Abstract
Chemical communication is central for the formation and maintenance of insect societies. Generally, social insects only allow nest-mates into their colony, which are recognized by their cuticular hydrocarbons (CHCs). Social parasites, which exploit insect societies, are selected to circumvent host recognition. Here, we studied whether chemical strategies to reduce recognition evolved convergently in slavemaking ants, and whether they extend to workers, queens and males alike. We studied CHCs of three social parasites and their related hosts to investigate whether the parasitic lifestyle selects for specific chemical traits that reduce host recognition. Slavemaker profiles were characterized by shorter-chained hydrocarbons and a shift from methyl-branched alkanes to n-alkanes, presumably to reduce recognition cue quantity. These shifts were consistent across independent origins of slavery and were found in isolated ants and those emerging in their mother colony. Lifestyle influenced profiles of workers most profoundly, with little effect on virgin queen profiles. We detected an across-species caste signal, with workers, for which nest-mate recognition is particularly important, carrying more and longer-chained hydrocarbons and males exhibiting a larger fraction of n-alkanes. This comprehensive study of CHCs across castes and species reveals how lifestyle-specific selection can result in convergent evolution of chemical phenotypes.
Keywords: host–parasite coevolution, odour, chemical strategies, cuticular hydrocarbons, social parasites, dulosis
1. Introduction
Recognition of group members is vital for the evolution and maintenance of sociality. Animals only benefit from altruistic behaviour when directed towards relatives [1]. In insects, especially in social ones, recognition and subsequent discrimination occurs through olfaction or contact chemoreception [2]. Besides their role as desiccation barriers, the low to non-volatile hydrocarbons on the insect's cuticle mediate chemical communication and form the basis for nest-mate, species, and sex recognition and fertility signalling [3].
Nest-mate recognition in ants is thought to be based on a comparison between the cuticular hydrocarbon (CHC) profile of another individual and a neuronal template of the colony odour [4]. CHC profiles of conspecifics typically include the same set of substances, so that social insects use quantitative variation to recognize nest-mates. Recognition cues are usually blended within a colony through trophallaxis and grooming, with the postpharyngeal gland serving as a reservoir, where CHCs obtained by grooming are mixed and re-distributed [5]. They are also affected by environmental factors such as nest material or food [6,7] and social parameters for instance queen signals, so that the colony odour is constantly updated [2].
Not all CHCs are used in nest-mate recognition. Straight-chained alkanes are especially effective to prevent desiccation and their production is influenced by temperature, humidity and task [8,9], but they rarely play an important role in recognition [4,10]. Insertion of double bonds or methyl-branching decreases anti-desiccation capacity [11,12], but increases information content [13,14]. Increasing the fraction of alkenes and methyl-alkanes in the profile facilitates chemical communication [15]. The insect CHC profile is therefore likely to be under differential selection, varying between castes [16,17], tasks [8,9,18], fertility states [19,20] and positions in the social hierarchy [21–23].
Compared to worker castes, males and young queens are subject to different selective forces. Sex-specific compounds are important for mate choice and sex discrimination [24]. While species-specific compounds allow species recognition, colony-specific profiles are important for inbreeding avoidance [25]. Queens communicate their fertility through their CHC profile and/or glandular secretions [2]. Besides these functions, CHCs experience selection from social parasites [26,27], which are thought to be a major driver of recognition cue diversity [28,29].
Ants often fall victim to social parasites such as inquilines or slavemaking ants, which invade host nests to exploit the workforce of another ant species [30]. Obligate slavemakers, such as our focal species, have lost the abilities to perform social tasks, such as brood care or nest defence and die when not fed by their slaves. During the raiding season in summer, they raid host colonies and steal brood, which will emerge in the slavemakers' nest to serve as slaves. It is crucial for slavemakers on raids, especially scouts searching for host nests, not to be recognized and attacked by host workers, which often outnumber them by 10 : 1 [31]. Strategies to avoid recognition include chemical insignificance, defined here as carrying almost no CHCs [32], chemical mimicry where they actively biosynthesize the hosts' CHCs, or chemical camouflage where they acquire the hosts' odour through grooming, trophallaxis or rubbing on nest material [32–35]. Chemical transparency is related to insignificance, where parasites carry mainly hydrocarbons irrelevant for recognition. Despite these strategies, raids are risky for slavemakers, often resulting in injury or death [31], as hosts are selected to counteract parasite adaptations. Beside many fascinating case studies, the influence of the parasitic lifestyle per se on the chemical profile compared to related non-parasitic species has not yet been systematically studied.
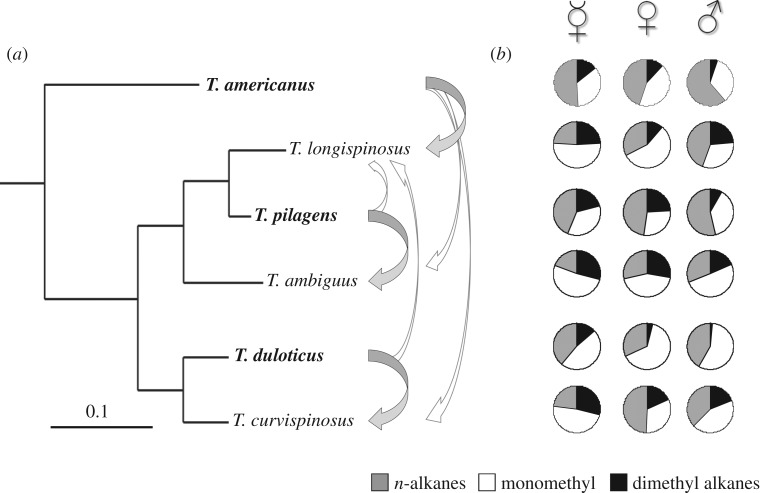
Here, we compare the chemical profiles of three North American slavemaking species and their three host species of the genus Temnothorax. The three slavemakers differ in evolutionary age and origin (fig. 1b within [36]; note T. pilagens [37] then undescribed was referred to as T. spp. and T. americanus changed its genus name from Protomognathus [38]). All of them raid host colonies for slaves, but their fighting strategies differ [31,39–41]. Temnothorax americanus, the oldest slavemaker in this clade, shows little evidence of chemical mimicry [30,41], but uses the Dufour's gland secretion to divert host aggression [42,43]. Temnothorax duloticus on the contrary, efficiently uses its sting [39]. Hosts recognize these parasites as enemies [41,44] and their raids are openly aggressive, often resulting in casualties among attackers and defenders [31,45]. A third slavemaking species, Temnothorax pilagens can perform peaceful raids facilitating the enslavement of adult workers [41]. These slavemakers are often not recognized because they use chemical camouflage, but in the cases where they are, they respond to host aggression by stinging host workers.
Figure 1.
(a) Phylogeny of Temnothorax slavemakers and hosts (adapted from fig. 2b in [36]). Slavemaking species are given in bold. Broad arrows indicate the main host species of each slavemaker; the thinner arrows indicate non-preferred hosts. (b) Mean substance class composition per lifestyle and caste.
Despite their different raiding strategies, we hypothesize that, owing to similar selection on their profiles, slavemakers should share CHC characteristics that increase the chance to remain unrecognized by hosts (electronic supplementary material, figure S1). Firstly, we predict slavemakers to exhibit fewer recognition substances, namely methyl-branched alkanes or alkenes. Secondly, they could exhibit a reduced amount of CHCs and/or higher proportions of n-alkanes to achieve chemical insignificance or transparency [46]. Recently, nine hydrocarbons associated with nest-mate recognition in Temnothorax longispinosus, one of the hosts studied here, were identified [26]. We predict that slavemakers carry less of those putative recognition substances to decrease the probability of host aggression. We were additionally interested in caste-specific differences in slavemakers and hosts as the CHC profile of workers, males and virgin queens should be adapted to their specific life histories and ecology. For example, sexuals should attract the other sex during the nuptial flight. As slavemaker workers and queens in contrast to host females need to invade host colonies for raids or colony foundation [47], they are expected to show chemical adaptations to circumvent host aggression. Slavemaking males do not directly interact with their host: they live only for a few days after leaving the maternal nest to participate in a mating flight and their profiles should mainly underlie sexual selection. Hence, if mate recognition is mediated by CHCs, signalling colony and species affiliation should be more important for males to avoid inbreeding or hybridization.
Here, we compare chemical profile compositions in all sexes and castes of an ant clade including both parasitic slavemaker (SM) and non-parasitic host (H) species. We are especially interested in how diverse selection pressures generated by the lifestyle and caste/sex in these groups are reflected in their CHC profiles. We investigated changes in the abundance of specific substance classes, which are known to have specific functions in the ants' communication system.
2. Material and methods
(a). Study system, collection and colony maintenance
In May–July 2013 we collected colonies of three slavemaker species, T. americanus, T. duloticus and T. pilagens and their three host species, T. longispinosus, Temnothorax curvispinosus and Temnothorax ambiguus from sites in New York (NY), Ohio (OH), West Virginia (WV) and Michigan (MI), USA (electronic supplementary material, table S1). These ants have small colonies with a few dozen workers and inhabit cavities in acorns or small sticks on the forest floor. Slavemaker colonies contain on average only two to five slavemakers and 30 slaves and are, in contrast to their facultative polygynous hosts, invariably monogynous [41,48]. Each slavemaker parasitizes multiple hosts, but shows a clear preference for a single host species [37,45] (electronic supplementary material, S1 for community composition and slavemaker preference). Host colonies were unparasitized at collection and are henceforth termed non-parasitic. Collection permits were obtained from preserves or we asked private landowners for permission to collect. Import and export licenses were not required for the transport of our study species.
Ant colonies were transferred in their natural nests to Ziploc bags and stored at 7°C during the field trip. In our laboratory, colonies were moved to artificial nests made of a plastic perimeter sandwiched between glass slides and placed into plastered boxes. All colony members were counted, after which the colonies were kept at room temperature, and fed weekly with crickets and honey.
(b). Collection of ants for chemical analyses
To exclude potential effects of fertility, age and environment on CHC profiles, we analysed ants that emerged in our laboratory under standardized conditions. All ants were 3–5 days old. For each species and study site (NY, WV, OH and MI), we used on average 14.8 colonies (range 5–31) and observed brood development. We noted the ants' emergence from the pupae and waited for 5 days for the chemical profile to fully develop. In a few cases, in which the cuticle darkened so strongly that we feared we might not be able to differentiate young individuals from older ones, we removed them after 3 or 4 days. We used two individuals per caste per colony whenever possible (total sample sizes: electronic supplementary material, table S1).
To exclude potential effects of nest-mates and/or slaves on ant hydrocarbon profiles through trophallaxis or grooming [32,34], we additionally let ant pupae emerge in isolation—a stressful situation for a social insect—and studied the effect of isolation on the CHC profile (see the electronic supplementary material, S3 for details). In total, we analysed 372 ants from original colonies and 246 ants that emerged in isolation (electronic supplementary material, table S1).
(c). Gas chromatography-mass spectrometry analyses
We killed ants by freezing and extracted CHCs in 0.5 ml hexane for 10 min, added 100 ng n-C18 as internal standard, and analysed all samples using gas chromatography-mass spectrometry (GC-MS) (Agilent Technologies, GC: Agilent 7890A; MS: Agilent 5975; electronic supplementary material, S2 for details). Peak areas were obtained using the software MSD ChemStation E.02.02 (Agilent). Chemical substances were identified based on diagnostic ions and retention indices. We included all hydrocarbons longer than C20 that occurred in at least 20% of all samples in any one species x caste combination with an abundance of at least 0.1%.
(d). Statistical analyses
Firstly, CHC composition was compared between species with a multivariate analysis of variance (PERMANOVA, 10 000 permutations; software Primer 6.0, Primer-E Ltd) based on Bray–Curtis similarity (BCS). We randomly chose one individual per colony to exclude effects of similarity between nest-mates. To test for local adaptation, we (i) compared similarities between slavemakers and their respective host species from the same locale or a different site. We expected that slavemakers are more similar to their sympatric host than to allopatric host species and more similar to their preferred host than towards co-occurring but less-preferred host species. Furthermore (ii), we applied a comprehensive test over all six species to test whether CHC composition of slavemakers systematically differed from non-parasitic species, using a PERMANOVA that included caste, lifestyle (slavemaker or non-parasitic) and species (nested in lifestyle) as fixed factors, with interactions allowed, and study site as a random factor.
Secondly, we tested whether specific chemical traits differed between slavemakers and non-parasitic species. These traits were the percentage of n-alkanes, monomethyl-alkanes and dimethyl-alkanes, the mean chain-length, the absolute CHC quantity (quantified based on the internal standard) and the percentage of putatively recognition-relevant hydrocarbons [26]. We detected eight of those nine putative recognition CHCs previously identified in T. longispinosus [26], the exception being 3-MeC31. Systematic trait differences between slavemakers and non-parasitic species might indicate chemical strategies of social parasites other than mimicry. We tested each trait as dependent variable in a separate linear mixed-effect model with caste and lifestyle (parasitic versus non-parasitic species) as fixed factors (interaction allowed) and colony ID (nested in species and study site) as a random factor. These analyses were based on two individuals per species, caste and colony. We removed non-significant model terms in a backward stepwise procedure so that only significant terms were retained in the final model. In a subsequent analysis, we performed similar statistics for individuals that emerged in isolation (electronic supplementary material, tables S13 and S14). All results are based on the analysis of individuals from their original colonies, with the exception of the section on social environment.
Thirdly, we tested the above traits in a phylogenetic context. For each of the castes, we calculated averages of each chemical trait (i.e. six data points per caste and trait), and calculated phylogenetic independent contrasts (PIC) of the trait (command pic, package ape) based on the phylogeny in [36]. We calculated the PICs of the trait lifestyle (slavemaking/non-parasitic), and tested for correlation between each trait and the lifestyle PICs using lmorigin (package ape) [49,50].
To analyse whether non-parasitic species show higher variance between colonies to hinder slavemakers matching host profiles, we compared BCS between and within colonies. We tested whether males show higher within-colony, but lower between-colony variance than workers or virgin queens. Males should not be under selection to exhibit colony-specific odours (except for inbreeding avoidance), but should rather exhibit species-specific profiles for sexual communication. For this, we calculated within-colony BCS by choosing two individuals per colony, caste and species. For between-colony data, we calculated BCS of one caste of each species per colony towards a randomly chosen individual of the same caste and species, but a different colony from the same locale. Chemical similarities were analysed using linear mixed models (LMMs) with lifestyle, caste and origin (intra-versus allocolonial) as fixed factors, with interactions allowed. Species nested in study site were included as random factors. All analyses were performed in R v. 3.0.2.
3. Results
(a). Chemical composition and the absence of chemical resemblance
The cuticular chemistry of the six closely related species was qualitatively the same: the identical set of 35 CHCs was detected on workers of all six species. Sexuals largely shared these hydrocarbons, with a few of them lacking in virgin queens and males of some species (electronic supplementary material, table S2; figure 1b). No species-specific hydrocarbons were detected. Nevertheless, when comparing the quantitative CHC composition, slavemakers were chemically not only invariably distinct from their sympatric hosts (PERMANOVAs all p < 0.0001; electronic supplementary material, table S3), but they were also not more similar to them than to allopatric host populations (electronic supplementary material, table S4). Hence, beyond the fact that all species carry that same hydrocarbon set, we found no evidence that slavemakers resemble their hosts more than any other species in this closely related taxon.
(b). Chemical differences between slavemakers and non-parasitic species
We analysed whether slavemakers exhibit common features in their chemical profile, which sets them apart from non-parasitic species. Indeed, the composition of cuticular chemicals differed among lifestyles (PERMANOVA: slavemaker versus non-parasitic species; pseudo-F1 = 10.89, p = 0.0001; electronic supplementary material, figure S2), castes (pseudo-F2 = 11.17, p = 0.0001) and among species (nested in lifestyle: pseudo-F4 = 5.24, p = 0.0001). Some of the caste-specific differences interacted with lifestyle (caste × lifestyle: pseudo-F2 = 7.13, p = 0.0001) or were species-specific (caste × species: pseudo-F8 = 3.52, p = 0.0001; electronic supplementary material, table S5; pairwise comparisons: electronic supplementary material, tables S6 and S7). A repetition of the analyses using ‘sexuals versus workers’ instead of ‘caste’ showed across-species chemical differences associated with being a sexual (male or virgin queen) or a worker (pseudo-F = 14.65, p = 0.0001; electronic supplementary material, table S8; pairwise comparisons electronic supplementary material, tables S9 and S10), next to the lifestyle (pseudo-F = 12.60, p = 0.0001) and species differences (pseudo-F = 4.10, p = 0.0001).
(c). Effect of lifestyle on chemical traits
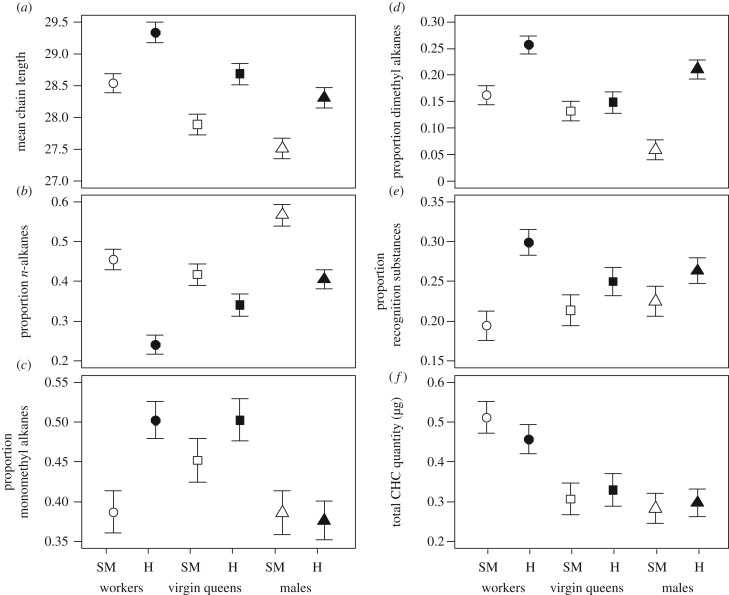
In all three castes, the chemical profiles of slavemakers contained higher relative abundances of shorter-chained hydrocarbons compared with those of non-parasitic species (statistical results: table 1 and figure 2a; electronic supplementary material, table S11). Furthermore, slavemakers of all castes possessed a greater relative abundance of n-alkanes than non-parasitic species (figure 2b). Methyl-branched alkanes were often relatively less abundant in slavemakers than in non-parasitic species, but these effects were caste-specific. Slavemaker workers had a lower abundance of monomethyl-alkanes than non-parasitic workers, whereas slavemaker virgin queens and males did not carry less than sexuals of non-parasitic species (figure 2c). Additionally, slavemaker workers and males exhibited a lower abundance of dimethyl-alkanes than non-parasitic species, whereas this effect of lifestyle was missing in virgin queens (figure 2d). Finally, slavemaker workers carried a lower abundance of putative recognition substances than non-parasitic workers, whereas slavemaker sexuals did not differ from non-parasitic species (figure 2e). The total CHC quantity did not differ between lifestyles, that is, slavemakers did not carry less hydrocarbons (figure 2f).
Table 1.
Model selection results from linear mixed models analysing the mean chain-length, the total CHC quantity and the different substance classes in relation to lifestyle and caste. (Statistics indicated in italics were retained in the final model. L.Ratio, likelihood ratio.)
| caste × lifestyle |
caste |
lifestyle |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| L.Ratio | p-value | d.f. | L.Ratio | p-value | d.f. | L.Ratio | p-value | d.f. | |
| mean chain-length | 5.71 | 0.057 | 2 | 70.99 | <0.001 | 2 | 15.39 | <0.001 | 1 |
| total CHC quantity | 1.83 | 0.400 | 2 | 42.94 | <0.001 | 2 | 0.02 | 0.887 | 1 |
| proportion of n-alkanes | 11.71 | 0.003 | 2 | 64.16 | <0.001 | 2 | 14.73 | <0.001 | 1 |
| proportion of monomethyl-alkanes | 17.35 | <0.001 | 2 | 39.34 | <0.001 | 2 | 2.98 | 0.084 | 1 |
| proportion of dimethyl-alkanes | 23.82 | <0.001 | 2 | 33.37 | <0.001 | 2 | 18.74 | <0.001 | 1 |
| proportion of recognition substances | 12.87 | 0.002 | 2 | 2.85 | 0.240 | 2 | 7.43 | 0.006 | 1 |
Figure 2.
Influence of lifestyle and caste on different substance classes, including mean chain-length (a), relative abundance of n-alkanes (b), monomethyl-alkanes (c), dimethyl-alkanes (d) putative recognition substances (e) and total CHC quantity in microgram (f). Slavemakers are shown in white, non-parasitic species in black. Symbols represent the different castes (workers, circles; virgin queens, squares; males, triangles). The plots represent intercepts ±s.e. of the LMM summaries. Statistical results are given in table 1 and the electronic supplementary material, table S11.
PICs supported most of the above results. For workers, they confirmed the differences for n-alkanes, dimethyl-alkanes, putative recognition substances and chain-length and their absence for monomethyl-alkanes and absolute CHC quantities (electronic supplementary material, table S11). In males, only dimethyl-alkanes and mean chain-length differed, whereas PICs revealed little effects of lifestyle on the chemical profiles of virgin queens, except a trend towards a higher abundance of CHCs with shorter chain-length in slavemaker queens (p = 0.06; electronic supplementary material, table S11).
(d). Effect of caste within lifestyle
Workers of both lifestyles exhibited a higher relative abundance of long-chained hydrocarbons than virgin queens, and virgin queens more than males (figure 2a). Slavemaker and non-parasitic males carried a higher relative abundance of n-alkanes than workers and virgin queens, and in non-parasitic species, n-alkanes were relatively more abundant in the profile of virgin queens than of workers (figure 2b). Moreover, the profile of slavemaker queens exhibited a higher relative abundance of monomethyl-alkanes than workers and males, whereas in non-parasitic species, virgin queens and workers did not differ. However, workers and virgin queens of non-parasitic species carried relatively more monomethyl-alkanes than males (figure 2c). Workers and virgin queens of slavemakers both possessed similar abundances of dimethyl-alkanes, but both carried more than males. In non-parasitic species, workers possessed more dimethyl-alkanes than virgin queens and males, and males exhibited higher abundances than virgin queens (figure 2d). Slavemaker castes did not vary in the relative abundance of putative recognition substances [27], but within the non-parasitic species, workers carried relatively more recognition substances than virgin queens and males, whereas males and virgin queens did not differ (figure 2e). Absolute CHC quantities were generally highest in workers and were unaffected by lifestyle (figure 2f).
(e). The effect of social environment
The influence of lifestyle and caste on chemical profiles of isolated ants was similar to those of ants emerging in their original colony (electronic supplementary material, figure S3). Overall, social isolation led to chemical profiles with a higher relative abundance of shorter-chained hydrocarbons (likelihood ratio1 = 96.71, p < 0.001; electronic supplementary material, tables S13, S14 and figure S3a) and of n-alkanes (likelihood ratio1 = 29.81, p < 0.001; electronic supplementary material, tables S13, S14 and figure S3c). Other effects of social isolation were specific to lifestyle, caste and species (electronic supplementary material, S3; including figure S3, tables S13 and S14).
(f). Chemical diversity between and within colonies
Between-individual chemical similarity was affected by caste (likelihood ratio = 11.75, p = 0.003), origin (intra- versus allocolonial; likelihood ratio = 31.54, p < 0.001) and an interaction between lifestyle and caste (likelihood ratio = 11.27, p = 0.004). Only in males, intra- and intercolonial similarity differed between parasitic and non-parasitic lifestyle (electronic supplementary material, figure S4). As expected, members of the same colony were more chemically similar than ants from different colonies (pairwise test within LMM: t280 = −6.15, p < 0.001). For slavemakers, inter-individual similarity did not differ between castes (worker versus males: t280 = −0.30, p = 0.768, workers versus virgin queens: t280 = 0.54, p = 0.593; virgin queens versus males t280 = 0.25, p = 0.799), yet it differed for the non-parasitic species. Males showed lower similarity, within and between colonies, than workers and virgin queens (versus workers: t280 = 3.73, p < 0.001; versus virgin queens: t280 = 4.17, p < 0.001), whereas the latter did not differ (t280 = −0.85, p = 0.393, electronic supplementary material, figure S4).
4. Discussion
Here, we demonstrate that slavemaking species exhibit a characteristic chemical profile that clearly sets them apart from related non-parasitic species. Interestingly, the qualitative CHC composition was the same in all six species, which is unusual even for closely related species [51–53] (but see [54]). Nevertheless, as quantitative differences are sufficient for reliable discrimination, recognition of nest-mates and heterospecifics is not hampered in this clade [55]. Moreover, this qualitative similarity is unlikely to represent chemical mimicry, as the species within each lifestyle also carry similar CHCs, but have no incentive to mimic each other. Quantitative traits that are characteristic for slavemakers included a higher relative abundance of short hydrocarbons and of n-alkanes, whereas methyl-branched alkanes were relatively less abundant. Hence, slavemakers carry less of the CHCs known to be important in recognition [13,14] including the putative recognition substances described for T. longispinosus [26]. Instead, they possess more n-alkanes, which are thought to have little recognition value [56]. Slavery arose several times independently in this clade (figure 1a; [36]), so that chemical similarities in slavemaker profiles cannot have a common origin. Rather, similar selection pressures to avoid host recognition have probably led to convergent evolution of chemical traits in slavemakers. To the best of our knowledge, such general strategies that are similar across multiple interaction systems, and yield similar effects on CHC composition across species, have only been shown in mutualistic associations so far [57].
Myrmecophiles and social parasites frequently use chemical mimicry to overcome their hosts' recognition system [32]. They can chemically mimic even hosts from a different order, such as ant-mimicking lycaenid butterflies or spiders [58,59]. Our focal slavemakers do not employ mimicry, but exhibit profiles distinct from their hosts, as has been reported for other social parasites [60–62] and as evident by the aggressive responses of their hosts [40,45,63]. Here T. pilagens is an exception, which can raid undetected by its hosts, presumably using chemical camouflage during raids [41]. Just as Polyergus queens change their chemical strategies from insignificance to camouflage [64], T. pilagens might adapt its profile during the raiding season. The lack of mimicry in slavemakers may be owing to host defences [40,65,66]: the host T. longispinosus has evolved counter-adaptations [47,65,67], including chemical profile diversification, preventing the parasite from matching all host colonies [26]. With few exceptions [58], chemical mimicry allows adaptation only to a single host. Not surprisingly, each of our three focal slavemakers shows a clear preference for a sole host species. Yet, we found no indication for chemical resemblance of local host populations in our data, albeit there is evidence for local adaptation on a behavioural level [45,68]. A reduction in recognition cues through higher relative abundances of n-alkanes is a more versatile strategy, as it facilitates the exploitation of multiple host species. All of our focal slavemakers can indeed exploit several host species, and they occasionally enslave workers of different host species in the same nest [41,48]. They may profit from the absence of slavemaker-specific hydrocarbons, such that the differences to their host species are only quantitative. Combined with lower relative abundances of putative recognition cues, this can decrease the risk of being recognized and attacked by hosts. Although T. americanus and T. duloticus raiders often encounter fierce counterattacks by host defenders [40,47], host aggression varies and affects raiding outcomes.
High abundances of n-alkanes have previously been reported for socially parasitic queens of leaf-cutting ants, and eggs of the social parasite Vespa dybowskii, and have been interpreted as chemical transparency facilitating host colony entry by the absence of recognition labels [46,69]. N-alkanes may indeed ‘dilute’ other substances, thus weakening the ants' recognition label. Profiles harbouring few recognition cues might be difficult to interpret by host workers [70], thus reducing attacks. Hosts may be forced to integrate such parasites, because discriminating against individuals deficient of recognition cues would lead to rejection of young ants that have just developed their hydrocarbon profile [32]. In turn, the higher relative abundance of methyl-alkanes in hosts might be a counter-adaptation to enhance their discriminative ability against slavemakers [26] next to more diverse chemical profiles [26,27]. Slavemakers possessed similar total CHC quantities as their hosts, and thus were not chemically insignificant sensu [32].
On average, CHCs of slavemakers were shorter than those of non-parasitic species. A plausible explanation is that a certain fluidity of the CHC layer is required to allow diffusion across the cuticle [71]. To maintain fluidity despite a higher relative abundance of n-alkanes, slavemakers need overall shorter chain-lengths, since longer-chained n-alkanes would aggregate too tightly and thus reduce CHC fluidity [72,73]. This hypothesis is corroborated by the correspondence of relative n-alkane abundance and average chain-length in the different castes and species (figure 2a,b). The shorter chain-lengths contrast with the extremely long ones (more than C40) reported for mutualistically associated parabiotic ants [57] and, partly, in an incipient leaf-cutting ant social parasite ([62], but see [46]), whose profiles are characterized by longer-chained CHCs than non-associated species. Longer chain-length may lead to lower detectability of the CHC profiles, and hence hamper recognition [57,74].
The lifestyle of slavemaker females clearly differs from that of their non-parasitic hosts, while this is not true for males. Only slavemaker workers and queens interact with hosts, which led us to expect a signal of parasitic lifestyle to be present only in females, especially because parasite pressure on one sex can lead to sex-specific adaptations [75]. However, the characteristic chemical composition of slavemakers was especially pronounced in workers and males. Indeed, after applying phylogenetic independent contrasts, lifestyle only slightly affected virgin queen hydrocarbon chemistry (albeit there was a trend for a reduction in CHC length). This is surprising as young slavemaking queens have to takeover host colonies single handily. However, as ant queens strongly alter their profile within hours following copulation [76], it is possible that slavemaker queens first exhibit an attractive profile for males before adapting it for host colony invasion. The cuticular chemistry of males instead clearly encoded a signal of parasitic lifestyle. Male profiles could be dragged along due to strong selection on worker profiles. Another explanation would be that males need similar profiles to females in order to be cared for by slaves. Slavemaker males differed from their non-parasitic counterparts in another trait. Compared to slavemakers, host males showed lower chemical similarities (i.e. higher diversity) both to nest-mates and non-nest-mates, which was not the case for females. As parasitized host populations are chemically more diverse than unparasitized ones, we predicted higher chemical diversity in host females compared with slavemakers. However, we found this effect only in males. Because the haploid males lack allelic variation in contrast to the diploid females, chemical variation owing to different alleles might be more pronounced in males, while they are likely to be balanced out in heterozygous females.
Specific compounds that are characteristic for queens (queen pheromones) have been identified in various species [54,77,78]. However, our study is one of a few that revealed systematic chemical differences between castes across multiple species. Overall, workers possessed higher CHC quantities, and a higher average chain-length than queens or males. As workers are usually smaller than queens, the difference in CHC amounts probably underestimates the real difference corrected for body surface. The higher CHC quantity can be explained by the need for waterproofing, as workers spend more time outside the nest, thus being exposed to drier conditions. Secondly, workers possessed more dimethyl-alkanes than queens and males, at least in non-parasitic species. Workers encounter non-nest-mates much more frequently than queens and males. Thus, by exposing larger quantities of recognition cues (dimethyl-alkanes), they facilitate nest-mate recognition, which is less important for queens and males. The multivariate analyses on the entire CHC composition detected an overall signal of caste across species and lifestyle pointing to the importance of signalling caste and/or to similar selection pressures through caste-specific lifestyles. This contradicts somewhat earlier studies on ants and Drosophila that revealed species differences to be more pronounced than sex differences [79]. The profiles of males and queens are mainly under sexual selection [24], but mate choice in ants might also depend on other pheromones, e.g. volatile glandular secretions. Profiles of Temnothorax virgin queens and males also clearly encoded species identity (electronic supplementary material, table S6). In contrast to [79], however, we did not find sex-specific compounds, which agree with other studies on social insects that only detected quantitative sex differences [80–82].
Most of our results obtained from individuals in functioning colonies were also found in individuals that emerged in isolation. This indicates that the chemical traits are largely genetically determined, and do not depend on individual CHC acquisition from nest-mates. Although the effects of lifestyle and caste were similar across individuals from nests and isolated ants, social isolation itself had a dramatic effect on hydrocarbon profiles. Isolated slavemakers and their hosts both possessed drastically increased proportions of n-alkanes, which may be a way to compensate for the lack of nest-mate care. Newly emerged insects often increase their amount of n-alkanes within the first days, but also adjust it throughout their lifetime [83].
5. Conclusion
Our results demonstrate that lifestyle-specific differences in selection pressure between slavemaking ants and their hosts, but also between workers and sexuals result in characteristic signals in their chemical profiles. Increased n-alkane abundances and a concomitant decrease in recognition cues seem to represent a chemical transparency strategy that evolved three times convergently in this clade. Furthermore, castes differed in chemical traits, especially absolute CHC quantities and relative dimethyl-alkane abundances, which may be related to differences in waterproofing requirements and—for non-parasitic species—to exposing cues to facilitate nest-mate recognition. To the best of our knowledge, this is the first study to demonstrate how chemical profiles systematically respond to these diverse selective pressures, yielding similar differences between castes and lifestyles across multiple species.
Supplementary Material
Acknowledgements
We thank Barbara Feldmeyer and Evelien Jongepier for helping in the field and Austin Alleman for constructive comments on the manuscript. Thanks to Patricia Kleeberg, who designed the electronic supplementary material, figure S1.
Ethics
We followed the Association for the Study of Animal Behaviour guidelines [84] and legal and institutional rules.
Data accessibility
All raw data presented here is provided in the electronic supplementary material.
Authors' contributions
I.K. and S.F. designed the experimental set-up. I.K. collected and analysed the samples and processed data for statistical analyses. I.K. and F.M. analysed the data. I.K. wrote a first draft and all authors revised it.
Competing interests
We have no competing interests.
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (Fo 298/9-2) and the Huyck Preserve in New York, USA.
References
- 1.Hamilton WD. 1987. Kinship, recognition, disease, and intelligence: constraints of social evolution. In Animal societies: theories and facts (eds Ito Y, Brown JL, Kikkawa J), pp. 81–102. Tokyo, Japan: Japanese Scientific Societies. [Google Scholar]
- 2.Leonhardt SD, Menzel F, Nehring V, Schmitt T. 2016. Ecology and evolution of communication in social insects. Cell 164, 1277–1287. ( 10.1016/j.cell.2016.01.035) [DOI] [PubMed] [Google Scholar]
- 3.Blomquist GJ, Bagnères A-G.. 2010. Introduction: history and overview of insect hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 3–18. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Bagnères A-G, Blomquist GJ. 2010. Site of synthesis, mechanism of transport and selective deposition of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 75–99. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Sorvari J, Theodora P, Turillazzi S, Hakkarainen H, Sundström L. 2008. Food resources, chemical signaling, and nest mate recognition in the ant Formica aquilonia. Behav. Ecol. 19, 441–447. ( 10.1093/beheco/arm160) [DOI] [Google Scholar]
- 7.Bos N, Grinsted L, Holman L. 2011. Wax on, wax off: nest soil facilitates indirect transfer of recognition cues between ant nestmates. PLoS ONE 6, e19435 ( 10.1371/journal.pone.0019435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner D, Tissot M, Gordon D. 2001. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 27, 1805–1819. ( 10.1023/A:1010408725464) [DOI] [PubMed] [Google Scholar]
- 9.Martin SJ, Drijfhout FP. 2009. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J. Chem. Ecol. 35, 368–374. ( 10.1007/s10886-009-9612-x) [DOI] [PubMed] [Google Scholar]
- 10.Dani FR, Jones GR, Corsi S, Beard R, Pradella D, Turillazzi S. 2005. Nestmate recognition cues in the honey bee: differential importance of cuticular alkanes and alkenes. Chem. Senses 30, 477–489. ( 10.1093/chemse/bji040) [DOI] [PubMed] [Google Scholar]
- 11.Gibbs AG. 1998. Water-proofing properties of cuticular lipids. Am. Zool. 38, 471–482. ( 10.1093/icb/38.3.471) [DOI] [Google Scholar]
- 12.Gibbs AG. 2002. Lipid melting and cuticular permeability: new insights into an old problem. J. Insect Physiol. 48, 391–400. ( 10.1016/S0022-1910(02)00059-8) [DOI] [PubMed] [Google Scholar]
- 13.Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S. 2001. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 62, 165–171. ( 10.1006/anbe.2001.1714) [DOI] [Google Scholar]
- 14.Cristina Lorenzi M, Cervo R, Bagnères A-G. 2011. Facultative social parasites mark host nests with branched hydrocarbons. Anim. Behav. 82, 1143–1149. ( 10.1016/j.anbehav.2011.08.011) [DOI] [Google Scholar]
- 15.Steiger S, Schmitt T, Schaefer MH. 2011. The origin and dynamic evolution of chemical information transfer. Proc. R. Soc. B 278, 970–979. ( 10.1098/rspb.2010.2285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tentschert J, Bestmann HJ, Heinze J. 2002. Cuticular compounds of workers and queens in two Leptothorax ant species: a comparison of results obtained by solvent extraction, solid sampling, and SPME. Chemoecology 12, 15–21. ( 10.1007/s00049-002-8322-4) [DOI] [Google Scholar]
- 17.Ayasse M, Birnbaum J, Tengö J, van Doorn A, Taghizadeh T, Francke W. 1999. Caste- and colony-specific chemical signals on eggs of the bumble bee, Bombus terrestris L. (Hymenoptera: Apidae). Chemoecology 9, 119–126. ( 10.1007/s000490050042) [DOI] [Google Scholar]
- 18.Greene MJ, Gordon DM. 2003. Social insects: cuticular hydrocarbons inform task decisions. Nature 423, 32 ( 10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 19.Liebig J, Peeters C, Oldham NJ, Markstadter C, Hölldobler B. 2000. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. R. Soc. Lond. B 97, 4124–4131. ( 10.1073/pnas.97.8.4124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hefetz A. 2007. The evolution of hydrocarbon pheromone parsimony in ants (Hymenoptera: Formicidae): interplay of colony odor uniformity and odor idiosyncrasy. Myrmecol. News 10, 59–68. [Google Scholar]
- 21.Ayasse M, Marlovits T, Tengö J, Taghizadeh T, Francke W. 1995. Are there pheromonal dominance signals in the bumblebee Bombus hypnorum L. (Hymenoptera: Apidae)? Apidologie 26, 163–180. ( 10.1051/apido:19950301) [DOI] [Google Scholar]
- 22.Heinze J, Stengl B, Sledge MF. 2002. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 52, 59–65. ( 10.1007/s00265-002-0491-1) [DOI] [Google Scholar]
- 23.Monnin T, Ratnieks FLW, Jones GR, Beard R. 2002. Pretender punishment induced by chemical signalling in a queenless ant. Nature 419, 61–65. ( 10.1038/nature00932) [DOI] [PubMed] [Google Scholar]
- 24.Thomas ML, Simmons LW. 2008. Sexual dimorphism in cuticular hydrocarbons of the Australian field cricket Teleogryllus oceanicus (Orthoptera: Gryllidae). J. Insect Physiol. 54, 1081–1089. ( 10.1016/j.jinsphys.2008.04.012) [DOI] [PubMed] [Google Scholar]
- 25.Martin SJ, Helanterä H, Kiss K, Lee YR, Drijfhout FP. 2009. Polygyny reduces rather than increases nestmate discrimination cue diversity in Formica exsecta ants. Insectes Soc. 56, 375–383. ( 10.1007/s00040-009-0035-z) [DOI] [Google Scholar]
- 26.Jongepier E, Foitzik S. 2016. Ant recognition cue diversity is higher in the presence of slavemaker ants. Behav. Ecol. 27, 304–311. ( 10.1093/beheco/arv153) [DOI] [Google Scholar]
- 27.Martin SJ, Helanterä H, Drijfhout FP. 2011. Is parasite pressure a driver of chemical cue diversity in ants? Proc. R. Soc. B 278, 496–503. ( 10.1098/rspb.2010.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner A, West SA. 2007. Social evolution: the decline and fall of genetic kin recognition. Curr. Biol. 17, R810–R812. ( 10.1016/j.cub.2007.07.030) [DOI] [PubMed] [Google Scholar]
- 29.Crozier RH. 1986. Genetic clonal recognition abilities in marine invertebrates must be maintained by selection for something else. Evolution 40, 1100–1101. ( 10.2307/2408769) [DOI] [PubMed] [Google Scholar]
- 30.Brandt M, Foitzik S, Fischer-Blass B, Heinze J. 2005. The coevolutionary dynamics of obligate ant social parasite systems: between prudence and antagonism. Biol. Rev. 80, 251–267. ( 10.1017/S1464793104006669) [DOI] [PubMed] [Google Scholar]
- 31.Foitzik S, DeHeer CJ, Hunjan DN, Herbers JM. 2001. Coevolution in host-parasite systems: behavioural strategies of slave-making ants and their hosts. Proc. R. Soc. Lond. B 268, 1139–1146. ( 10.1098/rspb.2001.1627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenoir A, D'Ettorre P, Errard C, Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. ( 10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 33.Dettner K, Liepert C. 1994. Chemical mimicry and camouflage. Annu. Rev. Entomol. 39, 129–154. ( 10.1146/annurev.en.39.010194.001021) [DOI] [Google Scholar]
- 34.D'Ettorre P, Mondy N, Lenoir A, Errard C. 2002. Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc. R. Soc. Lond. B 269, 1911–1918. ( 10.1098/rspb.2002.2110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuneoka Y, Akino T. 2012. Chemical camouflage of the slave-making ant Polyergus samurai queen in the process of the host colony usurpation (Hymenoptera: Formicidae). Chemoecology 22, 89–99. ( 10.1007/s00049-012-0101-2) [DOI] [Google Scholar]
- 36.Beibl J, Stuart RJ, Heinze J, Foitzik S. 2005. Six origins of slavery in formicoxenine ants. Insectes Soc. 52, 291–297. ( 10.1007/s00040-005-0808-y) [DOI] [Google Scholar]
- 37.Seifert B, Kleeberg I, Feldmeyer B, Pamminger T, Jongepier E, Foitzik S. 2014. Temnothorax pilagens sp. n. – a new slave-making species of the tribe Formicoxenini from North America (Hymenoptera, Formicidae). Zookeys 368, 65–77. ( 10.3897/zookeys.368.6423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward P, Brady SG, Fisher BL, Schultz TR. 2015. The evolution of myrmicine ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 40, 61–81. ( 10.1111/syen.12090) [DOI] [Google Scholar]
- 39.Alloway TM. 1979. Raiding behaviour of two species of slavemaking ants, Harpagoxenus americanus (Emery) and Leptothorax duloticus Wesson (Hymenoptera: Formicidae). Anim. Behav. 27, 202–210. ( 10.1016/0003-3472(79)90140-4) [DOI] [Google Scholar]
- 40.Kleeberg I, Pamminger T, Jongepier E, Papenhagen M, Foitzik S. 2014. Forewarned is forearmed: aggression and information use determine fitness costs of slave raids. Behav. Ecol. 25, 1058–1063. ( 10.1093/beheco/aru084) [DOI] [Google Scholar]
- 41.Kleeberg I, Foitzik S. 2016. The placid slavemaker: avoiding detection and conflict as an alternative, peaceful raiding strategy. Behav. Ecol. Sociobiol. 70, 27–39. ( 10.1007/s00265-015-2018-6) [DOI] [Google Scholar]
- 42.Brandt M, Heinze J, Schmitt T, Foitzik S. 2006. Convergent evolution of the Dufour's gland secretion as a propaganda substance in the slave-making ant genera Protomognathus and Harpagoxenus. Insectes Soc. 53, 291–299. ( 10.1007/s00040-006-0871-z) [DOI] [Google Scholar]
- 43.Jongepier E, Kleeberg I, Foitzik S. 2015. The ecological success of a social parasite increases with manipulation of collective host behaviour. J. Evol. Biol. 28, 2152–2162. ( 10.1111/jeb.12738) [DOI] [PubMed] [Google Scholar]
- 44.Alloway TM. 1990. Slave-species ant colonies recognize slavemakers as enemies. Anim. Behav. 39, 1218–1220. ( 10.1016/S0003-3472(05)80797-3) [DOI] [Google Scholar]
- 45.Brandt M, Foitzik S. 2004. Community context and specialization influence coevolution between a slavemaking ant and its hosts. Ecology 85, 2997–3009. ( 10.1890/03-0778) [DOI] [Google Scholar]
- 46.Nehring V, Dani FR, Turillazzi S, Boomsma JJ, d'Ettorre P. 2015. Integration strategies of a leaf-cutting ant social parasite. Anim. Behav. 108, 55–65. ( 10.1016/j.anbehav.2015.07.009) [DOI] [Google Scholar]
- 47.Pamminger T, Modlmeier AP, Suette S, Pennings PS, Foitzik S. 2012. Raiders from the sky: slavemaker founding queens select for aggressive host colonies. Biol. Lett. 8, 748–750. ( 10.1098/rsbl.2012.0499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foitzik S, Achenbach A, Brandt M. 2009. Locally adapted social parasite affects density, social structure and life history of its ant hosts. Ecology 90, 1195–1206. ( 10.1890/08-0520.1) [DOI] [PubMed] [Google Scholar]
- 49.Legendre P, Desdevises Y. 2009. Independent contrasts and regression through the origin. J. Theor. Biol. 259, 727–743. ( 10.1016/j.jtbi.2009.04.022) [DOI] [PubMed] [Google Scholar]
- 50.Paradis E. 2011. Analysis of phylogenetics and evolution with R. Berlin, Germany: Springer. [Google Scholar]
- 51.Kather R, Martin SJ. 2012. Cuticular hydrocarbon profiles as a taxonomic tool: advantages, limitations and technical aspects. Physiol. Entomol. 37, 25–32. ( 10.1111/j.1365-3032.2011.00826.x) [DOI] [Google Scholar]
- 52.Morrison WR, Witte V. 2011. Strong differences in chemical recognition cues between two closely related species of ants from the genus Lasius (Hymenoptera: Formicidae). J. Evol. Biol. 24, 2389–2397. ( 10.1111/j.1420-9101.2011.02364.x) [DOI] [PubMed] [Google Scholar]
- 53.Pokorny T, Lunau K, Quezada-Euan JJG, Eltz T. 2014. Cuticular hydrocarbons distinguish cryptic sibling species in Euglossa orchid bees. Apidologie 45, 276–283. ( 10.1007/s13592-013-0250-5) [DOI] [Google Scholar]
- 54.Brunner E, Kroiss J, Trindl A, Heinze J. 2011. Queen pheromones in Temnothorax ants: control or honest signal? BMC Evol. Biol. 11, 55 ( 10.1186/1471-2148-11-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scharf I, Bauer S, Fischer-Blass B, Foitzik S. 2011. Impact of a social parasite on ant host populations depends on host species, habitat and year. Biol. J. Linn. Soc. 103, 559–570. ( 10.1111/j.1095-8312.2011.01638.x) [DOI] [Google Scholar]
- 56.Martin SJ, Vitikainen E, Helanterä H, Drijfhout FP. 2008. Chemical basis of nest-mate discrimination in the ant Formica exsecta. Proc. R. Soc. B 275, 1271–1278. ( 10.1098/rspb.2007.1708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menzel F, Schmitt T. 2012. Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues. Evolution 66, 896–904. ( 10.1111/j.1558-5646.2011.01489.x) [DOI] [PubMed] [Google Scholar]
- 58.Schlick-Steiner BC, Steiner FM, Höttinger H, Nikiforov A, Mistrik R, Schafellner C, Baier P, Christian E. 2004. A butterfly's chemical key to various ant forts: intersection-odour or aggregate-odour multi-host mimicry? Naturwissenschaften 91, 209–214. ( 10.1007/s00114-004-0518-8) [DOI] [PubMed] [Google Scholar]
- 59.Allan RA, Capon RJ, Brown WV, Elgar MA. 2002. Mimicry of host cuticular hydrocarbons by salticid spider Cosmophasis bitaeniata that preys on larvae of tree ants Oecophylla smaragdina. J. Chem. Ecol. 28, 835–848. ( 10.1023/A:1015249012493) [DOI] [PubMed] [Google Scholar]
- 60.Brandt M, Heinze J, Schmitt T, Foitzik S. 2005. A chemical level in the coevolutionary arms race between an ant social parasite and its hosts. J. Evol. Biol. 18, 576–586. ( 10.1111/j.1420-9101.2004.00867.x) [DOI] [PubMed] [Google Scholar]
- 61.Liu ZB, Yamane S, Yamamoto H, Wang QC. 2000. Nestmate discrimination and cuticular profiles of a temporary parasitic ant Lasius sp. and its host L. fuliginosus (Hymenoptera, Formicidae). J. Ethol. 18, 69–74. ( 10.1007/s101640070002) [DOI] [Google Scholar]
- 62.Lambardi D, Dani FR, Turillazzi S, Boomsma JJ. 2007. Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 61, 843–851. ( 10.1007/s00265-006-0313-y) [DOI] [Google Scholar]
- 63.Pamminger T, Scharf I, Pennings P, Foitzik S. 2011. Increased host aggression as an induced defence against slavemaking ants. Behav. Ecol. 22, 255–260. ( 10.1093/beheco/arq191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson CA, Vander Meer RK, Lavine B. 2001. Changes in the cuticular hydrocarbon profile of the slave-maker ant queen, Polyergus breviceps Emery, after killing a Formica host queen (Hymenoptera : Formicidae). J. Chem. Ecol. 27, 1787–1804. ( 10.1023/A:1010456608626) [DOI] [PubMed] [Google Scholar]
- 65.Jongepier E, Kleeberg I, Job S, Foitzik S. 2014. Collective defence portfolios of ant hosts shift with social parasite pressure. Proc. R. Soc. B 281, 20140225 ( 10.1098/rspb.2014.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleeberg I, Jongepier E, Job S, Foitzik S. 2015. Geographic variation in social parasite pressure predicts intraspecific but not interspecific aggressive responses in hosts of a slavemaking ant. Ethology 121, 694–702. ( 10.1111/eth.12384) [DOI] [Google Scholar]
- 67.Achenbach A, Foitzik S. 2009. First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evolution 63, 1068–1075. ( 10.1111/j.1558-5646.2009.00591.x) [DOI] [PubMed] [Google Scholar]
- 68.Achenbach A, Witte V, Foitzik S. 2010. Brood exchange experiments and chemical analyses shed light on slave rebellion in ants. Behav. Ecol. 21, 948–956. ( 10.1093/beheco/arq008) [DOI] [Google Scholar]
- 69.Martin SJ, Takahashi J, Ono M, Drijfhout FP. 2008. Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J. Insect Physiol. 54, 700–707. ( 10.1016/j.jinsphys.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 70.Cini A, Gioli L, Cervo R. 2009. A quantitative threshold for nestmate recognition in a paper social wasp. Biol. Lett. 5, 459–461. ( 10.1098/rsbl.2009.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geiselhardt S, Lamm S, Gack C, Peschke K. 2010. Interaction of liquid epicuticular hydrocarbons and tarsal adhesive secretion in Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). J. Comp. Physiol. 196, 369–378. ( 10.1007/s00359-010-0522-8) [DOI] [PubMed] [Google Scholar]
- 72.Gibbs A. 1995. Physical properties of insect cuticular hydrocarbons: model mixtures and lipid interactions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 112, 667–672. ( 10.1016/0305-0491(95)00119-0) [DOI] [Google Scholar]
- 73.Gibbs A, Pomonis JG. 1995. Physical properties of insect cuticular hydrocarbons: the effects of chain lengths, methyl-branching and unsaturation. Comp. Biochem. Physiol. 112, 243–249. ( 10.1016/0305-0491(95)00081-X) [DOI] [Google Scholar]
- 74.Menzel F, Blüthgen N, Schmitt T. 2008. Tropical parabiotic ants: highly unusual cuticular substances and low interspecific discrimination. Front. Zool. 5, 16 ( 10.1186/1742-9994-5-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dargent F, Rolshausen G, Hendry AP, Scott ME, Fussmann GF. 2016. Parting ways: parasite release in nature leads to sex-specific evolution of defence. J. Evol. Biol. 29, 23–34. ( 10.1111/jeb.12758) [DOI] [PubMed] [Google Scholar]
- 76.Oppelt A, Heinze J. 2009. Mating is associated with immediate changes of the hydrocarbon profile of Leptothorax gredleri ant queens. J. Insect Physiol. 55, 624–628. ( 10.1016/j.jinsphys.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 77.Kocher SD, Grozinger CM. 2011. Cooperation, conflict, and the evolution of queen pheromones. J. Chem. Ecol. 37, 1263–1275. ( 10.1007/s10886-011-0036-z) [DOI] [PubMed] [Google Scholar]
- 78.Oi CA, van Zweden JS, Oliveira RC, Van Oystaeyen A, Nascimento FS, Wenseleers T. 2015. The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. BioEssays 37, 808–821. ( 10.1002/bies.201400180) [DOI] [PubMed] [Google Scholar]
- 79.Billeter J-C, Atallah J, Krupp JJ, Millar JG, Levine JD. 2009. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991. ( 10.1038/nature08495) [DOI] [PubMed] [Google Scholar]
- 80.Chernenko A, Holman L, Helanterä H, Sundström L. 2012. Cuticular chemistry of males and females in the ant Formica fusca. J. Chem. Ecol. 38, 1474–1482. ( 10.1007/s10886-012-0217-4) [DOI] [PubMed] [Google Scholar]
- 81.Beibl J, D'Ettorre P, Heinze J. 2007. Cuticular profiles and mating preference in a slave-making ant. Insectes Soc. 54, 174–182. ( 10.1007/s00040-007-0929-6) [DOI] [Google Scholar]
- 82.Oppelt A, Spitzenpfeil N, Kroiss J, Heinze J. 2008. The significance of intercolonial variation of cuticular hydrocarbons for inbreeding avoidance in ant sexuals. Anim. Behav. 76, 1029–1034. ( 10.1016/j.anbehav.2008.05.020) [DOI] [Google Scholar]
- 83.de Renobales M, Blomquist GJ. 1983. A developmental study of the composition and biosynthesis of the cuticular hydrocarbons of Trichoplusia ni (Lepidoptera: Noctuidae). Insect Biochem. 13, 493–502. ( 10.1016/0020-1790(83)90007-0) [DOI] [Google Scholar]
- 84.Buchanan K, et al. 2012. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 83, 301–309. ( 10.1016/j.anbehav.2011.10.031) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data presented here is provided in the electronic supplementary material.