously produced steroid hormones can be classified into two main groups: corticosteroids (glucocorticosteroids and mineralocorticoids) and sex hormones (androgenic and oestrogenic compounds). These naturally occurring steroids are mainly synthesised in the adrenal gland; mineralocorticoids, glucocorticoids and sex hormones are synthesised in zona glomerulosa cells, zona fasciculate cells and zona reticularis cells, respectively. Any interference with the steroidogenic enzyme activity or steroid receptors could affect the multistep steroid synthesis pathway, resulting in steroidal or antisteroidal activity.(1)
In Singapore, traditional Chinese medicine (TCM) is one of the most predominant forms of complementary and alternative medicines used for a wide range of ailments, including cancer,(2,3) chronic pain,(4) dermatological conditions(5) and other chronic diseases.(6) TCM use is not confined to the Chinese population alone – it is also popular among the Indians and Malays.(7) In Singapore, orally administered Chinese herbal medicine can generally be classified into two main categories: Chinese proprietary medicine (CPM) and traditional medicinal material. CPM refers to manufactured products in finished dosage forms (e.g. pills, capsules and liquids), which contain active substances derived from any plant, animal or mineral, or any combination thereof; a detailed description can be found in the current edition of The Chinese Herbal Medicine Materia Medica.(8) On the other hand, traditional medicinal materials refer to “medicinal materials from plants, animals or minerals in their natural states, or in processed forms that have undergone simple processing, such as cutting or drying”, including raw herbs used in TCM.(9) Presently, the import and local sale of CPM in Singapore is subjected to premarketing approval by the Health Sciences Authority (HSA), whereas there is no licensing requirement for traditional medicinal materials.
In Singapore General Hospital, a Traditional Medicine Information Service (TMIS) was established in 2011 as an extension to the Drug Information Service of the Department of Pharmacy. TMIS handles enquiries on traditional medicines (such as TCM, Jamu and Ayurveda herbal medicine) from healthcare professionals and provides evidence-based information in response, by conducting literature searches and critically reviewing the available evidence. Of the many enquiries received pertaining to adverse reactions, TCM herbs or supplements in their raw or finished forms were often questioned on their possible steroidal activity – whether they contain steroids or cause adrenal insufficiency or Cushing’s syndrome, among other concerns such as their potential nephrotoxicity and hepatotoxicity.
We conducted a comprehensive search of databases, including PubMed, China National Knowledge Infrastructure and the HSA website, for evidence of herbs or herbal products that have been studied for their potential glucocorticoid, mineralocorticoid, androgenic or oestrogenic activity. This review aims to present the current published evidence on some commonly used TCM herbs that have been extensively studied or frequently questioned for their potential steroidal activity. They include herbs with compounds that are structurally similar to steroids, and those that interfere with steroid metabolism or have the ability to bind to steroid receptors. We also discuss the adulteration of herbal products with exogenously added steroids, as a review on TCM is incomplete without an elaboration on this problem. Through this paper, we hope to address the common concerns of local healthcare providers and to dispel some myths surrounding the steroidal activity of TCM herbs.
HERBS WITH CORTICOSTEROIDAL ACTIVITY
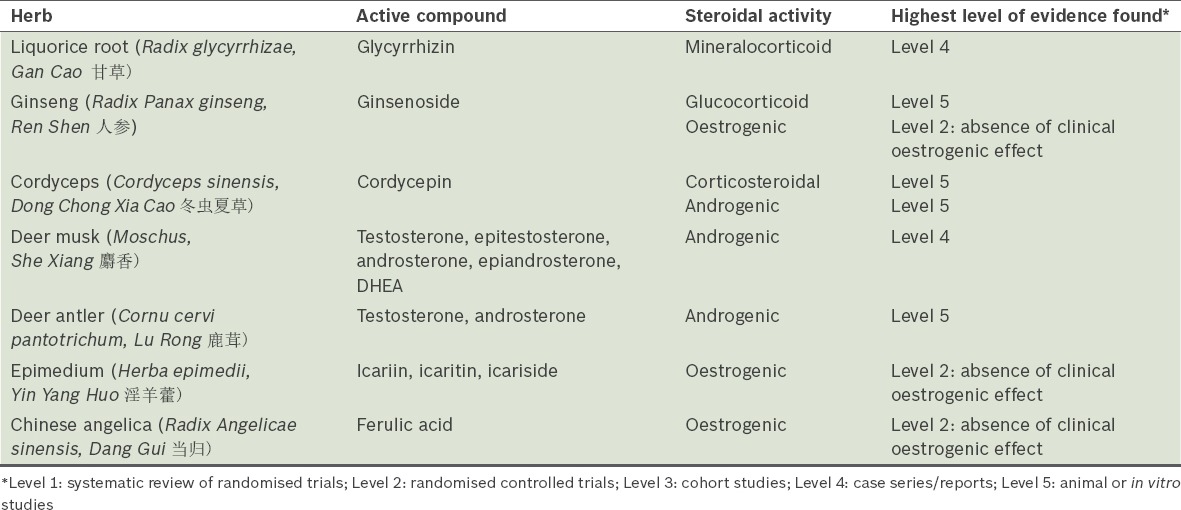
Liquorice root (Radix glycyrrhizae, Gan Cao 甘草)
Liquorice root is a commonly used herb in many TCM formulations due to its TCM property of ‘harmonising’ the various herbs, as well as for its ‘qi-tonifying’ property. It is the most studied herb in terms of its corticosteroidal activity. Glycyrrhizin, the principal active ingredient in liquorice, and its hydrolysed metabolite, 18β-glycyrrhetinic acid, are found to inhibit the oxidative function of 11β-hydroxysteroid dehydrogenase, thereby preventing the metabolism of cortisol. The excess cortisol could then bind to mineralocorticoid receptors, resulting in an apparent mineralocorticoid excess that manifests as fluid retention, hypertension and hypokalaemia.(10)
A few cases of pseudoaldosteronism caused by consumption of liquorice at high doses have been reported in the literature, demonstrating the clinical significance of the mineralocorticoid activity of the herb when consumed in large quantities.(11) Healthy volunteer studies that investigated the safe dose of liquorice reported that a daily dose of 2 mg/kg of glycyrrhizic acid did not have any effect on aldosterone level, blood pressure, potassium and fluid retention.(12) Translating this to a person who weighs 50 kg, the safe dose of glycyrrhizic acid would be 100 mg. The World Health Organization suggested that the consumption of 100 mg/day of glycyrrhizic acid is unlikely to cause adverse effects.(13) The usual dose of liquorice root in a TCM concoction is 3–10 g/day.(14) The difficulty in inferring the safe dose of liquorice root is confounded by the highly variable glycyrrhizic acid content among liquorice products of different preparations, as well as in liquorice roots harvested from different sources.(15) This is further complicated by the wide interindividual susceptibility to its side effects.(16) Therefore, while there are no clinical reports of liquorice causing Cushing’s syndrome, the possibility of it causing pseudoaldosteronism cannot be entirely excluded, especially in patients with prolonged consumption of liquorice-containing formulations.
Ginseng (Radix Panax ginseng, Ren Shen 人参)
Ginseng is a ‘qi-tonifying’ herb that is often used for general wellness in individuals with fatigue, or in patients recovering from a severe or prolonged bout of illness. It mainly consists of panaxynol and ginsenosides. Of the many ginsenosides that have been isolated from this herb, ginsenosides Rg1 and Re were found to be functional ligands of glucocorticoid receptor in an in vitro study.(17,18) Animal studies have shown different mechanisms of its effect on steroid synthesis with contradictory conclusions.(19,20) To date, no clinical reports have demonstrated that the consumption of ginseng causes Cushing’s syndrome. Further research on the whole herb is needed to understand its overall effect on steroidogenesis.
Cordyceps (Cordyceps sinensis, Dong Chong Xia Cao 冬虫夏草)
In TCM practice, cordyceps is highly regarded as a prized tonic that is indicated for people with poor respiratory health or general weakness. An animal study and an in vitro study reported that cordyceps extract has a stimulatory effect on the adrenal gland and increased the corticosterone level.(21,22) However, no clinical case reports or studies have reported on its corticosteroidal activity.
HERBS WITH ANDROGENIC ACTIVITY
Deer musk (Moschus, She Xiang 麝香)
Deer musk, known for its resuscitation or ‘mind-clearing’ property in TCM practice, is often used in proprietary medicine in combination with various other herbal ingredients. One such famous formulation is Angong Niuhuang Wan (安宫牛黄丸), which is used for comatose or convulsion patients. This dried secretion of the musk pod of adult male deer contains chemical constituents such as steroids, lipids and peptides. The androgenic content of deer musk was well studied as a result of an incident during the FIFA Women’s World Cup 2011, when five athletes tested positive for steroids in their urine. Investigations uncovered that steroidal components were present in deer musk-containing traditional medicine taken by the athletes. Gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry analyses on two samples of the traditional medicine and a specimen from a live musk deer detected steroidal components, including nine prohibited androgens (e.g. testosterone, epitestosterone, androsterone, epiandrosterone and dehydroepiandrosterone or DHEA), validating that the identified steroidal compounds were not exogenous but inherent in deer musk.(23,24)
Deer antler (Cornu cervi pantotrichum, Lu Rong 鹿茸)
Deer antler, also known as deer velvet, is a ‘kidney-yang tonifying’ herb that is normally used to treat cold extremities, fatigue, impotence, infertility and other health problems. Chemical profiling studies on different deer antler samples have found that it contains naturally occurring steroids such as testosterone, androsterone, oestradiol and progesterone.(25,26) While there are no published clinical reports on virilisation due to deer musk or deer antler consumption, the inherent presence of androgenic compounds in these two deer products is proven and may be clinically relevant with prolonged use.
Cordyceps (Cordyceps sinensis, Dong Chong Xia Cao 冬虫夏草)
Preclinical studies examining the effect of cordyceps extract and its constituent, cordycepin, have found that they stimulated mouse Leydig cell testosterone production and increased testosterone secretions as well as testis weight in mice.(27,28) However, corresponding clinical reports on these effects of cordyceps are lacking.
HERBS WITH OESTROGENIC ACTIVITY
Epimedium (Herba epimedii, Yin Yang Huo 淫羊藿)
Epimedium is classified as a ‘kidney-yang tonifying’ herb that is frequently used to treat disorders such as impotence, premature ejaculation and infertility, in combination with other herbs. Various preclinical studies have found that epimedium extract or its specific constituents (e.g. icaritin and icariside) have a selective oestrogen receptor modulator effect on breast cancer and uterine growth in nude mice.(29) Icariin, the major compound in epimedium, promotes oestrogen biosynthesis in human ovarian granulosa cells through aromatase, which converts androgens to oestrogens.(30) Another animal study found that icariin could exert anabolic effects on bone by activating oestrogen receptors (ER) and prevent ovariectomy-induced bone loss without inducing uterotrophic effects.(31)
These oestrogenic activities led to clinical studies using epimedium for menopausal symptoms. A randomised, placebo-controlled trial on 90 postmenopausal women, who consumed either 300 mL of aqueous herb extract or water daily for six months, reported significantly increased serum levels of oestradiol in the herbal group compared to the group that consumed only water. However, no recurrence of menstruation was reported in any of the subjects.(32) Another clinical trial on 50 late postmenopausal women, who were randomised to take either herb-derived phyto-oestrogen flavonoids (60 mg icariin, 15 mg genistein and 3 mg daidzein) or a placebo daily for 24 months, reported no significant increase in the treatment group’s serum oestradiol level. Interestingly, as with animal data, bone mineral density was maintained in the treatment group but was significantly reduced in the placebo group, without a concurrent hyperplasia effect on endometrium thickness.(33)
Ginseng (Radix Panax ginseng, Ren Shen 人参)
Ginseng is capable of restoring the oestrus cycle, interfering with the atrophy of reproductive target tissues caused by ovariectomy. These oestrogenic effects may be mediated by stimulating the biosynthesis of oestrogen in circulation and increasing the quantity of ER in the target organs.(34) It has also been suggested that the structural similarity between ginsenosides and oestradiol could be a contributing factor to the oestrogenic activity of ginseng.(35) An animal study reported the oestrogenic activity of ginseng on reproductive tissues in ovariectomised mice.(34) In a case report, a 12-year-old Korean-Japanese boy was diagnosed with gynaecomastia after taking 500 mg/day of red ginseng extract for one month for its strengthening effect; the gynaecomastia resolved upon cessation of the ginseng supplement. However, the boy’s oestradiol and testosterone levels were within normal levels.(36) A small number of case reports also showed an association between ginseng and postmenopausal vaginal bleeding and mastalgia. However, the exact source and ingredients consumed by the case subjects were not specified in these reports.(37,38)
In a placebo-controlled, double-blind clinical trial conducted in 72 postmenopausal women who were randomised to take either 3 g of red ginseng (including ginsenosides 60 mg/day) or a placebo daily for 12 weeks, no significant difference in serum oestradiol level was found between the treatment and placebo groups. However, subjects in the treatment group showed a significant improvement in menopausal symptoms, total cholesterol and low-density lipoprotein.(39)
Chinese angelica (Radix Angelicae sinensis, Dang Gui 当归)
Chinese angelica is a ‘blood-nourishing’ herb often used in gynaecological conditions such as perimenopausal syndrome and menstrual disorders. Chinese angelica extract or its constituent, ferulic acid, exhibits oestrogenic activity via various mechanisms: it stimulates the growth of both ER-positive and ER-negative breast cancer cells in vitro; competitively inhibits binding of oestradiol to ER in vitro; induces transcription activity in oestrogen-responsive cells in vitro; suppresses luteinising hormone (LH) secretion; and influences uterine growth and vaginal cytology in ovariectomised rats.(40,41) Its oestrogenic activity in humans is implicated by a case report of a 35-year-old man who developed gynaecomastia after ingesting ‘Dong Quai’ pills daily for a month (specific dosage unknown). His hormonal results (oestradiol, testosterone, follicle-stimulating hormone and LH levels) were all within normal range. His gynaecomastia regressed completely three months after he discontinued the pills.(42) However, this oestrogenic activity was not demonstrated in a double-blind, randomised, placebo-controlled trial in which 71 postmenopausal women were randomised to take either 4.5 g Chinese angelica or a placebo daily for 24 weeks. The study showed that the herb did not significantly increase the serum oestradiol level compared with the placebo, nor did it promote endometrial proliferation or increase the maturation of vaginal epithelial cells.(43)
Table I summarises the aforementioned herbs, the steroidal compounds identified in these herbs, and the highest level of evidence supporting or refuting their steroidal activities.
Table I.
A summary of the herbs reviewed.
ADULTERATION OF HERBAL PRODUCTS WITH EXOGENOUS GLUCOCORTICOIDS
Adulteration involving the addition of undeclared drugs to herbal products is a real cause for concern in the safety of TCM usage. Adulteration of herbal products can potentially have a greater clinical impact than the inherent steroidal activities of the aforementioned herbs. Illegal products could be marketed as natural herbal products by adopting TCM herb names and listing herbal components as their ingredients, when they are in fact adulterated with undeclared, potent Western medicinal drug components. Among these adulterants – which include nonsteroidal anti-inflammatory drugs, oral hypoglycaemics, antihistamines and sildenafil – glucocorticoid is one of the most commonly found ingredients.(44,45) There have been cases of consumers who were misled into consuming these ‘natural and safe’ herbal products that carry TCM herb names in their product name or ingredient list, but ended up experiencing adverse events such as Cushing’s syndrome, due to adulteration with glucocorticoids.(46)
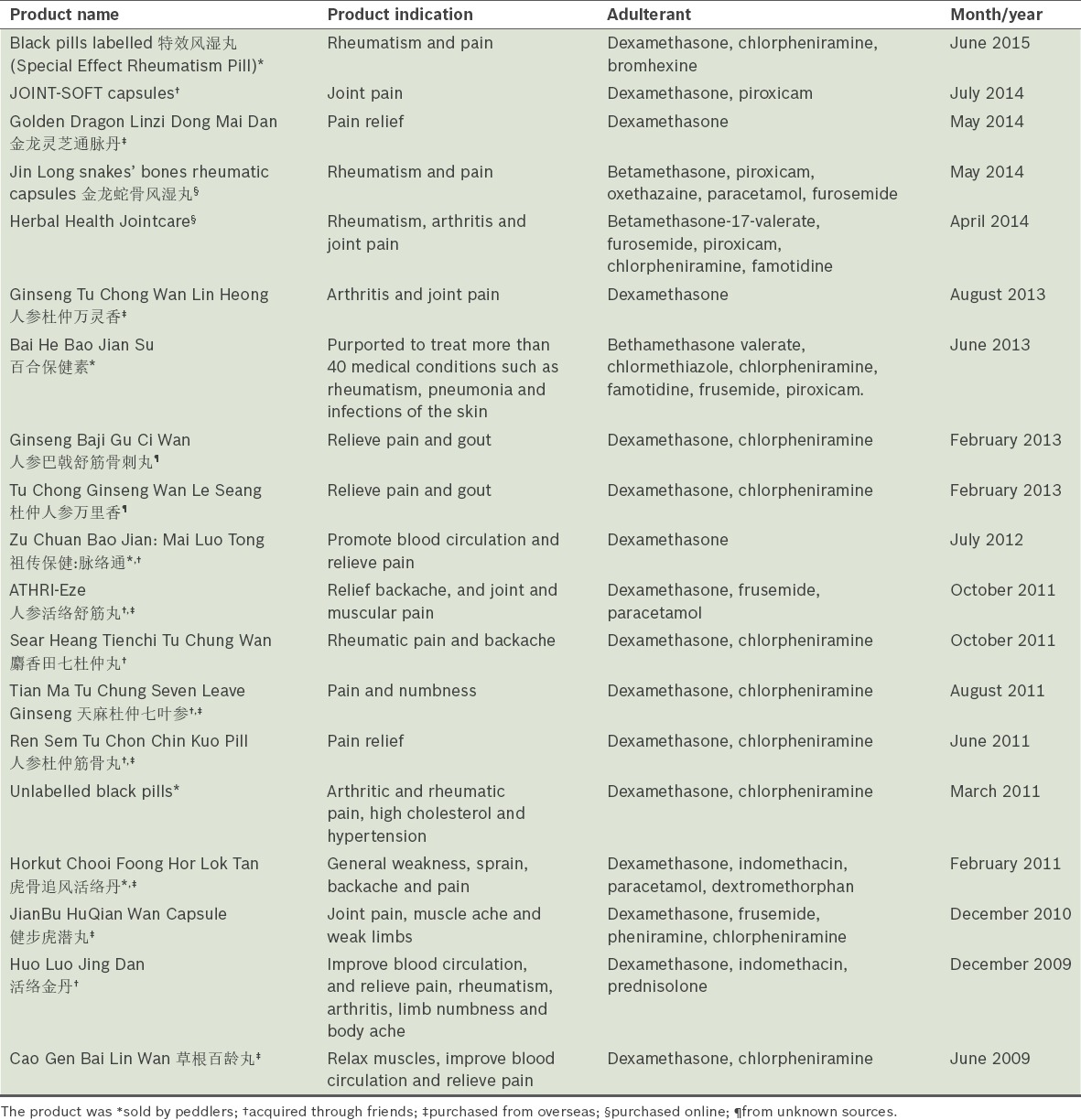
While most herbs do not naturally contain corticosteroids, synthetic steroidal components may be intentionally added to illegal products trying to masquerade as safe herbal products. This is illustrated by a case encountered by Singapore General Hospital’s TMIS in April 2014. A 67-year-old female patient with idiopathic thrombocytopenic purpura had consumed an herbal supplement called ‘Herbal Health Jointcare’ for her frozen shoulder and joint pain. She experienced rapid pain relief within two days, as did her friend who recommended the product. According to its official website, the herbal product contains multiple herbal components commonly used to treat arthritis pain and relieve joint inflammation caused by rheumatism. However, none of these herbs are known to contain naturally occurring steroids. This product aroused our suspicion and prompted reporting to the HSA, because it claimed to contain TCM herbs, yet was not found in the list of CPM registered with the HSA. Chemical analysis for common poisons in the product sample found that it was adulterated with five undeclared, potent medicinal components, one of which was betamethasone-17-valerate.
A search of the HSA’s illegal products online database and press release archive(47,48) yielded various other products sold under the guise of traditional or herbal medicine, which were found to be adulterated with undeclared glucocorticoids in 2009–2015. All these products were not locally registered CPM but mostly obtained from overseas, local peddlers or friends, and the legitimacy of their manufacturers was unknown. They usually incorporated herb names as part of their product names or claimed to contain natural, herbal ingredients. A striking pattern observed in these products is that the majority were indicated for pain relief and most were uncovered due to either adverse events or miraculous pain relief reported by consumers. Table II summarises the information on these illegal products.
Table II.
Illegal herbal products adulterated with steroids (2009–2015).
CONCLUSION
Based on the currently available evidence (as reviewed above and summarised in Table I), very few herbs used in TCM have clinically relevant steroidal activities. The few randomised controlled trials on ginseng, Chinese angelica and epimedium have shown an absence of clinically detectable oestrogenic activities, despite the presence of oestrogenic compounds in these herbs. Case reports have shown that liquorice exerts mineralocorticoid effects, while deer musk contains androgenic compounds. The present evidence on the steroidal activity of many other herbs is generally limited to in vitro and animal studies, which have yet to be corroborated by clinical studies for their clinical relevance. More often than not, it is the illegal products marketed under the guise of traditional or herbal medicine that contain steroids, especially glucocorticoids. The infiltration of such adulterated products into the market has not only put consumers’ health at risk, but also perpetuated and substantiated the widely held misconception that TCM products or herbs contain steroids, when the undeclared medicinal components in these products are the actual culprits.
In clinical practice, if a patient presents with adrenal insufficiency or Cushingoid features and gives a history of TCM herb or CPM consumption, it would be wise for the healthcare provider to probe further to find out specifically what the patient consumed. If a CPM product is involved, he should check whether it has been registered locally by looking for the label on the outer packaging (Fig. 1) or searching the HSA online search portal.(49) Extra caution should be taken when the herbal supplements are indicated for pain relief, as such products may be adulterated with glucocorticoids for their potent anti-inflammatory effect. Exaggerated product claims or a lack of ingredient labels on poor-quality packaging are signals of dubiety. If adverse events are associated with CPM products, reporting of suspected adverse events to the HSA(50) is strongly encouraged, even if causality has not been ascertained, because further investigation may lead to the discovery of products that pose a threat to public health, followed by appropriate regulatory action. Analysis of such cases is important in monitoring the safety of complementary and alternative medicine products, and improves our understanding of the benefits and risks associated with the use of such products.(46) Conversely, simply noting that the patient has taken ‘TCM’ and attributing the adverse effect to this without further investigation into the specific product may lead to a misperception of TCM while allowing the real culprit to go undetected, which could pose a serious public health risk.
Fig. 1.

Image shows the required label on the outer packaging of locally listed Chinese proprietary medicine.
REFERENCES
- 1.Chrousos G, Pavlaki AN, Magiakou MA. Glucocorticoid Therapy and Adrenal Suppression. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al., editors. Endotext [Internet] South Dartmouth, MA: MDText.com, Inc; 2000. [Google Scholar]
- 2.Shih V, Chiang JY, Chan A. Complementary and alternative medicine (CAM) usage in Singaporean adult cancer patients. Ann Oncol. 2009;20:752–7. doi: 10.1093/annonc/mdn659. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Chang P, Lee SC, et al. Complementary and alternative medicine among Singapore cancer patients. Ann Acad Med Singapore. 2010;39:129–35. [PubMed] [Google Scholar]
- 4.Tan MG, Win MT, Khan SA. The use of complementary and alternative medicine in chronic pain patients in Singapore: a single-centre study. Ann Acad Med Singapore. 2013;42:133–7. [PubMed] [Google Scholar]
- 5.See A, Teo B, Kwan R, et al. Use of complementary and alternative medicine among dermatology outpatients in Singapore. Australas J Dermatol. 2011;52:7–13. doi: 10.1111/j.1440-0960.2010.00709.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee GB, Charn TC, Chew ZH, Ng TP. Complementary and alternative medicine use in patients with chronic diseases in primary care is associated with perceived quality of care and cultural beliefs. Fam Pract. 2004;21:645–60. doi: 10.1093/fampra/cmh613. [DOI] [PubMed] [Google Scholar]
- 7.Lim MK, Sadarangani P, Chan HL, Heng JY. Complementary and alternative medicine use in multiracial Singapore. Complement Ther Med. 2005;13:16–24. doi: 10.1016/j.ctim.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Health Sciences Authority Singapore. Regulatory Framework. [Accessed July 13, 2015]. Available at: http://www.hsa.gov.sg/content/hsa/en/Health_Products_Regulation/Complementary_Health_Products/Chinese_Proprietary_Medicines/Overview/Regulatory_Framework.html .
- 9.Health Sciences Authority Singapore. Guidelines for Traditional Medicinal Materials. [Accessed July 13, 2015]. Available at: http://www.hsa.gov.sg/content/hsa/en/Health_Products_Regulation/Complementary_Health_Products/Guidelines_for_Traditional_Medicinal_Materials.html .
- 10.Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.) its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46:167–92. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Celik MM, Karakus A, Zeren C, et al. Licorice induced hypokalemia, edema, and thrombocytopenia. Hum Exp Toxicol. 2012;31:1295–8. doi: 10.1177/0960327112446843. [DOI] [PubMed] [Google Scholar]
- 12.van Gelderen CE, Bijlsma JA, van Dokkum W, Savelkoul TJ. Glycyrrhizic acid: the assessment of a no effect level. Hum Exp Toxicol. 2000;19:434–9. doi: 10.1191/096032700682694251. [DOI] [PubMed] [Google Scholar]
- 13.Food and Agriculture Organization of the United Nations; World Health Organization. Evaluation of certain food additives. World Health Organ Tech Rep Ser. 2005;928:1–156. [PubMed] [Google Scholar]
- 14.Miao MS, Zhu FP, Zhu PS. Shiyong Zhongyao Dulixue. 1st ed. Shanghai: Second Military Medical University Publisher; 2007. [Google Scholar]
- 15.Su M, Zhang J, You YY, et al. [Content of active ingredient in Glycyrrhiza uralensis Fisch from different origins] Cent South Pharm. 2014;12:1022–4. Chinese. [Google Scholar]
- 16.Sigurjónsdóttir HA, Franzson L, Manhem K, et al. Liquorice-induced rise in blood pressure: a linear dose-response relationship. J Hum Hypertens. 2001;15:549–52. doi: 10.1038/sj.jhh.1001215. [DOI] [PubMed] [Google Scholar]
- 17.Lee YJ, Chung E, Lee KY, et al. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–40. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- 18.Leung KW, Leung FP, Huang Y, Mak NK, Wong RN. Non-genomic effects of ginsenoside-Re in endothelial cells via glucocorticoid receptor. FEBS Lett. 2007;581:2423–8. doi: 10.1016/j.febslet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 19.Kim IH, Kim SK, Kim EH, et al. Korean Red Ginseng Up-regulates C21-Steroid Hormone Metabolism via Cyp11a1 Gene in Senescent Rat Testes. J Ginseng Res. 2011;35:272–82. doi: 10.5142/jgr.2011.35.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa E, Nakagawa S, Miyate Y, et al. Inhibitory effect of protopanaxatriol ginseng metabolite M4 on the production of corticosteroids in ACTH-stimulated bovine adrenal fasciculata cells. Life Sci. 2013;92:687–93. doi: 10.1016/j.lfs.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Leu SF, Chien CH, Tseng CY, Kuo YM, Huang BM. The in vivo effect of Cordyceps sinensis mycelium on plasma corticosterone level in male mouse. Biol Pharm Bull. 2005;28:1722–5. doi: 10.1248/bpb.28.1722. [DOI] [PubMed] [Google Scholar]
- 22.Wang SM, Lee LJ, Lin WW, Chang CM. Effects of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplets in cultured rat adrenocortical cells. J Cell Biochem. 1998;69:483–9. doi: 10.1002/(sici)1097-4644(19980615)69:4<483::aid-jcb9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Thevis M, Schänzer W, Geyer H, et al. Traditional Chinese medicine and sports drug testing: identification of natural steroid administration in doping control urine samples resulting from musk (pod) extracts. Br J Sports Med. 2013;47:109–14. doi: 10.1136/bjsports-2012-090988. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Wang J, Liu X, Xu Y, He Z. Influences of musk administration on the doping test. Steroids. 2013;78:1047–52. doi: 10.1016/j.steroids.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Ling Y, Luo F, Li Q, Bao Y, Zhang Y. [Assay of estradiol and testosterone in pilose antler] Chin Trad Herbal Drugs. 1999;30:499–501. Chinese. [Google Scholar]
- 26.Lu C, Wang M, Mu J, et al. Simultaneous determination of eighteen steroid hormones in antler velvet by gas chromatography-tandem mass spectrometry. Food Chem. 2013;141:1796–806. doi: 10.1016/j.foodchem.2013.04.104. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CC, Huang YL, Tsai SJ, Sheu CC, Huang BM. In vivo and in vitro stimulatory effects of Cordyceps sinensis on testosterone production in mouse Leydig cells. Life Sci. 2003;73:2127–36. doi: 10.1016/s0024-3205(03)00595-2. [DOI] [PubMed] [Google Scholar]
- 28.Leu SF, Poon SL, Pao HY, Huang BM. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci Biotechnol Biochem. 2011;75:723–31. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 29.Indran IR, Zhang SJ, Zhang ZW, et al. Selective estrogen receptor modulator effects of epimedium extracts on breast cancer and uterine growth in nude mice. Planta Med. 2014;80:22–8. doi: 10.1055/s-0033-1360112. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Lu D, Guo J, et al. Icariin from Epimedium brevicornum Maxim promotes the biosynthesis of estrogen by aromatase (CYP19) J Ethnopharmacol. 2013;145:715–21. doi: 10.1016/j.jep.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 31.Mok SK, Chen WF, Lai WP, et al. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor-dependent osteoblastic functions in UMR 106 cells. Br J Pharmacol. 2010;159:939–49. doi: 10.1111/j.1476-5381.2009.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan FF, Liu Y, Liu YF, Zhao YX. Herba Epimedii water extract elevates estrogen level and improves lipid metabolism in postmenopausal women. Phytother Res. 2008;22:1224–8. doi: 10.1002/ptr.2451. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G, Qin L, Shi Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J Bone Miner Res. 2007;22:1072–9. doi: 10.1359/jbmr.070405. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Ding J, Ma XP, et al. Treatment with Panax ginseng antagonizes the estrogen decline in ovariectomized mice. Int J Mol Sci. 2014;15:7827–40. doi: 10.3390/ijms15057827. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Gray SL, Lackey BR, Tate PL, Riley MB, Camper ND. Mycotoxins in root extracts of American and Asian ginseng bind estrogen receptors alpha and beta. Exp Biol Med (Maywood) 2004;229:560–8. doi: 10.1177/153537020422900615. [DOI] [PubMed] [Google Scholar]
- 36.Kakisaka Y, Ohara T, Tozawa H, et al. Panax ginseng: a newly identified cause of gynecomastia. Tohoku J Exp Med. 2012;228:143–5. doi: 10.1620/tjem.228.143. [DOI] [PubMed] [Google Scholar]
- 37.Greenspan EM. Ginseng and vaginal bleeding. JAMA. 1983;249:2018. [PubMed] [Google Scholar]
- 38.Palmer BV, Montgomery AC, Monteiro JC. Gin Seng and mastalgia. Br Med J. 1978;6122:1284. doi: 10.1136/bmj.1.6122.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SY, Seo SK, Choi YM, et al. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: a double-blind randomized controlled trial. Menopause. 2012;19:461–6. doi: 10.1097/gme.0b013e3182325e4b. [DOI] [PubMed] [Google Scholar]
- 40.Circosta C, Pasquale RD, Palumbo DR, Samperi S, Occhiuto F. Estrogenic activity of standardized extract of Angelica sinensis. Phytother Res. 2006;20:665–9. doi: 10.1002/ptr.1928. [DOI] [PubMed] [Google Scholar]
- 41.Lau CB, Ho TC, Chan TW, Kim SC. Use of dong quai (Angelica sinensis) to treat peri- or postmenopausal symptoms in women with breast cancer: is it appropriate? Menopause. 2005;12:734–40. doi: 10.1097/01.gme.0000184419.65943.01. [DOI] [PubMed] [Google Scholar]
- 42.Goh SY, Loh KC. Gynaecomastia and the herbal tonic “Dong Quai”. Singapore Med J. 2001;42:115–6. [PubMed] [Google Scholar]
- 43.Hirata JD, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril. 1997;68:981–6. doi: 10.1016/s0015-0282(97)00397-x. [DOI] [PubMed] [Google Scholar]
- 44.Low MY, Zheng Y, Li L, et al. Safety and quality assessment of 175 illegal sexual enhancement products seized in red-light districts in Singapore. Drug Saf. 2009;32:1141–6. doi: 10.2165/11316690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Yee SK, Chu SS, Xu YM, Choo PL. Regulatory control of Chinese Proprietary Medicines in Singapore. Health Policy. 2005;71:133–49. doi: 10.1016/j.healthpol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Patel DN, Low WL, Tan LL, et al. Adverse events associated with the use of complementary medicine and health supplements: an analysis of reports in the Singapore Pharmacovigilance database from 1998 to 2009. Clin Toxicol (Phila) 2012;50:481–9. doi: 10.3109/15563650.2012.700402. [DOI] [PubMed] [Google Scholar]
- 47.Health Sciences Authority Singapore. Illegal Health Product Database Search. [Accessed July 13, 2015]. Available at: http://www.hsa.gov.sg/pub/IllegalDrug/ProductListing.aspx .
- 48.Health Sciences Authority Singapore. Press Releases. [Accessed July 13, 2015]. Available at: http://www.hsa.gov.sg/content/hsa/en/News_Events/Press_Releases.html .
- 49.Health Sciences Authority Singapore. PZ4970 Infosearch - Chinese Proprietary Medicine. [Accessed July 26, 2015]. Available at: http://eservice.hsa.gov.sg/prism/common/enquirepublic/SearchCPProduct.do?action=load .
- 50.Health Sciences Authority Singapore. Report Adverse Events related to Health Products. [Accessed July 13, 2015]. Available at: http://www.hsa.gov.sg/content/hsa/en/Health_Products_Regulation/Safety_Information_and_Product_Recalls/Report_Adverse_Events_related_to_health_products.html .


