Abstract
INTRODUCTION
This study aimed to analyse the concordance rate, sensitivity, specificity, positive predictive value (PPV) and negative predictive value of core needle biopsy (CNB) and subsequent surgical specimen (SS) in assessing levels of oestrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2/neu). It also evaluated the revised American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines for ER/PgR positivity.
METHODS
We analysed the breast cancer database of KK Women’s and Children’s Hospital, Singapore, from 1 June 2005 to 30 December 2012. Invasive breast cancer patients who had CNB and subsequent SS were included.
RESULTS
A total of 560 patients were included. The concordance of ER, PgR and HER2/neu positivity between CNB and SS was 96.1%, 89.1% and 96.8%, respectively. When the ‘ER ≥ 10% positive’ group was compared with the ‘ER ≥ 1% positive’ group, specificity increased from 79.7% to 92.5% and PPV increased from 93.9% to 97.5%. When the ‘PgR ≥ 10% positive’ group was compared with the ‘PgR ≥ 1% positive’ group, specificity increased from 84.2% to 89.3% and PPV improved from 89.7% to 92.9%. The revised ASCO/CAP guidelines decreased discordant results by > 50% for ER and by 18.2% for PgR.
CONCLUSION
CNB has high concordance with SS in the evaluation of the molecular profile of invasive breast cancer. Thus, molecular evaluation does not need to be repeated with SS except for ER-, PgR- and HER2/neu-negative CNB results. The revised ASCO/CAP guidelines resulted in more precise ER and PgR status on CNB.
Keywords: accuracy of core needle biopsy, ASCO/CAP guidelines, biological profile ER PgR HER2/neu, breast carcinoma, concordance between core needle biopsy and surgical specimen
INTRODUCTION
Breast cancer is the most common cancer affecting women in Singapore; 9,284 cases were diagnosed from 2010 to 2014.(1) Although the incidence of breast cancer in Singapore is increasing at an average rate of 5% annually, the global mortality rates for breast cancer have significantly decreased since the 1970s and survival rates have also increased; this could be due to better screening, early detection and improved treatment.(2,3)
Core needle biopsy (CNB) has been well established as an important tool in cancer diagnosis. It is commonly performed before the start of any treatment. CNB is considered the method of choice for tissue sampling and is a part of the triple assessment for breast cancer. It is accurate in diagnosing breast carcinoma and has a specificity of 96%–100%.(4) Molecular profiling of cancer, an important aspect in cancer treatment, can also be determined using CNB. Profiling includes ascertaining the levels of oestrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2/neu) in the cancer cells. The molecular profile of breast cancer is critical in the management of patients with breast carcinoma. ER level is a powerful predictive factor for response to endocrine treatment and long-term outcome.(5) HER2/neu overexpression has been associated with a worse prognosis, and is also a predictor of response to trastuzumab and/or pertuzumab treatment.(6)
Surgical specimen (SS) has traditionally been considered the gold standard for assessing the predictive and prognostic factors for breast cancer. The results obtained from CNB, on the other hand, may be ambiguous and not representative of the whole tumour, as the sample obtained is small and the distribution of antigens could be varied within the tumour (i.e. tumour heterogeneity); moreover, CNB is prone to crush or edge artefacts. Nonetheless, a recent meta-analysis has suggested that CNB could replace SS in determining ER, PgR and HER2/neu status.(7)
The American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) has estimated that 20% of overall ER testing might be inaccurate due to variability in the post-analytical processes.(8) Based on this finding, in June 2010, ASCO/CAP recommended increasing testing reliability by lowering the positivity threshold of both ER- and PgR-positive cells from ≥ 10% to ≥ 1%.(8) The purpose of the present study was twofold: to deduce the concordance rate between CNB and SS for determining ER, PgR and HER2/neu status; and to compare the specificity and sensitivity of the old and new thresholds for determining ER and PgR status.
METHODS
Data archived in the breast cancer database of KK Women’s and Children’s Hospital (KKH), Singapore, from 1 June 2005 to 30 December 2012 (91 months) was collected prospectively and analysed. A total of 560 invasive breast cancer patients who underwent CNB and subsequent SS histology to determine their ER, PgR and HER2/neu status were included in the study. The Institutional Review Board of KKH approved the study protocol. Excluded from the study were patients (a) whose histology results showed noninvasive carcinoma; (b) whose histological processing was done in other hospitals; (c) whose CNB/SS receptor status was unavailable or remained equivocal; and (d) who had undergone neoadjuvant chemotherapy (i.e. a known confounding factor).
From June 2005 to December 2010 (67 months), both the ER and PgR data of 286 patients was available. From January 2011 to December 2012 (24 months), during which the new ASCO/CAP guidelines were used, the ER and PgR data of 264 and 262 patients, respectively, was available. The CNB and SS HER2/neu data of 468 patients was available from June 2005 to December 2012 (91 months).
All CNBs were performed using an automated biopsy gun or a vacuum-assisted biopsy system (both from Bard, Covington, GA, USA), with a 22-mm throw and 14-gauge needle. A radiologist or surgeon performed the CNB using a handheld technique, with or without ultrasonography guidance. The number of core samples obtained during CNB (usually 4–6) was left to the discretion of the radiologist or surgeon.
Immunohistochemistry (IHC) assays are usually used to determine the ER, PgR and HER2/neu status of breast cancers. IHC is superior to ligand binding assays because of its simplicity and reproducibility.(9,10) In the present study, the ER, PgR and HER2/neu status of breast cancer was determined using IHC. Paraffin sections of the tissues were fixed for 6–48 hours with 10% neutral buffered formalin. They were then stained and studied for the intensity/percentage of staining for ER, PgR and HER2/neu. The following antibodies were used for detection of ER, PgR and HER2/neu: CONFIRM anti-ER (SP 1); CONFIRM anti-PgR (1E2); and PATHWAY anti-HER2/neu (4B5) (all from Ventana Medical Systems Inc, Tucson, AZ, USA). The ultraView Universal DAB autostainer and Benchmark ULTRA detection system (Ventana Medical Systems Inc, Tucson, AZ, USA) were used.
HER2/neu analysis was based on non-equivocal IHC results; if the IHC results were equivocal, the HER2/neu test was repeated using SS. If the results remain equivocal after repeat SS, fluorescence in situ hybridisation (FISH) was performed. This was in accordance with the hospital’s protocol to reduce the patient’s cost for histological testing (as the patient would need to pay for the additional FISH test, if performed). Statistical analysis of the HER2/neu equivocal results on CNB is not presented in this article.
Prior to June 2010, ER and PgR status was considered to be positive (1+) if ≥ 10% of the tumour nuclei were immunoreactive. In June 2010, ASCO/CAP revised the guidelines on ER and PgR status, and the revised guidelines were implemented in our hospital in January 2011. The revised guidelines state that: (a) ER and PgR status was considered to be positive (1+) if ≥ 1% of the tumour nuclei were immunoreactive; (b) ER and PgR status was considered to be negative if < 1% of the tumour nuclei were immunoreactive, even in the presence of positive internal epithelial elements; and (c) ER and PgR status was considered to be uninterpretable if no tumour nuclei were immunoreactive and the internal epithelial elements lacked nuclear staining. HER2/neu status interpretation was done according to the ASCO/CAP 2010 guidelines.
The concordance, sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) were calculated by using CNB as the test assessment and SS as the gold standard. Exact 95% confidence interval was calculated based on the binomial distribution.
RESULTS
The clinicopathological characteristics of the 560 patients who were treated in our hospital are summarised in Table I. Breast cancer was most prevalent in the 51–60 years age group and the median age of the patients was 52 years. Invasive ductal carcinoma was the most common type of breast cancer (82.7%). The ER and PgR results were divided into two groups for analysis: (a) Group 1 consisted of patients whose ER and PgR data was collected from June 2005 to December 2010; and (b) Group 2 consisted of patients whose ER and PgR data was collected from January 2011 to December 2012 (i.e. after implementation of the revised ASCO/CAP guidelines).
Table I.
Clinicopathological characteristics of the patients (n = 560).
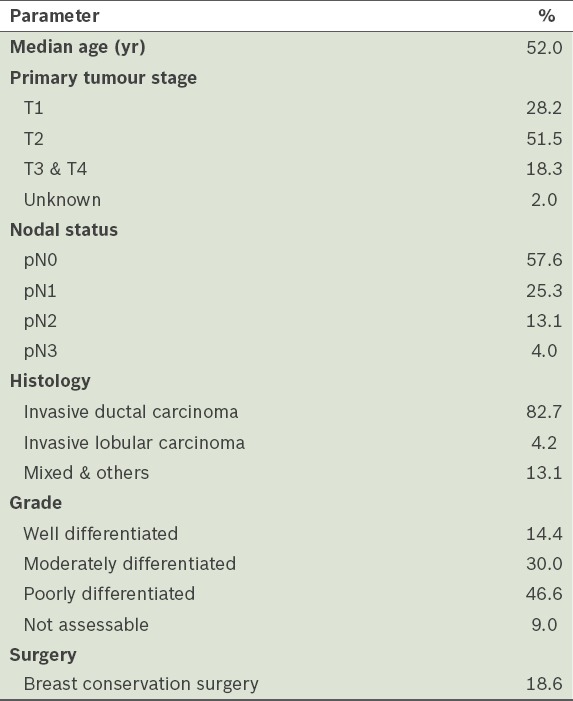
For ER, Group 1 had 286 patient samples and Group 2 had 264 patient samples (Table II). Among the patient samples, 75.9% in Group 1 were found to be ER-positive, while 74.6% in Group 2 were ER-positive. The diagnostic statistics for ER status are shown in Table III. Comparing the ER status of Group 1 and Group 2, the specificity and PPV were found to have increased, from 79.7% to 92.5% and from 93.9% to 97.5%, respectively. In other words, the evaluation of ER-positive patients became easier after the revised ASCO/CAP guidelines (i.e. ASCO/CAP 2010) were implemented in our hospital. In Group 1, 22 patients had discordant results between CNB and SS (concordance rate was 94.8%). CNB was found to be better than SS in predicting ER positivity – 19 SS results that were reported to be ER-negative were found to be ER-positive on CNB, while only three ER-negative CNB samples turned out to be ER-positive on SS histology. The concordance rate between CNB and SS was higher in Group 2 than in Group 1 (97.4% vs. 94.8%). After the change in guidelines, CNB was found to be more reliable than SS in predicting ER positivity.
Table II.
Concordance between core needle biopsy (CNB) and surgical specimen (SS) for oestrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2/neu) status.
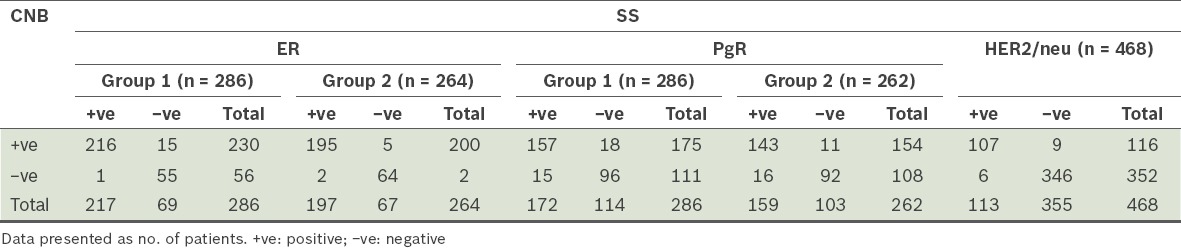
Table III.
Diagnostic statistics of the patients’ oestrogen receptor (ER) status according to time of data collection.
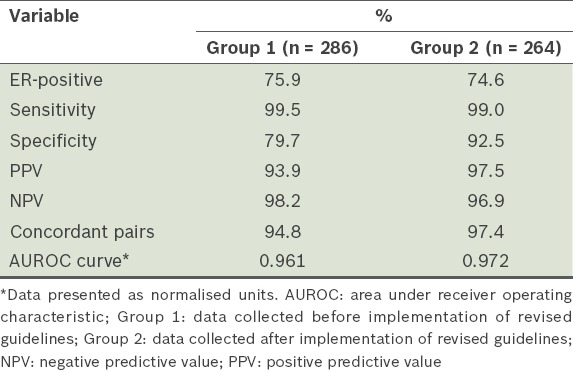
There were 286 patient samples for PgR in Group 1 and 262 patient samples for PgR in Group 2 (Table II). Among the patient samples, 60.1% in Group 1 were found to be PgR-positive, while 60.7% in Group 2 were PgR-positive. The diagnostic statistics for PgR status are shown in Table IV. Comparing the PgR status of Group 1 with that of Group 2, there was a marginal increase in specificity and PPV, from 84.2% to 89.3% and from 89.7% to 92.9%, respectively. Thus, after the revised ASCO/CAP guidelines were implemented in our hospital, there was greater ease in the evaluation of PgR-positive patients. A total of 60 patients with discordant PgR results were noted in the two groups – on SS histology, 29 PgR-positive tumours found on CNB turned out to be PgR-negative, while 31 tumours that were PgR-negative on CNB were PgR-positive. The concordance rate for PgR status increased marginally, from 88.5% to 89.7%, after the revised ASCO/CAP guidelines were implemented.
Table IV.
Diagnostic statistics of the patients’ progesterone receptor (PgR) status according to time of data collection.
A total of 468 patient samples were analysed for HER2/neu status. HER2/neu positivity was 24.2%. The diagnostic statistics for HER2/neu status are shown in Table V. The concordance rate between CNB and SS for HER2/neu status was 96.8%, indicating that CNB was as reliable as SS in predicting HER2/neu positivity on IHC.
Table V.
Diagnostic statistics of the patients’ human epidermal growth factor receptor 2 (HER2/neu) status (n = 468).

DISCUSSION
Breast conservation surgery is becoming increasingly popular among women with breast cancer. Many patients opt for neoadjuvant chemotherapy to shrink the size of the tumour, so as to facilitate breast conservation surgery. Accurate determination of the molecular profile of the tumour is important to predict which patient groups would benefit the most from neoadjuvant chemotherapy.
Although SS has traditionally been considered the gold standard for molecular profiling, ASCO and CAP jointly recommend the preferential use of CNB samples for ER and PgR testing; this recommendation is based on their empirical observations that CNB samples are more likely to fix better in formalin than SS samples.(11) For SS, the time from the interruption of the blood supply to the initiation of fixation is longer. The resected specimens are not immediately sliced and fixed, resulting in poor fixation of the tumour. In our hospital, fixation protocols are more standardised for CNB than for SS; the samples obtained from CNB are immediately fixed in formalin for lab analysis, while the time from resection to fixation is usually much longer for surgically excised specimens. It is known that preanalytical variables, such as prolonged time to fixation, surface fixation or prolonged fixation in neutral buffered formalin, can lead to negative ER/PgR results. In the present study, we did not analyse the fixation time, as there is variable time delay at the different stages of specimen preparation (e.g. marking, weighing, photographing and transporting of the specimen); there is also a lack of data on fixation time in KKH’s database.(12-16)
There are, however, disadvantages associated with the use of CNB samples for molecular profiling. Previous studies have reported a tendency toward upscoring in CNB as compared to SS; this finding is probably due to the better fixation that is achieved with CNB as compared to SS.(17) CNB samples are also prone to crush artefacts, which may lead to false positive results. Furthermore, CNB may not accurately reflect the biological profile of the tumour, as sampling error may occur due to the heterogeneous distribution of the antigens within the tumour.
Previous studies have shown variable concordance rates between CNB and SS, ranging from 61% to 99% for ER, 61% to 91% for PgR and 64% to 96% for HER2/neu.(18) In the present study, concordance rates between CNB and SS for ER and HER2/neu were high (96.1% and 96.8%, respectively); for PgR, the concordance rate was only 89.1%. The overall rate of PgR positivity was 75.3%.
Our analysis of the discordant results for ER showed 22 patients with discordant results in Group 1 (i.e. the group that was scored based on the old ASCO/CAP guidelines). CNB was found to be better than SS at predicting ER positivity, which can likely be attributed to better processing and fixation associated with CNB. In the present study, about 3.4% of patients would have benefited from endocrine therapy because of the higher specificity of CNB; these patients would have missed the benefit if only SS results were used for therapy decisions. If the ER status is negative on CNB, it should be repeated using subsequent SS to confirm ER negativity.
The discordance between CNB and SS for ER status was reduced by > 50% (from 15 to seven) after the revised ASCO/CAP guidelines were implemented. The revised guidelines, which changed the threshold for determining ER status, resulted in increased PPV, NPV and specificity. In other words, it made CNB more accurate and reliable for predicting ER status.
Probable explanations for the discordant findings include tumour heterogeneity, variation in tissue processing and fixation, inter- and intraobserver variability, sampling error, and delay in the exposure of the centre of SS sample to formalin; all of these could have resulted in false negative results. Douglas-Jones et al noted that 35% lower staining was seen in SS as compared to CNB when tissue assay was done on the same specimen.(19) Immunoreactivity for both ER and PgR was significantly higher in CNB than in SS. Douglas-Jones et al also noted that the periphery of the tumours generally stained more intensely than the centre in SS. This variation was not seen in CNB, suggesting that such findings may be an artefact problem in SS.(19) Formalin fixation induces the formation of crosslinks between proteins and nucleic acids, and this is necessary for IHC analysis. Irregular and/or inadequate formalin fixation due to delayed fixation, under-fixation or over-fixation can reduce the reliability of IHC staining.
In the present study, the overall concordance between CNB and SS for PgR status was 89.1%. A total of 60 patients had discordant PgR results in the two groups – 29 PgR-positive tumours on CNB turned out to be PgR-negative on SS histology and 31 tumours that were PgR-negative on CNB turned out to be PgR-positive on SS histology. Hence, CNB can miss 5.5% of PgR-positive patients if the PgR study is done using CNB only. Although consideration for endocrine treatment is generally not based on PgR status alone, PgR expression is still used as a prognostic index.(20,21)
In most previous studies, as well as in the present study, the concordance rate between CNB and SS for PgR is relatively low; hence, the results obtained should be used with caution. The higher discordance rate seen for PgR status as compared to ER status might be due to the higher tumour heterogeneity for PgR. As the expression of PgR is often dependent on an intact signal pathway and the histological grade of the tumour, PgR frequently shows a more heterogeneous spread within the tumour cells.(22) It is thought that poorly differentiated tumours are more often PgR-negative, and that if a tumour is PgR-positive, it will not be easily influenced by external signals.(22)
In the present study, the number of discordant results for PgR was reduced from 33 to 27 (i.e. 18.2% reduction in discordance rate) after the revised ASCO/CAP guidelines were implemented. There was only a marginal increase in the specificity, PPV and concordance rates for PgR after the revised guidelines were implemented.
The concordance between CNB and SS for HER2/neu was 96.8% in the present study. Overall, 15 patients had discordant results – nine tumours that were HER2/neu-positive on CNB turned out to be HER2/neu negative on SS histology and six tumours that were HER2/neu negative on CNB turned out to be HER2/neu-positive on SS histology. The HER2/neu positivity rate was 24.2%. CNB has high concordance with SS and is accurate in predicting HER2/neu status. If the results are equivocal, FISH needs to be done to confirm HER2/neu status.
Based on the findings of the present study, the revised ASCO/CAP guidelines for ER and PgR positivity have resulted in greater accuracy in the interpretation of CNB results. It decreased the discordance rate between CNB and SS by > 50% for ER and by 18.2% for PgR. The revision also increased the specificity and PPV of CNB and increased the reliability of accurately predicting the biological profile of invasive breast cancer. Probable explanations for the improved accuracy of CNB with the use of the revised ASCO/CAP guidelines include: (a) ease of counting, as pathologists can now identify the tumour as ER-positive even if only 1% of the nuclei are immunoreactive; and (b) the role of tumour heterogeneity in hindering accurate diagnosis is reduced, as ≥ 1% positivity is taken as positive. ASCO/CAP had predicted that the revised guidelines would decrease the variation caused due to postanalytic variables. To improve CNB accuracy further, some studies have suggested that a minimum of five samples be taken (instead of a maximum of five samples) and that samples should be taken from the centre and periphery of the tumour.(23) Using tissue microarray analysis to overcome tumour heterogeneity problems may also further improve CNB results.
In the present study, the concordance between CNB and SS is high for ER and HER2/neu (96.1% and 96.8%, respectively). However, for PgR, it was only 89.1%. As the concordance for PgR is not high, caution needs to be exercised when interpreting CNB PgR results. The discordant results for ER were reduced by > 50% after the revised ASCO/CAP guidelines were implemented, while the discordant results for PgR decreased by 18.2% after the implementation of the revised ASCO/CAP guidelines. In other words, the revised ASCO/CAP guidelines have made the interpretation of CNB results more accurate and reliable in predicting both ER and PgR status. Since about 75% of breast cancers are hormone receptor-positive, the ability to avoid a repeat hormonal study with SS will reduce the cost of medical expenditure for both the patient and hospital.
To conclude, the present study provides evidence that CNB provides an accurate evaluation of the molecular profile of invasive breast cancer, especially ER and HER2/neu status. It also shows that evaluation of the molecular profile of breast cancer does not need to be repeated in SS, except in cases where CNB is negative for ER, PgR or HER2/neu and when cancer is shown to be weakly positive. In these cases, repeat tests are done with SS to prevent patients from missing out on the potential benefits of targeted therapy.
REFERENCES
- 1.National Registry of Diseases Office (NRDO), Health Promotion Board, Singapore. Singapore Cancer Registry Interim Annual Report Trends in Cancer Incidence in Singapore. 2010-2014. [Accessed February 14, 2017]. Available at: https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/cancer-trends-2010-2014_interim-annual-report_final-(public).pdf?sfvrsn=0 .
- 2.Lacey JV, Jr, Devesa SS, Brinton LA. Recent trends in breast cancer incidence and mortality. Environ Mol Mutagen. 2002;39:82–8. doi: 10.1002/em.10062. [DOI] [PubMed] [Google Scholar]
- 3.Kohler BA, Sherman RL, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, 1975-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verkooijen HM Core Biopsy After Radiological Localisation (COBRA) Study Group. Diagnostic accuracy of stereotactic large-core needle biopsy for nonpalpable breast disease: results of a multicenter prospective study with 95% surgical confirmation. Int J Cancer. 2002;99:853–9. doi: 10.1002/ijc.10419. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 6.Konecny G, Pauletti G, Pegram M, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–53. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Yuan Y, Gu Z, Shen K. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;134:957–67. doi: 10.1007/s10549-012-1990-z. [DOI] [PubMed] [Google Scholar]
- 8.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denley H, Pinder SE, Elston CW, Lee AH, Ellis IO. Preoperative assessment of prognostic factors in breast cancer. J Clin Pathol. 2001;54:20–4. doi: 10.1136/jcp.54.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller-Holzner E, Fink V, Frede T, Marth C. Immunohistochemical determination of HER2 expression in breast cancer from core biopsy specimens: a reliable predictor of HER2 status of the whole tumor. Breast Cancer Res Treat. 2001;69:13–9. doi: 10.1023/a:1012281221647. [DOI] [PubMed] [Google Scholar]
- 12.Nkoy FL, Hammond ME, Rees W, et al. Variable specimen handling affects hormone receptor test results in women with breast cancer: a large multihospital retrospective study. Arch Pathol Lab Med. 2010;134:606–12. doi: 10.5858/134.4.606. [DOI] [PubMed] [Google Scholar]
- 13.Diaz L, Sneige N. Estrogen receptor analysis for breast cancer: current issues and keys to increasing testing accuracy. Adv Anat Pathol. 2005;12:10–9. doi: 10.1097/00125480-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Fergenbaum JH, Garcia-Closas M, Hewitt SM, et al. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev. 2004;13:667–72. [PubMed] [Google Scholar]
- 15.Layfield LJ, Gupta D, Mooney EE. Assessment of Tissue Estrogen and Progesterone Receptor Levels: A Survey of Current Practice, Techniques, and Quantitation Methods. Breast J. 2000;6:189–196. doi: 10.1046/j.1524-4741.2000.99097.x. [DOI] [PubMed] [Google Scholar]
- 16.Pertschuk LP, Kim YD, Axiotis CA, et al. Estrogen receptor immunocytochemistry: the promise and the perils. J Cell Biochem Suppl. 1994;19:134–7. [PubMed] [Google Scholar]
- 17.Arnedos M, Nerurkar A, Osin P, et al. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC) Ann Oncol. 2009;20:1948–52. doi: 10.1093/annonc/mdp234. [DOI] [PubMed] [Google Scholar]
- 18.Thakkar JP, Mehta DG. A review of an unfavorable subset of breast cancer: estrogen receptor positive progesterone receptor negative. Oncologist. 2011;16:276–85. doi: 10.1634/theoncologist.2010-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douglas-Jones AG, Collett N, Morgan JM, Jasani B. Comparison of core oestrogen receptor (ER) assay with excised tumour: intratumoral distribution of ER in breast carcinoma. J Clin Pathol. 2001;54:951–5. doi: 10.1136/jcp.54.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hefti MM, Hu R, Knoblauch NW, et al. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013;15:R68. doi: 10.1186/bcr3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix M, Toillon RA, Leclercq G. Stable ‘portrait’ of breast tumors during progression: data from biology, pathology and genetics. Endocr Relat Cancer. 2004;11:497–522. doi: 10.1677/erc.1.00758. [DOI] [PubMed] [Google Scholar]
- 23.Ozdemir A, Voyvoda NK, Gultekin S, et al. Can core biopsy be used instead of surgical biopsy in the diagnosis and prognostic factor analysis of breast carcinoma? Clin Breast Cancer. 2007;7:791–5. doi: 10.3816/cbc.2007.n.041. [DOI] [PubMed] [Google Scholar]


