Abstract
Species engaged in multiple, simultaneous mutualisms are subject to trade-offs in their mutualistic investment if the traits involved in each interaction are overlapping, which can lead to conflicts and affect the longevity of these associations. We investigate this issue via a tripartite mutualism involving an ant plant, two competing ant species and a fungus the ants cultivate to build galleries under the stems of their host plant to capture insect prey. The use of the galleries represents an innovative prey capture strategy compared with the more typical strategy of foraging on leaves. However, because of a limited worker force in their colonies, the prey capture behaviour of the ants results in a trade-off between plant protection (i.e. the ants patrol the foliage and attack intruders including herbivores) and ambushing prey in the galleries, which has a cascading effect on the fitness of all of the partners. The quantification of partners' traits and effects showed that the two ant species differed in their mutualistic investment. Less investment in the galleries (i.e. in fungal cultivation) translated into more benefits for the plant in terms of less herbivory and higher growth rates and vice versa. However, the greater vegetative growth of the plants did not produce a positive fitness effect for the better mutualistic ant species in terms of colony size and production of sexuals nor was the mutualist compensated by the wider dispersal of its queens. As a consequence, although the better ant mutualist is the one that provides more benefits to its host plant, its lower host–plant exploitation does not give this ant species a competitive advantage. The local coexistence of the ant species is thus fleeting and should eventually lead to the exclusion of the less competitive species.
Keywords: allomerus, ant–plant–fungus interaction, dispersal, Hirtella, mutualism, species coexistence
1. Introduction
Most mutualistic interactions involve multiple partners that can compete for the same resource and/or interact synergistically, enhancing the benefits for all [1]. Evaluating mutualisms in a community context thus appears important to understanding the maintenance, stability and dynamics of these interactions [2,3]. Such understanding amounts to quantifying both the net outcomes arising from the mutualistic traits and the population dynamics of the partners [4,5]. The conceptual framework provided by the consumer–resource approach enabled mutualisms to be considered exchanges of services and rewards in which competition and population dynamics are important drivers behind the evolutionary diversification and specialization of such interactions [6,7].
Obligate ant–plant mutualisms have proved to be interesting systems for studying issues related to species coexistence and exploitation [8–10]. They are quite diversified and share a common interaction pattern, with ant–plants (i.e. myrmecophytes) providing the ants with nesting cavities (i.e. domatia) and, in most cases, food through the production of extrafloral nectar and/or food bodies. In exchange, the ants protect the plant from herbivores, competitors and pathogens [11,12]. Ants can also provide their host myrmecophytes with nutrients through prey remains and faeces (i.e. myrmecotrophy) [13]. Although ant–plant symbioses are organized in compartmentalized networks, most often multiple ant species can interact with a given plant species rather than there being purely species-specific associations [14,15]. As a consequence, ant species are engaged in a competitive lottery for host occupation (i.e. there is no supplanting of already established colonies) and stable coexistence requires niche differentiation, dispersal limitation or the spatial structuring of populations [5,16,17]. Moreover, ant–plant symbioses also involve other mutualistic partners such as hemipterans or fungi [18,19]. Conflicts resulting from trade-offs in mutualistic investment or synergies can thus arise among all of these interacting species (see [20]).
Here, we investigate mutualistic traits and trade-offs in mutualistic investment and their consequences for the longevity of and species coexistence in a tripartite interaction involving a myrmecophyte, two ant species which compete to occupy its domatia and a fungus they cultivate. In French Guiana, Hirtella physophora (Chrysobalanaceae) is mainly associated with the ant Allomerus decemarticulatus (Myrmicinae) although, in some areas, Allomerus octoarticulatus competes for host–plant occupation. These two ant species also cultivate a specific fungus, Trimmatostroma sp. (Chaetothyriales), that they use to build galleries under the stems of their host plant to capture prey [21,22]. By interacting with plant tissues, the fungus also improves nutrient uptake by the host plant [23]. Yet, the use of galleries, as a derived and innovative strategy for prey capture versus the more typical foraging on leaves, is subject to a trade-off related to the limited worker force in the colonies. So, we hypothesized that variations in the ants' prey capture strategy might affect the outcomes obtained by the partners. To this end, we quantified the partners' traits and effects related to the interactions. The consequences of the variations in outcomes resulting from differential investment are discussed in terms of the longevity of the mutualism and how this can affect the coexistence of closely related ant species competing for the same host plant species.
2. Material and methods
(a). Study site and species
This study was conducted between 2008 and 2016 in a total of five areas of old growth, primary forest in French Guiana: La Montagne des Singes (MdS) near Kourou (05°04′21″ N, 052°04′44″ W), Saül (Sau) (03°36′36″ N, 053°11′58″ W), Petit Saut (PtS) (05°04′35″ N, 053°01′15″ W) and on two Hirtella populations located in the Kaw area (Camp Patawa (Pat) 04°32′27″ N, 052°09′03″ W and Chutes Fourgassier (Fou) 04°37′40″ N, 052°18′22″ W). The H. physophora populations studied differed in the ratio of their associated ant species (see details in the electronic supplementary material). Plant identification was confirmed by M.-F. Prévost after comparison with voucher specimens deposited at the Cayenne Herbarium (http://publish.plantnet-project.org/project/caypub), the ants were identified by J.H.C. Delabie at the Laboratorio de Mirmecologia, Cocoa Research Centre, Ilhéus, Bahia, Brazil where voucher specimens were deposited and the ITS-EF1α DNA sequences of the fungal haplotypes are accessible from GenBank (see [21]).
Hirtella physophora is a long-lived myrmecophyte that grows strictly in the understory of terrafirme Amazonian forests, mostly in patches located on the uppermost parts of hillsides [24,25]. Its leaves bear extrafloral nectaries and a pair of pouches (domatia) at the base of each lamina that house ant colonies [26].
At the mature stage of colony development, both A. decemarticulatus and A. octoarticulatus inhabit individual plants and each plant hosts a single colony. It should be noted that the A. octoarticulatus species found on H. physophora is a different species than the one associated with Cordia nodosa, although the two species cannot be distinguished based only on morphological differences (see the electronic supplementary material, figure S1). All Allomerus species are specialist plant–ants inhabiting a variety of myrmecophytic hosts [27]. Concerning the A. octoarticulatus and A. decemarticulatus species studied thus far, the workers protect their host plant from defoliators through their predatory behaviour [9,28,29]. On the other hand, these two ant species are also known to impose costs on their host plants through their castration behaviour, which favours the vegetative growth of the plant and thus the production of more nesting space [30–33].
(b). Quantification of the partners' traits and effects
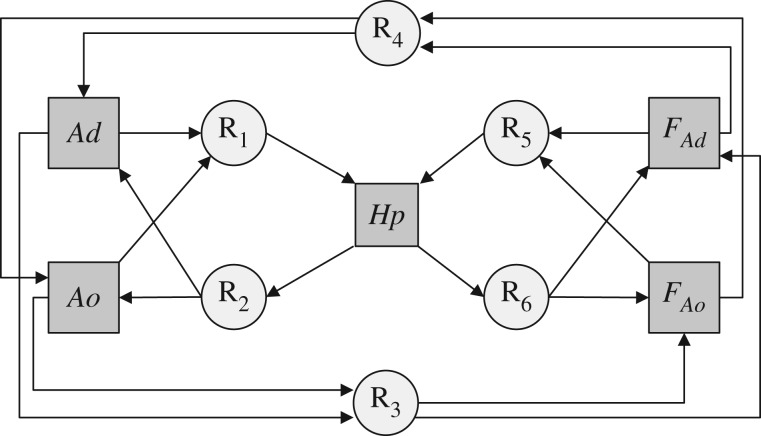
The interactions between H. physophora, Allomerus ants and their associated fungus can be summarized by a consumer–resource approach in which the partners exchange rewards such as resources and/or services (figure 1) [1]. The exchanged resources or services were quantified through the determination of species' traits and effects, including both positive and negative effects. Trait measurements and experiments were performed only on host plants hosting a single, mature ant colony to avoid any confounding factor owing to the former or simultaneous presence of another ant species.
Figure 1.
Interactions and reward exchanges between the host plant, the ants and their associated fungus. Grey squares and white circles indicate the focal species and the rewards (R1–R6), respectively. Ad, Allomerus decemarticulatus; Ao, A. octoarticulatus; Hp, Hirtella physophora; FAd and FAo, A. decemarticulatus- and A. octoarticulatus-associated fungus, respectively.
R1, which represents the net effects of ant symbionts on their host plant (figure 1), was assessed via measurements of standing herbivory, ant patrolling activity on leaves, the time needed to discover an alien insect and castration intensity (table 1).
Table 1.
Ant, plant and fungal traits measured and their relationships with the exchanged resources or services (figure 1). (Sampled populations and the number of individuals are provided. MdS, Montagne des Singes; Fou, Fourgassier; Pat, Patawa; Sau, Saül; PtS, Petit Saut; Ad, Allomerus decemarticulatus; Ao, Allomerus octoarticulatus.)
| rewards | traits | population | no. individuals (Ad/Ao) |
|---|---|---|---|
| R1 | standing herbivory | MdS, Fou, Pat, Sau, PtS | 584 (398/186) |
| R1 | patrolling intensity | MdS | 26 (13/13) |
| R1 | time to discover insects | MdS | 26 (13/13) |
| R1 | castration intensity | MdS | 26 (13/13) |
| R2 | vegetative growth | MdS | 26 (13/13) |
| R2 | flowering effort | MdS | 26 (13/13) |
| R2 | domatia volume/leaf area | MdS, Fou, Pat, Sau, PtS | 478 (296/182) |
| R2 | ant colony size | MdS, PtS | 65 (35/30) |
| R2 | attraction to new leaves | MdS, PtS | 40 (20/20) |
| R3 | gallery construction | MdS, Fou, Pat, Sau, PtS | 584 (398/186) |
| R3 | fungal nutrition | MdS | 14 (7/7) |
| R4 | gallery use | MdS | 30 (15/15) |
| R5 | plant nutrition | MdS | 14 (7/7) |
| R6 | fungal nutrition | MdS | 14 (7/7) |
Standing herbivory was determined as the mean percentage of herbivory on the two to three most recently mature leaves. Expanding leaves are the most vulnerable to herbivores [29] and thus quantifying herbivory at their next developmental stage provides a good estimation of the impact of herbivores and thus ant protection, without it being necessary to assess herbivory rates. Herbivory was estimated as a categorical variable with five categories: 0 = no herbivory, 1 = less than 10% of the leaf area lost, 2 = between 10 and 25%, 3 = between 25% and 50% and 4 = more than 50% of the leaf area lost. Statistical comparisons were conducted using generalized linear mixed modelling (GLMM) with plant identification (ID) nested within population as a random effect.
Ant patrolling activity was determined by counting the number of ant workers patrolling on both sides of two mature, but still tender, leaves per plant between 13.00 and 15.00, a timeframe that corresponds to the species' main period of activity [29,34]. Statistical comparisons were conducted, using GLMM with plant ID as a random effect.
The time needed to locate alien arthropods on the leaves was assessed, using soldiers of the termite, Cavitermes tuberosus. One individual was gently deposited in the middle of one leaf per plant halfway between the central vein and the leaf margin, and the time it took to be discovered by a worker was recorded. Putative differences in data dispersion between the two ant species were assessed using the Ansari–Bradley test, a non-parametric alternative to the F-test.
The castration intensity was quantified by comparing the flower-to-bud and fruit-to-flower ratios. The buds, flowers in bloom and fruits per plant were counted each week during one period of reproduction (January–June 2009). Comparisons between the two ant species were made using generalized linear models (GLM) with the number of flowers/fruits per inflorescence considered the ‘successes’ and the initial number of buds/flowers minus the number of flowers/fruits the ‘failures’ in a binomial distribution.
R2 represents the rewards the host plant provides to the ant colonies; i.e. the myrmecophytic traits (figure 1). The plant provides the ants with domatia and extrafloral nectar. Because the hosting capacity of the plant is influenced by domatia volume rather than by the number or surface area of the extrafloral nectaries [35], we focused only on the former trait, as well as on vegetative growth (i.e. the number of domatia) and the size and structure of the ant colonies (table 1). Plant reproduction was also quantified by comparing the flower-to-bud and fruit-to-flower ratios from data on castration intensity using GLM.
Vegetative growth was determined by recording the number of new leaves produced each month during nine months. Comparisons between plants inhabited by either A. decemarticulatus or A. octoarticulatus were made using the Mann–Whitney rank test (MW) for unpaired data.
Domatia volume (mm3) was estimated from measurements made on the two to three most recently mature leaves per plant and based on the volume of an ellipsoid for which the three elliptic radii were equal to the length, width and height of the domatium. Leaf area (LA, cm2) was calculated for the same leaves using leaf length (L) and width (W) using the formula: LA = 0.79689 (LW) − 3.1303. This formula derives from the relationships between LA, L and W previously determined from digital pictures of 126 H. physophora leaves of various sizes using ImageJ software. Statistical comparisons were conducted using GLMM with plant ID nested within population as a random effect.
The size and structure of the ant colonies were calculated from 30 different A. octoarticulatus colonies, whereas data for the A. decemarticulatus colonies were taken from Orivel et al. [24]. All of the leaves and stems on each plant individual were cut off and placed into a plastic bag, and then preserved in 70% ethanol. Each domatium was then dissected, and the number of workers and sexuals was recorded. The relationships between the total number of workers or sexuals and the number of domatia were assessed using linear regressions. Linear models were forced to the origin in order to fit the biological assumption (i.e. when the plants bear no domatium, the number of workers or sexuals is thought to be null). Interspecific differences in the number of workers and sexuals per colony were determined by an ANCOVA, using the number of domatia as the covariate.
In addition, we tested the chemical basis of the biotic protection by quantifying the ant behaviour towards hexane extracts from the leaf surface, fractions and synthetic compounds. Details on these methods are presented in the electronic supplementary material. Briefly, groups of 30 ant workers were confronted with the extract and a control (hexane) placed on pieces of filter paper and the number of workers in contact with the paper was scored each minute for 20 min.
R3 corresponds to the rewards provided by the ants to the fungus and was investigated in terms of gallery construction and nutrition (figure 1). The investment in gallery construction by the two ant species was measured as the proportion of host–plant stems covered by galleries. It was estimated as a categorical variable with four categories: 1, less than 20% of the stems covered by galleries; 2, between 20% and 50%; 3, between 50% and 80% and 4, more than 80%. Statistical comparisons were conducted using GLMM with plant ID nested within population as a random effect.
Nutrient transfer from the ants to the fungus and the plant was investigated via the quantification of the natural abundances of δ13C and δ15N and 15N-labelling for neighbouring individuals from a single population (MdS) where the two ant species occur in sympatry, thus avoiding site-related variations in isotopic signatures. Details on the methods of the experimental labelling are presented in the electronic supplementary material. For natural abundances of δ13C and δ15N, samples of each associated partner were collected before the 15N enrichment procedure (i.e. negative controls) and processed and analysed the same way as were the enriched samples.
R4 is the rewards provided by the fungus to the ants (figure 1), and it was quantified via the use of the galleries by determining the percentage of ant-occupied holes. To do so, between 13.00 and 15.00, we took pictures of the part of the gallery located at the first basal branch and then counted the number of empty and ant-occupied holes for each picture (mean number of holes ± s.e. = 54.5 ± 20.4). The percentages of ant-occupied holes were compared between ant species using GLM, with the presence of ants considered the ‘successes’ and their absence the ‘failures’ in a binomial distribution.
R5 corresponds to the rewards provided by the fungus to the plant (figure 1). Leroy et al. [23] showed that the A. decemarticulatus-associated fungus acts as a trophic mediator, and we thus investigated the nutrient transfer from the fungus to the plant in the same manner as for R3 (see the electronic supplementary material).
R6 are the rewards provided by the plant to the fungus (figure 1). The substrate on which the fungus grows includes plant tissues chewed by the ants on the inner side of domatia, suggesting potential nutritional benefits (i.e. carbon) for the fungus. However, the experimental enrichments of the plants in 13C did not succeed, so that no estimation of the trophic fluxes could be made. Nevertheless, the natural abundances of δ13C provided some insights into the use of plant tissues as a carbon source by the fungus.
(c). Experimental host–plant colonization
The two ant species are competing for the occupation of the same host plant species, so that their coexistence at the population level depends on both the colonization and dispersal abilities of the founding queens. Colonization abilities were investigated by determining the identity of the founding queens that colonized plants in the field, whereas dispersal abilities were assessed by measuring their alitrunk volume (see below).
Ten H. physophora from which the ant colonies were removed were successfully transferred and placed in the Montagne des Singes area where both Allomerus species are present. Note that winged sexuals are produced throughout the year in these species (J. Orivel 2003–2015, personal observation). After eight weeks, we opened every domatia and collected every founding queen (n = 26).
In addition, a snapshot sampling of founding queens was conducted in the same area. Based on a previous census, the plants devoid of mature ant colonies were sampled for the presence of founding queens. Only queens having recently colonized plants were considered along with incipient colonies. A total of 118 founding queens were collected.
If workers were already present, then the species ID was determined morphologically by counting the number of antennal segments; otherwise, the queens were assigned to A. decemarticulatus or A. octoarticulatus after amplification of a 608 bp fragment of the cytochrome oxidase subunit I (COI) with primers LCO1490 and HCO2198 [36]. DNA extraction or amplification failed in six and four samples from the recolonization experiment and the snapshot survey, respectively.
(d). Dispersal abilities
The dispersal abilities of the founding queens of both Allomerus species were estimated from 18 A. decemarticulatus and 16 A. octoarticulatus queens collected during the snapshot survey of host plants by measuring their alitrunk volume as a proxy of wing muscle mass. The volumes were estimated by considering the alitrunks as rectangular parallelepipeds with length, width and depth as the edges. Measurements were performed with a stereomicroscope equipped with an ocular micrometer. Differences in alitrunk volume between the two species were assessed using GLM.
All statistical analyses were conducted using R [37]. GLMM was conducted with the lmer function in the lme4 package [38]. When needed, Shapiro–Wilk, Breusch–Pagan and Durbin–Watson tests were conducted to test for the normality of the residuals, homoscedasticity and non-autocorrelation, respectively, using functions in the lmtest package [39].
3. Results
(a). Ant effects on plant traits
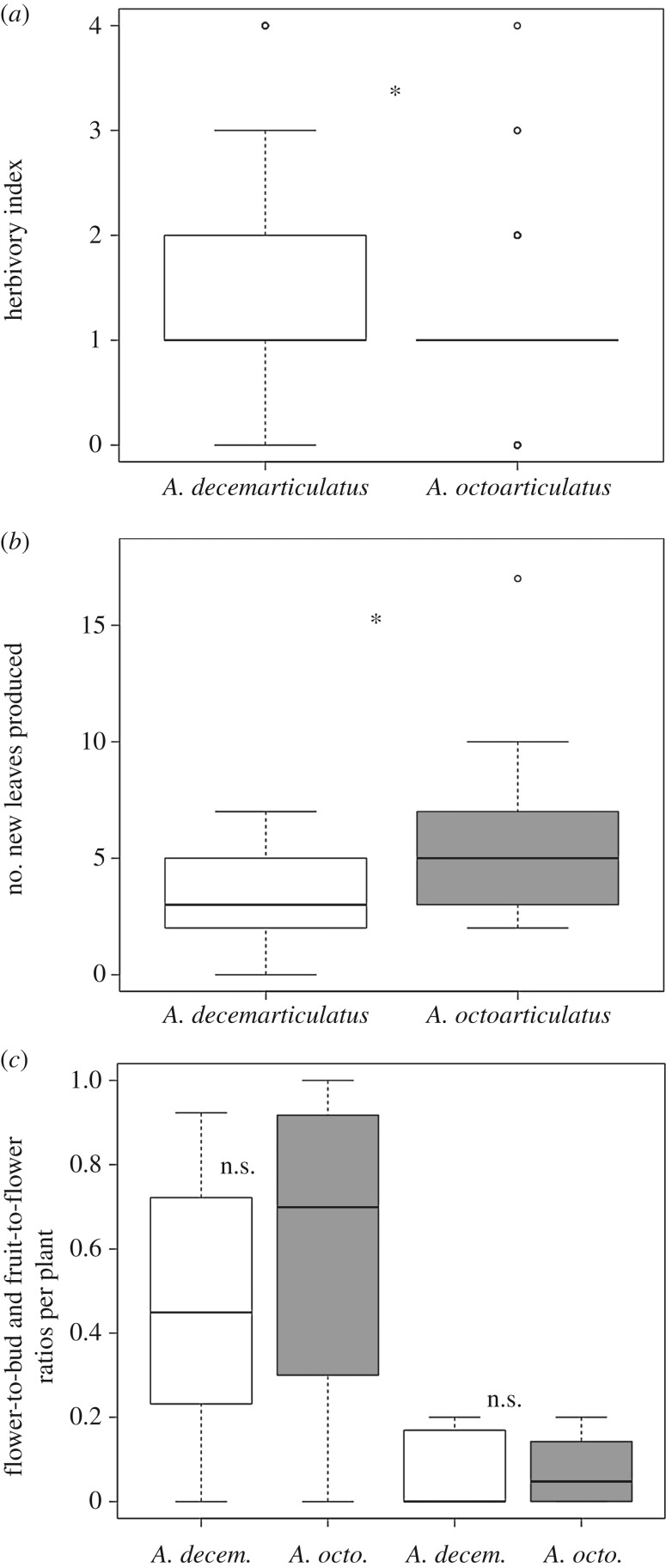
The plants inhabited by A. octoarticulatus were less prone to phytophagy than were those inhabited by A. decemarticulatus (figure 2a; GLMM, ant species (A. octoarticulatus): estimate =−0.47, s.e. = 0.07, z-value = −6.38, p < 0.0001). No difference among populations was able to be highlighted. Moreover, those plants associated with A. octoarticulatus produced significantly more leaves than did those associated with A. decemarticulatus (figure 2b; GLMM, ant species (A. octoarticulatus): estimate = 3.08, s.e. = 1.28, t-value = 2.39, p = 0.0249).
Figure 2.
(a) Ant efficiency in protecting Hirtella physophora leaves expressed as an index of herbivory. The index varies from 0 (no damage on the most recently mature leaves) to 5 (>50% herbivory). (b) Hirtella physophora leaf production during nine months depending on the associated ant species. (c) Impact of ant activity on the flowering and fruiting success of H. physophora expressed as flower-to-bud and fruit-to-flower ratios. Box and whisker plots represent, top to bottom, the 90th percentile, 75th percentile, median, 25th percentile and 10th percentile, and open circles indicate outliers. n.s. or asterisks indicate the absence or presence of a significant difference between species, respectively.
Both the flowering and fruiting successes of the plants were quite low but did not differ significantly according to the inhabiting ant species, highlighting the similar negative effect of the ants on the host's reproduction (figure 2c; GLMM, flowering success, ant species (A. octoarticulatus): estimate = 0.08, s.e. = 0.21, z-value = 0.41, p = 0.68; fruiting success, ant species (A. octoarticulatus): estimate = −0.97, s.e. = 0.52, z-value = −1.85, p = 0.06).
(b). Ant traits
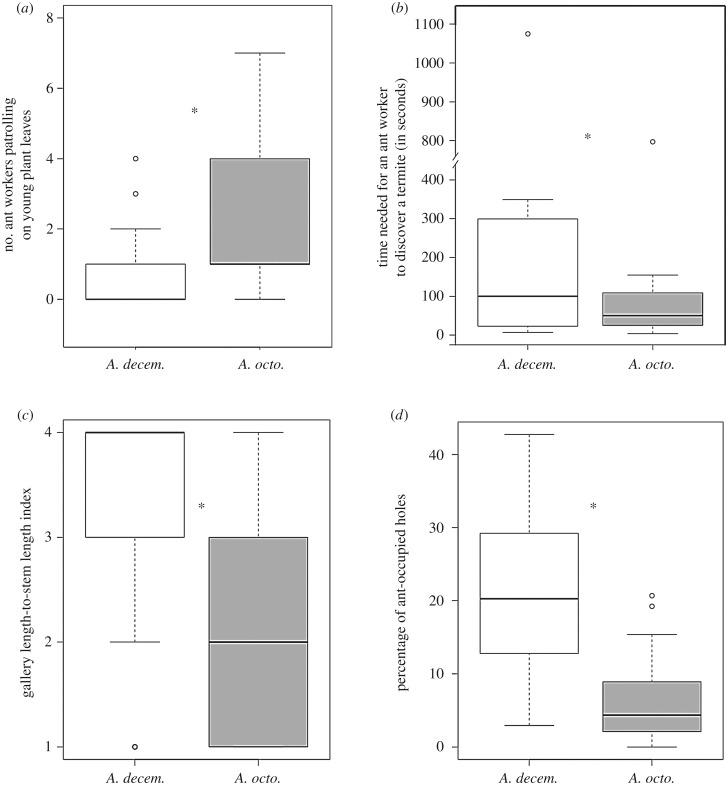
The number of workers patrolling the leaves was higher for A. octoarticulatus- than for A. decemarticulatus-inhabited plants (figure 3a; GLMM, ant species (A. octoarticulatus): estimate = 1.07, s.e. = 0.35, z-value = 3.09, p = 0.002). As a consequence, the time needed by A. octoarticulatus workers to find an alien arthropod on H. physophora foliage was shorter and less variable than for A. decemarticulatus (figure 3b; Ansari–Bradley test, p = 0.042).
Figure 3.
(a) Ant patrolling activity on Hirtella physophora leaves expressed as the number of workers per leaf. (b) Ant efficiency in discovering alien arthropods on H. physophora leaves expressed as the time needed for workers to find a termite. (c) Ant investment in gallery construction expressed as a gallery length-to-stem length index. The index varies from 1 (<20% of the stems covered by galleries) to 4 (>80%). (d) Gallery use by ants expressed as the percentage of ant-occupied holes. Box and whisker plots represent, top to bottom, the 90th percentile, 75th percentile, median, 25th percentile and 10th percentile, and open circles indicate outliers. ‘n.s.’ or asterisks indicate the absence or presence of a significant difference between ant species, respectively.
The proportion of host plant stem covered by galleries was lower for A. octoarticulatus- than for A. decemarticulatus-inhabited plants (figure 3c; GLMM, ant species (A. octoarticulatus): estimate = −1.87, s.e. = 0.08, z-value = −21.82, p < 0.001). Moreover, the proportion of holes occupied in these galleries was also lower for A. octoarticulatus than for A. decemarticulatus (figure 3d; GLMM, ant species (A. octoarticulatus): estimate = −1.54, s.e. = 0.18, z-value = −8.46, p < 0.001).
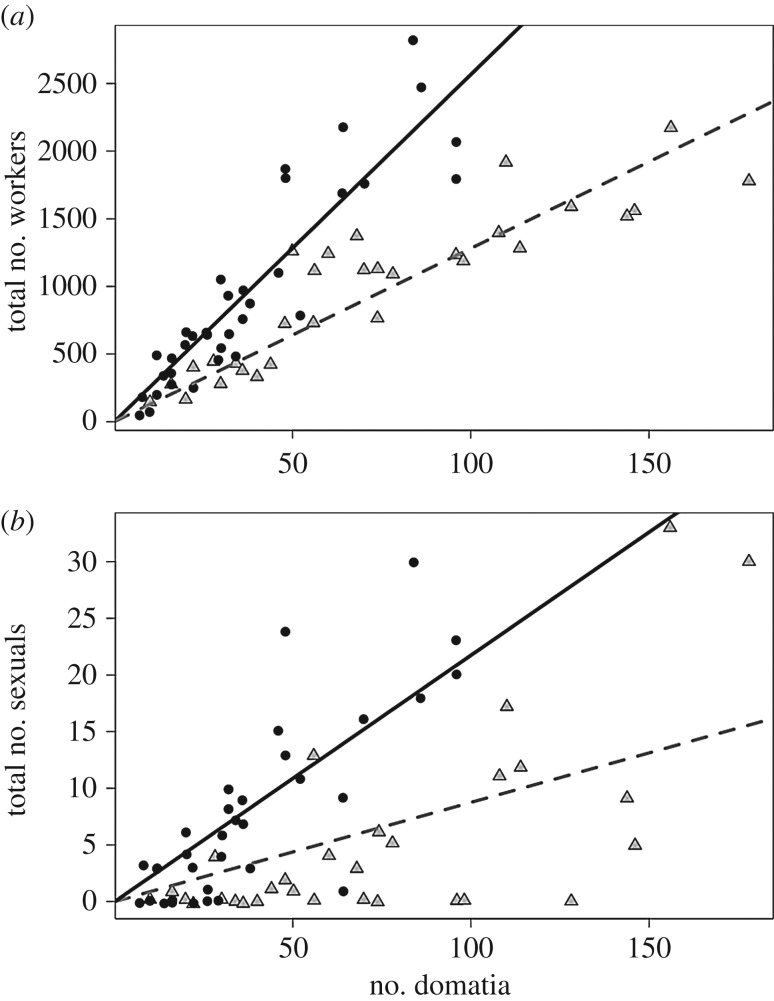
For each ant species, the total number of workers and sexuals per colony significantly increased with plant size (figure 4). The estimated regression slopes significantly differed for workers and sexuals (workers: ANCOVA, plant size/ant species F = 134.7, p < 0.0001; sexuals: ANCOVA, plant size/ant species F = 65.9, p < 0.0001). Moreover, both the number of workers per colony and ant species ID had significant effects on the number of sexuals produced (ANCOVA, F = 160.6, p < 0.0001 and F = 3.9, p = 0.026, respectively).
Figure 4.
(a) Linear regressions of the number of domatia on H. physophora by the total number of workers (a) or sexuals (b) for A. decemarticulatus (black circles) and A. octoarticulatus (grey triangles) colonies. For A. octoarticulatus, the linear regression is represented by a dotted grey line and, for A. decemarticulatus, the linear regression is represented by a continuous black line. Domatia number was positively related to the total number of workers or sexuals in the A. decemarticulatus and A. octoarticulatus colonies (workers: (A. octoarticulatus): y = 12.81x, R2 = 0.94, F = 511.9, p < 0.001; (A. decemarticulatus): y = 25.66x, R2 = 0.93, F = 497.3, p < 0.001; sexuals: (A. octoarticulatus): y = 0.09x, R2 = 0.56, F = 39.37, p < 0.001; (A. decemarticulatus): y = 0.22x, R2 = 0.80, F = 144.9, p < 0.001).
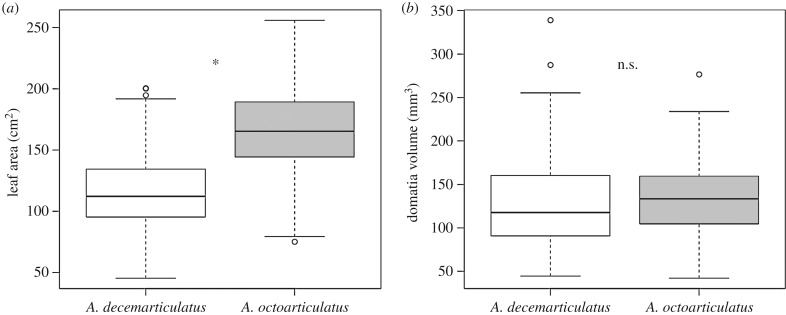
The leaf area of H. physophora was significantly larger for A. octoarticulatus- than for A. decemarticulatus-associated plants (figure 5a; GLMM, ant species (A. octoarticulatus): estimate = 1.17, s.e. = 0.10, z-value = 11.37, p < 0.0001). On the contrary, the identity of the associated ant species did not have any effect on domatia volume (figure 5b; GLMM, ant species (A. octoarticulatus): estimate = −0.14, s.e. = 0.10, z-value = −1.32, p = 0.184).
Figure 5.
(a) Leaf area (cm2) and (b) domatia volume (mm3) of Hirtella physophora leaves according to the associated ant species. Box and whisker plots represent, top to bottom, the 90th percentile, 75th percentile, median, 25th percentile and 10th percentile, and open circles indicate outliers. n.s. or asterisks indicate the absence or presence of a significant difference between ant species, respectively.
(c). Nutrient transfer
The natural abundances of 15N and 13C allowed the three partners to be clearly distinguished (figure 6). Allomerus octoarticulatus had significantly higher δ15N compared with A. decemarticulatus (MW, U = 8, p < 0.05). When associated with A. octoarticulatus, the host plant had significantly higher δ15N compared with those associated with A. decemarticulatus (MW, U = 11, p < 0.05). On the contrary, no difference was observed in the fungus when associated with either A. octoarticulatus or A. decemarticulatus (MW, U = 21, p = 0.710). Conversely, the δ13C values for the fungus were significantly higher when associated with A. octoarticulatus compared with when the fungus was associated with A. decemarticulatus (MW, U = 0, p < 0.001), whereas both the ants and the host plants showed similar values (A. octoarticulatus versus A. decemarticulatus: MW, U = 9, p = 0.053; A. octoarticulatus- versus A. decemarticulatus-associated plants: MW, U = 21, p = 0.279). While the percentage of total nitrogen did not differ significantly between the two ant species (MW, U = 35, p = 0.463, see the electronic supplementary material, figure S3), both the fungus and the host plant were richer in nitrogen when associated with A. octoarticulatus (fungus: MW, U = 1.5, p < 0.01; host plant: MW, U = 8, p < 0.05). No difference in the percentage of total carbon was observed.
Figure 6.
Natural abundance (mean ± s.e.) of δ15N and δ13C for each of the three partners involved in the interaction. Grey triangles represent the values for Allomerus octoarticulatus and its associated fungus and plant, and black circles represent the values for A. decemarticulatus and its associated fungus and plant. n.s. or asterisks indicate the absence or presence of a significant difference between species, respectively.
The 15N labelling experiment resulted in a significant increase in δ15N for all of the three associated partners compared with the negative controls (see details in the electronic supplementary material). No difference in 15N translocation existed between the two systems studied, demonstrating a similar functioning.
(d). Chemical basis of leaf protection
Crude leaf extracts from young H. physophora leaves were the only ones that attracted A. decemarticulatus workers, whereas A. octoarticulatus did not respond to any leaf extracts (see the electronic supplementary material, table S1). The fractionation of the crude extracts coupled with behavioural tests and gas chromatography–mass spectrometry analyses showed that α- and β-tocopherol were the only two putative candidates explaining the attractiveness of young leaves (see the electronic supplementary material for details). If the mixture of both compounds did not induce an ant response, when added to a crude extract from an old leaf (not attractive and not containing tocopherol), then the attractiveness for workers was highly significant (see the electronic supplementary material, table S4). Similar results were obtained with α-tocopherol only, whereas β-tocopherol alone with an extract from an old leaf did not appear to attract the ants.
(e). Host plant colonization and dispersal abilities
The experimental colonization of host plants enabled the collection of a total of 26 founding queens. While about 15% of the plants in the area were inhabited by A. octoarticulatus, all of the founding queens assigned to species (n = 20) were identified as A. decemarticulatus based on worker morphology or DNA sequence at the COI region. The snapshot survey of founding queens in the same area yielded a slightly different picture with 15.8% (18 out of 114) of the queens assigned to A. octoarticulatus either through morphological identification or barcoding, a ratio similar to that of mature colonies at the plant population level.
The alitrunk volume of founding queens did not differ between the two species (mean ± s.d.: 2.8565 × 10−3 ± 0.395 × 10−3 mm3 versus 2.8648 × 10−3 ± 0.224 × 10−3 mm3 for A. octoarticulatus and A. decemarticulatus, respectively; estimate = 8.344 × 10−6, s.e. = 1.121 × 10−4, t-value = 0.074, p = 0.94). As a consequence, it was inferred that both species have similar dispersal abilities.
4. Discussion
Altogether our results demonstrate that interspecific differences in partner quality induce variations in the outcomes experienced by the host plant. Such context dependency/conditionality in species interactions, whether mutualistic or not, has been documented in a number of studies, although those studies mainly focused on whether the outcomes were positive or negative (i.e. mutualistic or parasitic) [40,41]. Here we have shown that the emergence of an innovative trait (i.e. the building and use of galleries for prey capture) in ants translates into contrasting effects for their host plant. This trait being related both to the interaction with a third partner (i.e. the fungus) and to one of the key benefits for the host plant in ant–plant interactions (i.e. biotic protection), the observed conditionality results from a trade-off in partner investment. Finally, partner specificity, control over a mutualistic partner and competitive advantage are likely to act as regulating factors enabling both the interaction to remain mutualistic and the continued coexistence of the species.
(a). Ant traits and the consequences for partners' rewards
In ant–plant interactions, the protection of plants by ants is most often the indirect result of foraging and territorial activities on host leaves, which are by-products of the ‘selfish’ activities of the colonies [11,42]. Allomerus species have developed an alternative to this classic foraging behaviour, thanks to an investment in the construction of galleries they use to ambush prey and, in the species studied thus far, the galleries are reinforced by the mycelium of a specific fungus [21,22]. The differences in investment in gallery construction (and thus fungal cultivation) and in the use of the galleries induce variations in the outcomes experienced by the host plant. Indeed, because of a limited worker force in the ant colonies, both predation strategies (i.e. patrolling on leaves or ambushing in the galleries) are subject to a trade-off. The higher number of patrolling A. octoarticulatus workers significantly reduces the time needed to discover alien arthropods resulting in the better defence of the host plant compared with plants associated with A. decemarticulatus. Hence, these two contrasting actions produce different rewards for their hosts (R1 in figure 1). Nonetheless, it should be noted that, although its presence is less beneficial for the plant, A. decemarticulatus remains a mutualist, as the net outcome experienced by H. physophora from the interaction is positive. These ants also protect the foliage by patrolling on leaves, so that host plants have a better level of fitness when associated with A. decemarticulatus than when devoid of ants [24,29].
The fitness of both ant species (R2 in figure 1) should be qualitatively affected in a similar way by the differential growth rate of their host plant as their colony growth is highly correlated with plant size. However, because the limitations to colony growth and reproduction do not vary similarly between the ant species, there is no fitness gain from the greater vegetative growth of A. octoarticulatus-associated H. physophora. Such a difference in vegetative growth might also result from variations in plant castration intensity [8,31–33]. The flowering and fruiting successes were, however, similar, regardless of the associated ant species.
The absence of a fitness effect on A. decemarticulatus deriving from the lower biotic protection it provides to its host plant is probably the result of a better adaptation to the partner. First, the higher offspring production might result from physiological differences in the queens of both ant species and/or better food exploitation. Both species are of a similar size and show the same monoandrous and monogynous colony structure [43]. Moreover, they feed at the same trophic level with δ15N values of 4–6‰ that reflect their omnivorous diet [44], so that this difference in higher offspring production does not result from their diet. It seems then reasonable to affirm that the higher offspring production experienced by A. decemarticulatus compared with A. octoarticulatus is a direct consequence of the construction and use of a highly effective trap. Food supplementation and colony size are indeed two important factors in the higher production of sexual offspring [45]. Second, if A. octoarticulatus appears to be a specialist inhabitant of H. physophora locally, the species is a generalist over its distribution range (see the electronic supplementary material) and this, combined with the lack of attractiveness of the leaf extracts, speaks in favour of the better adaptation of A. decemarticulatus to its host plant. The role of tocopherol in inducing leaf patrolling in A. decemarticulatus can also be interpreted as a control mechanism developed by H. physophora, similar to the retaliation mechanism demonstrated by this system against floral destruction [35].
The greater investment in gallery construction by A. decemarticulatus has a positive effect on the growth of the associated fungus and thus on the rewards it obtains (R3). This also positively affects the rewards the fungus provides to the ants in terms of gallery length (R4). Moreover, experimental 15N enrichments highlighted a similar global functioning for both A. decemarticulatus- and A. octoarticulatus-associated partners. Under natural conditions, comparisons of δ13C and δ15N signatures highlighted variations in the trophic fluxes between each partner. Both the fungus and the plant showed significantly higher nitrogen contents when associated with A. octoarticulatus. Thus, A. octoarticulatus transfers more nitrogen to its associated fungus and, because the latter retains very little nitrogen and transfers most of it to the plant [23], the plant also benefits from the higher supply of nitrogen arising from the presence of the ants. Moreover, part of the nitrogen is thought to be cycling from exchanges between the partners, which might explain the higher δ15N values for A. octoarticulatus and its associated plants than for the A. decemarticulatus system (see also [46]). Such a difference in functioning is of importance as the higher nitrogen content in A. octoarticulatus-associated plants translates into phenotypic differences, with a higher leaf area compared with A. decemarticulatus-associated plants. Because leaf area is correlated with nutrient availability [47,48], these traits, among others, underline the performance of the plants (see [49]). It should be noted, however, that no variation in domatia volume was noted between A. octoarticulatus- and A. decemarticulatus-inhabited plants. This highlights the differential resource allocations by the plant, which might be linked to the different developmental pathways between the lamina and the domatia [26]. It also echoes the retaliation mechanism used against too virulent cheaters and the control of mutualistic investment (and thus rewards) to the ants [35].
(b). Coexistence mechanisms
The stable coexistence of competing mutualistic species in systems driven by replacement competition requires niche differentiation, the spatial structuring of populations and/or a dispersal–fecundity trade-off [5,8]. If none of these stabilization mechanisms exists, then the species are involved in a classic competitive lottery in which stable coexistence cannot occur [5]. The two Allomerus species studied here rely entirely on the same host plant, so that there is no niche differentiation, at least locally. Nor does the higher fecundity of A. decemarticulatus appear to be compensated in A. octoarticulatus by better dispersal abilities and a competitive advantage for host–plant occupation. Indeed, the queens of both ant species are the same size and the ratio of founding queens reflects the ratio of mature colonies in the population studied. As a consequence, stable coexistence appears impossible and the observed sympatry between the two ant species locally should eventually lead to the competitive exclusion of A. octoarticulatus.
Even if A. octoarticulatus appears to be a specialist of H. physophora at the scale of this study, this ant can inhabit a variety of host–plant species over its distribution range, highlighting its non-specialist character globally. Such a generalist trait might explain the local maladaptation of the species to H. physophora probably resulting from a recent host shift [50]. On the other hand, coevolutionary processes seem to have induced the better exploitation by the partners in the A. decemarticulatus/H. physophora interaction than in the association between A. octoarticulatus and H. physophora. In conclusion, both trade-offs in mutualistic investment and coevolutionary history are shaping the global outcome of the interaction. Although the better-adapted mutualist is not the one that provides its host the most benefits, its competitive advantage appears to be the most important mechanism for the maintenance of the interaction.
Supplementary Material
Acknowledgements
We thank Susanne Renner and Guillaume Chomicki for inviting us to contribute to this Proceedings B Special Feature on ‘ant interactions with their biotic environments’. We are also grateful to the Laboratoire Environnement de Petit Saut for its logistical assistance, to A. Yockey-Dejean for proofreading the manuscript, to H.L. Vasconcelos and T.J. Izzo for providing us with samples of Allomerus species from Brazil, to D.W. Yu for the Allomerus specimen from Peru, to R. Boulay for the Monomorium subopacum specimen, and to J. Mener, M. Duvignau and I. Henry for their help during fieldwork.
Data accessibility
Datasets are available via Dryad (doi:10.5061/dryad.hm872 [51]), and DNA sequences are available at GenBank through accession numbers KX585770-KX585863.
Authors' contributions
All of the authors contributed to the data acquisition and participated in editing the manuscript. J.O. coordinated the study and drafted the manuscript. J.O., P.J.M., J.L. O.R. and C.L. participated in the data analysis. P.J.M. and F.P. carried out molecular laboratory work, O.R. and J.O. carried out the chemical ecology study, and C.L. and J.L. carried out the stable isotope analysis. All of the authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
Financial support for this study was provided by the Programme Amazonie II of the French Centre National de la Recherche Scientifique, the Programme Convergence 2007–2013, Région Guyane from the European Community (project Bi-Appli, 115/SGAR-DE/2011/052274), a fellowship from the Fondation pour la Recherche sur la Biodiversité (research agreement no. AAP-IN-2009-050). J.L.'s financial support was provided through a PhD fellowship from the Fond Social Européen. This work has also benefited from ‘Investissement d'Avenir’ grants managed by the Agence Nationale de la Recherche (CEBA, ref. ANR-10-LABX-25-01 and TULIP, ref. ANR-10-LABX-0041).
References
- 1.Afkhami ME, Rudgers JA, Stachowicz J. 2014. Multiple mutualist effects: conflict and synergy in multispecies mutualisms. Ecology 95, 833–844. ( 10.1890/13-1010.1) [DOI] [PubMed] [Google Scholar]
- 2.Stanton ML. 2003. Interacting guilds: moving beyond the pairwise perspective on mutualisms. Am. Nat. 162, 10–23. ( 10.1086/378646) [DOI] [PubMed] [Google Scholar]
- 3.Bascompte J, Jordano P. 2014. Mutualistic networks, p. 206 Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Bruna EM, Izzo TJ, Inouye BD, Uriarte M, Vasconcelos HL. 2011. Asymmetric dispersal and colonization success of Amazonian plant-ants queens. PLoS ONE 6, e22937 ( 10.1371/journal.pone.0022937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CT, Inouye BD. 2010. Mutualism between consumers and their shared resource can promote competitive coexistence. Am. Nat. 175, 277–288. ( 10.1086/650370) [DOI] [PubMed] [Google Scholar]
- 6.Jones EI, Bronstein JL, Ferrière R. 2012. The fundamental role of competition in the ecology and evolution of mutualisms. Ann. NY Acad. Sci. 1256, 66–88. ( 10.1111/j.1749-6632.2012.06552.x) [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain SA, Holland JN. 2008. Density-mediated, context-dependent consumer-resource interactions between ants and extrafloral nectar plants. Ecology 89, 1364–1374. ( 10.1890/07-1139.1) [DOI] [PubMed] [Google Scholar]
- 8.Szilágyi A, Scheuring I, Edwards DP, Orivel J, Yu DW. 2009. The evolution of intermediate castration virulence and ant coexistence in a spatially structured environment. Ecol. Lett. 12, 1–11. ( 10.1111/j.1461-0248.2009.01382.x) [DOI] [PubMed] [Google Scholar]
- 9.Frederickson ME. 2005. Ant species confer different partner benefits on two neotropical myrmecophytes. Oecologia 143, 387–395. ( 10.1007/s00442-004-1817-7) [DOI] [PubMed] [Google Scholar]
- 10.Heil M, Gonzales-Teuber M, Clement LW, Kautz S, Verhaagh M, Silva Bueno JC. 2009. Divergent investment strategies of Acacia myrmecophytes and the coexistence of mutualists and exploiters. Proc. Natl Acad. Sci. USA 106, 18 091–18 096. ( 10.1073/pnas.0904304106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heil M, McKey D. 2003. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 34, 425–453. ( 10.1146/annurev.ecolsys.34.011802.132410) [DOI] [Google Scholar]
- 12.Rico-Gray V, Oliveira PS. 2007. The ecology and evolution of ant-plant interactions, p. 331 Chicago, IL: University of Chicago Press. [Google Scholar]
- 13.Mayer VE, Frederickson ME, McKey D, Blatrix R. 2014. Current issues in the evolutionary ecology of ant-plant symbioses. New Phytol. 202, 749–764. ( 10.1111/nph.12690) [DOI] [PubMed] [Google Scholar]
- 14.Guimaraes PR, Rico-Gray V, Oliveira PS, Izzo TJ, dos Reis SF, Thompson JN. 2007. Interaction intimacy affects structure and coevolutionary dynamics in mutualistic networks. Curr. Biol. 17, 1797–1803. ( 10.1016/j.cub.2007.09.059) [DOI] [PubMed] [Google Scholar]
- 15.Fonseca CR, Ganade G. 1996. Asymetries, compartments and null interactions in an Amazonian ant-plant community. J. Anim. Ecol. 65, 339–347. ( 10.2307/5880) [DOI] [Google Scholar]
- 16.Yu DW, Wilson HB. 2001. The competition-colonization trade-off is dead; long Live the competition-colonization trade-off. Am. Nat. 158, 49–63. ( 10.1086/320865) [DOI] [PubMed] [Google Scholar]
- 17.Yu DW, Wilson HB, Frederickson ME, Palomino W, De la Colina R, Edwards DP, Balareso AA. 2004. Experimental demonstration of species coexistence enabled by dispersal limitation. J. Anim. Ecol. 73, 1102–1104. ( 10.1111/j.0021-8790.2004.00877.x) [DOI] [Google Scholar]
- 18.Voglmayr H, Mayer V, Maschwitz U, Moog J, Djiéto-Lordon C, Blatrix R. 2011. The diversity of ant-associated black yeasts: insights into a newly discovered world of symbiotic interactions. Fungal Biol. 115, 1077–1091. ( 10.1016/j.funbio.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 19.Lapola DM, Bruna EM, de Willink CG, Vasconcelos HL. 2005. Ant-tended Hemiptera in Amazonian myrmecophytes: patterns of abundance and implications for mutualism function (Hymenoptera: Formicidae). Sociobiology 46, 433–442. [Google Scholar]
- 20.Pringle EG, Dirzo R, Gordon DM. 2011. Indirect benefits of symbiotic coccoids for an ant-defended myrmecophytic tree. Ecology 92, 37–46. ( 10.1890/10-0234.1) [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-González MX, Malé PJG, Leroy C, Dejean A, Gryta H, Jargeat P, Quilichini A, Orivel J. 2011. Specific, non-nutritional association between an ascomycete fungus and Allomerus plant-ants. Biol. Lett. 7, 475–479. ( 10.1098/rsbl.2010.0920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dejean A, Solano PJ, Ayrolles J, Corbara B, Orivel J. 2005. Arboreal ants build traps to capture prey. Nature 434, 973 ( 10.1038/434973a) [DOI] [PubMed] [Google Scholar]
- 23.Leroy C, Séjalon-Delmas N, Jauneau A, Ruiz-González MX, Gryta H, Jargeat P, Corbara B, Dejean A, Orivel J. 2011. Trophic mediation by a fungus in an ant-plant mutualism. J. Ecol. 99, 583–590. ( 10.1111/j.1365-2745.2010.01763.x) [DOI] [Google Scholar]
- 24.Orivel J, Lambs L, Malé PJG, Leroy C, Grangier J, Otto T, Quilichini A, Dejean A. 2011. Dynamics of the association between a long-lived understory myrmecophyte and its specific associated ants. Oecologia 165, 369–376. ( 10.1007/s00442-010-1739-5) [DOI] [PubMed] [Google Scholar]
- 25.Solano PJ, Durou S, Corbara B, Quilichini A, Cerdan P, Belin Depoux M, Delabie JHC, Dejean A. 2003. Myrmecophytes of the understory of French Guianian rainforests: their distribution and their associated ants. Sociobiology 41, 605–614. [Google Scholar]
- 26.Leroy C, Jauneau A, Quilichini A, Dejean A, Orivel J. 2010. Comparative structure and ontogeny of the foliar domatia in three neotropical myrmecophytes. Am. J. Bot. 97, 557–565. ( 10.3732/ajb.0900207) [DOI] [PubMed] [Google Scholar]
- 27.Fernandez F. 2007. The myrmicine ant genus Allomerus Mayr (Hymenoptera: Formicidae). Caldasia 29, 159–175. [Google Scholar]
- 28.Romero GQ, Izzo TJ. 2004. Leaf damage induces ant recruitment in the Amazonian ant-plant Hirtella myrmecophila. J. Trop. Ecol. 20, 675–682. ( 10.1017/S0266467404001749) [DOI] [Google Scholar]
- 29.Grangier J, Dejean A, Malé PJG, Orivel J. 2008. Indirect defense in a highly specific ant-plant mutualism. Naturwissenschaften 96, 57–63. ( 10.1007/s00114-008-0398-4) [DOI] [PubMed] [Google Scholar]
- 30.Izzo TJ, Vasconcelos HL. 2002. Cheating the cheater: domatia loss minimizes the effects of ant castration in an Amazonian ant-plant. Oecologia 133, 200–205. ( 10.1007/s00442-002-1027-0) [DOI] [PubMed] [Google Scholar]
- 31.Malé PJG, Leroy C, Dejean A, Quilichini A, Orivel J. 2012. An ant symbiont directly and indirectly limits its host plant's reproductive success. Evol. Ecol. 26, 55–63. ( 10.1007/s10682-011-9485-7) [DOI] [Google Scholar]
- 32.Yu DW, Pierce NE. 1998. A castration parasite of an ant-plant mutualism. Proc. R. Soc. Lond. B 265, 375–382. ( 10.1098/rspb.1998.0305) [DOI] [Google Scholar]
- 33.Frederickson ME. 2009. Conflict over reproduction in an ant-plant symbiosis: why Allomerus octoarticulatus ants sterilize Cordia nodosa trees. Am. Nat. 173, 675–681. ( 10.1086/597608) [DOI] [PubMed] [Google Scholar]
- 34.Malé PGJ, Leroy C, Lusignan L, Petitclerc F, Quilichini A, Orivel J. 2015. The reproductive biology of the myrmecophyte, Hirtella physophora, and the limitation of negative interactions between pollinators and ants. Arthropod-Plant Interact. 9, 23–31. ( 10.1007/s11829-014-9352-x) [DOI] [Google Scholar]
- 35.Malé PJG, Ferdy JB, Leroy C, Roux O, Lauth J, Avilez A, Dejean A, Quilichini A, Orivel J. 2014. Retaliation in response to castration promotes a low level of virulence in an ant-plant mutualism. Evol. Biol. 41, 22–28. ( 10.1007/s11692-013-9242-7) [DOI] [Google Scholar]
- 36.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299. [PubMed] [Google Scholar]
- 37.R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 38.Bates D, Maechler M. 2009. lme4: linear mixed-effects models using S4 classes. (R package version 0.999375-32 ed.). Vienna, Austria: R Foundation for Statistical Computing.
- 39.Zeileis A, Hothorn T. 2002. Diagnostic checking in regression relationships. R News 2, 7–10. [Google Scholar]
- 40.Thompson JN. 1988. Variation in interspecific interactions. Annu. Rev. Ecol. Syst. 19, 65–87. ( 10.1146/annurev.es.19.110188.000433) [DOI] [Google Scholar]
- 41.Chamberlain SA, Bronstein JL, Rudgers JA. 2014. How context dependent are species interactions? Ecol. Lett. 17, 881–890. ( 10.1111/ele.12279) [DOI] [PubMed] [Google Scholar]
- 42.Leimar O, Connor RC. 2003. By-product benefits, reciprocity, and pseudoreciprocity in mutualism. In Genetic and cultural evolution of cooperation (ed. Hammerstein P.), pp. 203–222. Cambridge, MA: The MIT Press. [Google Scholar]
- 43.Malé PJG, Leroy C, Humblot P, Dejean A, Quilichini A, Orivel J. 2016. Limited gene dispersal and spatial genetic structure as stabilizing factors in an ant-plant mutualism. J. Evol. Biol. 29, 2519–2529. ( 10.1111/jeb.12980) [DOI] [PubMed] [Google Scholar]
- 44.Davidson DW, Cook SC, Snelling RR, Chua TH. 2003. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972. ( 10.1126/science.1082074) [DOI] [PubMed] [Google Scholar]
- 45.Sorvari J, Hakkarainen H. 2007. The role of food and colony size in sexual offspring production in a social insect: an experiment. Ecol. Entomol. 32, 11–14. ( 10.1111/j.1365-2311.2006.00861.x) [DOI] [Google Scholar]
- 46.Fischer RC, Wanek W, Richter A, Mayer V. 2003. Do ants feed plants? A 15N labelling study of nitrogen fluxes from ants to plants in the mutualism of Pheidole and Piper. J. Ecol. 91, 126–134. ( 10.1046/j.1365-2745.2003.00747.x) [DOI] [Google Scholar]
- 47.Fonseca CR, Overton JM, Collins B, Westoby M. 2000. Shifts in trait-combinations along rainfall and phosphorus gradients. J. Ecol. 88, 964–977. ( 10.1046/j.1365-2745.2000.00506.x) [DOI] [Google Scholar]
- 48.Ackerly DD, Knight CA, Weiss SB, Barton K, Starmer KP. 2002. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 130, 449–457. ( 10.1007/s004420100805) [DOI] [PubMed] [Google Scholar]
- 49.Poorter L, Bongers F. 2006. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87, 1733–1743. ( 10.1890/0012-9658(2006)871733:LTAGPO%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 50.Thompson JN, Nuismer SL, Gomulkiewicz R. 2002. Coevolution and maladaptation. Integr. Comp. Biol. 42, 381–387. ( 10.1093/icb/42.2.381) [DOI] [PubMed] [Google Scholar]
- 51.Orivel J, Malé P-J, Lauth J, Roux O, Petitclerc F, Dejean A, Leroy C. 2017. Trade-offs in an ant–plant–fungus mutualism. Dryad Digital Repository. ( 10.5061/dryad.hm872) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Orivel J, Malé P-J, Lauth J, Roux O, Petitclerc F, Dejean A, Leroy C. 2017. Trade-offs in an ant–plant–fungus mutualism. Dryad Digital Repository. ( 10.5061/dryad.hm872) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets are available via Dryad (doi:10.5061/dryad.hm872 [51]), and DNA sequences are available at GenBank through accession numbers KX585770-KX585863.