Abstract
Immune cells, particularly macrophages, play critical roles in the hypoxia-induced inflammatory response. The small GTPase RhoB is usually rapidly induced by a variety of stimuli and has been described as an important regulator of cytoskeletal organization and vesicle and membrane receptor trafficking. However, it is unknown whether RhoB is involved in the hypoxia-induced inflammatory response. Here, we investigated the effect of hypoxia on the expression of RhoB and the mechanism and significance of RhoB expression in macrophages. We found that hypoxia significantly upregulated the expression of RhoB in RAW264.7 cells, mouse peritoneal macrophages, and the spleen of rats. Hypoxia-induced expression of RhoB was significantly blocked by a specific inhibitor of hypoxia-inducible factor-1α (HIF-1α), c-Jun N-terminal kinase (JNK), or extracellular-signal regulated protein kinase (ERK), indicating that hypoxia-activated HIF-1α, JNK, and ERK are involved in the upregulation of RhoB by hypoxia. Knockdown of RhoB expression not only significantly suppressed basal production of interleukin-1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) in normoxia but also more markedly decreased the hypoxia-stimulated production of these cytokines. Furthermore, we showed that RhoB increased nuclear factor-kappa B (NF-κB) activity, and the inhibition of NF-κB transcriptional activity significantly decreased the RhoB-increased mRNA levels of IL-1β, IL-6, and TNF-α. Finally, we demonstrated that RhoB enhanced cell adhesion and inhibited cell migration in normoxia and hypoxia. Taken together, these results suggest that RhoB plays an important role in the hypoxia-induced activation of macrophages and the inflammatory response.
Keywords: hypoxia, macrophages, NF-κB, proinflammatory cytokines, RhoB
Introduction
Hypoxia is a causative factor in a large number of diseases, such as pulmonary edema, cancer, and ischemic diseases. However, hypoxia also influences the subsequent inflammatory and immune reactions that co-determine the outcome of the disease.1,2,3,4 The idea that hypoxia and inflammation are intertwined at the clinical, cellular, and molecular levels has gained prevailing acceptance.4 Immune cells, particularly macrophages, play a critical role in the hypoxia-induced inflammatory response, either in the defense against anaerobic infection or sterile damage in innate immunity or in the regulation of T lymphocytes in adaptive immunity.5 The hypoxic microenvironment appears to alter the biological behavior and function of macrophages by regulating the expression of genes involved in processes such as metabolism, motility, apoptosis, and the secretion of cytokines and chemokines.6,7,8,9,10,11,12 Hypoxic stress can affect several independent transcriptional regulators related to the adaptive and inflammatory responses, in which hypoxia-inducible transcription factor-1 (HIF-1) and nuclear factor-kappa B (NF-κB) play the central roles in orchestrating inflammatory responses and hypoxic stress.6,13,14,15 However, the precise molecular mechanism responsible for the regulation of macrophage function during hypoxia remains elusive.
RhoB is a member of the Rho subfamily of small GTPases that has two interconvertible forms: an inactive GDP-bound form and an active GTP-bound form. RhoB is distinguished from other members of the Rho subfamily (RhoA and RhoC) by its subcellular localization on endosomes, the plasma membrane and in the nucleus and its rapid turnover and post-translational modification with lipid moieties (geranylgeranyl and farnesyl groups and palmitate).16,17,18,19 RhoB is a key regulator of diverse cellular processes, such as cytoskeleton organization and vesicle and membrane receptor trafficking.20,21,22 As an immediate-early response gene, RhoB is rapidly induced by a wide variety of stimuli, including genotoxic and non-genotoxic stress, growth factors, and cytokines, thereby modulating cellular responses, such as cell proliferation, survival, and apoptosis.21,23,24,25,26 The effect of hypoxia on RhoB has been studied by different groups with inconsistent results. It has been reported that hypoxia upregulates the expression of Cdc42, Rac1, and RhoA but not RhoB, in renal carcinoma Caki-1 cells.27 Another group reported that hypoxia does not upregulate the expression of RhoB, rather it activates RhoB in glioblastoma U87 cells.28 Nevertheless, a recent report demonstrated that hypoxia upregulates the expression of RhoB (but not RhoA) and activates RhoB in human pulmonary artery endothelial and smooth muscle cells and that RhoB activation is required for increased endothelial permeability and for the proliferative response to hypoxic stress.26 It is unclear whether the regulation of RhoB by hypoxia is dependent on the cellular context or whether hypoxia regulates the expression of RhoB in macrophages.
Recently, increasing evidence as suggested that RhoB plays a role in the immune and inflammatory responses. RhoB-null mice have been reported to display a thymic atrophy phenotype at an early age.29 Changes in cell shape and adhesive and migratory capacities have been observed in macrophages derived from the bone marrow of RhoB-null mice.30 In vitro studies have shown that RhoB is involved in the lipopolysaccharide (LPS)-mediated surface expression of major histocompatibility complex class II molecules in dendritic cells.31 Moreover, RhoB regulates tumor necrosis factor alpha (TNF-α) receptor trafficking and mediates the TNF-α-triggered proinflammatory response of vascular endothelial cells by elevating intercellular adhesion molecule-1 (ICAM-1) expression, as well as the production of interleukin 6 (IL-6) and IL-8.21 Our previous study found that LPS induces RhoB expression in vivo and in vitro, a process involved in LPS-induced NF-κB activation and secretion of TNF-α and nitric oxide in RAW264.7 cells.32 Because hypoxia regulates macrophage functions during inflammation, it is worthwhile to investigate whether RhoB's role in the hypoxia-induced inflammatory response occurs through its effects on the function of macrophages.
In the present study, we investigated the induction effect of hypoxia on RhoB expression in the murine macrophage cell line RAW264.7, peritoneal macrophages, and spleen tissue of rats. We found that hypoxia upregulated the expression of RhoB in vitro and in vivo. Furthermore, we explored the mechanism underlying the hypoxia-induced expression of RhoB and the possible regulatory role of RhoB in the biological functions and behaviors of macrophages, including proinflammatory cytokine production, cell adhesion and migration, under normoxic and hypoxic conditions. The results of this study facilitate the understanding of the role of RhoB in the regulation of macrophage function under physiologic and pathophysiologic conditions.
Materials and methods
Chemicals and plasmids
The p38 inhibitor (SB203580), extracellular-signal regulated protein kinase (ERK) inhibitor (PD98059), and c-Jun N-terminal kinase (JNK) inhibitor (SP600125) and cobalt chloride (CoCl2) were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). The HIF-1α inhibitor (400083) was purchased from Calbiochem (Darmstadt, Germany). The NF-κB inhibitor (Bay11-7082) was purchased from Selleckchem (Houston, TX, USA). The concentrations of these inhibitors were determined according to previous studies in macrophages.8,33,34 The wild-type RhoB (RhoB-wt) expression vector (pcDNA3-RhoB; RhoB-wt), the constitutively activated RhoB mutant containing a point mutation in the GTPase domain (G14 V; RhoB-V14), and the empty vector pcDNA3.1 were generously provided by Dr. Prendergast.35 The RhoB-RNA interference (RhoB-RNAi) plasmid was constructed as described previously.32 Ambion (Austin, TX, USA) supplied the negative-control plasmid, which encodes a hairpin small interfering RNA (siRNA) with limited homology to any known sequences in the human, mouse, and rat genomes. The adenoviral RhoB shRNA plasmid (Ad-RhoB-RNAi) and adenoviral control (Ad-control) were obtained from Obio Technology Corp. (Shanghai, China). The pGL3-NF-κB-luc construct, containing two copies of wild-type NF-κB-luc-responsive elements, was kindly provided by Dr. Xu.36 The pRL-SV40-luc vector was obtained from Promega (Beijing, China).
Cell culture and hypoxic exposure
The murine macrophage cell line RAW264.7 was cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco, New York, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, Utah, USA) and maintained in an incubator with a humidified atmosphere of 5% CO2 at 37 °C. For the hypoxic exposure experiment, the cells were incubated in an anaerobic system (1% O2, 5% CO2, 94% N2; Forma Scientific, USA), and control cells were cultured under normal oxygen tension (20% O2, 5% CO2) for the indicated times.
Isolation of mouse peritoneal macrophages
Male BALB/C mice, at the age of 6–8 weeks, were housed in accordance with the guidelines of the Institutional Animal Care Committee of the Second Military Medical University in Shanghai, China. Macrophages were harvested from peritoneal lavage fluid, as described previously.37 Cells were cultured in high-glucose DMEM containing 10% heat-inactivated FBS at 37 °C for 3 h, followed by washing to remove the nonadherent cells. The remaining adherent monolayer macrophages were cultured overnight in complete medium; then, cells were exposed to hypoxia, as described for RAW264.7 cells.
Animals and hypoxic exposure
The study protocol was approved by the Institutional Animal Care Committee of the Second Military Medical University in Shanghai, China. The animal care facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Sprague Dawley male rats, weighing between 200 g and 250 g, were purchased from the Shanghai Laboratory Animal Commission (Shanghai, China). All animals were acclimatized in our animal laboratory for at least 7 days before the experiment, being fed a standard laboratory chow and water. Randomly selected rats were put in a normobaric hypoxia chamber (40 L; Yangyuan Hyperbaric Oxygen Chamber Company, Shanghai, China) and flushed with 8% O2 (a gas mixture of 8% O2 and 92% N2) for the indicated times.38 After hypoxic exposure, the animals were immediately anesthetized and killed. The spleens were removed for RhoB detection.
Transient transfection and adenoviral infection
RAW264.7 cells were seeded in duplicate into a 12-well plate (6 × 105 cells well−1) or 6-well plate (1.2 × 106 cells well−1). After overnight culture, the cells were transiently transfected with 1.6 or 4.0 μg well−1 of pcDNA3.1, RhoB-wt, RhoB-V14, negative-control plasmid or RhoB-RNAi plasmid using Lipofectamine 2000 Transfection Reagent (Invitrogen), according to the manufacturer's instructions. For adenoviral transient infection, the indicated adenoviruses (MOI 1:100) were added to mouse peritoneal macrophages (1.0 × 106 cells well−1) for 48 h without obvious cytopathic effects, and the knockdown efficiency was determined by quantitative real-time PCR.
RNA extraction and quantitative real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and 2 μg total RNA was reverse transcribed using Reverse Transcription Reagents (MBI Fermentas, Vilnius, Lithuania) following the manufacturer's protocol. Quantitative real-time PCR was performed in triplicate using a SYBR Green PCR Master Mix (Toyobo, Japan) on a Mastercycler ep realplex (Eppendorf, Germany). The primer sequences used were as follows: RhoB (mouse): 5′-CTGGCCCGCATGAAGCA-3′ and 5′-AGGCAGTCTGGTGGTGTCC-3′; RhoB (rat): 5′-TGCTGATCGTGTTCAGTAAG-3′ and 5′-AGCACATGAGAATGACGTCG-3′; IL-1β (mouse): 5′-CAAATCTCGCAGCAGCACA-3′ and 5′-TCATGTCCTCATCCTGGAAGG-3′; IL-6 (mouse): 5′-TAGTCCTTCCTACCCCAATTTCC-3′and 5′-TTGGTCCTTAGCCACTCCTTC-3′; TNF-α (mouse): 5′-GACGTGGAACTGGCAGAAGAG-3′ and 5′-TTGGTGGTTTGTGAGTGTGAG-3′; β-actin (mouse): 5′-CTGTATGCCTCTGGTCGTAC-3′ and 5′-TGATGTCACGCACGATTTCC-3′; and GAPDH (rat): 5′-ATGGTGGGTATGGGTCAGAAG-3′ and 5′-TGGCTGGGGTGTTGAAGGTC-3′. Thermal cycling conditions consisted of an initial denaturing step (95 °C, 2 min), followed by 40 cycles of denaturing (95 °C, 15 s), annealing (60 °C, 15 s), and extending (72 °C, 45 s). The mRNA levels were normalized to β-actin or GAPDH (internal control) and their relative quantities were determined using the 2ΔΔCT formula.
Preparation of the nuclear and cytoplasmic extracts
The nuclear and cytoplasmic protein fractions were extracted using the NE-PER extraction reagents kit (Thermo Fisher Scientific) according to the manufacturer's protocol. Nuclear and cytoplasmic protein extracts were used for western blot analysis.
Western blot analysis
The total cell lysates were prepared with 1× SDS lysis buffer with 100 mM dithiothreitol and 2 µg mL−1 protease inhibitors containing 0.1 mM leupeptin, aprotinin, and pepstatin. After electrophoresis, proteins were transferred to a nitrocellulose membrane, blocked with 5% nonfat milk, and probed overnight with primary antibodies against RhoB (sc-180, Santa Cruz, TX, USA), β-actin (A5441, Sigma-Aldrich Chemicals, St. Louis, MO, USA), HIF-1α (H-206, Santa Cruz, TX, USA), p65/RelA (PC138, Calbiochem, Darmstadt, Germany), Lamin B1, phosphorylated JNK (p-JNK), JNK, phosphorylated ERK (p-ERK), ERK, phosphorylated p38 (p-p38), or p38 mitogen-activated protein kinases (MAPK) (Cell Signaling, Boston, MA, USA). The membranes were washed three times and incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000, Rockland Immunochemicals, Limerick, PA, USA) for 2 h. Finally, blots were detected by ECL chemiluminescence (Pierce, Rockford, IL, USA). Protein bands were quantified with ImageJ software (NIH, USA) using β-actin as an internal control.
ELISA assay
Commercially obtained ELISA kits (BioLegend, San Diego, CA, USA) were used to quantify the levels of mouse IL-1β, IL-6, and TNF-α released from cells into the culture medium under various experimental conditions. The cell culture supernatant was collected and prepared, and the assay was carried out following the manufacturer's instructions.
Luciferase assay
Cells were plated in triplicate into a 24-well plate at a density of 3 × 105 cells well−1 for overnight culture; then, cultures were transiently co-transfected with 200 ng of pGL3-NF-κB-luc along with 600 ng of RhoB-wt, RhoB-V14, pcDNA3.1, RhoB-RNAi plasmid, or negative-control plasmid using Lipofectamine 2000 Transfection Reagent (Invitrogen). One nanogram of pRL-SV40-luc per well was co-transfected to normalize the transfection efficiency. Following transfection for 36 h, cells were left unexposed or exposed to hypoxia for another 12 h. The luciferase activity was determined by the dual luciferase assay system (Promega). Results were normalized against the internal renilla control and presented as fold induction over the control.
Cell adhesion assay
Cell adhesive ability was determined by a cell adhesion assay.39 Following transient transfection, cells were incubated under normoxic or hypoxic culture conditions for another 12 h. Then, cells were digested in a single cell suspension, and approximately 8 × 104 cells were seeded onto 96-well plates and incubated at 37 °C under normoxic culture conditions for 1 h. The plates were gently washed twice with PBS to remove the nonadherent cells. The remaining cells were incubated in medium supplemented with 50 μg mL−1 methylthiazole tetrazolium for 3 h. Cells were then solubilized by adding 0.5× volumes of dimethyl sulfoxide. The absorbance was measured at 570 nm.
Cell migration assay
Cell migratory ability was assessed in vitro using trans-well chambers (24-well insert; pore size, 8 µm; Corning, Toledo, Ohio, USA). Following transient transfection, approximately 8 × 104 cells in serum-free medium were plated in the upper chamber, and medium supplemented with 10% serum was used as a chemoattractant in the lower chamber. Then, cells were incubated under normoxic and hypoxic conditions for 20 h. The non-migrating cells on the upper surface of the membrane were removed by a cotton swab. Cells on the lower surface of the membrane were then fixed with cold methanol and stained with 0.1% crystal violet. Cell migration was quantified by counting stained cells in five randomly selected fields at 100× magnification with a light microscope.
Statistical analysis
Quantitative data are shown as the means ± SD. Differences between the two groups were analyzed using the Student's t-test. A value of P < 0.05 was considered statistically significant.
Results
Hypoxia induces RhoB expression in macrophages
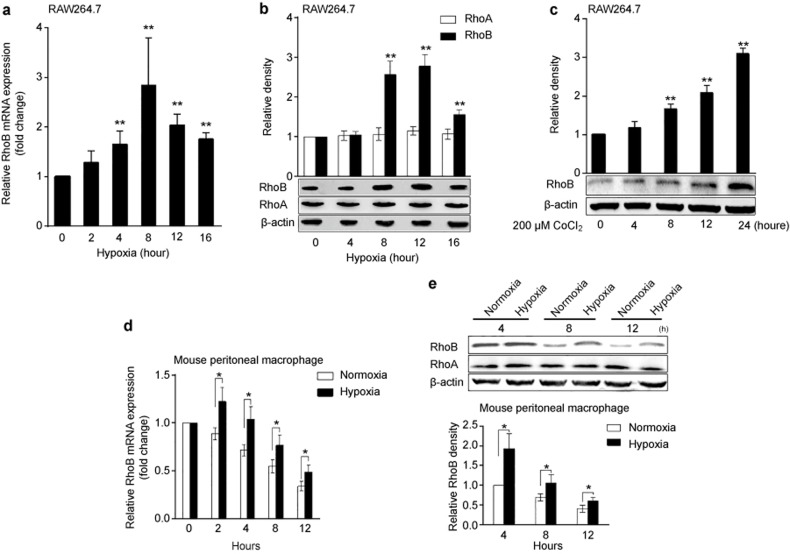
We first investigated the effect of hypoxia (1% O2) on RhoB expression in murine macrophage RAW264.7 cells and mouse peritoneal macrophages. The results showed that hypoxia significantly induced the mRNA and protein expression of RhoB in RAW264.7 cells, reaching a maximal 2.9-fold (P < 0.01) increase at 8 h and a 2.8-fold (P < 0.01) increase at 12 h compared with the normoxic control (Figure 1a and b). Hypoxia had no significant effect on the protein expression of RhoA in RAW264.7 cells (Figure 1b). Furthermore, the treatment of RAW264.7 cells with 200 μM CoCl2, a chemical inducer of hypoxia, significantly increased the RhoB protein level in a time-dependent manner (Figure 1c). The effect of hypoxia on the expression of RhoB in mouse peritoneal macrophages was also observed. As shown in Figure 1d and e, although the mRNA and protein levels of RhoB declined over time in normoxia, hypoxia upregulated the mRNA and protein expression of RhoB at the indicated time points. Hypoxic exposure for 4 h induced a maximal 1.9-fold (P < 0.05) increase in the RhoB protein level compared with the corresponding normoxic control in mouse peritoneal macrophages. No significant changes in the RhoA protein level were observed in mouse peritoneal macrophages under hypoxic conditions.
Figure 1.
Upregulation of RhoB expression by hypoxia in RAW264.7 cells and mouse peritoneal macrophages. RAW264.7 cells and mouse peritoneal macrophages were incubated in normoxic or hypoxic conditions (1% O2) for the indicated time periods; then, the mRNA (a and d) and protein (b and e) expression of RhoA or RhoB were measured by qRT-PCR and western blotting, respectively. (c) RAW264.7 cells were incubated with 200 µM CoCl2 for the indicated times, and then the protein expression of RhoB was measured as described in the section “Materials and Methods.” Mouse β-actin was used as a normalization control for qRT-PCR, and β-actin was used as a loading control for western blots. *P < 0.05, **P < 0.01 versus the normoxic control or 0.
Hypoxia significantly increases RhoB expression in the spleen of rats
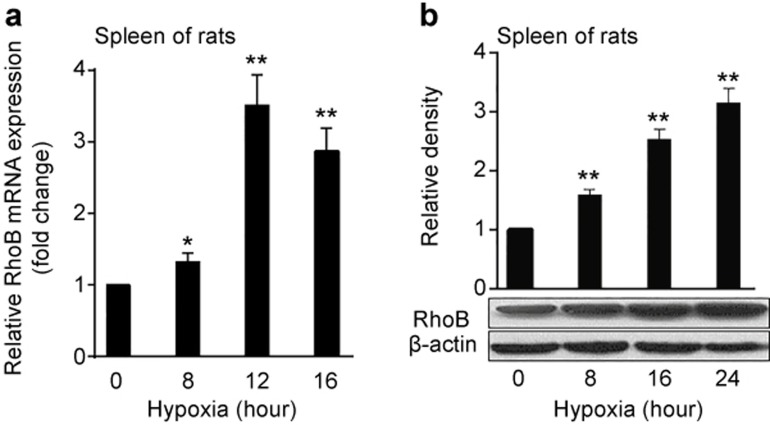
Given that splenic macrophages play a pivotal role in inflammatory and immune reactions,40 we further investigated the effect of hypoxia on RhoB expression in the spleen tissue of rats. The rats were exposed to normoxia and hypoxia (8% O2) for different times, and then the mRNA and protein levels of RhoB in the spleen tissue were examined by qRT-PCR and western blot, respectively. As shown in Figure 2a, the mRNA level of RhoB began to increase after exposure to hypoxia for 8 h and reached a maximal 3.5-fold increase compared with the normoxic group after hypoxic exposure for 12 h (P < 0.01). A similar result was observed at the protein level for RhoB, reaching a maximal 3.1-fold increase compared with the normoxic group after hypoxic exposure for 24 h (P < 0.01; Figure 1b). These data indicate that hypoxia significantly induced the expression of RhoB in a time-dependent manner in vivo.
Figure 2.
Upregulation of RhoB expression by hypoxia in the spleen tissue of rats. Adult male Sprague Dawley rats were put in a normal pressure hypoxia chamber filled with 8% O2 and 92% N2 for 8 h (n = 6), 16 h (n = 6), or 24 h (n = 6) at random; the control group (n = 6) remained in a normoxic environment. The mRNA (a) and protein (b) levels of RhoB in the spleens of rats were assessed by qRT-PCR and western blotting, respectively. Rat GAPDH was used as a normalization control for qRT-PCR, and β-actin was used as a loading control for western blots. RhoB protein expression was quantified by densitometric analysis. The results were expressed as fold changes compared to the normoxia levels. Each bar represents the mean ± SD. *P < 0.05, **P < 0.01 versus the normoxic group.
HIF-1α, ERK, and JNK activity are involved in the induction of RhoB expression by hypoxia in RAW264.7 cells
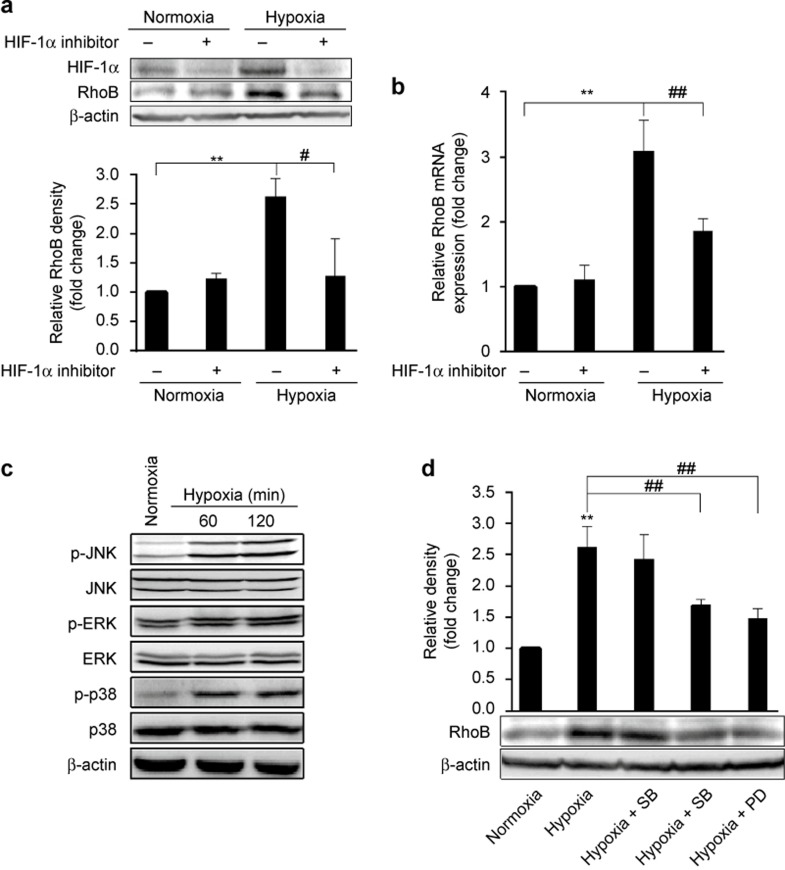
We further investigated the mechanism underlying hypoxia-induced expression of RhoB in RAW264.7 cells. HIF-1 is a transcription factor induced by hypoxia that possesses two subunits, HIF-1α and HIF-1β. Hypoxia increases the protein level of HIF-1α by stabilizing the HIF-1α protein, whereas HIF-1β is constitutively expressed.41 The results showed that hypoxia increased the protein level of HIF-1α (Figure 3a). The treatment of cells with a specific HIF-1α inhibitor markedly decreased the RhoB mRNA and protein levels induced by hypoxia (Figure 3a and b), indicating that HIF-1α contributed to the upregulation of RhoB in response to hypoxia.
Figure 3.
Role of HIF-1α and the MAPK family in the induction of RhoB by hypoxia (a and b) RAW264.7 cells were exposed to hypoxia for 8 h or 12 h in the presence or absence of HIF-1α inhibitor (100 μM); then, the RhoB mRNA level and the HIF-1α and RhoB protein levels were measured by qRT-PCR and western blotting, respectively. (c) RAW264.7 cells were exposed to hypoxia for the indicated times, and p-JNK, JNK, p-ERK, ERK, p-p38, and p38 were measured by western blot. (d) Cells were pre-incubated with or without SB203580 (SB, 10 μM), SP600125 (SP, 20 μM), or PD98059 (PD, 20 μM) for 1 h; then, the cells were exposed to hypoxia for 12 h. RhoB protein was measured by western blotting and quantified by densitometric analysis; β-actin was used as a normalization control. The results were expressed as the fold change compared to normoxic conditions. Each bar represents the mean ± SD. **P < 0.01 versus the normoxic control, #P < 0.05, ##P < 0.01 versus the hypoxic control.
Hypoxia reportedly activates kinases of the MAPK family, such as ERK, JNK, and p38 MAPK, in multiple cell types.42,43,44,45 However, it is unknown whether these kinases are involved in the induction of RhoB by hypoxia. We demonstrated that hypoxia increased the phosphorylated levels of JNK, ERK, and p38 MAPK (Figure 3c), indicating that hypoxia led to the activation of these kinases. Then, we observed the effect of a specific inhibitor of p38 MAPK (SB203580), ERK (PD098059), or JNK (SP600125) on the hypoxia-induced expression of RhoB. The inhibition of JNK and ERK but not p38 MAPK activity caused an obvious inhibitory effect on the induction of RhoB protein by hypoxia (Figure 3d). These data indicate that ERK and JNK activity is also involved in the upregulation of RhoB expression by hypoxia in RAW264.7 cells.
Upregulation of RhoB contributes to the augmentation of proinflammatory cytokine production in macrophages under hypoxic conditions
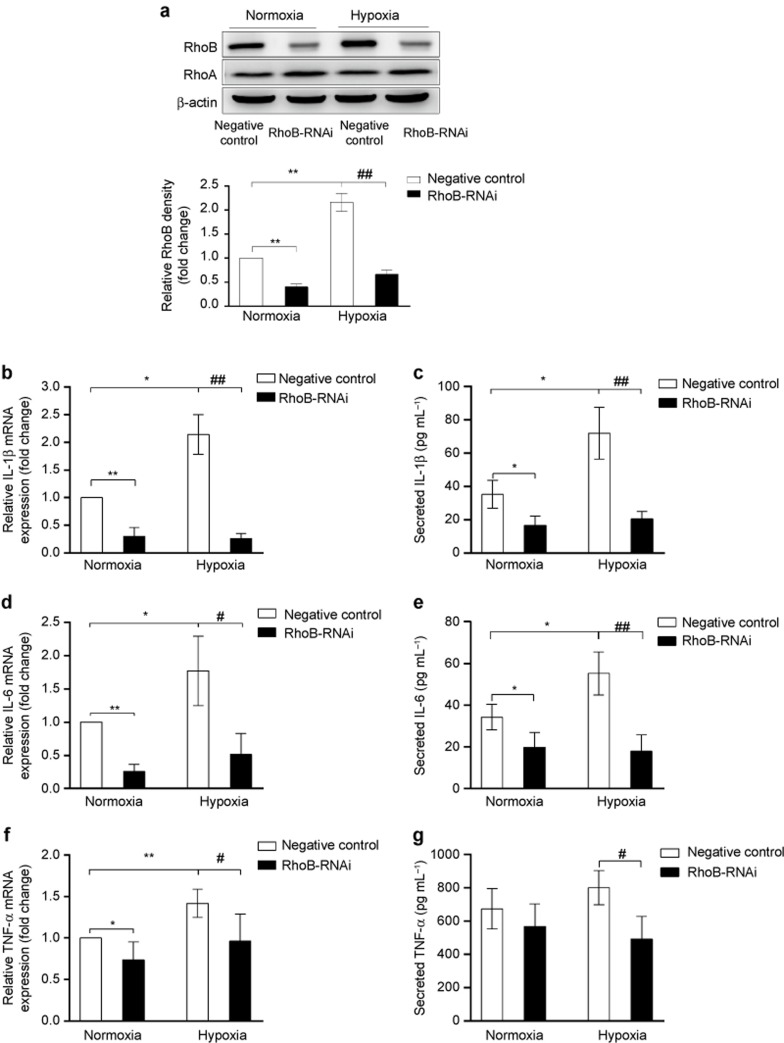
We next investigated the relationship between the upregulation of RhoB by hypoxia and proinflammatory cytokine production in macrophages by silencing the expression of RhoB. The knockdown of RhoB but not RhoA expression in RAW264.7 cells, using specific RhoB-RNAi in conditions of normoxia and in hypoxia was confirmed by western blotting (Figure 4a). The mRNA expression of IL-1β, IL-6, and TNF-α was determined by qRT-PCR, and the secretion of these cytokines in culture medium supernatants was detected by ELISA. As shown in Figure 4b–g, hypoxia significantly increased the expression of IL-1β, IL-6, and TNF-α in control-transfected cells. The knockdown of RhoB expression not only significantly suppressed the basal mRNA levels of IL-1β, IL-6, and TNF-α, as well as the basal secretion of IL-1β and IL-6 in normoxia but also more markedly decreased the hypoxia-stimulated production of these cytokines. Moreover, we found that RhoB silencing also significantly decreased the mRNA levels of IL-1β, IL-6, and TNF-α in primary peritoneal macrophages under normoxic and hypoxic conditions (Supplementary Figure S1b–d). These results indicate that the upregulation of RhoB by hypoxia contributed to the increased proinflammatory cytokine production in macrophages under hypoxic condition.
Figure 4.
Effect of RhoB knockdown on proinflammatory cytokine induction by hypoxia in RAW264.7 cells RAW264.7 cells were transfected with a negative-control plasmid or a RhoB-RNAi plasmid. After transfection for 36 h, cells were incubated in hypoxic (1% O2) or normoxic conditions for another 12 h. Knockdown of RhoB expression was monitored at the protein level by western blotting (a), and the mRNA levels of IL-1β (b), IL-6 (d), and TNF-α (f) were assessed by qRT-PCR. The supernatants were collected after culture medium renewal for 36 h and analyzed for IL-1β (c), IL-6 (e), and TNF-α (g) by ELISA; β-actin was used as a normalization control for qRT-PCR and as a loading control for western blotting. RhoB protein expression was quantified by densitometric analysis. The values are expressed as the fold change compared to the normoxic control and represent the mean of three independent experiments. Each bar represents the mean ± SD. *P < 0.05, **P < 0.01 versus the negative control in conditions of normoxia, #P < 0.05, ##P < 0.01 versus the negative control in hypoxic conditions.
RhoB promotes the production of proinflammatory cytokines by activating NF-κB in RAW264.7 cells
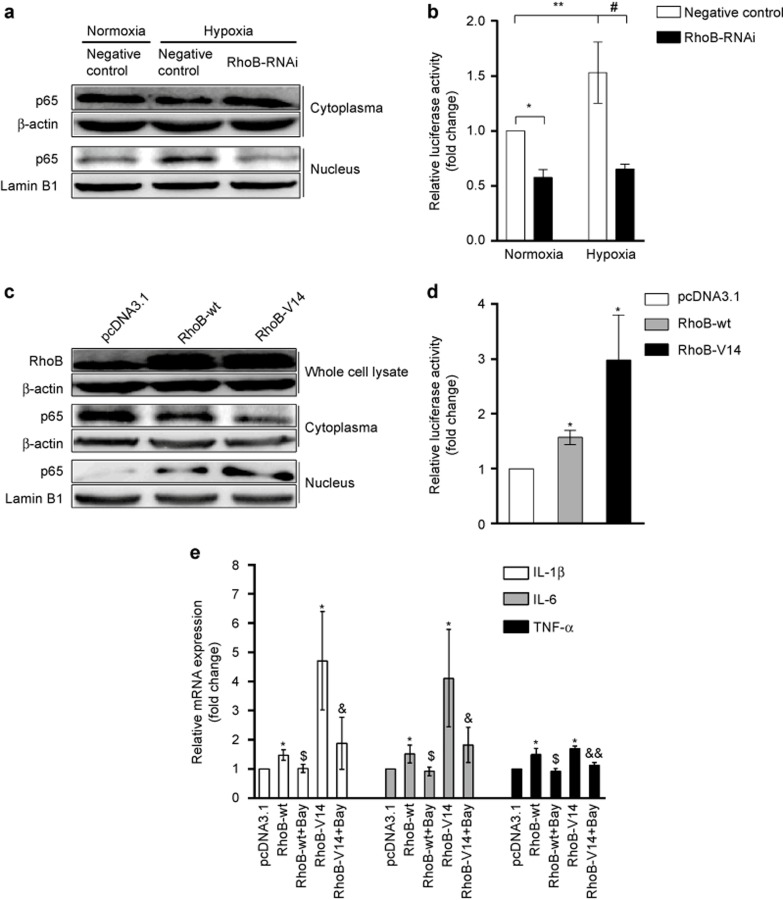
NF-κB signaling reportedly plays an important role in the hypoxia-induced inflammatory response, including proinflammatory cytokine production.4 Therefore, we investigated the impact of RhoB on p65 nuclear translocation and NF-κB transcriptional activity in RAW264.7 cells. The results showed that hypoxia increased p65 nuclear translocation and luciferase activity of NF-κB in the control transfectant. The suppression of RhoB expression with RhoB-RNAi significantly reduced hypoxia-induced p65 nuclear translocation and inhibited luciferase activity of NF-κB in normoxic and hypoxic conditions (Figure 5a and b). Moreover, the overexpression of RhoB-wt transfectant and the constitutive activation of RhoB (RhoB-V14 transfectant) increased p65 nuclear translocation and the transcriptional activity of NF-κB in normoxic conditions (Figure 5c and d). The increase in the luciferase activity of NF-κB was 1.57-fold (P < 0.05) and 2.97-fold (P < 0.05) higher in the RhoB-wt and RhoB-V14 transfectants compared with the control transfectant, respectively (Figure 5d). These data indicate that RhoB increased NF-κB signaling in RAW264.7 cells.
Figure 5.
Effect of RhoB on NF-κB activity in normoxic or hypoxic conditions in RAW264.7 cells (a) RAW264.7 cells were transfected with the indicated plasmids for 36 h, and then cells were incubated in hypoxic or normoxic conditions for another 12 h. The nuclear and cytoplasmic proteins were extracted to detect the p65 protein by western blotting. (b) NF-κB transcriptional activity was measured with a luciferase reporter system. (c) RAW264.7 cells were transfected with the indicated plasmids for 48 h in normoxic conditions; then, the total proteins, nuclear proteins, and cytoplasmic proteins were extracted for detection by western blotting. (d) NF-κB transcriptional activity was measured with a luciferase reporter system. (e) Following transfection with the indicated plasmid for 24 h, the cells were treated with 5 μM Bay11-7082 (Bay) or vehicle for another 12 h; then, the mRNA levels of IL-1β, IL-6, and TNF-α were measured by qRT-PCR; β-actin was used as a normalization control for qRT-PCR and as a total and cytoplasmic loading control for western blots. Lamin B1 was used as a nuclear loading control for western blots. The values were expressed as the fold change relative to the control and represent the mean of three independent experiments. Each bar represents the mean ± SD. *P < 0.05, **P < 0.01 versus the corresponding control in conditions of normoxia, #P < 0.05 versus the negative control in conditions of hypoxia, $P < 0.05 versus RhoB-wt transfectants, &P < 0.05, &&P < 0.01 versus RhoB-V14 transfectants.
We further examined whether RhoB promotes the expression of IL-1β, IL-6, and TNF-α through the activation of NF-κB. The results showed that overexpression of RhoB-wt and the constitutive activation of RhoB significantly increased the mRNA levels of IL-1β, IL-6, and TNF-α in normoxia. The inhibition of NF-κB trans-activation using Bay11-7082 (Bay), a specific NF-κB inhibitor, significantly reversed the upregulating effect of RhoB on the mRNA levels of IL-1β, IL-6, and TNF-α (Figure 5e). These data indicate that RhoB promotes the expression of proinflammatory cytokines by activating NF-κB in RAW264.7 cells.
RhoB promotes cell adhesion and inhibits cell migration in RAW264.7 cells in normoxia and hypoxia
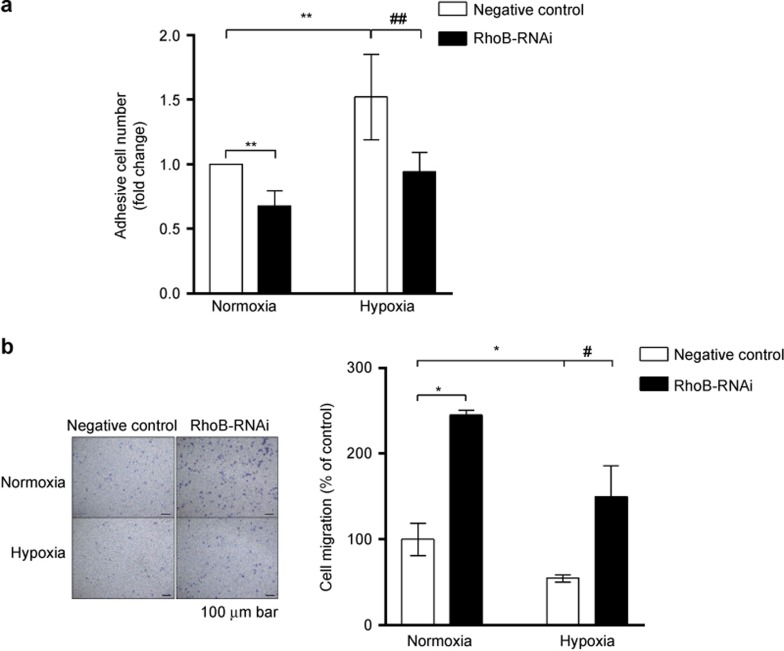
Finally, we investigated the effect of RhoB on the adhesive and migratory capacity of RAW264.7 cells. As shown in Figure 6a, hypoxic preconditioning for 12 h induced a 1.52-fold (P < 0.01) increase in the cell adhesive capacity compared with the normoxic control transfectant RAW264.7 cells. The suppression of RhoB expression with RhoB-RNAi not only abrogated hypoxic preconditioning-induced adhesive enhancement but also significantly inhibited cell adhesion (67% of the level of the control transfectant, P < 0.01) in normoxia. Hypoxia exerted an inhibitory effect on the cell migration of the control transfectants of RAW264.7 cells (55% of the normoxic control, P < 0.05; Figure 6b). The knockdown of RhoB expression significantly promoted cell migration in both normoxic and hypoxic conditions (Figure 6b). To further evaluate the role of RhoB in cell adhesion and migration in macrophages, we investigated the effect of RhoB overexpression on cell adhesion and migration in RAW264.7 cells under normoxic conditions. The results showed that the overexpression of RhoB increased the cell adhesive ability and attenuated the cell migratory ability in normoxic conditions (Supplementary Figure S2b and c). These results indicate that RhoB played an important role in enhancing macrophage adhesion and inhibiting macrophage migration in normoxic and hypoxic conditions.
Figure 6.
Effect of RhoB silencing on cell adhesion and migration under normoxic or hypoxic conditions in RAW264.7 cells (a) RAW264.7 cells were transfected with negative-control plasmid or RhoB-RNAi plasmid. After transient transfection for 36 h, the cells were incubated in hypoxic or normoxic conditions for another 12 h. Then, a cell adhesion assay was performed as described in the section “Materials and Methods.” (b) After transient transfection for 36 h, 8 × 104 cells were seeded into the upper trans-well chambers and incubated in normoxic or hypoxic conditions for another 20 h. The graph (left) shows representative images (100× magnification) of three independent experiments. Scale bar, 100 μm. The values were expressed as the fold change relative to the control and represent the mean of three independent experiments. Each bar represents the mean ± SD. *P < 0.05, **P < 0.01 versus the negative control in conditions of normoxia, #P < 0.05, ##P < 0.01 versus the negative control in conditions of hypoxia.
Discussion
Tissue hypoxia can induce an inflammatory response, and macrophages play a key part in the hypoxia-induced inflammatory response. However, it is unclear whether RhoB is involved in the regulation of macrophage function during hypoxia. In this study, we investigated the effect of hypoxia on the expression of RhoB and its mechanism and significance in macrophages. We found that hypoxia significantly induced RhoB expression in vitro in murine macrophage RAW264.7 cells and mouse peritoneal macrophages and in vivo in the spleen tissue of rats. It is well known that HIF-1α plays a central role in hypoxic stress by modulating gene profiles. In our study, hypoxia increased the protein level of HIF-1α, and treatment with a HIF-1α inhibitor markedly reduced the mRNA and protein levels of RhoB induced by hypoxia, indicating that HIF-1α contributed to the upregulation of RhoB in RAW264.7 cells. Skuli et al. reported that silencing RhoB with siRNA induces the degradation and inhibition of the transcriptional activity of HIF-1α by the proteasome in U87 hypoxic cells, indicating that RhoB stabilizes HIF-1α.28 Conversely, our results showed that HIF-1α was involved in hypoxia-induced RhoB expression. These data suggest that a positive regulation loop exists between HIF-1α and RhoB under hypoxic conditions.
Previous studies demonstrated that several types of stresses, including hypoxic stress, can induce the activation of MAPK signal transduction pathways.42,43,44,45 Fritz et al. demonstrated that UVC-induced expression of RhoB is independent of ERK, JNK, and p38 MAPK in fibroblast cells.46 However, our previous studies found that activation of the p38 MAPK pathway contributes to heat stress-induced expression of RhoB in A549 cells.47 It is unknown whether these kinases are involved in the upregulation of RhoB by hypoxia. Here, we demonstrated that hypoxia-activated ERK, JNK, and p38 MAPK in RAW264.7 cells. The treatment of cells with inhibitors of JNK or ERK but not p38 MAPK markedly reduced the hypoxia-induced expression of RhoB, indicating that ERK and JNK activities were involved in the upregulation of RhoB expression by hypoxia in RAW264.7 cells.
Studies conducted by both us and others have demonstrated that hypoxia augments the proinflammatory cytokine production in macrophages.9,12,33 In this study, we found that hypoxia-induced RhoB expression was involved in the hypoxia-induced production of IL-1β, IL-6, and TNF-α in macrophages. In our previous studies, we found that RhoB is involved in basal NF-κB activation in ovarian cancer cells and LPS-induced NF-κB activation in RAW264.7 cells.32,48 Here, we further found that RhoB promoted NF-κB and p65 nuclear translocation, the transcriptional activity of NF-κB, and the expression of IL-1β, IL-6, and TNF-α under normoxic and hypoxic conditions. Inhibiting the transcriptional activity of NF-κB significantly decreased the RhoB-induced increases in the mRNA levels of IL-1β, IL-6, and TNF-α. These data indicate that RhoB contributed to the expression of proinflammatory cytokines through the activation of NF-κB signaling in RAW264.7 cells. However, Rodriguez et al. reported that the activation of NF-κB by RhoB is ROCK I-dependent and is associated with the direct phosphorylation of the p65 transactivation domain rather than the increased nuclear translocation of p65 in 293T and HeLa cells.49 The underlying mechanism responsible for the activation of NF-κB signaling by RhoB in macrophages is unclear and requires further investigation.
In addition to regulating proinflammatory cytokine production, we found that RhoB promoted macrophage adhesion to the ECM and inhibited macrophage migration in normoxic and hypoxic conditions. These findings reveal that RhoB not only contributed to the regulation of macrophage functions physiologically, including promoting the production of proinflammatory cytokines, enhancing macrophage adhesion and inhibiting macrophage migration, but also played an important role in the activation of macrophages and the hypoxia-induced inflammatory response. Consistently, recent in vitro studies have shown that macrophages derived from the bone marrow of RhoB-null mice reduced cell adhesion to ICAM-1 and glass, which is associated with reduced cell surface expression of the β2 and β3 integrins.30 The activation of RhoB by hypoxia also positively modulates integrin-based adhesion in glioblastoma cells.50 With regard to the regulation of macrophage motility, it was reported that hypoxia inhibits cell migration in human THP-1 monocytic cells and human primary macrophages.51 Here, we demonstrated that RhoB inhibited macrophage migration in conditions of normoxia and hypoxia, indicating that RhoB is a negative regulator of macrophage motility. The upregulation of RhoB enhances adhesion and inhibits migration in macrophages under hypoxic conditions, which may partially explain how macrophages infiltrate hypoxic sites of tissues: cells migrate along a chemotactic gradient until they reach an area of hypoxia, where they are rapidly prevented from further progressing.
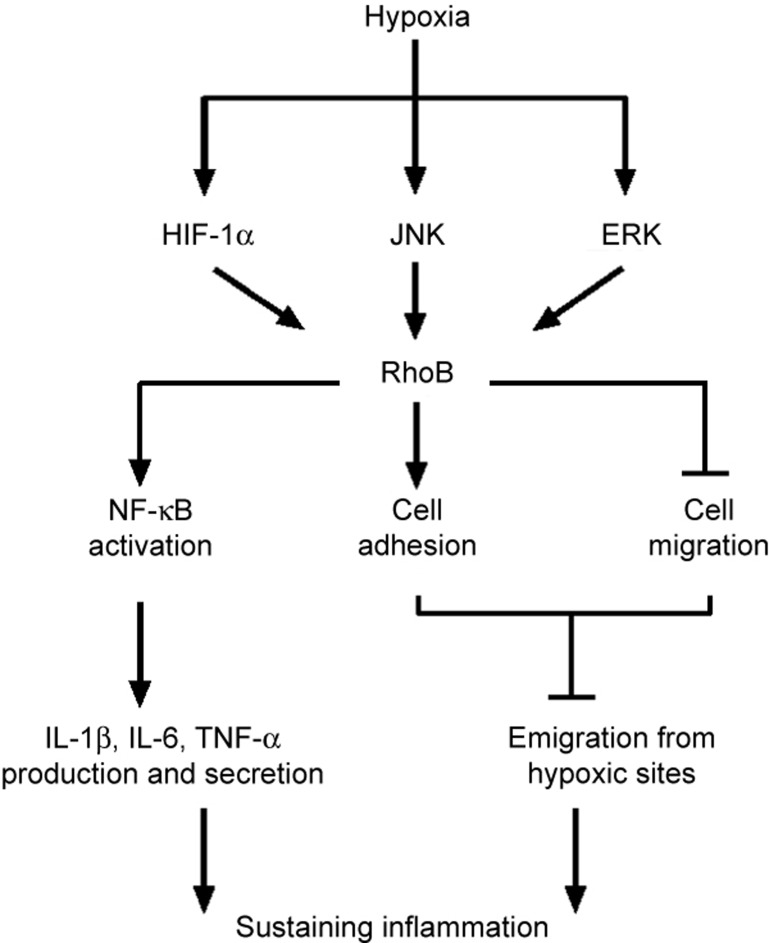
In summary, we found that hypoxia upregulated the expression of RhoB in macrophages. The transcription factor HIF-1α and the ERK and JNK kinases were involved in the induction of RhoB by hypoxia in RAW264.7 cells. RhoB promoted the production of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, by activating NF-κB signaling, enhancing macrophage adhesion and inhibiting macrophage migration under hypoxic conditions, all of which sustained the inflammation (Figure 7). These findings provide a novel mechanism by which RhoB links macrophage function to the hypoxia-induced inflammatory response.
Figure 7.
A proposed model for the signaling roles and functions of RhoB in hypoxic macrophages. This schema summarizes the main signaling roles and functions of RhoB that we have identified in the regulation of macrophage function under hypoxic conditions.
Acknowledgments
This work is supported by grants from the National Basic Research Program “973” (2006CB504100). We would like to thank Prof. Wei-gang Xu (Department of Diving Medicine, Faculty of Naval Medicine, Second Military Medical University, Shanghai, China) for providing the hypoxia chamber for rats and the anaerobic system for cells. We would also like to thank Prof. Liang-Nian Song (Department of Medicine, Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, USA) for providing the English editing in this paper.
Footnotes
Supplementary information of this article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
The authors have no financial conflicts of interest.
Supplementary Information
References
- Basnyat B, Murdoch DR. High-altitude illness. Lancet 2003; 361: 1967–1974. [DOI] [PubMed] [Google Scholar]
- Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009; 33: 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl) 2007; 85: 1295–1300. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 2011; 364: 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun 2014; 6: 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int Immunol 2013; 25: 67–75. [DOI] [PubMed] [Google Scholar]
- Safronova O, Pluemsampant S, Nakahama K, Morita I. Regulation of chemokine gene expression by hypoxia via cooperative activation of NF-kappaB and histone deacetylase. Int J Biochem Cell Biol 2009; 41: 2270–2280. [DOI] [PubMed] [Google Scholar]
- Ramkhelawon B, Yang Y, van Gils JM, Hewing B, Rayner KJ, Parathath S et al. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol 2013; 33: 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco EJ, Sukhova GK, Quillard T, Libby P. Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ Res 2014; 115: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco MC, Puppo M, Blengio F, Fraone T, Cappello P, Giovarelli M et al. Monocytes and dendritic cells in a hypoxic environment: spotlights on chemotaxis and migration. Immunobiology 2008; 213: 733–749. [DOI] [PubMed] [Google Scholar]
- Hempel SL, Monick MM, Hunninghake GW. Effect of hypoxia on release of IL-1 and TNF by human alveolar macrophages. Am J Respir Cell Mol Biol 1996; 14: 170–176. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol 2005; 175: 6257–6263. [DOI] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008; 453: 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappa B in hypoxic inflammation. J Physiol 2008; 586: 4055–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 2009; 27: 693–733. [DOI] [PubMed] [Google Scholar]
- Lebowitz PF, Prendergast GC. Functional interaction between RhoB and the transcription factor DB1. Cell Adhes Commun 1998; 6: 277–287. [DOI] [PubMed] [Google Scholar]
- Wherlock M, Gampel A, Futter C, Mellor H. Farnesyltransferase inhibitors disrupt EGF receptor traffic through modulation of the RhoB GTPase. J Cell Sci 2004; 117: 3221–3231. [DOI] [PubMed] [Google Scholar]
- Gerald D, Adini I, Shechter S, Perruzzi C, Varnau J, Hopkins B et al. RhoB controls coordination of adult angiogenesis and lymphangiogenesis following injury by regulating VEZF1-mediated transcription. Nat Commun 2013; 4: 2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki E, Papadimitriou E, Tajadura V, Ridley AJ, Stournaras C, Kardassis D. Transcriptional regulation of the small GTPase RhoB gene by TGF{beta}-induced signaling pathways. FASEB J 2010; 24: 891–905. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21: 247–269. [DOI] [PubMed] [Google Scholar]
- Kroon J, Tol S, van Amstel S, Elias JA, Fernandez-Borja M. The small GTPase RhoB regulates TNFα signaling in endothelial cells. PLoS One 2013; 8: e75031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Duhadaway JB, Prendergast GC, Laury-Kleintop LD. RhoB regulates PDGFR-beta trafficking and signaling in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2007; 27: 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G, Kaina B. Transcriptional activation of the small GTPase gene RhoB by genotoxic stress is regulated via a CCAAT element. Nucleic Acids Res 2001; 29: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G, Kaina B, Aktories K. The ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J Biol Chem 1995; 270: 25172–25177. [DOI] [PubMed] [Google Scholar]
- Jahner D, Hunter T. The ras-related gene RhoB is an immediate-early gene inducible by v-Fps, epidermal growth factor, and platelet-derived growth factor in rat fibroblasts. Mol Cell Biol 1991; 11: 3682–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D et al. Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circ Res 2012; 110: 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte S, Desrosiers RR, Beliveau R. HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J Cell Sci 2003; 116: 2247–2260. [DOI] [PubMed] [Google Scholar]
- Skuli N, Monferran S, Delmas C, Lajoie-Mazenc I, Favre G, Toulas C et al. Activation of RhoB by hypoxia controls hypoxia-inducible factor-1alpha stabilization through glycogen synthase kinase-3 in U87 glioblastoma cells. Cancer Res 2006; 66: 482–489. [DOI] [PubMed] [Google Scholar]
- Bravo-Nuevo A, O'Donnell R, Rosendahl A, Chung JH, Benjamin LE, Odaka C. RhoB deficiency in thymic medullary epithelium leads to early thymic atrophy. Int Immunol 2011; 23: 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AP, Ridley AJ. RhoB affects macrophage adhesion, integrin expression and migration. Exp Cell Res 2007; 313: 3505–3516. [DOI] [PubMed] [Google Scholar]
- Kamon H, Kawabe T, Kitamura H, Lee J, Kamimura D, Kaisho T et al. TRIF-GEFH1-RhoB pathway is involved in MHCII expression on dendritic cells that is critical for CD4 T-cell activation. EMBO J 2006; 25: 4108–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Wang Y, Diao F, Lu J. RhoB is involved in lipopolysaccharide-induced inflammation in mouse in vivo and in vitro. J Physiol Biochem 2013; 69: 189–197. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ma YY, Song XL, Cai HY, Chen JC, Song LN et al. Upregulations of glucocorticoid-induced leucine zipper by hypoxia and glucocorticoid inhibit proinflammatory cytokines under hypoxic conditions in macrophages. J Immunol 2012; 188: 222–229. [DOI] [PubMed] [Google Scholar]
- Luan H, Zhang Q, Wang L, Wang C, Zhang M, Xu X et al. OM85-BV induced the productions of IL-1beta, IL-6, and TNF-alpha via TLR4- and TLR2-mediated ERK1/2/NF-kappaB pathway in RAW264.7 cells. J Interferon Cytokine Res 2014; 34: 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast GC. Actin' up: RhoB in cancer and apoptosis. Nat Rev Cancer 2001; 1: 162–168. [DOI] [PubMed] [Google Scholar]
- Xu H, An H, Yu Y, Zhang M, Qi R, Cao X. Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J Biol Chem 2003; 278: 36334–36340. [DOI] [PubMed] [Google Scholar]
- Brisseau GF, Grinstein S, Hackam DJ, Nordstrom T, Manolson MF, Khine AA et al. Interleukin-1 increases vacuolar-type H+-ATPase activity in murine peritoneal macrophages. J Biol Chem 1996; 271: 2005–2011. [DOI] [PubMed] [Google Scholar]
- Vivona ML, Matthay M, Chabaud MB, Friedlander G, Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: reversal by beta-adrenergic agonist treatment. Am J Respir Cell Mol Biol 2001; 25: 554–561. [DOI] [PubMed] [Google Scholar]
- Chen YX, Wang Y, Fu CC, Diao F, Song LN, Li ZB et al. Dexamethasone enhances cell resistance to chemotherapy by increasing adhesion to extracellular matrix in human ovarian cancer cells. Endocr Relat Cancer 2010; 17: 39–50. [DOI] [PubMed] [Google Scholar]
- Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity 2013; 39: 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol 2004; 51: 563–585. [PubMed] [Google Scholar]
- Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M et al. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 2000; 468: 53–58. [DOI] [PubMed] [Google Scholar]
- Muller JM, Krauss B, Kaltschmidt C, Baeuerle PA, Rupec RA. Hypoxia induces c-fos transcription via a mitogen-activated protein kinase-dependent pathway. J Biol Chem 1997; 272: 23435–23439. [DOI] [PubMed] [Google Scholar]
- Guma M, Rius J, Duong-Polk KX, Haddad GG, Lindsey JD, Karin M. Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc Natl Acad Sci USA 2009; 106: 8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand RJ, Gribar SC, Li J, Kohler JW, Branca MF, Dubowski T et al. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1alpha-dependent manner. J Leukoc Biol 2007; 82: 1257–1265. [DOI] [PubMed] [Google Scholar]
- Fritz G, Kaina B. RhoB encoding a UV-inducible Ras-related small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. J Biol Chem 1997; 272: 30637–30644. [DOI] [PubMed] [Google Scholar]
- Li YD, Liu YP, Cao DM, Yan YM, Hou YN, Zhao JY et al. Induction of small G protein RhoB by non-genotoxic stress inhibits apoptosis and activates NF-kappaB. J Cell Physiol 2011; 226: 729–738. [DOI] [PubMed] [Google Scholar]
- Chen YX, Li ZB, Diao F, Cao DM, Fu CC, Lu J. Up-regulation of RhoB by glucocorticoids and its effects on the cell proliferation and NF-kappaB transcriptional activity. J Steroid Biochem Mol Biol 2006; 101: 179–187. [DOI] [PubMed] [Google Scholar]
- Rodriguez PL, Sahay S, Olabisi OO, Whitehead IP. ROCK I-mediated activation of NF-kappaB by RhoB. Cell Signal 2007; 19: 2361–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C et al. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res 2009; 69: 3308–3316. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Balkwill FR. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation – a potential mechanism. Eur J Immunol 2001; 31: 480–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.