Abstract
γδT cells are a conserved population of innate lymphocytes with diverse structural and functional heterogeneity that participate in various immune responses during tumor progression. γδT cells perform potent immunosurveillance by exerting direct cytotoxicity, strong cytokine production and indirect antitumor immune responses. However, certain γδT-cell subsets also contribute to tumor progression by facilitating cancer-related inflammation and immunosuppression. Here, we review recent observations regarding the antitumor and protumor roles of major structural and functional subsets of human γδT cells, describing how these subsets are activated and polarized, and how these events relate to subsequent function in tumor immunity. These studies provide insights into the manipulation of γδT-cell function to facilitate more targeted approaches for tumor therapy.
Keywords: antitumor, γδT cells, protumor, subsets, tumor immunity
Introduction
γδT cells, which are innate-like T lymphocytes characterized by T-cell receptors (TCRs) composed of γ and δ chains, are widely distributed in the peripheral blood (PB) and mucosal tissues.1 γδT cells rapidly recognize exogenous pathogens and endogenous stress-induced ligands in a major histocompatibility complex (MHC)-unrestricted manner and initiate adaptive immunity, acting as a first line of immune defense.2 Activated γδT cells exhibit multiple effector functions, including cytotoxicity against infected or tumor cells, cytokine and chemokine production, antigen-presenting functions and regulatory abilities,3 thus allowing them to participate in an array of diseases, including infection, allergy, autoimmunity and cancer.4, 5, 6
Human γδT cells contribute to the immune response against a subset of tumors of hematological and epithelial origin, and many clinical trials have been conducted to test the use of γδT cells in adoptive cell therapy.7 However, human γδT cells have diverse physiological roles in tumor immunity, owing to their wide-ranging structural subsets, which are defined by their TCR repertoire and functional heterogeneity driven by differential environmental stimulation.8, 9 Recent reports have described the diverse responses of human γδT cells to tumors.10 For example, γδT cells exert cytotoxicity toward tumor cells via the NKG2D pathway;11 however, they also develop a regulatory profile by expressing interleukin-10 (IL-10) and tumor growth factor (TGF)-β, thereby exerting suppressive effects on antitumor responses.12 Moreover, our previous studies have indicated that human PB Vδ1 T cells demonstrate favorable cytotoxicity against colon cancer,13 whereas γδT17 cells with Vδ1 TCR usage in colon cancer tissue promote tumor progression.14
Therefore, understanding γδT-cell subset-specific responses during tumor immunity is vital to rationally exploit the antitumor activity of γδT cells while avoiding their tumor-promoting effects during tumor therapy. In this review, we summarize research progress regarding the major structural and functional subsets of human γδT cells and their effects on tumor immunity, and we describe the clinical implications for tumor therapy involving the manipulation of γδT-cell function.
Structural subsets and γδt-cell activation
Generally, human γδT cells are divided into two major structural subsets according to their TCR δ chain usage: Vδ1 and Vδ2 T cells.15 In terms of TCR γ chain usage, Vδ1 T cells are predominantly associated with the VγI gene family (Vγ2/3/4/5/8), whereas the majority of Vδ2 T cells coexpress VγII (Vγ9).16 γδT subsets exhibit distinct developmental properties, tissue localization and activation modes.1, 17, 18
Vγ9Vδ2 γδt cells
γδT-cell development primarily occurs in the fetal thymus, and subsets arise through rearrangements at distinct phases of thymic ontogeny.19 Vδ2 subsets are generated in the thymi at 8.5–15 weeks in human embryos, with gene rearrangements of Vδ2 to Dδ3 and of Vγ1.8 or Vγ9 to Jγ1.19 Human Vδ2 T cells, which are almost exclusively paired with the Vγ9 chain (also termed Vγ9Vδ2 γδT cells), are predominant in the PB (>70%),15 and are uniquely activated by phosphoantigens produced by microbes and transformed cells. Exposure to (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), an intermediate metabolite of microbial isoprenoid biosynthesis20 and Isopentenyl pyrophosphate (IPP), which is generated by transformed mammalian cells via the the mevalonate pathway, leads to TCR-dependent activation of Vγ9Vδ2 T cells,21 thus enabling them to rapidly respond to exogenous infection or endogenous transformed cells. Moreover, aminobisphosphonates such as zoledronic acid combined with low-dose IL-2 selectively activate and expand Vγ9Vδ2 T cells in vitro.22 Phosphoantigens interact with specific proteins rather than being directly recognized by the TCR.23 F1-ATPase expressed on tumor cells has been defined as an antigen-recognition molecule for phosphoantigen-mediated stimulation of human Vγ9Vδ2 T cells.24 Butyrophilin3A1 is another essential phosphorylated antigen-presenting modality of Vγ9Vδ2 T-cell activation.25, 26, 27 In addition to phosphoantigens, human MutS homolog 2, a DNA repair-related protein ectopically expressed on tumor cells, is recognized by Vγ9Vδ2 T cells via the TCR.28
Toll-like receptors (TLRs) and natural killer receptors (NKRs) have been reported to co-stimulate human Vγ9Vδ2 T cells in combination with TCR stimulation.29, 30 Pathogen-associated molecular patterns derived from microbes trigger Vγ9Vδ2 T-cell activation via TLRs and promote cytokine and chemokine production.29 Moreover, human Vγ9Vδ2 T cells also recognize stress-induced MHC class I chain-related antigens A and B (MICA/B) as well as MIC-A-related UL16-binding proteins (ULBPs) upregulated by transformed or infected cells via NKG2D.11 Another NKR involved in Vγ9Vδ2 T-cell activation, DNAM-1, binds to its ligand, nectin-like-5, which is expressed on tumor cells, and consequently exerts cytotoxic effects.31 Vγ9Vδ2 T cells also respond to superantigens such as staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin (TSST)-1.32, 33 The above evidence has demonstrated that Vγ9Vδ2 T cells respond to a variety of ligands, although these represent only a few defined antigens; these responses suggest implications for the clinical management of these cells.
Vδ1 γδt cells
Vδ1 TCR gene rearrangement occurs 4–6 months after birth and involves the joining of Vδ1 to Dδ1 or Dδ2 and the joining of upstream Vγ gene segments, including Vγ2, 3, 5 and 8, to Jγ2.19 Unlike Vγ9Vδ2 T cells, human Vδ1 T cells primarily reside in the gut epithelia, dermis, spleen and liver, and are involved in maintaining epithelial tissue integrity.1 Vδ1 T cells constitute less than 30% of γδT cells in PB and contain diverse paired Vγ chains.15, 16 During HIV infection, Vδ1 T-cell numbers are increased, and the normal ratio of Vδ2/Vδ1 T cells is inverted, thus suggesting the potential involvement of Vδ1 T cells in antiviral immunity.34 Ligand recognition by Vδ1 T cells remains largely uncharacterized, although CD1 family proteins are recognized by Vδ1 T cells. Both PB and tissue Vδ1 T cells recognize CD1c35, 36, 37 and the lipid-presenting MHC-like molecule CD1d via the TCR.38 Two recent studies have explored the structural basis of the recognition of lipid antigens by the Vδ1 TCR via CD1d-presenting molecules.39, 40 In addition to the CD1 family, human intestinal epithelial Vδ1 T cells respond to stress-induced MICA/B through the synergistic actions of TCR and NKG2D.41, 42 Specifically, in a manner analogous to Vγ9Vδ2 T cells, Vδ1 T cells respond to tumor cells by overexpressing MICA/B and ULBPs via NKG2D.43, 44 Moreover, Vδ1 T cells are activated by the superantigen SE but respond exclusively to SEB rather than SEA.45 A unique feature of Vδ1 T-cell activation is the recognition of B7-H6, a B7 family member exclusively expressed on tumor cells, by NKp30, thereby exerting antitumor effects.46, 47
Non-vδ1 and non-vγ9vδ2 γδt cells
Human Vδ3 T cells compose the majority of non-Vδ1 and non-Vγ9Vδ2 γδT cells and are found in healthy PB, the liver48 and in patients with cytomegalovirus (CMV) infection,49 HIV infection50 and B-cell leukemia.51 Vδ3 T cells, paired with Vγ2 or Vγ3,50 respond to CD1d and express the degranulation marker CD107a.52 A Vγ4Vδ5+ T-cell clone has been reported to recognize stressed human cells via TCR binding to endothelial protein C receptor.53 Furthermore, Vδ4, Vδ6, Vδ7 and Vδ8 T cells have been detected in the PB of lymphoma patients;54 however, further studies are required to evaluate γ chain pairings and how these subsets are activated. Studies examining the activation of γδT-cell subsets are highlighted in Table 1.
Table 1. Structural subsets of human γδT cells.
| Structural subset | Paired Vγ gene usage | Distribution | Activation stimulus and/or γδTCR ligands | References |
|---|---|---|---|---|
| Vδ1 | Vγ2/3/4/5/8/9 | PB, skin, gut, spleen, liver | MICA/B; ULBPs; B7-H6; CD1c; CD1d; SEB | 35, 39, 44, 45, 46 |
| Vδ2 | Vγ9 | PB | Phosphoantigens; F1-ATPase; BTN3A1; hMSH2; MICA/B; ULBPs; SEs; TSST-1; Nectin-like-5; | 20, 24, 27, 28, 31, 32, 33 |
| Vδ3 | Vγ2/3 | PB, liver | CD1d | 50, 52 |
| Vδ5 | Vγ4 | PB | EPCR | 53 |
Abbreviations: BTN3A1, butyrophilin3A1; EPCR, endothelial protein C receptor; hMSH2, human MutS homolog 2; MHC, major histocompatibility complex; MICA/B, MHC class I chain-related antigens A and B; PB, peripheral blood; ULBP, UL16-binding protein; SE, staphylococcal superantigens; TSST-1, toxic shock syndrome toxin-1.
Functional subsets and γδt-cell polarization
γδT cells share pleiotropic functions with conventional αβ T cells.55 Each functional subset is induced through the stimulation of resting γδT cells by different polarization factors in vitro.56
Ifn-γ-producing γδt cells
Human circulating γδT cells are driven to produce interferon (IFN)-γ in the presence of IPP by IL-12 and anti-IL-4 antibodies, whereas these cells are polarized and become IL-4-producing cells when exposed to IPP plus IL-4 and anti-IL-12 antibodies,57 which mediate anti-infection responses. Moreover, activation of an IFN-γ-producing response in the absence of IL-4 detection is promoted by nonpeptide antigens plus IL-21.58 Similarly, IL-2 and IL-21 drive γδT cells toward an IFN-γ-producing phenotype characterized by increased CD56 expression and enhanced cytolytic responses.59, 60 IL-2 and IL-15 signals drive human γδT-cell differentiation toward cytotoxic IFN-γ-producing subsets in the absence of TCR activation.61
Antigen-presenting γδ t cells
γδT cells also display functional plasticity in terms of indirect anti-infection or antitumor responses.62, 63 Bovine γδT cells present antigens to CD4+ αβT cells.64 Microbial infections induce professional antigen-presenting cell (APC) functions of human tonsillar γδT cells characterized by the expression of co-stimulatory molecules such as MHC-II, CD80, CD86 and CD40, thereby initiating adaptive immune responses by CD4+ and CD8+ αβT cells.65 Furthermore, γδT-APCs process soluble protein for cross-presentation on MHC-I and induce CD8+ αβT-effector cell responses more efficiently than monocyte-derived dendritic cells (DCs).66
Follicular b helper γδt cells
Follicular T helper (TFH) cells have critical roles in adaptive immunity via interactions with B cells.67 Vermijlen D et al.56 have reported IL-21-induced expression of the follicular B-cell-attracting chemokine CXCL13/BCA-1 on γδT cells, thus resulting in a TFH-associated phenotype. The transcriptional suppressor Bcl-6 is an indispensable regulator of TFH lineage commitment.67 γδTFH cells polarized by HMB-PP and IL-21 exhibit TFH-like activity accompanied by the expression of the transcriptional repressors Bcl-6, ICOS, CD40L, CXCR5, IL-21 R, CD244, CXCL10 and CXCL13, which, in maturing B cells, facilitate the production of high-affinity antibodies against foreign antigens.68, 69
Regulatory γδt cells
γδT cells also exert immunosuppressive and regulatory activities during immune responses. Casetti et al.70 have reported the induction of Foxp3+ regulatory γδT (γδTreg) cells by TGF-β1 and IL-15, accompanied by antigen stimulation, which inhibits the proliferation of anti-CD3 and anti-CD28 antibody-stimulated PBMCs. Indeed, in vitro-expanded Vδ1 T cells stimulated by an anti-human TCR Vδ1 antibody with TGF-β1 predominantly express Foxp3, CD25, glucocorticoid-induced TNFR family-related protein and CTLA4, all of which suppress CD4+ T cell proliferation.71 Tumor-infiltrating γδTreg cells are induced by IP-10 secreted by breast cancer cells, thereby suppressing T-cell responses and DC maturation.72 These regulatory γδT cells lack the expression of Foxp3, GIRT and CD25, and their suppressive activity does not occur via TGF-β or IL-10.73 Recently, we have identified a novel γδTreg subset exhibiting CD39 expression that accounts for 60% of γδT17 cells and is polarized by TGF-β, thus resulting in stronger immunosuppression than CD4+ Treg cells in the context of human colorectal cancer (unpublished data). These CD39+ γδTreg cells suppress the activity of human CD3+ T cells in an adenosine-dependent manner (unpublished data).
Il-17-producing γδt cells
γδT17 cells broadly participate in inflammatory responses, having pathogenic roles during infection and autoimmune diseases.74 Differentiation into γδ17 T cells requires high levels of RAR-related orphan receptor C (RORC) and aryl hydrocarbon receptor (AHR) expression but low levels of T-bet expression, which is efficiently induced by coordinated stimulation by phosphoantigens and cytokines, including IL-1β, TGF-β, IL-6 and IL-23.75 Fresh human cord blood γδT cells cultured with IL-7 plus TCR agonists for 1 week and stimulated by PMA and ionomycin for 6 h were polarized into IL-17 producers.76 IL-6, IL-1β and TGF-β are required to generate γδT17 cells in neonates.77 In addition, IL-23 is highly important for γδT17 cell maturation and growth.78 In a previous study, we have identified that γδT17 cells polarized in human colorectal cancer tissue under stimulation by IL-23 derived from inflammatory DCs.14 Table 2 summarizes studies investigating the polarization of γδT-cell subsets with distinct functions.
Table 2. Functional subsets of human γδT cells.
| Functional subsets | Polarization | References |
|---|---|---|
| IFN-γ-producing γδT | IPP+IL-12+IL-4 antibody; IL-2+IL-21; nonpeptide antigens+IL-21; IL-2+IL-15 | 57, 58, 59, 60, 61 |
| IL-4-producing γδT | IPP+IL-4+IL-12 antibody | 57 |
| γδT-APC | Microbial product | 6 |
| γδTFH | IL-21; HMB-PP+IL-21 | 56, 68 |
| γδTreg | TGF-β+IL-15; Vδ1 TCR antibody+ TGF-β1; IP-10 | 70, 71, 72, 73 |
| γδT17 | IL-7+TCR agonists; IL-23; phosphoantigens+IL-1β+TGF-β+IL-6+IL-23; IL-6+IL-1β+TGF-β | 14, 75, 76, 77 |
Abbreviations: APC, antigen-presenting cell; γδTreg, regulatory γδT; HMB-PP, (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate; IFN, interferon; IL, interleukin; IPP, Isopentenyl pyrophosphate; TCR, T-cell receptor; TGF, tumor growth factor.
The role of γδt-cell subsets in tumor immunity
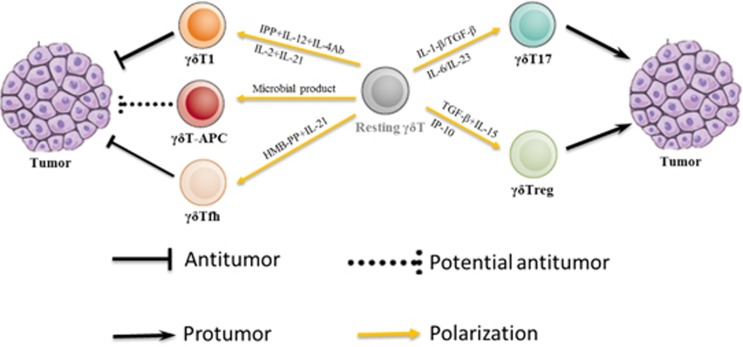
Differentially polarized γδT-cell subsets exhibit functionally diverse responses to tumors, thus potentially leading to antitumor or protumor responses (Figure 1).
Figure 1.
Polarization and responses of human γδT-cell subsets to tumors.
Antitumor effects
The first report of tumor surveillance by γδT cells described a potential association between the increased frequency of γδT cells and improved disease-free survival of leukemia patients who received αβT-cell-depleted bone marrow transplants.79 Recently, intratumoral γδT cells have been demonstrated to be the most significant predictors of favorable survival across various cancer types.80 γδT cells display cytotoxicity against hematopoietic and solid tumors in an MHC-independent manner.8 Although their activation mechanisms differ, both Vδ2 and Vδ1 subsets exert potent antitumor effects.8 One common γδT-cell-mediated killing pattern involves tumor cell recognition via receptor–ligand interactions. TCR is strongly implicated in controlling Vγ9Vδ2 T-cell cytotoxicity via the recognition of phosphoantigens that are overexpressed in tumor cells and mediate tumor cell lysis.81 NKG2D binds to MICA/B and ULBPs and induces Vγ9Vδ2 T-cell cytotoxicity against hemopoietic and epithelial tumors.11, 30, 82, 83, 84 Vγ9Vδ2 T cells are induced to produce IFN-γ and kill hepatocellular carcinoma cells via the interaction of DNAM-1 and nectin-like-5.31 γδT cells also exhibit strong cytotoxicity against myeloma cells via NKp44.85 Furthermore, CD56+ γδT cells are capable of killing squamous cell carcinoma of the head and neck, a process that is likely to be mediated by the enhanced expression of granzyme B and upregulated degranulation.86
Similarly to NK cells, γδT cells induce antibody-dependent cell-mediated cytotoxicity (ADCC) effects, thus resulting in the lysis of tumor cells. According to Tokuyama H et al.,87 CD16+ Vγ9Vδ2 T cells recognize monoclonal antibody-coated lymphoma, chronic lymphocytic leukemia (CLL) and breast cancer cells via CD16 and exert ADCC-dependent cytotoxicity. γδT cells mediate ADCC against B-lineage acute lymphoblastic leukemia via CD19 antibodies.88 In several other studies, γδT cells have also been shown to mediate ADCC effects against tumor cells via CD16 in the presence of therapeutic antitumor monoclonal antibodies.89, 90, 91
Moreover, γδT cells have antitumor roles by modulating other effector cells. For instance, Vγ9Vδ2 T cells process endogenous antigens along the MHC-I peptide presentation pathway, which may promote antitumor adaptive immunity via the cross-presentation of tumor antigens.65 Vγ9Vδ2 T cells activated by HMB-PP promote Th1 responses by inducing DC maturation and IL-12 secretion, which may facilitate antitumor immunity.92 IPP-expanded Vγ9Vδ2 T cells induce NK cells to recognize and kill tumors that are usually resistant to NK cytolysis by increasing NKG2D expression on their surface through CD137L co-stimulation.93 Phosphoantigen-activated APC-like Vγ9Vδ2 T cells present glycolipid antigens to invariant NKT cells in a CD1d-restricted and α-GalCer-dependent manner, and subsequently initiate antitumor responses.94 Together, these results suggest that Vγ9Vδ2 T cells exert antitumor effects primarily through direct killing, ADCC-dependent cytolysis and by regulating the functions of other innate and adaptive immune cells.
The dramatic expansion of Vδ1 T cells, which usually compose a minor proportion of PB γδT cells, has been observed in solid organ transplant recipients who had developed CMV infection,95, 96 and the long-term expansion of effector Vδ1 T cells is a specific blood signature of CMV infection.97 Anti-CMV-reactive Vδ1 T cells recognize intestinal tumor epithelial cells. After recognition, Vδ1 T cells release IFN-γ and tumor necrosis factor-α (TNF-α) and exert FasL-, TNF-α-independent and perforin-dependent cytotoxicity against target cells.98 CMV-induced Vδ1 T cells demonstrate better antitumor potential and are associated with reduced cancer risk in kidney transplant recipients.99 Expanded Vδ1 T cells expressing CD8αα after CMV reactivation after allogeneic stem cell transplantation recognize both CMV-infected cells and primary leukemic blasts.100 In contrast, ex vivo-expanded Vδ1 T cells mediate the killing of glioblastoma cells in a CMV-independent manner.101 Furthermore, CMV infection also decreases tumor immunogenicity by downregulating the expression of NKG2D ligands and ULBPs.102, 103 Together, these results indicate that CMV infection is closely associated with the antitumor immunity of Vδ1 T cells, although the mechanism underlying the recognition of CMV-infected cells and tumor cells by Vδ1 T cells requires further study.
In addition to CMV-associated antitumor activity, both circulating and tumor-infiltrating Vδ1 T cells respond to malignancies of hematological and epithelial origin. Circulating Vδ1 T cells contribute to the antitumor response against low-grade non-Hodgkin lymphoma (NHL) by recognizing ULBPs on lymphoma cells.44 Moreover, Vδ1 T cells, but not Vγ9Vδ2 T cells, have been detected in ULBP-positive lymph nodes in NHL patients.44 In our previous study, we have found that ex vivo-expanded human PB Vδ1 T cells demonstrate more potent killing of colon cancer cells than Vγ9Vδ2 T cells via cytolytic receptor–ligand interactions.13 Moreover, human Vδ1 T cells have been reported to inhibit tumor metastases independently of primary tumor control in a xenograft model of colon cancer.104 Tumor-infiltrating Vδ1 T cells isolated from colorectal cancer exert cytotoxicity against autologous and allogeneic cancer cells via the recognition of cell surface antigens shared by epithelial tumors.105 With proper induction, In vitro-re-activated tumor-infiltrating Vδ1 T cells isolated from melanoma produce TNF-α and IFN-γ, and act in a cytolytic manner against tumor cells.106, 107 Ex vivo-expanded Vδ1 T cells isolated from various solid tumors demonstrate stronger cytotoxicity against tumor cell lines and/or freshly isolated tumor cells compared with Vγ9Vδ2 T cells.105, 108, 109, 110, 111, 112 Notably, in a previous study, the majority of Vδ1 T-cell lines exerted robust cytotoxic responses against the melanoma cell line A375, whereas only two of eight Vδ2 T-cell lines demonstrated clear cytotoxic activity against A375, which was enhanced by pretreating target cells with zoledronate.107 Thus, although both structural subsets of γδT cells exert antitumor effects, Vδ1 T cells are potentially better killers than Vγ9Vδ2 T cells, at least in the context of certain tumors.
Protumor effects
Although γδT cells demonstrate potent antitumor capacity, paradoxically they also exert protumor effects by promoting noncytotoxic inflammation and regulatory functions that subvert cytotoxic antitumor immunity. Intratumoral γδT cell numbers are positively associated with advanced tumor stages and are inversely correlated with breast cancer prognosis.113 γδT cells are essential producers of IL-17, both in mice and humans.75, 114 Furthermore, IL-17 mediates inflammatory responses in tumor immunity. In our previous review, we have described how IL-17 promotes colorectal cancer progression.115 According to recent studies, γδT17 cells exert tumor-promoting effects in mice by facilitating angiogenesis.114, 116 γδT17 cells also promote breast cancer metastasis because mice treated with γδT-cell-depleting agents or anti-γδTCR antibodies are profoundly protected against pulmonary and lymph node metastases.117 However, there have been few studies investigating the role of human γδT17 cells in tumor immunity. In our previous study, we have found that tumor-infiltrating γδT17 cells induced by tumor-elicited inflammation promote tumor progression via the secretion of IL-17, IL-8, tumor necrosis factor-α (TNF-α) and granulocyte-macrophage colony-stimulating factor (GM-CSF), thereby forming an immunosuppressive microenvironment in human colorectal cancer.14 Furthermore, γδT17 cells are the predominant producers of IL-17 in lung cancer (unpublished data), thus indicating their crucial role in IL-17-related inflammatory responses in tumor immunity. In a murine ovarian cancer model, γδT17 cells have been found to accumulate during later stages of tumor progression.118 We have also demonstrated a positive correlation between γδT17 cell numbers and advancing tumor stages of human colorectal cancer.14
γδT cells possess potential regulatory roles in the control of tumor immune responses. For example, according to Peng et al.,73 tumor-infiltrating γδT cells in breast cancer contribute to the formation of an immunosuppressive microenvironment by suppressing naive and effector T cells and impairing DC maturation and function. In addition, γδTreg cells derived from breast cancer induce the immunosenescence of naive and effector T cells and DCs, and this immunosuppressive activity is further amplified by the senescent cells themselves.119 Moreover, our group has identified a novel γδTreg subset in human colorectal cancer that promotes an immunosuppressive microenvironment via a metabolism-related mechanism (unpublished data). Thus, certain γδT cell subsets behave as immunosuppressive cells and promote tumor progression in specific cancers. However, more studies focusing on the polarization mechanisms of protumor γδT cells in human tumor microenvironments (TMEs) are needed.
Clinical implications
Given their potent MHC-unrestricted antitumor effector activities, γδT cells are attractive candidates for antitumor immunotherapies. The cytotoxic features of the Vδ1 and Vδ2 subsets have been investigated.8 Preclinical and clinical studies have paved the way for Vγ9Vδ2 T-cell-mediated immunotherapy, given the high-frequency and broad antitumor properties of this cell type.120 Clinical-scale expansion of Vγ9Vδ2 T cells via direct stimulation by phosphoantigens or the induction of agonist accumulation with aminobisphosphonates makes Vγ9Vδ2 T-cell-based cancer immunotherapy feasible.120 Phase I and II clinical trials have been conducted in patients with various tumor types, and objective tumor responses have been observed.7 Given the accumulating evidence supporting the cytotoxic functions of Vδ1 subsets in basic research,13, 121, 122 Vδ1 T cells may be a potent tool for clinical manipulation in cancer immunotherapy, and efforts have been put forth to explore strategies for clinical-grade expansion. Intriguingly, IL-4 promotes the proliferation of Vδ1 T cells and simultaneously inhibits Vδ2 T-cell growth,123 thus providing a novel basis to develop preferential expansion approaches for Vδ1 T cells. Recently, Almeida et al.124 have reported a robust two-step protocol for the selective expansion of Vδ1 T cells up to 2000-fold, and cellular products demonstrated strong cytotoxicity in vitro and therapeutic potential in xenograft models of CLL. Clinical trials are necessary to ascertain the safety and efficacy of Vδ1 T cells to move forward with autologous or allogeneic cell therapies for both hematological and solid tumors.
Immunosuppressive functions of γδT cells infiltrating breast cancer and colorectal cancer TMEs have been described.14, 73 The emerging evidence supporting protumor roles for specific γδT-cell subsets potentially poses an obstacle to the development of future therapies.125 Although knowledge of γδT-cell function in the TME has gradually increased, it remains a challenge to determine whether the inflammatory and regulatory features of γδT cells in the tumor-infiltrating lymphocytes are intrinsic or induced by inflammatory factors in the TME. To achieve successful therapeutic effects, it may be better to identify immunosuppressive functional subsets and eliminate them from a population of adoptive γδT cells before transfer or to combine γδT-cell-based adoptive immunotherapy with a strategy targeting the TME to prevent potential polarization into tumor-promoting subsets.
Concluding remarks
There are no clear boundaries between the structural and functional subsets of γδT cells, and it is possible to polarize Vδ2 T cells into nearly all functional subsets. However, efforts should be made to further distinguish between Vδ1 and Vδ2 subsets, which may differ substantially in terms of their localization and demonstrate context-dependent plasticity and function. To date, no one-to-one correspondence between a specific TCR structure and a specific effector γδT-cell type has been reported. A myriad of evidence indicates either antitumor effects or tumor-promoting activities for γδT cells in tumor immunity. The dual role of γδT cells is closely associated with their complex surrounding microenvironment, which influences γδT-cell polarization. Our group has identified the ability of ex vivo-expanded Vδ1 T cells to exert favorable killing activity against colon cancer, whereas γδT17 cells in colon cancer tissue, the majority of which demonstrate Vδ1 TCR usage, promote the formation of an immunosuppressive TME and thus exert a tumor-promoting role. Therefore, deciphering the mechanisms underlying the development, tissue tropism, ligands and immune responses of γδT-cell subsets should elucidate their effects in tumor immunity, thus providing sufficient evidence for the application of γδT-cell subsets for antitumor adoptive immunotherapy or for targeting certain inflammatory or regulatory γδT-cell subsets for tumor therapy.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81520108024, JH; 81572952, QW; 81602692, DW; 81472640, FQ and 81572800, PW) and the Natural Science Foundation of Zhejiang Province (LY15H160041, PW).
Footnotes
Author contributions
DW and PW contributed to the literature collection and manuscript writing. Fuming Qiu contributed to manuscript polishing. JH and QW participated in the design and review of the manuscript.
The authors declare no conflict of interest.
References
- Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10: 467–478. [DOI] [PubMed] [Google Scholar]
- Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol 2014; 32: 121–155. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, He W. The multifunctionality of human Vgamma9Vdelta2 gammadelta T cells: clonal plasticity or distinct subsets? Scand J Immunol 2012; 76: 213–222. [DOI] [PubMed] [Google Scholar]
- Paul S, Shilpi, Lal G. Role of gamma-delta (gammadelta) T cells in autoimmunity. J Leukoc Biol 2015; 97: 259–271. [DOI] [PubMed] [Google Scholar]
- Zheng R, Yang Q. The role of the gamma delta T cell in allergic diseases. J Immunol Res 2014; 2014: 963484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C, Massaia M, Davey MS, Eberl M. Human gammadelta T-cell responses in infection and immunotherapy: common mechanisms, common mediators? Eur J Immunol 2012; 42: 1668–1676. [DOI] [PubMed] [Google Scholar]
- Hannani D, Ma Y, Yamazaki T, Dechanet-Merville J, Kroemer G, Zitvogel L. Harnessing gammadelta T cells in anticancer immunotherapy. Trends Immunol 2012; 33: 199–206. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Kalyan S, Oberg HH, Wesch D. Human Vdelta2 versus non-Vdelta2 gammadelta T cells in antitumor immunity. Oncoimmunology 2013; 2: e23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont V, Sanchez F, Laprevotte E, Michaud HA, Gros L, Eliaou JF et al. Plasticity of gammadelta T Cells: impact on the anti-tumor response. Front Immunol 2014; 5: 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol 2015; 15: 683–691. [DOI] [PubMed] [Google Scholar]
- Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol 2007; 66: 320–328. [DOI] [PubMed] [Google Scholar]
- Kuhl AA, Pawlowski NN, Grollich K, Blessenohl M, Westermann J, Zeitz M et al. Human peripheral gammadelta T cells possess regulatory potential. Immunology 2009; 128: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wu P, Wu X, Ye J, Wang Z, Zhao S et al. Ex vivo expanded human circulating Vdelta1 gammadeltaT cells exhibit favorable therapeutic potential for colon cancer. Oncoimmunology 2015; 4: e992749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wu D, Ni C, Ye J, Chen W, Hu G et al. GammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014; 40: 785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C, Tambussi G, Ferrini S, Ciccone E, Varese P, Mingari MC et al. Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J Exp Med 1988; 168: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesch D, Hinz T, Kabelitz D. Analysis of the TCR Vgamma repertoire in healthy donors and HIV-1-infected individuals. Int Immunol 1998; 10: 1067–1075. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 2013; 13: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legut M, Cole DK, Sewell AK. The promise of gammadelta T cells and the gammadelta T cell receptor for cancer immunotherapy. Cell Mol Immunol 2015; 12: 656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel MS, Yssel H, Brocklehurst C, Spits H. A distinct wave of human T cell receptor gamma/delta lymphocytes in the early fetal thymus: evidence for controlled gene rearrangement and cytokine production. J Exp Med 1990; 172: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett 2001; 509: 317–322. [DOI] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003; 197: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Izumi T, Fujieda N, Kondo A, Morishita T, Matsushita H et al. Expansion of human peripheral blood gammadelta T cells using zoledronate. J Vis Exp 2011, 3791–3182. [DOI] [PMC free article] [PubMed]
- Allison TJ, Winter CC, Fournie JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature 2001; 411: 820–824. [DOI] [PubMed] [Google Scholar]
- Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 2005; 22: 71–80. [DOI] [PubMed] [Google Scholar]
- Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 2014; 40: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 2012; 120: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Henry O, Distefano MD, Wang YC, Raikkonen J, Monkkonen J et al. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells. J Immunol 2013; 191: 1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Chen H, Mo C, Cui L, He W. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J Biol Chem 2012; 287: 16812–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg HH et al. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand J Immunol 2009; 70: 245–255. [DOI] [PubMed] [Google Scholar]
- Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol 2005; 175: 2144–2151. [DOI] [PubMed] [Google Scholar]
- Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur J Immunol 2009; 39: 1361–1368. [DOI] [PubMed] [Google Scholar]
- Morita CT, Li H, Lamphear JG, Rich RR, Fraser JD, Mariuzza RA et al. Superantigen recognition by gammadelta T cells: SEA recognition site for human Vgamma2 T cell receptors. Immunity 2001; 14: 331–344. [DOI] [PubMed] [Google Scholar]
- Kalyan S, Chow AW. Human peripheral gammadelta T cells potentiate the early proinflammatory cytokine response to staphylococcal toxic shock syndrome toxin-1. J Infect Dis 2004; 189: 1892–1896. [DOI] [PubMed] [Google Scholar]
- De Paoli P, Gennari D, Martelli P, Basaglia G, Crovatto M, Battistin S et al. A subset of gamma delta lymphocytes is increased during HIV-1 infection. Clin Exp Immunol 1991; 83: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med 2000; 191: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature 1989; 341: 447–450. [DOI] [PubMed] [Google Scholar]
- Faure F, Jitsukawa S, Miossec C, Hercend T. CD1c as a target recognition structure for human T lymphocytes: analysis with peripheral blood gamma/delta cells. Eur J Immunol 1990; 20: 703–706. [DOI] [PubMed] [Google Scholar]
- Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol 2012; 42: 2505–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT et al. CD1d-lipid antigen recognition by the gammadelta TCR. Nat Immunol 2013; 14: 1137–1145. [DOI] [PubMed] [Google Scholar]
- Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D et al. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity 2013; 39: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998; 279: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci USA 2011; 108: 2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res 2004; 64: 9172–9179. [DOI] [PubMed] [Google Scholar]
- Catellani S, Poggi A, Bruzzone A, Dadati P, Ravetti JL, Gobbi M et al. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood 2007; 109: 2078–2085. [DOI] [PubMed] [Google Scholar]
- Maeurer M, Zitvogel L, Elder E, Storkus WJ, Lotze MT. Human intestinal V delta 1+ T cells obtained from patients with colon cancer respond exclusively to SEB but not to SEA. Nat Immun 1995; 14: 188–197. [PubMed] [Google Scholar]
- Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J Exp Med 2011; 208: 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011; 118: 992–1001. [DOI] [PubMed] [Google Scholar]
- Kenna T, Golden-Mason L, Norris S, Hegarty JE, O'Farrelly C, Doherty DG. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin Immunol 2004; 113: 56–63. [DOI] [PubMed] [Google Scholar]
- Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 2010; 116: 2164–2172. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Hinz T, Dobmeyer T, Mentzel U, Marx S, Bohme A et al. Clonal expansion of Vgamma3/Vdelta3-expressing gammadelta T cells in an HIV-1/2-negative patient with CD4 T-cell deficiency. Br J Haematol 1997; 96: 266–271. [DOI] [PubMed] [Google Scholar]
- Bartkowiak J, Kulczyck-Wojdala D, Blonski JZ, Robak T. Molecular diversity of gammadelta T cells in peripheral blood from patients with B-cell chronic lymphocytic leukaemia. Neoplasma 2002; 49: 86–90. [PubMed] [Google Scholar]
- Mangan BA, Dunne MR, O'Reilly VP, Dunne PJ, Exley MA, O'Shea D et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vdelta3 T cells. J Immunol 2013; 191: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T et al. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol 2012; 13: 872–879. [DOI] [PubMed] [Google Scholar]
- Wang L, Xu M, Wang C, Zhu L, Hu J, Chen S et al. The feature of distribution and clonality of TCR gamma/delta subfamilies T cells in patients with B-cell non-Hodgkin lymphoma. J Immunol Res 2014; 2014: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of gammadelta T-cell subsets in mouse and human. Immunology 2012; 136: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K et al. Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J Immunol 2007; 178: 4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cell Immunol 2001; 212: 110–117. [DOI] [PubMed] [Google Scholar]
- Garcia VE, Sieling PA, Gong J, Barnes PF, Uyemura K, Tanaka Y et al. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J Immunol 1997; 159: 1328–1335. [PubMed] [Google Scholar]
- Urban EM, Li H, Armstrong C, Focaccetti C, Cairo C, Pauza CD. Control of CD56 expression and tumor cell cytotoxicity in human Vgamma2Vdelta2 T cells. BMC Immunol 2009; 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thedrez A, Harly C, Morice A, Salot S, Bonneville M, Scotet E. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human V gamma 9V delta 2 T cells for adoptive immunotherapy. J Immunol 2009; 182: 3423–3431. [DOI] [PubMed] [Google Scholar]
- Ribot JC, Ribeiro ST, Correia DV, Sousa AE, Silva-Santos B. Human gammadelta thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J Immunol 2014; 192: 2237–2243. [DOI] [PubMed] [Google Scholar]
- Caccamo N, Battistini L, Bonneville M, Poccia F, Fournie JJ, Meraviglia S et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol 2006; 177: 5290–5295. [DOI] [PubMed] [Google Scholar]
- Devilder MC, Maillet S, Bouyge-Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol 2006; 176: 1386–1393. [DOI] [PubMed] [Google Scholar]
- Collins RA, Werling D, Duggan SE, Bland AP, Parsons KR, Howard CJ. Gammadelta T cells present antigen to CD4+ alphabeta T cells. J Leukoc Biol 1998; 63: 707–714. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005; 309: 264–268. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA 2009; 106: 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29: 621–663. [DOI] [PubMed] [Google Scholar]
- Bansal RR, Mackay CR, Moser B, Eberl M. IL-21 enhances the potential of human gammadelta T cells to provide B-cell help. Eur J Immunol 2012; 42: 110–119. [DOI] [PubMed] [Google Scholar]
- Caccamo N, Todaro M, La Manna MP, Sireci G, Stassi G, Dieli F. IL-21 regulates the differentiation of a human gammadelta T cell subset equipped with B cell helper activity. PLoS One 2012; 7: e41940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A et al. Cutting edge: TGF-beta1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol 2009; 183: 3574–3577. [DOI] [PubMed] [Google Scholar]
- Hua F, Kang N, Gao YA, Cui LX, Ba DN, He W. Potential regulatory role of in vitro-expanded Vdelta1 T cells from human peripheral blood. Immunol Res 2013; 56: 172–180. [DOI] [PubMed] [Google Scholar]
- Ye J, Ma C, Wang F, Hsueh EC, Toth K, Huang Y et al. Specific recruitment of gammadelta regulatory T cells in human breast cancer. Cancer Res 2013; 73: 6137–6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007; 27: 334–348. [DOI] [PubMed] [Google Scholar]
- Hou L, Wang T, Sun J. Gammadelta T cells in infection and autoimmunity. Int Immunopharmacol 2015; 2: 887–891. [DOI] [PubMed] [Google Scholar]
- Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G et al. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood 2011; 118: 129–138. [DOI] [PubMed] [Google Scholar]
- Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells. Proc Natl Acad Sci USA 2012; 109: 17549–17554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol 2010; 184: 7268–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna TJ, Davidson SI, Duan R, Bradbury LA, McFarlane J, Smith M et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive gamma/delta T cells in patients with active ankylosing spondylitis. Arthritis Rheum 2012; 64: 1420–1429. [DOI] [PubMed] [Google Scholar]
- Lamb LS Jr, Henslee-Downey PJ, Parrish RS, Godder K, Thompson J, Lee C et al. Increased frequency of TCR gamma delta+T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother 1996; 5: 503–509. [DOI] [PubMed] [Google Scholar]
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015; 21: 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fang Z, Morita CT, Vgamma2Vdelta2 T. Cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol 2010; 184: 6209–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girlanda S, Fortis C, Belloni D, Ferrero E, Ticozzi P, Sciorati C et al. MICA expressed by multiple myeloma and monoclonal gammopathy of undetermined significance plasma cells Costimulates pamidronate-activated gammadelta lymphocytes. Cancer Res 2005; 65: 7502–7508. [DOI] [PubMed] [Google Scholar]
- Lanca T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood 2010; 115: 2407–2411. [DOI] [PubMed] [Google Scholar]
- Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood 2009; 114: 310–317. [DOI] [PubMed] [Google Scholar]
- von Lilienfeld-Toal M, Nattermann J, Feldmann G, Sievers E, Frank S, Strehl J et al. Activated gammadelta T cells express the natural cytotoxicity receptor natural killer p 44 and show cytotoxic activity against myeloma cells. Clin Exp Immunol 2006; 144: 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR et al. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res 2008; 14: 4232–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, So HF et al. V gamma 9V delta 2T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs—rituximab and trastuzumab. Int J Cancer 2008; 122: 2526–2534. [DOI] [PubMed] [Google Scholar]
- Seidel UJ, Vogt F, Grosse-Hovest L, Jung G, Handgretinger R, Lang P. gammadelta T cell-mediated antibody-dependent cellular cytotoxicity with CD19 antibodies assessed by an impedance-based label-free real-time cytotoxicity assay. Front Immunol 2014; 5: 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 2009; 113: 4875–4884. [DOI] [PubMed] [Google Scholar]
- Braza MS, Klein B, Fiol G, Rossi JF. gammadelta T-cell killing of primary follicular lymphoma cells is dramatically potentiated by GA101, a type II glycoengineered anti-CD20 monoclonal antibody. Haematologica 2011; 96: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capietto AH, Martinet L, Fournie JJ. Stimulated gammadelta T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol 2011; 187: 1031–1038. [DOI] [PubMed] [Google Scholar]
- Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vgamma9Vdelta2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunol Immunother 2010; 59: 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 2010; 116: 1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiders FL, Prodohl J, Ruben JM, O'Toole T, Scheper RJ, Bonneville M et al. CD1d-restricted antigen presentation by Vgamma9Vdelta2-T cells requires trogocytosis. Cancer Immunol Res 2014; 2: 732–740. [DOI] [PubMed] [Google Scholar]
- Dechanet J, Merville P, Berge F, Bone-Mane G, Taupin JL, Michel P et al. Major expansion of gammadelta T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J Infect Dis 1999; 179: 1–8. [DOI] [PubMed] [Google Scholar]
- Puig-Pey I, Bohne F, Benitez C, Lopez M, Martinez-Llordella M, Oppenheimer F et al. Characterization of gammadelta T cell subsets in organ transplantation. Transpl Int 2010; 23: 1045–1055. [DOI] [PubMed] [Google Scholar]
- Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 2008; 112: 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 2005; 201: 1567–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzi L, Levaillant Y, Jamai A, Pitard V, Lassalle R, Martin K et al. Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol 2010; 21: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S et al. GammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 2013; 27: 1328–1338. [DOI] [PubMed] [Google Scholar]
- Knight A, Arnouk H, Britt W, Gillespie GY, Cloud GA, Harkins L et al. CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vdelta1+ gammadelta T cells. PLoS One 2013; 8: e68729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett NJ, Ashiru O, Morgan FJ, Pang Y, Okecha G, Eagle RA et al. Intracellular sequestration of the NKG2D ligand ULBP3 by human cytomegalovirus. J Immunol 2010; 185: 1093–1102. [DOI] [PubMed] [Google Scholar]
- Rolle A, Mousavi-Jazi M, Eriksson M, Odeberg J, Soderberg-Naucler C, Cosman D et al. Effects of human cytomegalovirus infection on ligands for the activating NKG2D receptor of NK cells: up-regulation of UL16-binding protein (ULBP)1 and ULBP2 is counteracted by the viral UL16 protein. J Immunol 2003; 171: 902–908. [DOI] [PubMed] [Google Scholar]
- Devaud C, Rousseau B, Netzer S, Pitard V, Paroissin C, Khairallah C et al. Anti-metastatic potential of human Vdelta1(+) gammadelta T cells in an orthotopic mouse xenograft model of colon carcinoma. Cancer Immunol Immunother 2013; 62: 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeurer MJ, Martin D, Walter W, Liu K, Zitvogel L, Halusczcak K et al. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med 1996; 183: 1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia M, Ellebaek E, Andersen MH, Straten PT, Svane IM. Analysis of Vdelta1 T cells in clinical grade melanoma-infiltrating lymphocytes. Oncoimmunology 2012; 1: 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A, Toia F, La Mendola C, Orlando V, Meraviglia S, Rinaldi G et al. Characterization of human gammadelta T lymphocytes infiltrating primary malignant melanomas. PLoS One 2012; 7: e49878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA 1999; 96: 6879–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Niu H, He W, Ba D. Antitumor activity of expanded human tumor-infiltrating gammadelta T lymphocytes. Int Arch Allergy Immunol 2001; 125: 256–263. [DOI] [PubMed] [Google Scholar]
- Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F. Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol 1995; 154: 3932–3940. [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Pupa SM, Mernard S, Zocchi R. Killing of laminin receptor-positive human lung cancers by tumor infiltrating lymphocytes bearing gammadelta(+) t-cell receptors. J Natl Cancer Inst 1996; 88: 436–441. [DOI] [PubMed] [Google Scholar]
- Kitayama J, Atomi Y, Nagawa H, Kuroda A, Mutoh T, Minami M et al. Functional analysis of TCR gamma delta+ T cells in tumour-infiltrating lymphocytes (TIL) of human pancreatic cancer. Clin Exp Immunol 1993; 93: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol 2012; 189: 5029–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol 2010; 40: 1927–1937. [DOI] [PubMed] [Google Scholar]
- Wu D, Wu P, Huang Q, Liu Y, Ye J, Huang J. Interleukin-17: a promoter in colorectal cancer progression. Clin Dev Immunol 2013; 2013: 436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos B. Promoting angiogenesis within the tumor microenvironment: the secret life of murine lymphoid IL-17-producing gammadelta T cells. Eur J Immunol 2010; 40: 1873–1876. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522: 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rei M, Goncalves-Sousa N, Lanca T, Thompson RG, Mensurado S, Balkwill FR et al. Murine CD27(-) Vgamma6(+) gammadelta T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci USA 2014; 111: E3562–E3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA et al. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol 2013; 190: 2403–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol 2006; 18: 539–546. [DOI] [PubMed] [Google Scholar]
- Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y, Felizardo TC et al. Human Vdelta1 gammadelta T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy 2011; 13: 753–764. [DOI] [PubMed] [Google Scholar]
- Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 2012; 14: 1110–1118. [DOI] [PubMed] [Google Scholar]
- Mao Y, Yin S, Zhang J, Hu Y, Huang B, Cui L et al. A new effect of IL-4 on human gammadelta T cells: promoting regulatory Vdelta1 T cells via IL-10 production and inhibiting function of Vdelta2 T cells. Cell Mol Immunol 2016; 13: 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AR, Correia DV, Fernandes-Platzgummer A, da Silva CL, Gomes da Silva M, Anjos DR et al. Delta One T cells for immunotherapy of chronic lymphocytic leukemia: clinical-grade expansion/ differentiation and preclinical proof-of-concept. Clin Cancer Res 2016. pii: clincanres.0597. [DOI] [PubMed]
- Rei M, Pennington DJ, Silva-Santos B. The emerging protumor role of gammadelta T lymphocytes: implications for cancer immunotherapy. Cancer Res 2015; 75: 798–802. [DOI] [PubMed] [Google Scholar]