Abstract
Across species, oxytocin, an evolutionarily ancient neuropeptide, facilitates social communication by attuning individuals to conspecifics' social signals, fostering trust and bonding. The eyes have an important signalling function; and humans use their salient and communicative eyes to intentionally and unintentionally send social signals to others, by contracting the muscles around their eyes and pupils. In our earlier research, we observed that interaction partners with dilating pupils are trusted more than partners with constricting pupils. But over and beyond this effect, we found that the pupil sizes of partners synchronize and that when pupils synchronously dilate, trust is further boosted. Critically, this linkage between mimicry and trust was bound to interactions between ingroup members. The current study investigates whether these findings are modulated by oxytocin and sex of participant and partner. Using incentivized trust games with partners from ingroup and outgroup whose pupils dilated, remained static or constricted, this study replicates our earlier findings. It further reveals that (i) male participants withhold trust from partners with constricting pupils and extend trust to partners with dilating pupils, especially when given oxytocin rather than placebo; (ii) female participants trust partners with dilating pupils most, but this effect is blunted under oxytocin; (iii) under oxytocin rather than placebo, pupil dilation mimicry is weaker and pupil constriction mimicry stronger; and (iv) the link between pupil constriction mimicry and distrust observed under placebo disappears under oxytocin. We suggest that pupil-contingent trust is parochial and evolved in social species in and because of group life.
Keywords: oxytocin, pupil dilation, social decisions, economic game, eye signal, mimicry
1. Introduction
Pivotal to social life is the ability to trust others—to have a positive expectation that others will cooperate and not exploit us [1–3]. Sometimes, assessments of trustworthiness derive from an elaborate evaluation of the risks involved and the extent to which possible benefits outweigh potential losses [4,5]. Often, trust is intuitive, affect-based and reflecting a ‘gut feeling’ based on the partner's physical features [6–10]. Across species, such ‘gut feeling’ may derive from a variety of sources, such as partners' bodily scents (in rodents [11,12]; in humans [13]), posture (in rodents [14]; in humans [15,16,17,18]) and emotional vocalizations (in rodents [19]; in chimpanzees [20]; in humans [21]).
One important yet understudied physical characteristic that may be used to form intuitive assessments of the partner's trustworthiness is the eye. In humans, the eye has a morphology that is unique among primates [22,23]. The eyes are crucially important during social communication and provide information to regulate interaction, express intimacy, exercise social control, and facilitate service and task goals [24]. Not surprisingly, the making of eye-contact provides a powerful mode of establishing each other's emotions and intentions [25], which can influence trust-based decisions [26].
During eye-contact, pupil sizes tend to synchronize across partners so that dilating pupils induce pupil dilation in the partner, and constricting pupils increase pupil constriction in the partner [27]. This pupil mimicry is already present during the first months of life [28] and is an evolutionarily old phenomenon shared with other species [29]. Recently, pupil mimicry in humans has been shown to relate to intuitive assessments of a partner's trustworthiness [26], as Dutch participants played trust-games with partners of whom just the eye region was visible and in which the pupils were manipulated to change in size. Results showed that participants' own pupils dilated synchronously with their partner's pupils. Importantly, this correlated with the extent to which participants trusted their partner, especially when partners also were from Caucasian descent (henceforth ingroup). With partners from Japanese descent (henceforth outgroup), there was no linkage whatsoever between pupil mimicry and trust.
Although pupil mimicry reflects an autonomic response that emerges outside conscious awareness and deliberate control, the mechanisms that link pupil mimicry to trust remain poorly understood [26]. One possibility is that the linkage is conditioned by oxytocin, an evolutionary ancient neuropeptide that acts as hormone and neurotransmitter. This possibility follows from two lines of evidence. First, the making of eye contact fosters the release of oxytocin in humans as well as in other species including dogs [30]. Furthermore, in closely bonded partners such as parents and their offspring, oxytocin levels tend to synchronize. This holds for humans [31], as well as for family groups of cooperatively breeding marmoset monkeys [32]. Second, oxytocin is intimately involved in the regulation of social bonding, affiliation and prosocial behaviour, again across a wide range of mammalian species. For example, oxytocin boosts pair-bond formation and paternal behaviour in prairi voles [33,34], ‘tend-and-defend’ patterns of affiliation in chimpanzees [35,36], and social approach and affiliation with conspecifics in dogs [37]. In humans, oxytocin increases sensitivity to one's partner's emotion expressions [38–40].
While eye-contact between partners promotes the release of oxytocin, and oxytocin levels synchronize during close partner interactions and appear to facilitate pro-social exchange and affiliation, there is evidence also that these effects of oxytocin are ingroup bounded [41]. In both humans and chimpanzees, oxytocin increases trust and cooperation with familiar others and members of one's ingroup [35,41–45]. At the same time, oxytocin appears to upregulate defensive shielding and vigilance vis-à-vis outsiders and unfamiliar others. This tendency has been observed in humans [43], marmosets [46], California mice [47], female rats [48], prairie voles [49] and wild chimpanzees [36]. For example, one study demonstrated that prairie voles show increased partner-directed grooming toward familiar but not unfamiliar conspecifics that experienced an unobserved stressor, but that blocking the oxytonergic circuitry abolished this partner-directed response [49]. Also, Samuni et al. [36] showed stronger patterns of oxytocin-mediated ingroup affiliation among wild chimpanzees prior to intergroup fighting.
Taken together, there is reason to assume that the pupil dilation mimicry–trust linkage that emerges with ingroup partners is conditioned by oxytocin. We tested this possibility here, with healthy males and females. We focused on humans because of the catching morphology of the human eye, which sets it apart from most other species [22], and because humans have frequent encounters with unfamiliar others. We included both male and female subjects because animal studies show sex differences in how oxytocin influences behaviour [50,51]. Thus, a more exploratory goal of the present study was to examine possible sex differences in the interplay between pupil mimicry, oxytocin and ingroup trust (see also [52–54]).
2. Methods
In two separate sessions, participants received oxytocin or placebo and made investment decisions in incentivized trust games with different virtual ingroup or outgroup partners. Per game or trial, participants could invest €0, €2, €4 or €6 in their partner, knowing that investments would be tripled (i.e. €2 becomes €6 for the trustee), and that by the end of the experiment their earnings would be paid out in the form of a bonus. They did not receive feedback regarding trustees' payback decisions during the experiment. Prior to decision-making, participants viewed a short clip of their partner's eye region in which pupils dilated, constricted or remained static.
(a). Participants
Fifty-nine students (22 years old; 28 males) without neurological or psychiatric history participated in two 1 h sessions with two weeks in-between. The sample size is comparable with our earlier study on pupil mimicry [26] as well as with studies on oxytocin and human decision-making [43,44,55,56]. In both sessions participants were placed in the role of investor, yet in one session they received oxytocin and in the other placebo (double-blind, randomized cross-over). All participants were born and raised in the Netherlands, with Dutch parents.
(b). Medication
Before the experiment, participants completed a medical screening, and we invited those without a significant neurological or psychiatric history, who did not use prescription-based medication, smoked less than five cigarettes per day and did not report drug or alcohol abuse. Eligible participants were assigned to a session and instructed to refrain from smoking or drinking (except water) for 2 h before the experiment. At the beginning of each session, participants self-administered a single intranasal dose of 24 IU oxytocin (Syntocinon spray, Novartis; three puffs per nostril, each with 4 IU oxytocin, with 2 min interval between puffs) or placebo. To avoid any subjective effects (for example, olfactory effects) other than those caused by oxytocin, the placebo contained all the active ingredients except for the neuropeptide. Placebos were delivered in the same bottles as Syntocinon. Thus, participants and experimenters were ignorant about treatment conditions [42].
(c). Stimuli
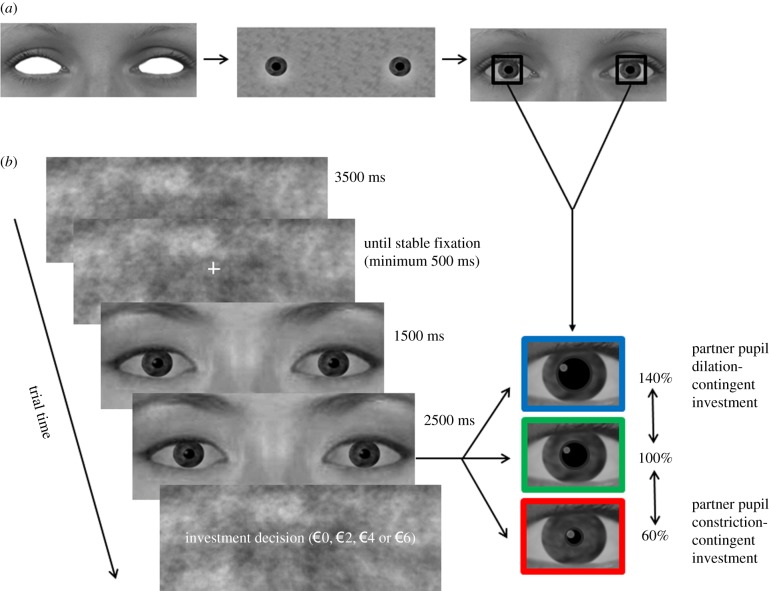
The stimulus material was similar to that used in our previous study [26]. Pictures of the eye region of Dutch (ingroup) and Japanese (outgroup) actors were included. Within the eye region, an artificial pupil was added to dynamically change in size. Specifically, after static presentation for 1500 ms, the pupil remained either static or dilated or constricted within the physiological range over 1500 ms. In the last second, the pupils were static. The eye images appeared life-size on the computer screen. We verified that images of the partners reflected ingroup/outgroup differences. (figure 1; electronic supplementary materials).
Figure 1.
(a) Stimulus characteristics and (b) sample trial sequence. To create partner stimuli, we removed the eyes from pictures of the eye regions of faces and then added the same eye white, iris and pupil to each stimulus (independent of partner's group). In each trial, a scrambled image of a stimulus was presented for 4000 ms. The scrambled image was then replaced by the stimulus itself. In all conditions, the stimulus remained static for the first 1500 ms, but in the dilation and constriction conditions, the pupils gradually changed in size over the following 1500 ms and then remained at that size during the final 1000 ms (in the static condition, pupils remained at the same size throughout the trial). Finally, a screen appeared asking participants to decide to transfer €0, €2, €4 or €6 to their partner. (Online version in colour.)
(d). Procedure
Participants were seated individually and provided written informed consent. The experimenter then handed participants a nasal spray and left after they self-administered oxytocin or placebo. Because treatment effects tend to emerge especially after 30 min of loading time [57], participants continued with otherwise irrelevant questionnaires and survey. After 30 min elapsed, the actual experiment began. The participant sat at a distance of 60 cm from the computer screen and read instructions. They read that they had €6, of which they could invest €0, €2, €4 or €6 in partners. Each investment would be tripled. It was emphasized that the partners had participated earlier and indicated for each possible investment how much they would reciprocate (this was indeed the case, with additional participants acting as trustees in an earlier session, and participants' investment decisions were coupled to these partner decisions to calculate actual payoffs). Participants were further told that recordings had been made of these partners, that these recordings had been manipulated and that they would see them before they had to make their investment decision, to give them an idea about what sort of person they would interact with. They were further told that Dutch and Japanese students were participating and were asked to indicate via button-press whether they themselves were from the University of Amsterdam or from the University of Tokyo.
After participants had correctly answered three practice questions, they started with a nine-point calibration of the eye-tracker (EyeLink, SR Research, Ottawa, Canada; screen-type: ViewSonic VG732M, 1280 × 1024 pixels), followed by the start of the first trial. To minimize pupil constriction following new information that is presented on the screen, a trial started with the presentation of a unique Fourier-scrambled image of the actual stimulus. This scrambled image was presented for 3500 ms. After 3500 ms, a fixation cross appeared on top of the scrambled image and lasted until participants fixated properly. Next, an image of partners' eyes with dilating, static or constricting pupil size was presented for 4000 ms. After the offset of the image, participants were instructed to make an investment decision.
After the experiment, participants were asked whether they had noted anything special about the partners' eyes and what they thought the study was about. Although participants indicated being aware of the dynamics in partners' pupil size, none of them suspected that we were interested in pupil mimicry and its link with trust.
(e). Trustee decisions
Participant (investors) payments were based on back-transfer decisions (i.e. decisions about the amount they would transfer back to their partners) made by 15 other students (2 males, 13 females, mean age 24 years, range 18–55 years old) in the role of trustee, who were given a form with 10 investment decisions of others (€0–€10) and asked how much they would reciprocate given a certain investment. These back-transfer decisions were randomly chosen and paired to those made by participants in the main experiment, to calculate actual earnings. For each trial, we randomly drew a decision to calculate participants' earnings after the experiment was over.
(f). Statistical analyses
Data were analysed with linear mixed multi-level models, allowing the estimation of individual differences by modelling variances of slopes and intercepts. Model selection started with a full model including as fixed factors the partner's group (ingroup/outgroup), partner's pupil (dilating/static/constricting), participant's treatment (oxytocin/placebo) and their interactions. After each single removal of one factor, we compared the more parsimonious model with the more complex model with a log-likelihood ratio test (LLRT). If the result of the LLRT determined that the term under consideration did not increase model fit, it was removed; otherwise it was kept. After defining the fixed factors, model selection proceeded with the random factors. We first added four random factors (a random slope and intercept for each subject and for each subject × trial) and similar to the back-fitting process of the fixed factors, defined the random factors. Crucially, we were able to include time as a repeated factor with a first-order autoregressive (AR1) covariance structure to control for auto-correlation with regard to time in the pupillometry analyses. These models additionally included linear, quadratic and cubic polynomials as fixed and random factors to precisely model the slope of participants pupil size. Given the large number of factors, we focus on effects that include the factor partner pupil. Especially the pupillometry models contain a large number of fixed factors due to the interactions with the polynomials. For that reason, when modelling participants' pupil size, we additionally limit ourselves to effects that survive a threshold of p < 0.005 (full reports are provided in the electronic supplementary material, Results).
Pupil responses were analysed over the last 2500 ms of stimulus presentation (i.e. from the moment partners' pupils started to change in size). Pupil-size data were down-sampled to 100 ms timeslots and smoothed with a 10th-order low-pass Butterworth filter. The 500 ms just before the partners' pupils started to change were used as baseline and subtracted from subsequent data points.
3. Results
(a). Investments
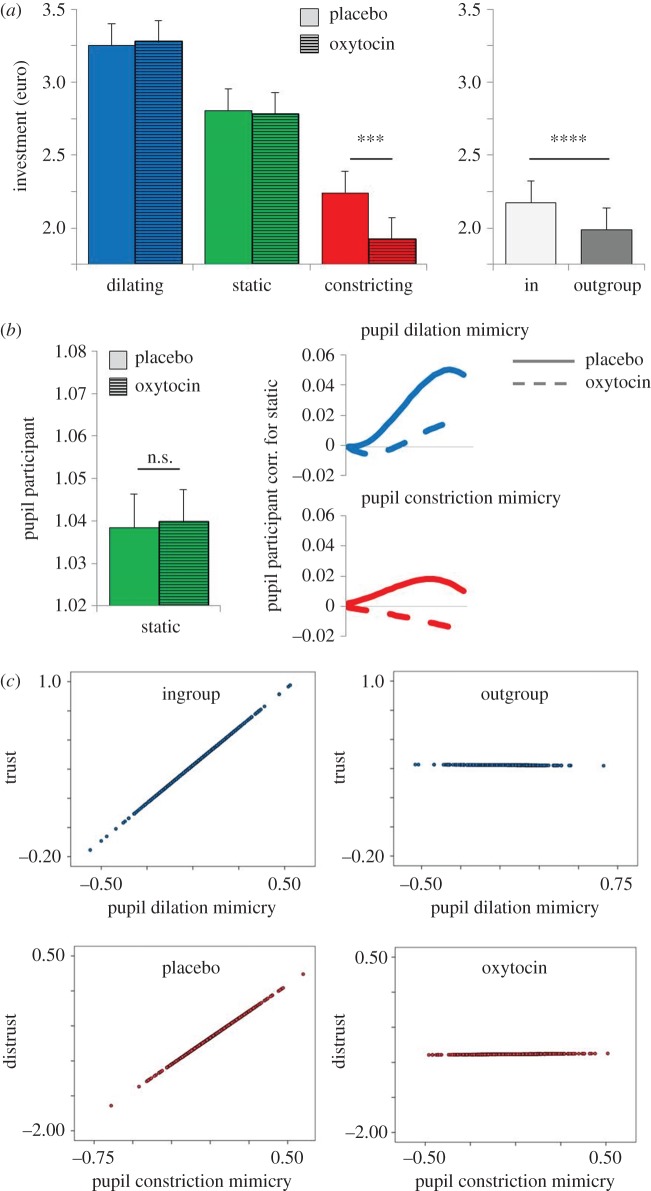
Effects of partner pupil (F2,5.213 = 247,184, p < 0.001) and group (F1,5.213 = 18,332, p < 0.001) showed that partners with dilating pupils and partners from the ingroup were trusted more than partners with static or constricting pupils and partners from the outgroup. The significant treatment × partner pupil interaction (F2,5.213 = 6.683, p = 0.001) showed that participants given oxytocin invested less in partners with constricting pupils than participants given placebo (figure 2a). This effect was further qualified by a treatment × sex participant × pupil partner interaction. The effect shown in figure 2a was present for male, but not for female participants (males p = 0.005; females p = 0.276). In addition, whereas males given oxytocin rather than placebo trusted partners with dilating pupils (p = 0.010), females trusted partners with dilating pupils less under oxytocin than under placebo (p = 0.031). There were no effects of treatment on investments when partner's pupils remained static (all ps > 0.602; electronic supplementary material, figure S1 and table S1).
Figure 2.
(a–c) All visualizations represent predicted data by the best-fitting statistical models. Error bars signify the standard error of the means. Dilation (constriction) mimicry (b,c) was measured by subtracting participants' pupil size when partners' pupils were static from their pupil size when partner's pupils dilated (constricted). Trust (distrust) in (c) denotes participants' investments in partners with dilating (constricting) pupils minus investments in partners with static pupils. *p < 0.05; **p < 0.01. (Online version in colour.)
(b). Pupil mimicry
To examine whether the current experiment replicates the results reported in [26], we computed their analytic model (i.e. first without the factors treatment and sex). As in that study, we find evidence for pupil mimicry, as is demonstrated by a main effect of partner pupil (F2,5630.400 = 9.731, p < 0.001) and a two-way interaction between pupil partner and the linear term (F2,82975.646 = 75.904, p < 0.001). Thus, participants' pupils were larger and dilated faster when observing a partner with dilating as compared with static or constricting pupils (electronic supplementary material, table S2). These effects were independent of looking times (electronic supplementary material, tables S8 and S9) or potential differences between the sexes or treatment groups in their level of tonic arousal (electronic supplementary material, table S10). With this successful replication of earlier findings, we proceeded with testing the moderating influence of oxytocin, and explored effects of partner and participant sex. Results are described separately for pupil dilation mimicry and for pupil constriction mimicry.
(c). Pupil dilation mimicry
A main effect of pupil partner showed that participants' pupils were larger when viewing partners with dilating as compared to static pupils (F1,3736.291 = 16.263, p < 0.001). A pupil partner × linear term interaction showed that participants' pupils also increased faster over stimulus presentation time than when viewing static pupils (F1,56039.191 = 111.880, p < 0.0001). A treatment × pupil partner interaction, in conjunction with the significant treatment × pupil partner × linear term interaction (F1,50754.286 = 16.839, p < 0.001 and F1,3736.808 = 8.877, p = 0.003) showed that pupil dilation mimicry was weaker under oxytocin as compared with placebo (figure 2b; electronic supplementary material, table S3). Effects of sex of partner and participant on pupil mimicry did not survive our statistical threshold and are reported in electronic supplementary material, table S3.
In a separate linear mixed multi-level model, we investigated the effects of pupil dilation mimicry, partner group, treatment and their interactions (fixed factors) on investment decisions (dependent variable). As in [26], we find that pupil dilation mimicry increased partner–pupil contingent trust in interactions with ingroup partners (F1,718.900 = 4.367, p = 0.037), but not in interactions with outgroup partners (F1,710.885 = 0.000, p = 0.989). Treatment did not modulate this general tendency, and nor did sex of partner or sex of participant (electronic supplementary material, tables S4 and S5).
(d). Pupil constriction mimicry
A pupil partner × quadratic term interaction showed that when viewing partners with constricting pupils, participants' pupils initially increased in size and then quickly decreased, resulting in a greater curvature of the slope as compared with when viewing partners with static pupils (F1,15.381 = 225966.099, p < 0.001). The treatment × pupil partner × linear term interaction, in conjunction with the treatment × pupil partner × quadratic term interaction, showed that pupil constriction mimicry was stronger under oxytocin as compared with placebo (F1,15.975 = 22590.950, p < 0.001; F1,9.794 = 225963.865, p = 0.002; figure 2b).
As noted, our design enabled us to explore effects of sex of partner and participant on pupil mimicry. We observed, first of all, a sex participant × sex partner × pupil partner interaction, showing that pupil constriction mimicry was stronger during interactions with a partner of the opposite as compared to the same sex (F1,9.040 = 225748.689, p = 0.003). Second, there was a sex participant × pupil partner × group partner × quadratic term interaction (F1,8.981 = 225972.102, p = 0.003). This effect was mainly driven by male participants whose pupils, after an initial increase in size, started to constrict following outgroup eyes with static pupils (electronic supplementary material, table S6).
In a separate model, we investigated the putative relationship between pupil constriction mimicry, partner group, treatment (fixed factors) and investments (dependent variable). Results showed an interaction between treatment and pupil constriction mimicry (F1,1441.258 = 7.053, p = 0.008), demonstrating that among participants given placebo, more constriction mimicry associated with lower trust (F1,716.611 = 8.445, p = 0.004); under oxytocin, this linkage disappeared (F1,735.116 = 0.002, p = 0.965; figure 2c; electronic supplementary material, table S7).
A summary of the key findings is provided in table 1.
Table 1.
Summary of results. Overview of the main results of the study. dil., dilating; con, constricting; n.s., not significant.
| fixed factors | investments/trust | dil. mimicry | con. mimicry |
|---|---|---|---|
| partner pupil | dil. > static > con. | dil. > static | n.s. |
| treatment | oxytocin = placebo | n.s. | n.s. |
| partner pupil × partner group | n.s. | n.s. | n.s. |
| partner pupil × treatment | oxytocin lowers trust in con. pupils | dil. mimicry: oxytocin < placebo | con. mimicry: oxytocin > placebo |
| partner pupil × partner sex | n.s. | n.s. | n.s. |
| partner pupil × participant sex | n.s. | n.s. | n.s. |
| treatment × pupil partner × sex participant | oxytocin boosts trust in dil. pupils in males, but lowers it in females | n.s. | n.s. |
| sex participant × sex partner × pupil partner | n.s. | n.s. | con. mimicry: opposite > same sex |
| pupil dil. mimicry—trust linkage | pupil dil. mimicry predicts trust in ingroup | ||
| pupil con. mimicry—distrust linkage | pupil con. mimicry predicts distrust under placebo |
4. Discussion
Across species, oxytocin can promote a wide range of affiliative behaviours including cooperation and trust [35,37,46], but depending on the context, can also have antisocial effects and reduce cooperation and trust [36,45,58,59]. The current study confirmed that quick and intuitive decisions to trust are influenced by (i) group membership of the trustee, (ii) trustee's pupil size and (iii) participants' tendency to mimic changes in trustees pupil size, and (iv) that both oxytocin and sex of participant and trustee further moderated these effects. We show that pupil size plays an important role during social interactions. Below we argue that pupil size may be a physiological marker of trust in other social species than humans as well.
On a daily basis, social animals decide quickly and intuitively whether or not to trust an interaction partner. Especially in humans, this is an important ability given that most people live in large cities where they know only a very small percentage of those they encounter in daily life. Yet although the way humans live today is unique compared with other animals, where unfamiliar individuals often pose genuine threats to the sorts of small, tightly bound groups of intimates, it is important to note that the human genome developed within much smaller, closely bound groups of people where encounters with strangers were more rare [60]. It stands to reason that both the neurocognitive mechanisms and the types of conspecifics' signals humans use when making trust decisions regarding strangers are shared with other social species.
In the instance of being confronted with a stranger, we mostly rely on the physical characteristics of the other. In humans and non-human primates one heuristic for whether to trust another individual is to categorize him or her as ingroup or outgroup, labels which often predict the tone of a social interaction and the behaviours employed [61,62]. Apart from physical characteristics pointing to familiarity and group membership, other characteristics trust decisions can be based upon are expressions of emotions, social intentions and interest. In that respect the eye region is most expressive and attracts most attention [22,23]. We use the eyes to quickly identify who is who [63], and who belongs to what group [64]; and although humans have particularly expressive eyes, this tendency is not limited to humans. For example, dogs also recognize conspecifics and humans by paying special attention to the eyes [65]. Apart from identity recognition, the eye region is crucially important during social interactions and owes its expressiveness to the fine muscles around the eyes and to the pupils, both expressing internal states of mind including emotions, social interest and trust [66]. Our recent research suggests that these positive signals are partly derived through pupil mimicry. That is, by looking into another's dilated pupils, our own pupils dilate in response, providing some sort of feedback signal that presumably helps us to trust the other person better [26]. In a study comparing humans and chimpanzees, Kret et al. [29] demonstrated that chimpanzees mimicked the pupil sizes of chimpanzees but not humans, and that humans mimicked the pupil sizes of humans but not chimpanzees. Thus, pupil mimicry, like other forms of mimicry [67], is not uniquely human but is present in at least one other species as well. Because pupil size can provide very useful information about the cognitive or emotional state of the expressor, it is likely to be a physiological marker of trust in a broader range of social species.
The current paper investigated the social and neurobiological boundaries of the relationship between pupil mimicry and trust in humans. Data replicated the effects reported in [26]. As observed earlier, participants trusted partners with dilating pupils more than partners with static or constricting pupils, and trusted partners with constricting pupils less than partners with static pupils (figure 2a). Moreover, participants' pupils mimicked the pupil size of their partner (figure 2b). Finally, pupil dilation mimicry correlated with higher investments in partners with dilating as compared with static pupils, and this effect was bound to interactions with the ingroup (figure 2c).
Data extend this earlier work in three ways. First, oxytocin led male but not female participants to withhold trust from partners with constricting pupils (figure 2a). This fits with recent accounts that in case of untrustworthy or unreliable partners, oxytocin can dampen trust [68]. Nature has never rewarded naivety and from an evolutionary perspective it can be inferred that oxytocin does not boost trust unconditionally, but rather that it increases vigilance and a stimulus-congruent ‘sharpening’ of perceived social signals (in humans [46,69,70]; in different species of rodents [46–49]). Along similar lines, Lambert et al. [71] observed that oxytocin improved humans' discriminatory ability of untrustworthy but not trustworthy faces. Previous studies in humans investigating the somewhat disputed link between oxytocin and trust mostly included male participants (i.e. [72]). In line with that earlier work, we here find that under oxytocin, male participants trusted partners with dilating pupils more than under placebo. However, as often observed in animal studies [50,51], an opposite pattern was observed for females who trusted these partners less under oxytocin than under placebo (although still more than partners with static or constricting pupils). Similarly, in humans Feng et al. [54] showed that oxytocin increased the salience of positive social cues in men, while decreasing their reward value in women (see also [73]).
A second key finding of the current study is that oxytocin weakened pupil dilation mimicry, and strengthened pupil constriction mimicry (figure 2b). This finding is similar to a recent study on facial mimicry where oxytocin increased the mimicry of angry faces but had no effect on the mimicry of happy faces [74]. Another recent study showed that oxytocin enhanced inter-brain synchrony during a social coordination but not a control task. This effect, however, was bound to male subjects and was not observed in females as they already had high baseline levels of synchrony [75]. Somewhat relatedly, a recent magneto-encefalogram (MEG) study showed that oxytocin modulated social brain processes differently in combat veterans than in controls when watching images probing social synchrony (in the case of this particular study, coordinated combat action [76]). Thus, oxytocin's effect on mimicry or synchronization may be valence- and saliency-dependent. Research on the effect of oxytocin on synchronous behaviour in other animals is scarce. One study in pigs found no effects of oxytocin on the mimicry of positive or negative emotions, yet some effects were found on the non-treated observing pigs [77].
Finally, whereas pupil dilation mimicry and ingroup partner–pupil contingent trust were not conditioned by oxytocin, oxytocin did condition pupil constriction mimicry and its link with distrust. Specifically, the link between constriction mimicry and lower levels of trust was present under placebo, and absent under oxytocin. Possibly, under oxytocin trust was already so low in interactions with partners with constricting pupils that constriction mimicry could not add much to it.
The current study included only humans, and although we presume similar processes are at stake in other social animals as well, comparative research is needed to confirm this presumption [78]. In a recent study, Engelmann et al. [79] tested chimpanzees on a trust game. The results demonstrate that in interacting with a conspecific, chimpanzees showed spontaneous trust in a novel context, flexibly adjusted their level of trust to the trustworthiness of their partner and developed patterns of trusting reciprocity over time. At least in some contexts, then, trust in reciprocity is not unique to humans, but rather has its evolutionary roots in the social interactions of humans' closest primate relatives. As trust and cooperation among human strangers is common [80,81] and evolutionarily advantageous [82], an important question for future studies is whether chimpanzees will trust unfamiliar conspecifics in a trust game and, if so, which cues they rely on when deciding to do so. In fact, our other closest living relatives, bonobos, show striking tolerance towards strangers and share food with them [83,84]. However, we lack experimental evidence regarding how trust develops and whether relationship formation differs between related species with diverse social backgrounds.
Another topic of interest for future studies is the usage of pupillary signals across species. In all mammals, pupillary responses are involuntary, and apart from responding automatically to light levels, also reflect cognitive emotional states (e.g. in macaques [85,86]). But the avian eye is different in this regard, as pupillary size is under voluntary control. Rapid fluctuations in pupil size are used in communication, and depending on the context, they indicate positive or negative excitement [87]. How and whether these pupillary signals are picked up by conspecifics and are used in social decisions is not known.
In summary, this study investigated the relationship between pupil mimicry and trust, and its socio-endocrine boundaries in humans. Oxytocin lowered trust extended to partners with constricting pupils and also enhanced the mimicry of this signal. Although oxytocin dampened pupil dilation mimicry, this had no effect on the level of trust that was extended to partners with dilating pupils. Whereas pupil dilation mimicry boosted trust in ingroup members, pupil constriction mimicry related to distrust in ingroup and outgroup partners alike, but only in the placebo condition. The results of the current study underline the importance of the eyes and their subtle and autonomic expressions, reflecting one's own and mirroring others' state of mind during social interactions. Moreover, this study demonstrates that group membership matters even at very basic levels of interaction, and that oxytocin treatment can profoundly change this fundamental relationship between pupil mimicry, on the one hand, and the emergence of interpersonal trust on the other. Pupil mimicry may be a particularly relevant tool for humans to use when trusting strangers, as interactions with strangers occur so frequently. Nonetheless, the mechanism itself may be phylogenetically older and shared across species.
Supplementary Material
Acknowledgements
We thank Luisa and Eliska Prochazkova and Christina Diatchkova for testing the subjects.
Ethics
The experiment received approval from the University Research Ethics Board (f 2013-WOP-2757). Procedures adhered to international standards of good research and clinical practice.
Data accessibility
Supporting data are available from https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/ZFXQI6 [88].
Authors' contributions
M.E.K. and C.K.W.D.D. conceived of the project; M.E.K. coordinated data collection and performed analyses; M.E.K. drafted the manuscript and C.K.W.D.D. provided critical revisions.
Competing interests
We declare we have no competing interests.
Funding
M.E.K. was supported by the Netherlands Science Foundation (VENI # 016-155-082) and C.K.W.D.D. by a Research Fellowship at the Netherlands Institute for Advanced Study, Wassenaar, the Netherlands.
References
- 1.Pruitt DG, Kimmel M. 1977. Twenty years of experimental gaming: critique, synthesis, and suggestions for the future. Annu. Rev. Psychol. 28, 363–392. ( 10.1146/annurev.ps.28.020177.002051) [DOI] [Google Scholar]
- 2.Bateson P. 2000. The biological evolution of cooperation and trust. In Trust: making and breaking cooperative relations (ed. Gambetta D.), pp. 14–30. Oxford, UK: Blackwell. [Google Scholar]
- 3.Brambilla M, Leach CW. 2014. On the importance of being moral: the distinctive role of morality in social judgment. Soc. Cogn. 32, 397–408. ( 10.1521/soco.2014.32.4.397) [DOI] [Google Scholar]
- 4.Bohnet I, Zeckhauser R. 2004. Trust, risk and betrayal. J. Econ. Behav. Organ. 55, 467–484. ( 10.1016/j.jebo.2003.11.004) [DOI] [Google Scholar]
- 5.Houser D, Schunk D, Winter J. 2010. Distinguishing trust from risk: an anatomy of the investment game. J. Econ. Behav. Organ. 74, 72–81. ( 10.1016/j.jebo.2010.01.002) [DOI] [Google Scholar]
- 6.Todorov A, Uleman JS. 2002. Spontaneous trait inferences are bound to actors’ faces: evidence from a false recognition paradigm. J. Pers. Soc. Psychol. 83, 1051–1065. ( 10.1037/0022-3514.83.5.1051) [DOI] [PubMed] [Google Scholar]
- 7.Fetchenhauer D, Groothuisch T, Pradela J. 2010. Not only states but traits—humans can identify permanent altruistic dispositions in 20 s. Evol. Hum. Behav. 31, 80–86. ( 10.1016/j.evolhumbehav.2009.06.009) [DOI] [Google Scholar]
- 8.Stirrat M, Perrett DI. 2010. Valid facial cues to cooperation and trust: male facial width and trustworthiness. Psychol. Sci. 21, 349–354. ( 10.1177/0956797610362647) [DOI] [PubMed] [Google Scholar]
- 9.Stewart LH, Ajina S, Getov S, Bahrami B, Todorov A, Rees G. 2012. Unconscious evaluation of faces on social dimensions. J. Exp. Psychol. Gen. 141, 715–727. ( 10.1037/a0027950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toscano H, Schubert TW. 2015. Judged and remembered trustworthiness of faces is enhanced by experiencing multisensory synchrony and asynchrony in the right order. PLoS ONE 10, e0145664 ( 10.1371/journal.pone.0145664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valenta JG, Rigby MK. 1968. Discrimination of the odor of stressed rats. Science 161, 599–601. ( 10.1126/science.161.3841.599) [DOI] [PubMed] [Google Scholar]
- 12.Kondoh K, Lu Z, Ye X, Olson DP, Lowell BB, Buck LB. 2016. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature 532, 103–106. ( 10.1038/nature17156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groot JHB, Smeets MAM, Kaldewaij A, Duijndam MJA, Semin GR. 2012. Chemosignals communicate human emotions. Psychol. Sci. 23, 1417–1424. ( 10.1177/0956797612445317) [DOI] [PubMed] [Google Scholar]
- 14.Pereira AG, Cruz A, Lima SQ, Moita MA. 2012. Silence resulting from the cessation of movement signals danger. Curr. Biol. 22, R627–R628. ( 10.1016/j.cub.2012.06.015) [DOI] [PubMed] [Google Scholar]
- 15.Kret ME, de Gelder B. 2010. Perceiving bodies in a social context. Exp. Brain Res. 203, 169–180. ( 10.1007/s00221-010-2220-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kret ME, Roelofs K, Stekelenburg J, de Gelder B. 2013. Emotional signals from faces, bodies and scenes influence observers’ face expressions, fixations and pupil-size. Front. Hum. Neurosci. 7, 810 ( 10.3389/fnhum.2013.00810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kret ME, Stekelenburg J, Roelofs K, de Gelder B. 2013. Perception of face and body expressions using electromyography, pupillometry and gaze measures. Front. Psychol. 4, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Valk JM, Wijnen J, Kret ME. 2015. Anger fosters action. Fast responses in a motor task involving approach movements towards angry faces and bodies. Front. Psychol. 6, 1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofer MA. 1996. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology 21, 203–217. ( 10.1016/0306-4530(95)00042-9) [DOI] [PubMed] [Google Scholar]
- 20.Slocombe KE, Zuberbühler K. 2007. Chimpanzees modify recruitment screams as a function of audience composition. Proc. Natl Acad. Sci. USA 104, 17 228–17 233. ( 10.1073/pnas.0706741104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauter D, Eisner F, Ekman P, Scott SK. 2010. Cross-cultural recognition of basic emotions through nonverbal emotional vocalizations. Proc. Natl Acad. Sci. USA 107, 2408–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi H, Kohshima S. 1997. Unique morphology of the human eye. Nature 387, 767–768. ( 10.1038/42842) [DOI] [PubMed] [Google Scholar]
- 23.Tomasello M, Hare B, Lehmann H, Call J. 2007. Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. J. Hum. Evol. 52, 314e320. ( 10.1016/j.jhevol.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 24.Emery NJ. 2000. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604. ( 10.1016/S0149-7634(00)00025-7) [DOI] [PubMed] [Google Scholar]
- 25.Senju A, Johnson MH. 2009. The eye contact effect: mechanisms and development. Trends Cogn. Sci. 13, 127–134. ( 10.1016/j.tics.2008.11.009) [DOI] [PubMed] [Google Scholar]
- 26.Kret ME, Fischer A, De Dreu CKW. 2015. Pupil-mimicry correlates with trust in in-group partners with dilating pupils. Psychol. Sci. 26, 1401–1410. ( 10.1177/0956797615588306) [DOI] [PubMed] [Google Scholar]
- 27.Harrison NA, Singer T, Rotshtein P, Dolan RJ, Critchley HD. 2006. Pupillary contagion: central mechanisms engaged in sadness processing. Soc. Cogn. Affect. Neurosci. 1, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fawcett C, Wesevich V, Gredebäck G. 2016. Pupillary contagion in infancy. Evidence for spontaneous transfer of arousal. Psychol. Sci. 27, 997–1003. ( 10.1177/0956797616643924) [DOI] [PubMed] [Google Scholar]
- 29.Kret ME, Tomonaga M, Matsuzawa T. 2014. Chimpanzees and humans mimic pupil-size of conspecifics. PLoS ONE 9, e104886 ( 10.1371/journal.pone.0104886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, Onaka T, Mogi K, Kikusui T. 2015. Oxytocin-gaze positive loop and the coevolution of human–dog bonds. Science 348, 333–336. ( 10.1126/science.1261022) [DOI] [PubMed] [Google Scholar]
- 31.Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. 2011. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav. Dev. 34, 569–577. ( 10.1016/j.infbeh.2011.06.008) [DOI] [PubMed] [Google Scholar]
- 32.Finkenwirth C, van Schaik C, Ziegler TE, Burkart JM. 2015. Strongly bonded family members in common marmosets show synchronized fluctuations in oxytocin. Physiol. Behav. 1, 246–251. ( 10.1016/j.physbeh.2015.07.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho MM, DeVries AC, Williams JR, Carter CS. 1999. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci. 113, 1071–1079. ( 10.1037/0735-7044.113.5.1071) [DOI] [PubMed] [Google Scholar]
- 34.Kenkel WM, Suboc G, Carter CS. 2014. Autonomic, behavioral and neuroendocrine correlates of paternal behavior in male prairie voles. Physiol. Behav. 128, 252–259. ( 10.1016/j.physbeh.2014.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbühler K. 2014. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 281, 20133096 ( 10.1098/rspb.2013.3096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuni L, Preisa A, Mundrya R, Deschnera T, Crockforda C, Wittiga RM. 2016. Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proc. Natl Acad. Sci. USA 114, 268–273. ( 10.1073/pnas.1616812114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero T, Nagasawa M, Mogi K, Hasegawa T, Kikusui T. 2014. Oxytocin promotes social bonding in dogs. Proc. Natl Acad. Sci. USA 111, 9085–9090. ( 10.1073/pnas.1322868111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leknes S, Wessberg J, Ellingsen DM, Chelnokova O, Olausson H, Laeng B. 2013. Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions. Soc. Cogn. Affect. Neurosci. 8, 741–749. ( 10.1093/scan/nss062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahrestani S, Kemp AH, Guastella AJ. 2013. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology 38, 1929–1936. ( 10.1038/npp.2013.86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Dreu CKW, Kret ME, Sauter DA. 2016. Assessing emotional vocalizations from cultural in-group and out-group depends on oxytocin. Soc. Psychol. Personality Sci. 7, 837–846. ( 10.1177/1948550616657596) [DOI] [Google Scholar]
- 41.De Dreu CKW, Kret ME. 2016. Oxytocin conditions intergroup relations through upregulated ingroup empathy, cooperation, conformity, and defense. Biol. Psychiatry 79, 165–173. ( 10.1016/j.biopsych.2015.03.020) [DOI] [PubMed] [Google Scholar]
- 42.De Dreu CKW, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SWW. 2010. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328, 1408–1411. ( 10.1126/science.1189047) [DOI] [PubMed] [Google Scholar]
- 43.De Dreu CKW, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA. 2012. Oxytocin modulates selection of allies in intergroup conflict. Proc. R. Soc. B 279, 1150–1154. ( 10.1098/rspb.2011.1444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Dreu CK, Shalvi S, Greer LL, Van Kleef GA, Handgraaf MJ. 2012. Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. PLoS One 7, e46751 ( 10.1371/journal.pone.0046751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbühler K, Deschner T. 2013. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 280, 20122765 ( 10.1098/rspb.2012.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mustoe A, Cavanaugh J, Harnisch AM, French J. 2015. Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Horm. Behav. 71, 83–90. ( 10.1016/j.yhbeh.2015.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinman MQ, et al. 2015. Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol. Psychiatry 80, 406–414. ( 10.1016/j.biopsych.2015.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosch OJ. 2013. Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Phil. Trans. R. Soc. B 368, 20130085 ( 10.1098/rstb.2013.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burkett JP, Andari E, Johnson ZV, Curry CD, de Waal FBM, Young LJ. 2016. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. ( 10.1126/science.aac4785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. 2009. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev. Neurosci. 31, 332–341. ( 10.1159/000216544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bales KL, Carter CS. 2003. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Horm. Behav. 44, 178–184. ( 10.1016/S0018-506X(03)00154-5) [DOI] [PubMed] [Google Scholar]
- 52.Daughters K, Manstead ASR, Ten Velden FS, De Dreu CKW. 2017. Oxytocin modulates third-party sanctioning of selfish and generous behavior within and between groups. Psychoneuroendocrinology 77, 18–24. ( 10.1016/j.psyneuen.2016.11.039) [DOI] [PubMed] [Google Scholar]
- 53.Rilling JK, et al. 2014. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39, 237–248. ( 10.1016/j.psyneuen.2013.09.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. 2015. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 9, 754–764. ( 10.1007/s11682-014-9333-9) [DOI] [PubMed] [Google Scholar]
- 55.De Dreu CKW, Scholte HS, Van Winden FAAM, Ridderinkhof KR. 2015. Oxytocin tempers calculated greed but not impulsive defense in predator–prey contests. Soc. Cogn. Affect. Neurosci. 5, 721–728. ( 10.1093/scan/nsu109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ten Velden FS, Daughters K, De Dreu CKW. In press Oxytocin promotes intuitive rather than deliberated cooperation with the ingroup. Horm. Behav. [DOI] [PubMed] [Google Scholar]
- 57.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. 2008. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58, 639–650. ( 10.1016/j.neuron.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 58.Brosnan SF, Leverett K, Heyler C, Flemmin T, Talbot CF, Zak PJ, Essler JL, Dougall P. 2015. Oxytocin reduces food sharing in capuchin monkeys by modulating social distance. Behaviour 152, 941–961. ( 10.1163/1568539X-00003268) [DOI] [Google Scholar]
- 59.Harrison N, Lopes PC, König B. 2017. Oxytocin administration during early pair formation delays communal nursing in female house mice. Anim. Behav. 123, 61–68. ( 10.1016/j.anbehav.2016.10.030) [DOI] [Google Scholar]
- 60.Henrich J, et al. 2010. Markets, religion, community size, and the evolution of fairness and punishment. Science 327, 1480–1484. ( 10.1126/science.1182238) [DOI] [PubMed] [Google Scholar]
- 61.Macy MW, Skvoretz J. 1998. The evolution of trust and cooperation between strangers: a computational model. Am. Sociol. Rev. 63, 638–660. ( 10.2307/2657332) [DOI] [Google Scholar]
- 62.Pokorny JJ, de Waal FBM. 2009. Monkeys recognize the faces of group mates in photographs. Proc. Natl Acad. Sci. USA 106, 21 539–21 543. ( 10.1073/pnas.0912174106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith ML, Volna B, Ewing L. 2016. Distinct information critically distinguishes judgments of face familiarity and identity. J. Exp. Psychol. Hum. Percept. Perform. 42, 1770–1779. ( 10.1037/xhp0000243) [DOI] [PubMed] [Google Scholar]
- 64.Adams RB, et al. 2010. Cross-cultural reading the mind in the eyes: an fMRI Investigation. J. Cogn. Neurosci. 22, 97–108. ( 10.1162/jocn.2009.21187) [DOI] [PubMed] [Google Scholar]
- 65.Somppi S, Törnqvist H, Hänninen L, Krause CM, Vainio O. 2014. How dogs scan familiar and inverted faces: an eye movement study. Anim. Cogn. 17, 793–803. ( 10.1007/s10071-013-0713-0) [DOI] [PubMed] [Google Scholar]
- 66.Kret ME. 2015. Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Front. Psychol. 6, 711 ( 10.3389/fpsyg.2015.00711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Waal FBM. 2008. Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 59, 279–300. ( 10.1146/annurev.psych.59.103006.093625) [DOI] [PubMed] [Google Scholar]
- 68.Mikolajczak M, Gross JJ, Lane A, Corneille O, de Timary P, Luminet O. 2010. Oxytocin makes people trusting, not gullible. Psychol. Sci. 21, 1072–1074. ( 10.1177/0956797610377343) [DOI] [PubMed] [Google Scholar]
- 69.Barazza JA, Zak PJ. 2013. Oxytocin instantiates empathy and produces prosocial behaviours. In Oxytocin, vasopressin and related peptides in the regulation of behaviour (eds Choleris E, Pfaff DW, Kavaliers M), pp. 331–342. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 70.Shamay-Tsoory SG, Abu-Akel A. 2016. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202. ( 10.1016/j.biopsych.2015.07.020) [DOI] [PubMed] [Google Scholar]
- 71.Lambert B, Declerck CH, Boone C. 2014. Oxytocin does not make a face appear more trustworthy but improves the accuracy of trustworthiness judgments. Psychoneuroendocrinology 40, 8 ( 10.1016/j.psyneuen.2013.10.015) [DOI] [PubMed] [Google Scholar]
- 72.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. 2005. Oxytocin increases trust in humans. Nature 435, 673–676. ( 10.1038/nature03701) [DOI] [PubMed] [Google Scholar]
- 73.Hoge EA, Anderson E, Lawson EA, Bui E, Fischer LE, Khadge SD, Barrett LF, Simon NM. 2014. Gender moderates the effect of oxytocin on social judgments. Hum. Psychopharmacol. Clin. Exp. 29, 299–304. ( 10.1002/hup.2402) [DOI] [PubMed] [Google Scholar]
- 74.Korb S, Malsert J, Strahearn L, Vuilleumier P, Niedenthal P. 2016. Sniff and mimic—intranasal oxytocin increases facial mimicry in a sample of men. Horm. Behav. 84, 64–74. [DOI] [PubMed] [Google Scholar]
- 75.Mu Y, Guo C, Han S. 2016. Oxytocin enhances interbrain synchrony during social coordination in male adults. Soc. Cogn. Affect. Neurosci. 11, 1882–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levy J, Goldstein A, Zagoory-Sharon O, Weisman O, Schneiderman I, Eidelman-Rothman M, Feldman R. 2016. Oxytocin selectively modulates brain response to stimuli probing social synchrony. Neuroimage 124, 923–930. ( 10.1016/j.neuroimage.2015.09.066) [DOI] [PubMed] [Google Scholar]
- 77.Reimert I, Bolhuis JE, Kemp B, Rodenburg TB. 2015. Emotions on the loose: emotional contagion and the role of oxytocin in pigs. Anim. Cogn. 18, 517–532. ( 10.1007/s10071-014-0820-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Rooijen R, Ploeger A, Kret ME. The dot-probe task to measure emotional attention: a suitable measure in comparative studies? Psychon. Bull. Rev. doi: 10.3758/s13423-016-1224-1. In press. [DOI] [PubMed] [Google Scholar]
- 79.Engelmann JM, Hermann E, Tomasello M. 2015. Chimpanzees trust conspecifics to engage in low-cost reciprocity. Proc R. Soc. B 282, 20142803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fehr E, Fischbacher U. 2003. The nature of human altruism. Nature 425, 785–791. ( 10.1038/nature02043) [DOI] [PubMed] [Google Scholar]
- 81.Gächter S, Falk A. 2002. Reputation and reciprocity: consequences for the labour relation. Scand. J. Econ. 104, 1–26. ( 10.1111/1467-9442.00269) [DOI] [Google Scholar]
- 82.Hill KR, Wood BM, Baggio J, Hurtado AM, Boyd RT. 2014. Hunter–gatherer inter-band interaction rates: Implications for cumulative culture. PLoS ONE 9, e102806 ( 10.1371/journal.pone.0102806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Itani G. 1990. Relations between unit-groups of bonobos at Wamba, Zaire: encounters and temporary fusions. Afr. Study Monogr. 11, 153–186. [Google Scholar]
- 84.Tan J, Hare B. 2013. Bonobos share with strangers. PLoS ONE 8, e51922 ( 10.1371/journal.pone.0051922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conway CA, Jones BC, DeBruine LM, Little AC, Sahraie A. 2008. Transient pupil constrictions to faces are sensitive to orientation and species. J. Vis. 8, 17.1–11. [DOI] [PubMed] [Google Scholar]
- 86.Ebitz RB, Platt ML. 2015. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron 85, 628–640. ( 10.1016/j.neuron.2014.12.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor DK, Lee VK, Strait KR. 2015. The laboratory bird. Boca Raton, FL: CRC Press. [Google Scholar]
- 88.Kret ME, De Dreu CKW. 2017. Data from: Pupil-mimicry conditions trust in partners: moderation by oxytocin and group membership. (https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/ZFXQI6) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kret ME, De Dreu CKW. 2017. Data from: Pupil-mimicry conditions trust in partners: moderation by oxytocin and group membership. (https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/ZFXQI6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Supporting data are available from https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/ZFXQI6 [88].