Abstract
Exosomes are nanoparticles of endocytic origin, secreted by a myriad of cell populations that are attracting increased attention by virtue of their ability to modulate cell-to-cell communications. They are also attracting attention in a variety of immunological issues, including autoimmunity and, in particular, their ability to regulate cytokine and chemokine activation. Primary biliary cirrhosis (PBC) is considered a model autoimmune disease, which has a highly focused cytotoxic response against biliary epithelial cells. We have isolated exosomes from plasma from 29 patients with PBC and 30 healthy controls (HCs), and studied the effect of these exosomes on co-stimulatory molecule expression and cytokine production in mononuclear cell populations using an ex vivo system. We also identified the microRNA (miRNA) populations in PBC compared to HC exosomes. We report herein that although exosomes do not change cytokine production, they do significantly alter co-stimulatory molecule expression on antigen-presenting populations. Further, we demonstrated that CD86 up-regulated expression on CD14+ monocytes, whereas CD40 up-regulated on CD11c+ dendritic cells by exosomes from patients with PBC. In addition, there were differences of miRNA expression of circulating exosomes in patients with PBC. These data have significant importance based on observations that co-stimulatory molecules play a differential role in the regulation of T-cell activation. Our observation indicated that aberrant exosomes from PBC selectively induce expression of co-stimulatory molecules in different subset of antigen-presenting cells. These alterations may involve in pathogenesis of autoimmune liver disease.
Keywords: autoimmunity, exosome, microRNA, primary biliary cirrhosis
Introduction
Exosomes are nanoparticles of endocytic origin secreted by most cells1 and engage in cell-to-cell communications by conveying cytosolic proteins and RNAs including messenger RNAs (mRNAs) and microRNAs (miRNAs) as their ‘cargo' to recipient cells.1,2,3 Exosomes not only maintain homeostasis in normal physiological condition such as transferrin receptor release during reticulocyte maturation4 and breast milk immunomodulation for infants,5 but are also involved in disease development including autoimmune diseases. However, the mechanism of how the exosomes participate in the induction and pathogenesis of autoimmune disease still remains unclear.6,7,8
Primary biliary cirrhosis (PBC) is a progressive liver-specific autoimmune disease characterized by chronic non-suppurative destructive cholangitis.9,10 The primary autoantigen of PBC has been identified as the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2).11,12,13,14,15 Data from human studies and animal models demonstrate that the pathogenesis of PBC involves not only autoreactive T cells and other immune cells16,17,18,19,20,21,22 but also biliary epithelial cells.12,18,19,23,24,25,26,27,28,29,30 Herein, we demonstrate that circulating exosomes from PBC could be taken up by antigen-presenting cells (APCs) and affect the expression of cell surface co-stimulatory molecules. We also demonstrate that circulating exosomes in PBC contain altered patterns of miRNA expression with elevated miRNA-451a and miRNA-642-3p compared to healthy adults. Our results demonstrate that altered circulating exosomes may contribute to the cascade of pathology in PBC.
Materials and methods
Study subjects and peripheral blood mononuclear cell isolation
Patients with PBC (n = 29), diagnosed according to internationally recognized diagnostic criteria,31,32 and healthy controls (HCs) (n = 30) were enrolled for this study. The clinical characteristics of the subjects are depicted in Table 1. All subjects provided written informed consent and the study was approved by the Institutional Review Board at the University of California, Davis, prior to the initiation of study. Peripheral blood mononuclear cells (PBMCs) and plasma were isolated from venous blood samples. PBMCs were isolated using Histopaque-1.077 (Sigma-Aldrich, St. Louis, MO, USA) density gradient centrifugation as described previously.23 The protocol used is illustrated in Figure 1.
Table 1. Clinical features.
| PBC (n = 29) | HC (n = 30) | |
|---|---|---|
| Age (range) | 63.5 (43–79) | 54.6 (30–79) |
| Gender (female/male) | 20/9 | 16/14 |
| Disease stage | ||
| Stage I–II | 18 | N/A |
| Stage III–IV | 8 | N/A |
| Unknown | 3 | N/A |
Figure 1.
Schematic illustration of the circulating exosome analysis protocol.
Exosome isolation
Exosomes were isolated using ExoQuick exosome precipitation kit according to the manufacturer's instruction (System Biosciences, Mountain View, CA, USA). Briefly, 500 μL of plasma was pretreated with thrombin at a final concentration of 5 U mL–1, incubated at room temperature for 5 min, and centrifuged at 10 000 rpm for 5 min to remove fibrin. Serum-like supernatant was mixed with ExoQuick (System Biosciences) and incubated for 30 min at 4 °C. After centrifuging at 1500g for 30 min, the exosome pellet was resuspended in 100 µL of phosphate-buffered saline for further analysis. The ExoQuick isolation method recovered around 100 nm exosomes and expressed CD63 and CD81 as measured using western blotting. The precipitated exosomes were either used for RNA isolation or resuspended in culture medium RPMI for culture with PBMCs. The protein concentration of exosomes was determined using the Bicinchoninic Acid Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
Antibodies and flow cytometry
Fluorochrome-conjugated monoclonal antibodies (mAbs) for CD19 (HIB19), CD80 (2D10), CD86 (IT2.2), HLA-DR (LN3), and CD40 (5C3) were purchased from eBioscience (San Diego, CA, USA), and mAb for CD3 (UCHT1), CD11c (Bu15), and CD14 (HCD14) were purchased from BioLegend (San Diego, CA, USA). For exosome uptake experiment, freshly isolated exosomes were labeled with lipophilic fluorochrome PKH-67 (Sigma-Aldrich).33 Freshly isolated PBMCs were cultured with autologous PKH-67-labeled exosomes at 37 °C for 16 h. The cells were then stained with antibodies for various cell subsets and analyzed by flow cytometry. For the detection of co-stimulatory molecules on APCs, freshly isolated 1 × 106 PBMCs were co-cultured with or without 600 µg autologous exosomes for 16 h in 24-well culture plate (Coning Incorporated, Corning, NY, USA). In our preliminary data, we noted that an exosome protein concentration of 500–600 µg mL−1 was sufficient to up-regulate expression of CD86 or HLA-DR on monocytes or dendritic cells (DCs). Therefore, 600 µg autologous exosomes were chosen for our subsequent co-stimulatory molecule expression study. After culture, the cells were stained with antibodies indicated for co-stimulatory molecules and immune cell subsets. The stained PBMCs were then analyzed by flow cytometry. The culture medium used in all the experiments was RPMI-1640 (GIBCO Invitrogen Life Technologies, Grand Island, NY, USA) supplemented with 10% exosome-free fetal bovine serum (FBS), which was prepared by ultracentrifugation of FBS at 25 000g for 4 h followed by filtration with a 0.22 µm pore size syringe filter.
Assay of in vitro cytokine production by monocyte
Monocytes were isolated from PBMCs by positive selection using anti-CD14 microbeads (Miltenyi Biotec, San Diego, CA, USA). The purity of the monocytic population was >95% as assessed by flow cytometry. Aliquots of monocytes (1 × 105/well) were cultured with various concentrations of freshly isolated autologous exosomes for 24–72 h at 5% CO2, 37 °C in 96-well culture plates. Cytokine production in the culture supernatants was measured by the human inflammatory cytokine CBA kit (BD Biosciences, San Jose, CA, USA).
RNA isolation and miRNA microarray
Total RNA was extracted and purified using mirVana PARISTM (Ambion, Austin, TX, USA) and checked for RNA integrity by an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). Labeling of miRNA molecular within total RNA was conducted by miRNA complete labeling and Hyb Kit (Agilent Technologies). The levels of miRNAs were measured by Agilent Human miRNA (8 × 60 K) V19.0 microarray (Agilent Technologies). Slides were hybridized with 100 ng Cy3-labeled RNA probes at 55 °C, 20 rpm for 20 h, washed, and scanned according to the manufacturer's instructions. Raw data were normalized by Quantile algorithm, Gene Spring Software 11.0 (Agilent Technologies). In our microarray analysis, a total of 2007 miRNAs were evaluated for both patients and HCs. The miRNAs with a level of 2-fold or larger difference between groups were considered differentially expressed.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Exosomal miRNAs were reverse transcribed using MultiScribe Reverse Transcriptase and miRNA-specific primers (Life Technologies, Carlsbad, CA, USA). The miRNAs were quantified by real-time PCR using TaqMan microRNA kits (Life Technologies) and the ABI 7900HT system, according to a standard protocol. The comparative cycle threshold (Ct) was used to evaluate the relative detection level of each miRNA in the sample, which was determined using the 2−ΔCt method. According to the results of microarray and TaqMan PCR, miR-1228-3p had a stable transcription level across PBC and healthy subjects with an overall coefficient of variation <5%. Therefore, miR-1228-3p was used as a normalization gene in this experiment.
Statistical analysis
Two-tailed unpaired Student's t-test and two-tailed paired t-test were used for data analysis, P values <0.05 were considered statistically significant.
Results
Exosomes uptake by APCs in patients with PBC
First, and for the purposes of control, we compared the ability of exosome uptake by circulating peripheral mononuclear cells in PBC to HCs. As expected, circulating exosomes were taken up efficiently by CD14+ monocytes, CD11c+ DCs, and CD19+ B cells in PBC and HC; in contrast, exosome uptake by CD3+ T cells was significantly less (Figure 2a). Importantly, and as expected, there were no significant differences of exosome uptake by various cell subsets that we detected in the PBC compared to the HC group (Figure 2b).
Figure 2.
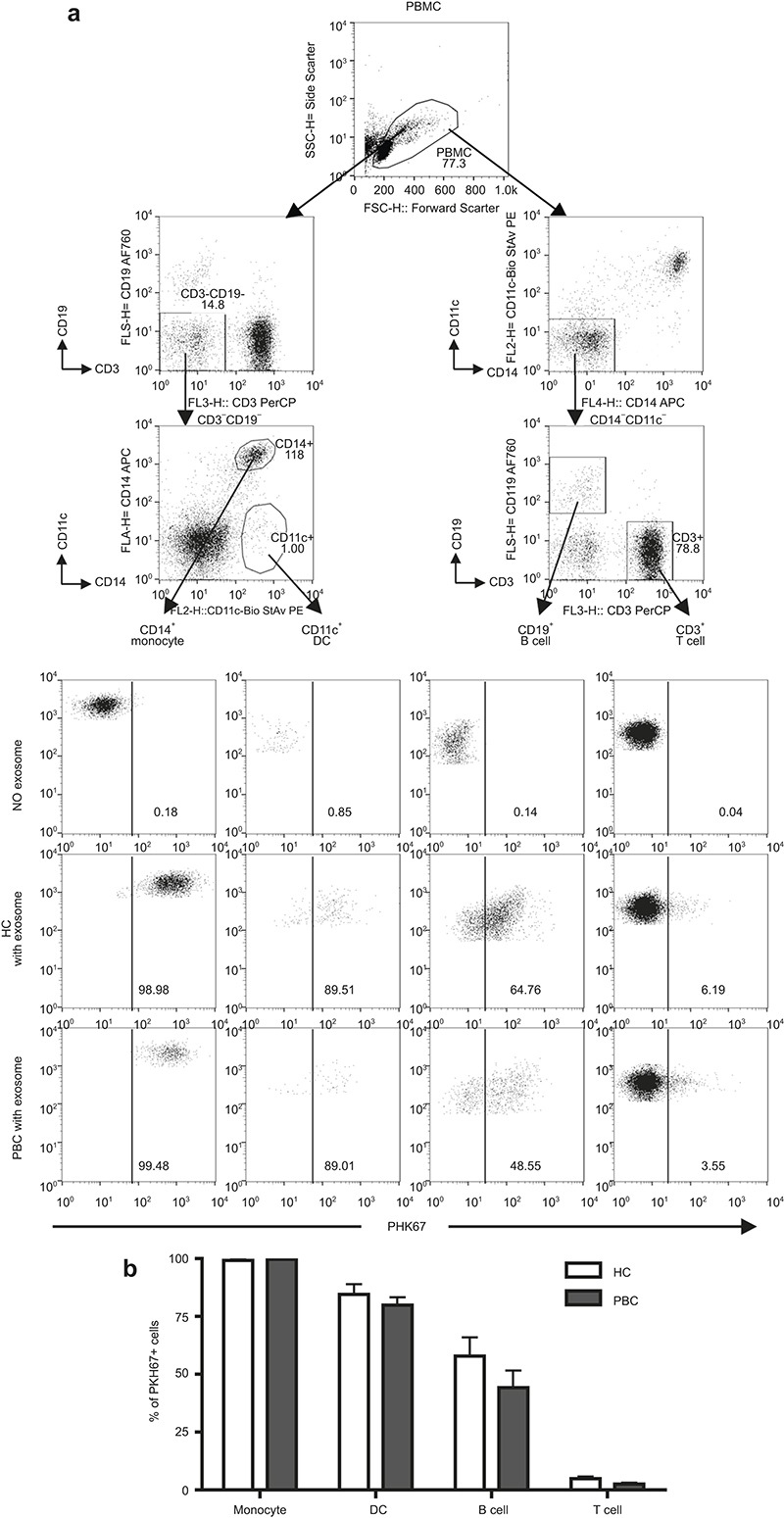
Uptake of exosomes by PBMCs. Exosomes were labeled with PKH-67 dye and cultured with freshly isolated 1 × 106 PBMCs for 16 h. (a) A representative pattern of PKH-67+ cells in each subpopulation was analyzed by flow cytometry. (b) Frequency of PKH-67+ cells in CD14+ monocytes, CD11c+ DCs, CD19+ B cells, and CD3+ T cells. Data are shown as mean ± SEM.
Exosomes modulate APC's co-stimulatory molecules in PBC versus HC
We thence compared the ability of exosomes to alter the phenotype of APCs, including the expression of co-stimulatory molecules CD80, CD86, and CD40, and HLA-DR on CD14+ monocytes, CD11c+ DCs, and CD19+ B cells following culture with or without autologous exosomes. There was no significant effect in the expression of co-stimulatory molecules on monocytes and DCs in the presence or absence of autologous exosomes in HC (Figure 3). Importantly, however, and in contrast, there were significant differences detected in select co-stimulatory molecules of mononuclear cell subsets following the addition of autologous exosomes in the PBC group. In CD14+ monocytes, the expression of CD86 was significantly increased, but CD80 and CD40 were decreased after cultured with exosomes. In CD11c+ DCs, however, the expression of CD40 was significantly increased by exosomes. In addition, although both CD80 and CD86 were down-regulated on both PBC and HC CD19+ B cells, CD40 expression was decreased only on B cells of patients with PBC (Figure 3). Of note, the expression of HLA-DR was not significantly changed in both group of PBC and HC. For further purposes of control, we analyzed these markers in freshly isolated PBMCs and cultured without addition of exosomes, there were no significant differences of the co-stimulatory molecule expression between the PBC and HC group (Figure 3 and data not shown). These data indicate that autologous exosomes selectively up-regulate co-stimulatory molecules in different APCs in patients with PBC.
Figure 3.
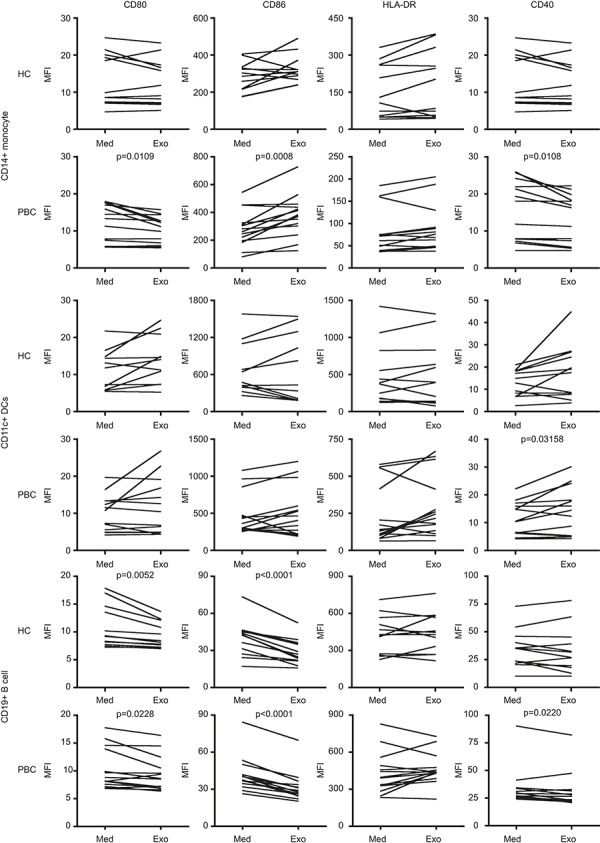
Circulating exosomes modulate co-stimulatory molecules on antigen-presenting cells in PBC. Freshly isolated 1 × 106 PBMCs were cultured with or without autologous circulating exosomes for 16 h. The expression of co-stimulatory and major histocompatibility complex (MHC)-II molecules on CD14+ monocytes, CD11c+ DCs, and CD19+ B cells in PBC (n = 14) were compared with HC (n = 12) by mean fluorescent intensity. The numbers indicate the P-values of MFI between groups of cells cultured with medium alone (Med) and exosomes (Exo).P-values were calculated by a two-tailed paired t-test.
Autologous exosomes did not elicit inflammatory cytokine production by monocytes in PBC
We next analyzed cytokine production by CD14+ monocytes. Essentially, freshly isolated CD14+ monocytes were co-cultured with autologous exosomes for 24–72 h. The inflammatory cytokines were analyzed by a CBA kit. The levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha (TNF-α) in the culture supernatants were not significant different in CD14+ monocytes cultured with and without exosomes (data not shown). Hence, the alteration of monocyte function by autologous exosomes likely occurs through changes in the pattern of co-stimulatory molecule expression, not cytokine production.
Exosome miRNA expression patterns
To compare miRNA expression in circulating exosomes, we first screened the expression profile of circulating exosomes using a miRNA expression array. As reflected in Figure 4a and Table 2, there were nine miRNAs significantly up-regulated and nine miRNAs significantly down-regulated in the PBC group compared to the HC.
Figure 4.
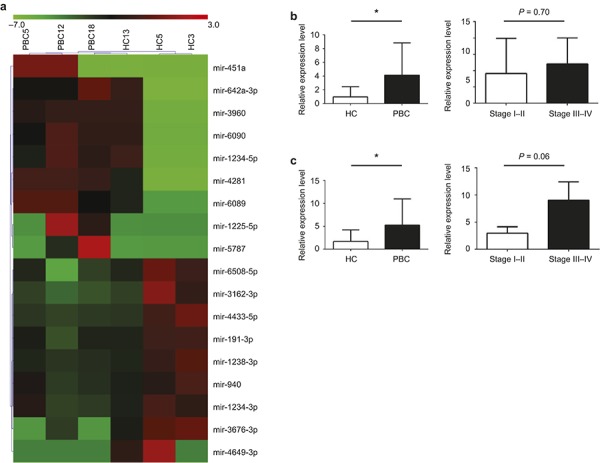
miRNAs expression of circulating exosomes are altered in PBC patients. (a) The profile of miRNA expression in small non-coding RNA isolated from plasma exosomes of PBC patients were compared to HCs by using miRNA expression array. The heat maps represent miRNA expression in serum exosomes from HC (n = 3) versus PBC (n = 3). Red indicates up-regulated miRNAs, whereas the green indicates down-regulated miRNAs. These results are summarized in Table 2. The relative expression of miRNA-451a (b) and miRNA-642-3p (c) in plasma exosomes from HC (n = 15) and PBC (n = 15) were analyzed by TaqMan PCR. The correlation analysis of miRNA-451a and miRNA-642-3p to the early stage (stage I–II) and late stage (stage III–IV) of patients with PBC are shown in the right panel of (b) and (c).P-values were calculated by unpaired t-test. *P <0.05.
Table 2. Differential expression of microRNAs between PBC and HCs.
| Up-regulated microRNAs in PBC | Down-regulated microRNAs in PBC |
|---|---|
| hsa-miR-451a | hsa-miR-940 |
| hsa-miR-5787 | hsa-miR-1234-3p |
| hsa-miR-1225-5p | hsa-miR-191-3p |
| hsa-miR-4281 | hsa-miR-1238-3p |
| hsa-miR-6089 | hsa-miR-4433-5p |
| hsa-miR-6090 | hsa-miR-6508-5p |
| hsa-miR-3960 | hsa-miR-3162-3p |
| hsa-miR-642a-3p | hsa-miR-3676-3p |
| hsa-miR-1234-5p | hsa-miR-4649-3p |
To verify this differential expression, we next determined the levels for each of these miRNAs using isolated exosomes from additional groups of patients with PBC and HC using a TaqMan PCR assay for individual miRNAs. We noted that two miRNAs, miRNA-451a and miRNA-642a-3p, were present at significantly higher levels in exosomes from PBC compared to HCs (Figure 4b and c). Further, we examined whether the levels of these exosomal miRNAs correlated with severity of disease. However, there were no significant differences detected in patients with PBC at earlier versus later stage of disease (Figure 4b and c), indicating that upregulation of these miRNAs are not dependent on disease severity.
Discussion
Exosomes are secreted intracellular microparticles that induce antigen-specific immune responses and potentially trigger inflammation. Importantly, exosomes from a variety of cell populations can mediate either immune activation or immune suppression based on the individual immunological microenvironment.34,35,36,37 In autoimmunity, it has also been suggested that exosomes play a role in the chronic inflammatory response and that the cellular origin of exosomes will differentially influence the immune response in autoimmune disease.6,38,39 Our data are important because they emphasize that co-stimulatory molecules play a pivotal role in APC-mediated immune responses, particularly T-cell activation and proliferation.40,41,42,43,44,45 In the study herein, we demonstrate the ability of autologous exosomes to be taken up by peripheral monocytes and DCs. Further, they have the potential to modulate the expression of co-stimulatory molecules on APCs from patients with PBC. Interestingly, as shown in Figure 2, exosomes significantly increase the expression of CD86 on CD14+ monocytes, but not on DCs or B cells. In contrast, the expression of CD40 is increased on DCs, but decreased when comparable analysis is performed on B cells in patients with PBC. These data suggest that exosomes regulate distinct co-stimulatory molecules on different APC subpopulations. Further study is necessary to focus on other co-stimulatory molecules, but these results imply that CD40 and CD86 are two key elements in exosome-induced APC activation in the evolution of the immune response in PBC. There are limitations in our data. First, it is unclear what the mechanism of action is that explains the activation of APCs in PBC. One possibility is that circulating exosomes in PBC may have more co-stimulatory molecules and indeed it has been shown that exosomes from activated DCs can have a higher level of expression of co-stimulatory molecules than immature DCs.46 In chronic inflammatory diseases (including PBC), there are increased levels of activated professional and non-professional APCs and such activated cells have the potential to secrete co-stimulatory molecule-enriched exosomes. When captured by DCs or monocytes, such exosomes could be retained on the surface of APCs and thus reflect increased density. Future efforts should focus on the ontogeny of expression of co-stimulatory molecules in circulating exosomes in patients at different stages of PBC and within a given patient over a longer period of observation. A second limitation is that future studies should focus on the mechanisms in PBC by which exosomes are internalized by phagocytosis or macropinocytosis.47,48 Exosomes carry autoantigens as reflected in multiple autoimmune disease.6,38 Once exosomes are internalized by DCs, autoantigens can be processed and further activated APCs via up-regulated co-stimulatory molecules. Indeed, we have found that exosomes harbor PDC-E2 (unpublished data), which is the major autoantigen in PBC. In addition, other exosome-carried molecules, such as miRNAs, which are aberrantly expressed in PBC, may also be involved in the regulation of co-stimulatory molecule expression on APCs.49
One issue is that in vivo, PBMC is constantly exposed to serum exosomes, and if serum exosomes do impose effects on APC functions as suggested by our in vitro data, the co-stimulatory molecule expression should be reflected in freshly isolated PBMCs. However, we did not detect significant difference of these molecules expressed on either peripheral monocytes or DCs between PBC and HC (data now shown), suggesting that circulating exosomes alone in vivo are not sufficient to activate the APCs. We note that the patients studied herein were under treatment with ursodeoxycholic acid, which has the potential to inhibit DC function.50,51 To further address this issue, future studies should evaluate the effect of exosomes on co-stimulatory molecule expression in naive patients.
We should note that exosomes can be produced by a variety of cell types. For example, exosomes derived from salivary gland epithelial cells contain the autoantigenic ribonucleoproteins in Sjögren's syndrome.6 We have previously demonstrated that apoptotic bodies from biliary epithelial cells contained the PBC-specific mitochondrial autoantigens.26 Hence, within the liver, it is likely that biliary epithelial cells may produce exosomes that are critical for the homeostasis of biliary function52,53 and we further hypothesize that exosomes released by damaged bile duct cells may augment this process. Indeed, since circulating exosomes can be internalized efficiently by APC (as shown in Figure 2), it is likely that such cells are involved in the processing and presentation of autoantigen to autoreactive T cells. In addition, circulating exosomes may also provide the critical MHC-PDC-E2 complex to DCs, leading to the activation of naïve T cells in the pathogenesis of PBC.
Professional APCs, particularly DCs, involved in the coordination of adaptive immune responses, are also regulated by miRNAs.54 One of our objectives was to investigate whether exosome carried molecule signaling, especially miRNAs, are altered in PBC and whether aberrant signaling is involved in the regulation of APC function. We note that activation of naive DCs by miRNAs up-regulate the expression of surface markers, including MHC II and the co-stimulatory molecules CD80, CD86, and CD40.55 Deficiency of miRNA-155 in activated DCs significantly decreases DC expression of MHC II, CD40, and CD86, leading to reduced levels of secreted IL-12p40, IL-12p35, and TNF-α.49 Our data on identification of 18 miRNAs aberrantly expressed in exosomes of PBC compared to HCs become an important tool for further study. Two miRNAs (miRNA-451a and miRNA-642a-3p) have been validated by using quantitative PCR analysis. miRNA-451a has been demonstrated to regulate DC cytokines in response to influenza infection.56 In rheumatoid arthritis, miRNA-451a expression in T cells correlates with peripheral IL-6 level, the erythrocyte sedimentation rate and disease severity scores.57 In addition, miRNA-642a regulates toll-like receptor 4 translation in monocytes.58
We speculate that differential modulation of co-stimulatory molecules will be found in a variety of autoimmune diseases and will not be disease-specific. We have focused on differential patterns in patients with PBC compared to HC. The field is currently in its infancy and we suggest that multiple other liver-specific autoimmune diseases should be included in future analysis. We further speculate that aberrant expression and/or function of miRNAs that are modulated by exosomes will influence the qualitative and quantitative nature of the continued immune response and, in particular, the activation of bystander cells. We are aware that in our ex vivo culture system, exosomes only affected the expression of co-stimulatory molecules on APCs; they did not elicit inflammatory cytokines in the culture supernatants. It will be important to assess whether the mRNA level of cytokines and related transcription factors are altered in the exosomes of PBC. Continued work will require functional correlation and, in particular, the clinical significance of altered co-stimulatory molecule expression at different stages of disease activity.
Acknowledgments
We thank Ms. Nikki Phipps for support in preparing this article. We also thank Mr. Sandeep Dhaliwal in preparing the blood samples. Financial support provided by National Institutes of Health grant, DK39588 (M. Eric Gershwin).
References
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581–593. [DOI] [PubMed] [Google Scholar]
- Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 1989; 74: 1844–1851. [PubMed] [Google Scholar]
- Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007; 179: 1969–1978. [DOI] [PubMed] [Google Scholar]
- Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: a source of autoantigenic ribonucleoproteins. Arthritis Rheum 2005; 52: 1517–1521. [DOI] [PubMed] [Google Scholar]
- Brouwer R, Vree Egberts WT, Hengstman GJ, Raijmakers R, van Engelen BG, Seelig HP et al. Autoantibodies directed to novel components of the PM/Scl complex, the human exosome. Arthritis Res 2002; 4: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Kim D, Han J, Kim Y, Lee M, Jin EJ. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med 2015; 15: 121–126. [DOI] [PubMed] [Google Scholar]
- Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev 2000; 174: 210–225. [DOI] [PubMed] [Google Scholar]
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005; 353: 1261–1273. [DOI] [PubMed] [Google Scholar]
- Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: convenient and inconvenient truths. Hepatology 2008; 47: 737–745. [DOI] [PubMed] [Google Scholar]
- Lleo A, Maroni L, Glaser S, Alpini G, Marzioni M. Role of cholangiocytes in primary biliary cirrhosis. Semin Liver Dis 2014; 34: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang FS, Chang C, Gershwin ME. Breach of tolerance: primary biliary cirrhosis. Semin Liver Dis 2014; 34: 297–317. [DOI] [PubMed] [Google Scholar]
- Dyson JK, Hirschfield GM, Adams DH, Beuers U, Mann DA, Lindor KD, Jones DE. Novel therapeutic targets in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol 2015; 12: 147–158. [DOI] [PubMed] [Google Scholar]
- Wang J, Budamagunta MS, Voss JC, Kurth MJ, Lam KS, Lu L et al. Antimitochondrial antibody recognition and structural integrity of the inner lipoyl domain of the E2 subunit of pyruvate dehydrogenase complex. J Immunol 2013; 191: 2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J et al. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest 2002; 109: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med 2002; 195: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sun Y, Zhang Z, Jia Y, Zou Z, Ding J et al. CXCR5(+) CD4(+) T follicular helper cells participate in the pathogenesis of primary biliary cirrhosis. Hepatology 2015; 61: 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang W, Leung PS, Bowlus CL, Dhaliwal S, Coppel RL et al. Ongoing activation of autoantigen-specific B cells in primary biliary cirrhosis. Hepatology 2014; 60: 1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda S, Tsuneyama K, Kikuchi K, Harada K, Nakanuma Y, Nakamura M et al. The role of natural killer (NK) and NK T cells in the loss of tolerance in murine primary biliary cirrhosis. Clin Exp Immunol 2012; 168: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yang GX, Tsuneyama K, Gershwin ME, Ridgway WM, Leung PS. Animal models of primary biliary cirrhosis. Semin Liver Dis 2014; 34: 285–296. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Yang GX, Zhang WC, Lu L, Tsuneyama K, Kronenberg M et al. Escherichia coli infection induces autoimmune cholangitis and anti-mitochondrial antibodies in non-obese diabetic (NOD).B6 (Idd10/Idd18) mice. Clin Exp Immunol 2014; 175: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology 2010; 52: 987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasatomi K, Noguchi K, Sakisaka S, Sata M, Tanikawa K. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol 1998; 29: 409–416. [DOI] [PubMed] [Google Scholar]
- Lleo A, Zhang W, McDonald WH, Seeley EH, Leung PS, Coppel RL et al. Shotgun proteomics: identification of unique protein profiles of apoptotic bodies from biliary epithelial cells. Hepatology 2014; 60: 1314–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong G, Zhong R, Lleo A, Leung PS, Bowlus CL, Yang GX et al. Epithelial cell specificity and apotope recognition by serum autoantibodies in primary biliary cirrhosis. Hepatology 2011; 54: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth MJ, Yokoi T, Gershwin ME. Halothane-induced hepatitis: paradigm or paradox for drug-induced liver injury. Hepatology 2014; 60: 1473–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Yang W, Yang JB, Jia YJ, Tang W, Gershwin ME et al. Systems biologic analysis of T regulatory cells genetic pathways in murine primary biliary cirrhosis. J Autoimmun 2015; 59: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology 2014; 59: 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Yang W, Yang YQ, Ma HD, Lu FT, Li L et al. Distinct from its canonical effects, deletion of IL-12p40 induces cholangitis and fibrosis in interleukin-2Rα(-/-) mice. J Autoimmun 2014; 51: 99–108. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ, American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology 2009; 50: 291–308. [DOI] [PubMed] [Google Scholar]
- Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010; 51: 660–678. [DOI] [PubMed] [Google Scholar]
- Rong GH, Yang GX, Ando Y, Zhang W, He XS, Leung PS et al. Human intrahepatic biliary epithelial cells engulf blebs from their apoptotic peers. Clin Exp Immunol 2013; 172: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 2015; 16: 24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Terme M, Flament C, Taieb J, André F, Novault S et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One 2009; 4: e4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li JJ, Yang JY, Wang DS, Zhao W, Song WJ et al.Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS One 2012; 7: e44045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected]. J Immunol 2009; 183: 5983–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MJ, Regn D, Bashratyan R, Dai YD. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes 2014; 63: 1008–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Ouyang S, Li Y, Xiao B, Yang H. Immature dendritic cell-derived exosomes: a promise subcellular vaccine for autoimmunity. Inflammation 2013; 36: 232–240. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996; 14: 233–258. [DOI] [PubMed] [Google Scholar]
- Dilioglou S, Cruse JM, Lewis RE. Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp Mol Pathol 2003; 75: 217–227. [DOI] [PubMed] [Google Scholar]
- Vasilevko V, Ghochikyan A, Holterman MJ, Agadjanyan MG. CD80 (B7-1) and CD86 (B7-2) are functionally equivalent in the initiation and maintenance of CD4+ T-cell proliferation after activation with suboptimal doses of PHA. DNA Cell Biol 2002; 21: 137–149. [DOI] [PubMed] [Google Scholar]
- Zhang P, Lewis JP, Michalek SM, Katz J. Role of CD80 and CD86 in host immune responses to the recombinant hemagglutinin domain of Porphyromonas gingivalis gingipain and in the adjuvanticity of cholera toxin B and monophosphoryl lipid A. Vaccine 2007; 25: 6201–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem 2002; 277: 7766–7775. [DOI] [PubMed] [Google Scholar]
- Fleischer J, Soeth E, Reiling N, Grage-Griebenow E, Flad HD, Ernst M. Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology 1996; 89: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005; 106: 216–223. [DOI] [PubMed] [Google Scholar]
- Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010; 11: 675–687. [DOI] [PubMed] [Google Scholar]
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 2011; 124: 447–458. [DOI] [PubMed] [Google Scholar]
- Dunand-Sauthier I, Santiago-Raber ML, Capponi L, Vejnar CE, Schaad O, Irla M et al. Silencing of c-Fos expression by micro RNA-155 is critical for dendritic cell maturation and function. Blood 2011; 117: 4490–4500. [DOI] [PubMed] [Google Scholar]
- Willart MA, van Nimwegen M, Grefhorst A, Hammad H, Moons L, Hoogsteden HC et al. Ursodeoxycholic acid suppresses eosinophilic airway inflammation by inhibiting the function of dendritic cells through the nuclear farnesoid X receptor. Allergy 2012; 67: 1501–1510. [DOI] [PubMed] [Google Scholar]
- Sombetzki M, Fuchs CD, Fickert P, Osterreicher CH, Mueller M, Claudel T et al. 24-nor-ursodeoxycholic acid ameliorates inflammatory response and liver fibrosis in a murine model of hepatic schistosomiasis. J Hepatol 2015; 62: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 2009; 136: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol 2010; 299: G990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner ML, Schnorfeil FM, Brocker T. MicroRNAs regulate dendritic cell differentiation and function. J Immunol 2011; 187: 3911–3917. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol 2006; 6: 476–483. [DOI] [PubMed] [Google Scholar]
- Rosenberger CM, Podyminogin RL, Navarro G, Zhao GW, Askovich PS, Weiss MJ, Aderem A. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol 2012; 189: 5965–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigielska-Czepiel K, van den Berg A, Jellema P, van der Lei RJ, Bijzet J, Kluiver J et al. Comprehensive analysis of miRNA expression in T-cell subsets of rheumatoid arthritis patients reveals defined signatures of naive and memory Tregs. Genes Immun 2014; 15: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yoshimura A, Kaneko T, Ukai T, Ozaki Y, Nakamura H et al. A single nucleotide polymorphism in 3′-untranslated region contributes to the regulation of toll-like receptor 4 translation. J Biol Chem 2012; 287: 25163–25172. [DOI] [PMC free article] [PubMed] [Google Scholar]


