Abstract
Movement and growth habit of climbing plants have attracted attention since the time of Charles Darwin; however, there are no reports on whether plants can choose suitable hosts or avoid unsuitable ones based on chemoreception. Here, I show that the tendrils of Cayratia japonica (Vitaceae) appear to avoid conspecific leaves using contact chemoreception for oxalates, which are highly concentrated in C. japonica leaves. The coiling experiments show that C. japonica has a flexible plastic response to avoid coiling around conspecific leaves. The coiling response is negatively correlated with the oxalate content in the contacted leaves. Experiments using laboratory chemicals indicate that the tendrils avoid oxalate-coated plastic sticks. These results indicate that the tendrils of C. japonica avoid coiling around a conspecific leaf based on contact chemoreception for oxalate compounds. The tendrils of climbing plants may function as a chemoreceptor system to detect the chemical cues of a contacted plant.
Keywords: chemical cue, climbing plant, decision making, species recognition, thigmomorphogenesis, plant behaviour
1. Introduction
To acquire information about surrounding plants, plants have evolved a range of sensory systems, including mechanoreception, photoreception and chemoreception of volatiles [1–7]. Tendrils of climbing plants are modified leaves, leaflets, shoots, and stipules and appear to have evolved independently in many families [8,9]. They can start coiling quickly after a minute mechanical stimulus of 1 mg or less [10–12]. Therefore, tendrils have been studied extensively as a model system for the mechanoreception of plants since the time of Charles Darwin [11,13]. However, to the best of my knowledge, there have been no studies on whether tendrils have the ability to sense the chemical cues of contacted objects.
Unlike most plant species, climbing plants can grow rapidly both vertically and horizontally [9]. Therefore, similar to animal species, climbing plants have opportunities to choose favourable hosts—in this case, the plants on which they can climb successfully. Because the colonization of a favourable habitat is a key event in the life history of climbing plants [14], a preference for suitable hosts and the avoidance of unsuitable hosts might be essential for their survival and reproduction. However, there has been no evidence that climbing plants can select suitable host plants and avoid unsuitable host plants by sensing the chemical cues of contacted objects (except for parasitic plants [15]), despite a long history of research on climbing plants [8,9,11,13,14].
For species that climb using tendrils, conspecific leaves (including the plant's own leaves) would typically be the most frequently contacted objects. However, coiling around conspecific leaves is likely to represent a non-adaptive behaviour, because a conspecific plant can act as a stronger competitor for space and light than other plant species [16–18]. Moreover, conspecifics might be unstable supports for coiling because a climbing plant itself is not stable as a host plant. Therefore, natural selection will favour a plastic response to avoid coiling around conspecific leaves in tendril climbers. In this study, I examined this idea in the tendrils of the perennial vine Cayratia japonica (Vitaceae). Because C. japonica often dominates its growing habitats [19–21], avoiding the surrounding leaves of conspecific plants might be essential for finding a suitable host plant to climb.
Here, I provide evidence that tendrils of climbing plants exhibit contact chemoreception for cues of conspecific leaves and achieve a flexible plastic response to avoid coiling around them. First, I determined that C. japonica tendrils avoid coiling around conspecific leaves. Then, I tried to identify the cues of the conspecific leaf that inhibited the coiling response of C. japonica tendrils. I noticed that the tendrils of C. japonica did not coil around the leaves of Oxalis debilis. Oxalis, as well as Cayratia species, contain high concentrations of oxalate compounds in their leaves [22,23]. Thus, I hypothesized that oxalate compounds are the cues of conspecific leaves that inhibited the coiling response of C. japonica tendrils. To test the hypothesis, I examined the correlation between leaf oxalate contents and coiling response to the leaf and observed the coiling response to oxalate-coated plastic sticks. Finally, I observed the coiling response of Momordica charantia (Cucurbitaceae) tendrils to the oxalate-coated plastic sticks to confirm that the oxalates are species-specific cues of C. japonica.
2. Material and methods
In April and May 2015, I collected 36 C. japonica rhizomes from four triploid populations on the Japanese islands: 16 rhizomes from Hirosaki University, Aomori Prefecture; seven rhizomes from the Tokyo University of Agriculture and Technology, Tokyo; four rhizomes from Kyoto University, Kyoto Prefecture; and nine rhizomes from Kyushu University, Fukuoka Prefecture. The rhizomes were cut into segments, 30–40 cm long, and transplanted individually into pots containing a commercial vermiculite product (‘Golden’ vermiculite; Iris Ohyama Co. Ltd., Sendai, Japan). These individuals were grown under natural conditions in an experimental field and used in the no-choice experiment. In addition, 30 rhizomes were collected from the Tokyo population in August 2015 and transplanted using the same method. These individuals were grown indoors at 25°C with a 16 L : 8 D photoperiod and were used in the other experiments. The plants were watered sufficiently. Plants of at least 20 cm in height and with at least one tendril were used. Each tendril was used only once.
(a). Coiling responses of C. japonica to conspecific and heterospecific leaves
I conducted three types of coiling experiments: no-choice, choice and uncoiling experiments (electronic supplementary material, figure S1). The no-choice experiment was designed to examine the tendril's ability to identify a conspecific leaf when it contacted a single specific object. The choice experiment was designed to examine the sensitivity and resolution of the discrimination to conspecific and heterospecific leaves when the tendril contacted both leaf types at the same time. The uncoiling experiment was designed to examine the ability to change or cancel a coiling response that had already begun.
(i). No-choice experiment
The no-choice experiment began on 15 July and concluded on 27 August 2015. At 8.00 h, I chose six to 10 plants with fully expanded tendrils and transferred them into the experimental room at the Tokyo University of Agriculture and Technology. The room temperature was controlled at 26 ± 3°C. The tendrils were then placed in contact with a leaf-wrapped bamboo stick, a conspecific stem, or a naked bamboo stick (electronic supplementary material, figure S1a). I used leaf-wrapped bamboo sticks instead of leaves alone, to control the shape of the contacted object. The fresh wrapping leaf was obtained from plants of the following species: C. japonica (Vitaceae) as a conspecific leaf, and Setaria viridis (Poaceae), Pueraria lobata (Fabaceae), Solidago canadensis (Asteraceae), Conyza sumatrensis (Asteraceae), and Boehmeria nivea (Urticaceae) as heterospecific leaves. These heterospecific species coexisted with C. japonica in its natural populations at the Tokyo University of Agriculture and Technology and were therefore potentially suitable host plants. All leaves were collected each morning during the study period from a natural population on the campus of Tokyo University of Agriculture and Technology and were kept fresh by placing them in a tub with water until the start of the experiment. In addition, I prepared two types of treatments for the conspecific leaves (dried and covered) to provide additional information on the characteristics of potential candidates for conspecific signals. For the dried leaves, the conspecific leaf-wrapped bamboo stick was dried at 35°C for 12 h. For the covering treatment, the conspecific leaf was covered with a widely spaced plastic mesh (approximately 3 mm). For the tendril placed in contact with a conspecific stem, the stem was randomly selected from a live plant from one of the four source populations. The coiling responses were scored as no change (less than 5°), slightly coiled (tendrils rotated from 10° to 175° around the object) or completely coiled (≥180° or more) 1, 2, 3, 4 and 5 h after contact. The experiment was completed within 5 h to prevent drying of the leaves. The diameter of the contacted objects ranged from 7 to 19 mm. In all experiments, tendrils that were naturally detached from an object were recorded as having 0° degree of coiling. For this experiment, we used total 209 tendrils from 53 plants of four source populations (73, 66, 9 and 60 tendrils for Aomori, Tokyo, Kyoto and Fukuoka populations, respectively).
(ii). Choice experiment
The choice experiment started on 1 December and was completed on 10 December 2015. The tendril was sandwiched between a stick wrapped with a conspecific leaf and a stick wrapped with a heterospecific leaf (electronic supplementary material, figure S1b). In this experiment, I used Se. viridis as a typical heterospecific species. The left and right positions for the conspecific and heterospecific leaves were chosen alternately. After sandwiching the tendril, I recorded the direction and degree of coiling at 5° increments at 15, 30, 45 and 60 min after contact. Coiling that exceeded 180° was recorded as 180°. We used total 20 tendrils from 20 plants of Tokyo population.
(iii). Uncoiling experiment
The uncoiling experiment started on 8 October and completed on 15 October 2015. First, I placed a bamboo stick in contact with a tendril (electronic supplementary material, figure S1c). When the tendril had coiled around the stick by 90° ± 10°, the tendril was assigned to one of three treatments: detaching, heterospecific or conspecific. In the detaching treatment, the slightly coiled tendrils were detached from the bamboo stick but not reattached to any other object. In the heterospecific treatment, the tendrils were detached from the bamboo stick and placed in contact with a stick wrapped with a heterospecific leaf. In the conspecific treatment, the tendrils were detached from the bamboo stick and placed in contact with a stick wrapped with a conspecific leaf. In the heterospecific and conspecific treatments, the leaf contacted the inner edge of the tendril's curve. In this experiment, I used Se. viridis as the heterospecific leaf. I recorded the coiling and uncoiling responses under five categories: completely uncoiled (less than 5°), uncoiled (10° to 80°), no change (85° to 95°), slightly coiled (100° to 175°) and completely coiled (≥180°) at 1, 2, 3, 4 and 5 h after the treatments. We used a total of 31 tendrils from 15 plants of the Tokyo population (12, 12 and 7 tendrils for conspecific, heterospecific and detaching treatments, respectively).
(b). Oxalate contents and coiling response
To elucidate the relationship between leaf oxalate contents and the coiling response of tendrils to the leaves, the amounts of soluble and total oxalates were quantified in the leaves of 10 plant species: Spinacia oleracea, O. debilis, Rumex japonicus, C. japonica, Colocasia esculenta, Se. viridis, So. canadensis, Co. sumatrensis, P. lobata and B. nivea. The first five species are known as high-oxalate plants. In addition, the oxalate contents of C. japonica stem were measured. The leaves of each species were collected from wild populations or commercially grown plants, and their fresh weight was measured. After the leaves were dried at 80°C for 24 h, their dry weights were measured. The dried samples were then ground into a powder using a Tissue Lyser II (Qiagen, Hilden, Germany) for 3 min at 25 Hz. Then, 8.0–13.0 mg of the samples was placed into a 15 ml centrifuge tube; 5 ml of distilled de-ionized water was added to one set of weighed samples, and 5 ml of 2 M hydrochloric acid was added to the other set. Extraction in water provides an estimate of the soluble oxalate content, whereas extraction in hydrochloric acid provides an estimate of the total oxalate content. The insoluble oxalate content is the difference between the total and soluble contents.
The tubes were placed in a water bath at 80°C for 15 min. The extract was then allowed to cool and centrifuged at 3000 r.p.m. (approximate centrifugal force of 3000g). The supernatant was filtered through a Whatman #2 filter paper into a 15 ml centrifuge tube for storage. The oxalate contents of all samples were estimated through an enzymatic reaction using a commercially available oxalate kit (EOXA-100 Oxalate Assay kit, Bioassay Systems, Hayward, CA, USA). Before oxalate determination, 100 µl of each extract in hydrochloric acid was transferred into a 1.5 ml tube and mixed with 100 µl of 2 M sodium hydroxide solution to neutralize it. Oxalate quantification was performed using a standard protocol (https://www.bioassaysys.com/Datasheet/EOXA.pdf, electronic supplementary material).
To quantify the coiling response of C. japonica to the leaves of the 10 plant species, I conducted a no-choice experiment using the same method described above. Coiling was recorded in increments of 5° at 1, 2, 3, 4 and 5 h after contact. Finally, I calculated the mean coiling angle, soluble oxalate content and insoluble oxalate content for the leaves of each species. The sample sizes of experiments measuring coiling and oxalate contents were 10 and five, respectively, for each of 10 plant species.
(c). Coiling response to chemicals
To identify substances that inhibited the coiling response of C. japonica tendrils, their coiling responses to several chemicals were compared: agarose, citric acid, calcium oxalate, potassium oxalate and sodium oxalate (Wako Pure Chemicals Co. Ltd., Osaka, Japan). Agarose and citric acid were chosen for the control treatments because agarose is pH-neutral and chemically stable, and citric acid, like oxalate, is an organic acid. Each chemical was powdered finely in a mortar and attached to the top 20 mm of a plastic stick coated with an adhesive (Daiso Sangyo Inc., Hiroshima, Japan). All tendrils were placed in contact either with one of the chemicals or with a plastic stick without any chemical or adhesive (as a control). I recorded the degree of coiling in increments of 5° at 15, 30, 45 and 60 min after contact. Tendrils with a degree of coiling of 180° or greater were recorded as 180°. The sample sizes were 11, 11, 10, 10, 16 and 17 for control, agarose, citric acid, calcium oxalate, potassium oxalate and sodium oxalate, respectively.
(d). Coiling response of a Cucurbitaceae tendril to oxalate
To examine the effect of oxalates on the coiling response of a tendril from another climbing species, a no-choice experiment was conducted (as described above) using commercially grown M. charantia (Cucurbitaceae). The degree of coiling was recorded in increments of 5° at 60 min after contact with agarose or calcium oxalate attached to a plastic stick. The sample size was 10 for both treatments.
(e). Statistical analysis
The results of the no-choice experiment of C. japonica tendrils were analysed using an ordinal logistic regression. The models were fitted using the clm function in the ‘ordinal’ package for the R software. The degree of coiling at 5 h after contact was adopted as the response variable. The following four comparisons were performed: between the conspecific fresh leaf and a fresh leaf from one of the heterospecific species, between the conspecific fresh leaf and the treated (dried and mesh-covered) leaves, between the conspecific fresh leaf and the conspecific stem, and between the conspecific fresh leaf and the bamboo stick. For comparisons between conspecific and heterospecific leaves, the type of contacted leaf was used as the explanatory variable. For comparisons between fresh and treated (dried and covered) conspecific leaves, the condition of the contacted leaf was used as the explanatory variable. This analysis was performed separately for each treatment. For comparisons between the conspecific leaf and the stem, the type of contacted plant part (stem or leaf) and the relationship between the source population of the tendril and the contacted stem or leaf (inter- or intra-population) were used as explanatory variables. For comparisons between the conspecific leaf and bamboo stick, the type of contacted object (conspecific leaf or stick) was used as the explanatory variable. The likelihood-ratio test was used to evaluate the significance of the explanatory variables in these analyses.
For the choice experiment, a repeated binomial test was used to determine whether the tendril preferred to coil around conspecific or heterospecific leaves. We used treatment (observed binomial data versus randomly generated binomial data), elapsed time, and treatment × time interaction as response variables. If no significant interaction was found, the interaction term was omitted from the model.
For the uncoiling experiment, a χ2 test was used to examine the effect of each treatment type (control, conspecific leaf, heterospecific leaf) on the coiling response at 5 h after contact.
To analyse the coiling response of C. japonica to the chemicals, the same method as described above for the no-choice experiment (ordinal logistic regression) was used, with the degree of coiling (i.e. the category) at 60 min after contact used as the response variable. The actual coiling angle was not used as the response variable because the data had unequal variances. However, the statistical results were the same when the actual coiling angle was used as the response variable (data not shown). The type of chemical was used as the explanatory variable. To identify the chemical that induced an avoidance response by the tendril, the coiling response to each chemical was compared separately with that of the control treatment. For the analysis of the coiling response of M. charantia to calcium oxalate, the same analysis was used.
To examine the effect of soluble and insoluble oxalate contents in the leaves of the 10 species on the coiling response of C. japonica, generalized linear models were fitted to the mean coiling angle for each species of contacted leaf as the response variable, and the mean soluble and insoluble oxalate contents, and their interaction, as the explanatory variables. For this analysis, a Gaussian distribution with the identity link function was applied.
3. Results
(a). Coiling responses of C. japonica to conspecific and heterospecific leaves
(i). No-choice experiment
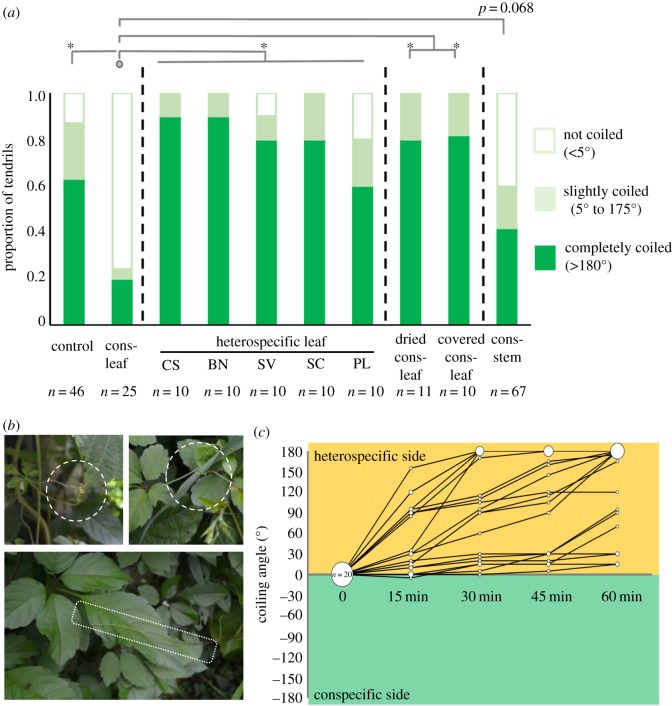
The tendrils were significantly less likely to coil around a conspecific fresh leaf than around a bamboo stick, heterospecific leaf, dried conspecific leaf and a covered conspecific leaf at 5 h after the start of the experiment (figure 1a, with temporal changes shown in the electronic supplementary material, figure S2, and table, S1). In fact, the tendrils that coiled around conspecific leaves were rarely observed in the field (personal communication; figure 2b bottom, a tendril that contacted with, but did not coil around the conspecific leaves; figure 2b top, tendrils that coiled around leaves of other species). There was a marginal difference between coiling responses to the conspecific leaf and stem (electronic supplementary material, table S1).
Figure 1.
Coiling response of Cayratia japonica to conspecific and heterospecific leaves. (a) The results of the no-choice experiment. The tendrils that contacted a bamboo stick (the control), a stick wrapped with a fresh conspecific (Cons) leaf, a fresh heterospecific leaf (CS, Conyza sumatrensis; BN, Boehmeria nivea; SV, Setaria viridis; SC, Solidago canadensis; and PL, Pueraria lobata), a dried conspecific leaf, a mesh-covered conspecific leaf, or a conspecific stem at 5 h after the start of the experiment. Asterisks indicate statistically significant differences (p < 0.01). (b) Observations in the field. The tendrils coiled around P. lobate (upper left) and Se. viridis (upper right) and did not coil around the conspecific leaf (bottom). (c) Temporal changes in the coiling angle in the choice experiment. Data are shown for Se. viridis as the heterospecific plant. Each line represents the coiling response of an individual tendril at 15, 30, 45 and 60 min after the start of the experiment. Circle size is proportional to the number of tendrils that achieved a given degree of coiling.
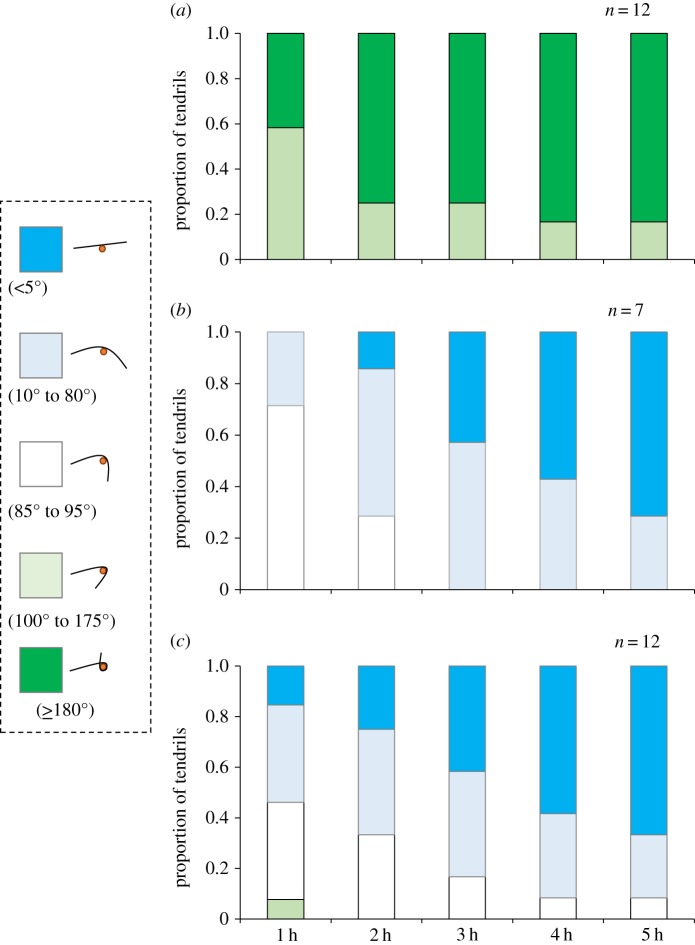
Figure 2.
Temporal changes in the degree of tendril coiling of Cayratia japonica in the uncoiling experiment. The tendril was detached from a bamboo stick and (a) placed in contact with a stick wrapped with a heterospecific leaf, (b) left detached, or (c) placed in contact with a stick wrapped in a conspecific leaf.
(ii). Choice experiment
The tendrils were significantly more likely to avoid coiling around a conspecific leaf, and they strongly preferred coiling around heterospecific leaves (χ21 = 113.65, p < 0.001; figure 1c). By the end of this experiment, none of the tendrils had coiled around the conspecific leaves. There is no significant effect of elapsed time on the result (χ21 = 1.29, p = 0.246).
(iii). Uncoiling experiment
The tendril response differed significantly among the three treatments (χ28 = 32.001, p < 0.001). All tendrils in the heterospecific treatment continued to coil around the heterospecific leaves (by at least 100°; figure 2a), whereas all tendrils in the conspecific and detached treatments stopped coiling and started uncoiling within 2 h (figure 2b,c). As a result, tendrils were significantly more likely to coil around a heterospecific leaf than a conspecific leaf within 5 h after the start of the experiment (χ24 = 24.0, p < 0.001).
(b). Oxalate content and coiling response
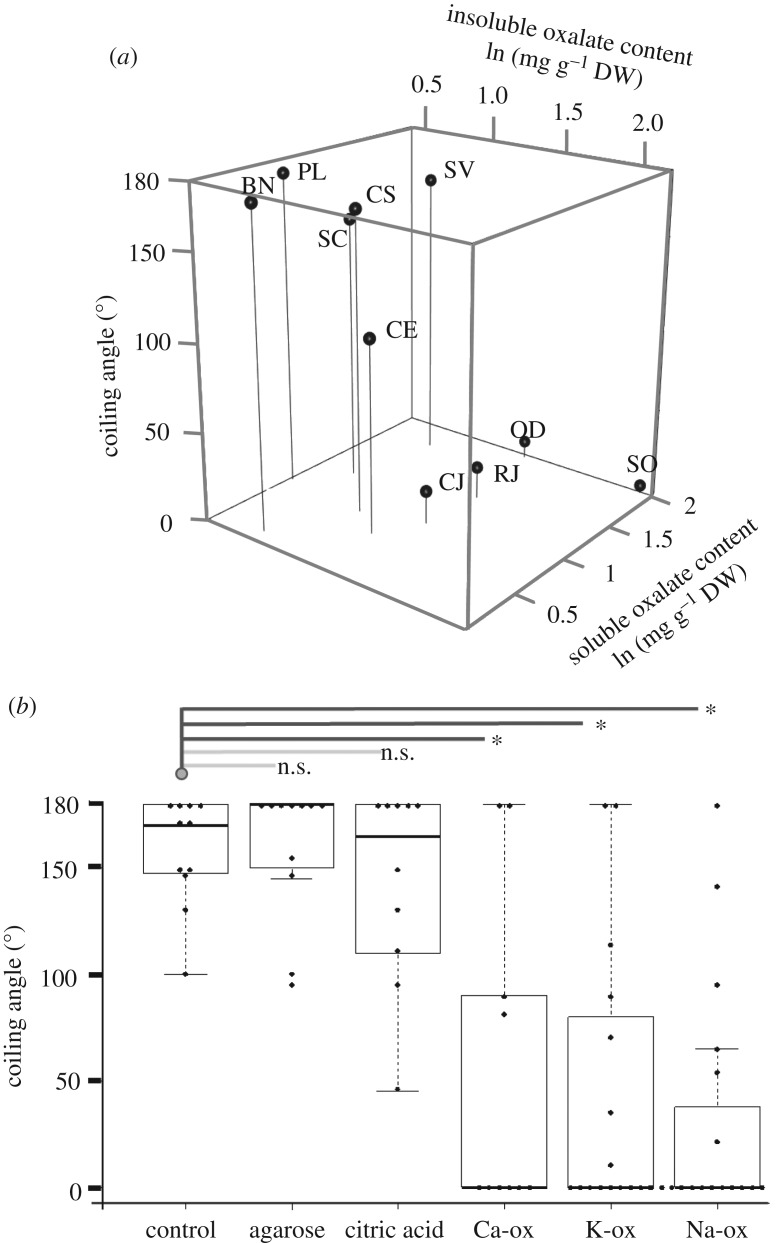
There are large variations in the soluble and insoluble oxalate contents in leaves of each plant species and stem of C. japonica (electronic supplementary material, table S2). The contents of both soluble and insoluble oxalates in the leaves of each plant were negatively correlated with the coiling response to the leaves (figure 3a). There was a significant interaction between soluble and insoluble oxalate content on the coiling response, suggesting that soluble and insoluble oxalates have a synergistic effect on the inhibition of the coiling response (electronic supplementary material, table S3).
Figure 3.
Coiling response to the leaves and chemicals. (a) The three-dimensional plot showing the relationships among the coiling responses of Cayratia japonica to fresh leaves from 10 species (SO, Spinacia oleracea; OD, Oxalis debilis; RJ, Rumex japonicas; CJ, Cayratia japonica; CE, Colocasia esculenta; SV, Setaria viridis; SC, Solidago Canadensis; CS, Conyza sumatrensis; PL, Pueraria lobate; BN, Boehmeria nivea) and the leaf contents of soluble and insoluble oxalates. Each dot represents the mean coiling angle, soluble oxalate content and insoluble oxalate content of each species. Details are provided in the electronic supplementary material, table S3. (b) The scatter box plot indicating the coiling angles of tendrils in response to a stick coated with agarose, citric acid, calcium oxalate (Ca-ox), potassium oxalate (K-ox), sodium oxalate (Na-ox) or without any chemical coating (the control) 1 h after contact. Asterisks indicate statistically significant differences (p < 0.01).
(c). Coiling response to chemicals
The tendrils were significantly less likely to coil around a stick coated with calcium oxalate, potassium oxalate or sodium oxalate than around the control (figure 3b; electronic supplementary material, table S4); by contrast, the agarose and citric acid coatings did not produce a significantly different response than the response to the control.
(d). Coiling response of a Cucurbitaceae tendril to oxalate
There was no differences in the coiling response of M. charantia tendrils between a stick coated with calcium oxalate and one coated with agarose (electronic supplementary material, figure S3, deviance = 0.32, p = 0.67).
4. Discussion
The results of this study provide evidence of a new plant sensing ability: tendrils of C. japonica appear to exhibit contact chemoreception for oxalate compounds and achieve a flexible plastic response to avoid coiling around a conspecific leaf. Tendrils of climbing plants are highly touch sensitive and start the coiling response immediately after the contact stimuli [11,12]. The results indicated that tendrils which encountered the cues of a conspecific leaf could terminate their coiling response and change it even after coiling had begun (figure 2). The tendrils might be able to sense both the stimuli of physical contact and the chemical cues of the conspecific leaves at the same time, and they can properly integrate these two kinds of information in order to avoid coiling around conspecifics.
Coiling avoidance based on the content of oxalate compounds would be an effective way to avoid coiling around a conspecific leaf, because the leaves of C. japonica have a relatively high oxalate content. On the other hand, this chemical-sensing ability may have a negative side-effect; the tendrils would also avoid coiling around the leaves of high-oxalate heterospecific plants. Plants in the Chenopodiaceae, Polygonaceae and Dioscoreaceae families generally contain high concentrations of oxalate compounds in their leaves, tubers, roots and stems [24,25]. Such avoidance would represent a cost for C. japonica because these heterospecific plants are potentially suitable host plants. However, despite the potential cost of ‘misrecognition’, conspecific avoidance would still be advantageous for finding a suitable host because this perennial vine often grows at a high population density in its natural habitat [19,21].
Oxalate compounds are one of the major groups of organic acids in plants and function as a physical defence against herbivores [26], but they also ameliorate the toxic effect of aluminium [27] and act as reducing agents [25]. The results of the present study suggest that they also function as a chemical cue that identifies conspecific leaves. It appears that the tendrils are not responding to the acidity of the oxalates, because the tendrils coiled normally around a stick coated with citric acid. In addition, the inhibition of coiling by oxalate compounds is unlikely to have been caused by toxic or harmful effects on the tendril because oxalate compounds had no effect on the coiling response of the tendrils of M. charantia (Cucurbitaceae) (electronic supplementary material, figure S3). Rather, it appears that the tendrils of C. japonica have a physiological mechanism that allows them to detect and respond to oxalate compounds on the leaves, although I found no previous reports on oxalate receptors in plants.
Parasitic vine Cuscuta pentagona uses volatile blends to locate several host plants [15]. The twining response of Convolvulus arvensis is induced by the volatile cues from injured conspecific leaves [28,29]. These studies implies the importance of the volatile cues on the avoiding response of the tendrils of C. japonica to conspecifics. However, given the fact that oxalates are non-volatile compounds and that the tendril coiled normally around a conspecific leaf covered by a mesh (figure 1a), I can conclude that the oxalates were sensed by contact chemoreception, not by the chemoreception of volatile compounds.
In a previous study, we reported that the tendrils of the perennial vine C. japonica exhibit a self-discrimination ability [18]; tendrils of C. japonica were more likely to coil around stems of neighbouring non-self-plants than around those of physiologically connected self-plants. Although the physiological mechanism responsible for this self-discrimination was not identified, it must be different or independent from the oxalate-based avoidance revealed in this study because the self-discrimination was based on physiological integration; the tendrils were more likely to coil around stems from a physiologically severed self-plant than around those of physiologically connected plants.
In animals, conspecific avoiding behaviour plays a critical role in foraging, mating, species interactions, and spatial distribution [30–34]. The avoiding of conspecific leaves and preference of heterospecifics in vine tendrils can also play an important role in several ecological interactions. For example, conspecific avoidance in vines will reduce intra-specific competition and increase interspecific competition for light resources. It may also affect the growth pattern of vines because vines that have the ability to avoid conspecific leaves might have an exclusive distribution to the conspecific plants. As a result, conspecific avoidance of vines may accelerate the spatial expansion of vine vegetation and cause a negative impact on surrounding heterospecific vegetation.
These findings raise new questions regarding the physiological and molecular background of the chemical-sensing ability of plants: how do the tendrils detect conspecific cues by contacting the leaf? How do the tendrils stop coiling and reverse the coiling response after they detect conspecific cues? Why did the dried conspecific leaf lose cues of a conspecific leaf (figure 1a)? Answering these questions will expand our knowledge of plants' chemical-sensing ability and management of tendril-bearing weeds and vegetables. In addition, examining the chemical-sensing ability of vine tendrils will provide important insights into several ecological interactions. For example, conspecific avoidance may influence the growth direction of climbing plants and the resulting spatial pattern within the community structure. Thus, further studies are required to evaluate the generality, physiological mechanisms and ecological implications of this phenomenon.
Supplementary Material
Supplementary Material
Acknowledgements
I thank Akira Yamawo for help with sample collection. I thank Yuuya Tachiki and Takashi Kuriwada for reading the manuscript and providing helpful feedback. I thank the members of the Institute for Sustainable Agro-ecosystem Services for the helpful discussion and the support of the research.
Data accessibility
Data are available in the Dryad Data Repository: http://dx.doi.org/10.5061/dryad.p342d [35].
Competing interests
The author declares no competing interests.
Funding
This work was supported by the Sekisui Chemical Grant Program.
References
- 1.Heil M, Karban R. 2010. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 25, 137–144. ( 10.1016/j.tree.2009.09.010) [DOI] [PubMed] [Google Scholar]
- 2.Karban R. 2015. Plant sensing and communication. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Karban R, Shiojiri K. 2009. Self-recognition affects plant communication and defense. Ecol. Lett. 12, 502–506. ( 10.1111/j.1461-0248.2009.01313.x) [DOI] [PubMed] [Google Scholar]
- 4.de Wit M, Kegge W, Evers JB, Vergeer-van Eijk MH, Gankema P, Voesenek LACJ, Pierik R. 2012. Plant neighbor detection through touching leaf tips precedes phytochrome signals. Proc. Natl Acad. Sci. USA 109, 14 705–14 710. ( 10.1073/pnas.1205437109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kegge W, Pierik R. 2010. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 15, 126–132. ( 10.1016/j.tplants.2009.11.007) [DOI] [PubMed] [Google Scholar]
- 6.Karban R, Baldwin IT. 1997. Induced responses to herbivory. Chicago, IL: University Of Chicago Press. [Google Scholar]
- 7.Ballaré CL, Scopel AL, Sánchez RA. 1990. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247, 329–332. ( 10.1126/science.247.4940.329) [DOI] [PubMed] [Google Scholar]
- 8.Isnard S, Silk W. 2009. Moving with climbing plants from Charles Darwin's time into the 21st century. Am. J. Bot. 96, 1205–1221. ( 10.3732/ajb.0900045) [DOI] [PubMed] [Google Scholar]
- 9.Putz F, Mooney H. 1991. The biology of vines. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Jaffe MJ, Galston AW. 1968. The physiology of tendrils. Annu. Rev. Plant Physiol. 19, 417–434. ( 10.1146/annurev.pp.19.060168.002221) [DOI] [Google Scholar]
- 11.Darwin C. 1875. On the movements and habits of climbing plants. J. Linn. Soc. London Bot. 9, 1–118. ( 10.1111/j.1095-8339.1865.tb00011.x) [DOI] [Google Scholar]
- 12.Braam J. 2005. In touch: plant responses to mechanical stimuli. New Phytol. 165, 373–389. ( 10.1111/j.1469-8137.2004.01263.x) [DOI] [PubMed] [Google Scholar]
- 13.Darwin C. 1880. The power of movement in plants. London, UK: John Murray. [Google Scholar]
- 14.Gianoli E. 2015. The behavioural ecology of climbing plants. AoB Plants 7, 1–11. ( 10.1093/aobpla/plv013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Runyon J, Mescher M, Moraes CD. 2006. Volatile chemical cues guide host location and host selection by parasitic plants. Science 313, 1964–1967. ( 10.1126/science.1131371) [DOI] [PubMed] [Google Scholar]
- 16.Farrer EC, Goldberg DE. 2011. Patterns and mechanisms of conspecific and heterospecific interactions in a dry perennial grassland. J. Ecol. 99, 265–276. ( 10.1111/j.1365-2745.2010.01734.x) [DOI] [Google Scholar]
- 17.Wassmuth BE, Stoll P, Tscharntke T, Thies C. 2009. Spatial aggregation facilitates coexistence and diversity of wild plant species in field margins. Perspect. Plant Ecol. Evol. Syst. 11, 127–135. ( 10.1016/j.ppees.2009.02.001) [DOI] [Google Scholar]
- 18.Fukano Y, Yamawo A. 2015. Self-discrimination in the tendrils of the vine Cayratia japonica is mediated by physiological connection. Proc. R. Soc. B 282, 20151379 ( 10.1098/rspb.2015.1379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West A, Richardson R, Arellano C, Burton M. 2010. Bushkiller (Cayratia japonica) growth in interspecific and intraspecific competition. Weed Sci. 58, 195–198. ( 10.1614/WS-09-051.1) [DOI] [Google Scholar]
- 20.West A. 2009. Biology and management of bushkiller (Cayratia japonica). See https://repository.lib.ncsu.edu.
- 21.Hiyane S, Yamashiro S, Daiku M, Degi K, Takaesu Y. 2015. Foliange applied herbicides control of Cayratia tenuifolia (Wight & Arn) Gagnep in sugarcane (in Japanese). Rep. Kyushu Br. Crop Sci. Soc. 81, 54–57. [Google Scholar]
- 22.Najmaddin C, Hussin K, Maideen H. 2011. Comparative anatomical study between Cayratia mollissima, Pterisanthes caudigera (Vitaceae) and Leea indica (Leeaceae). Am. J. Appl. Sci. 8, 839–842. ( 10.3844/ajassp.2011.839.842) [DOI] [Google Scholar]
- 23.Hodgkinson A. 1970. Determination of oxalic acid in biological material. Metab. Clin. Exp. 16, 547–557. [Google Scholar]
- 24.Siener R, Hönow R, Seidler A, Voss S, Hesse A. 2006. Oxalate contents of species of the Polygonaceae, Amaranthaceae and Chenopodiaceae families. Food Chem. 98, 220–224. ( 10.1016/j.foodchem.2005.05.059) [DOI] [Google Scholar]
- 25.Libert B, Franceschi VR. 1987. Oxalate in crop plants. J. Agric. Food Chem. 35, 926–938. ( 10.1021/jf00078a019) [DOI] [Google Scholar]
- 26.Konno K, Inoue TA, Nakamura M. 2014. Synergistic defensive function of raphides and protease through the needle effect. PLoS ONE 9, e91341 ( 10.1371/journal.pone.0091341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Z, Miyasaka SC. 1998. Oxalate exudation by taro in response to Al. Plant Physiol. 118, 861–865. ( 10.1104/pp.118.3.861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atala C, Gianoli E. 2008. Induced twining in Convolvulaceae climbing plants in response to leaf damage. Botany 86, 595–602. ( 10.1139/B08-037) [DOI] [Google Scholar]
- 29.Atala C, Quilodrán M, Molina-Montenegro MA. 2014. Induced twining in Ipomoea purpurea (L.) Roth.: response threshold and induction by volatiles and snail damage. Gayana Bot. 71, 181–187. ( 10.4067/S0717-66432014000200001) [DOI] [Google Scholar]
- 30.Reynolds AM. 2007. Avoidance of conspecific odour trails results in scale-free movement patterns and the execution of an optimal searching strategy. Europhys. Lett. 79, 30006 ( 10.1209/0295-5075/79/30006) [DOI] [Google Scholar]
- 31.Guy AG, Bohan DA, Powers SJ, Reynolds AM. 2008. Avoidance of conspecific odour by carabid beetles: a mechanism for the emergence of scale-free searching patterns. Anim. Behav. 76, 585–591. ( 10.1016/j.anbehav.2008.04.004) [DOI] [Google Scholar]
- 32.Franks NR, Dornhaus A, Hitchcock G, Guillem R, Hooper J, Webb C. 2007. Avoidance of conspecific colonies during nest choice by ants. Anim. Behav. 73, 525–534. ( 10.1016/j.anbehav.2006.05.020) [DOI] [Google Scholar]
- 33.Goulson D, Hawson S, Stout J. 1998. Foraging bumblebees avoid flowers already visited by conspecifics or by other bumblebee species. Anim. Behav. 55, 199–206. ( 10.1006/anbe.1997.0570) [DOI] [PubMed] [Google Scholar]
- 34.Persaud KN, Galef BG. 2003. Female Japanese quail aggregate to avoid sexual harassment by conspecific males: a possible cause of conspecific cueing. Anim. Behav. 65, 89–94. ( 10.1006/anbe.2002.2057) [DOI] [Google Scholar]
- 35.Fukano Y. 2017. Data from: Vine tendrils use contact chemoreception to avoid conspecific leaves. Dryad Digital Repository. ( 10.5061/dryad.p342d) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fukano Y. 2017. Data from: Vine tendrils use contact chemoreception to avoid conspecific leaves. Dryad Digital Repository. ( 10.5061/dryad.p342d) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available in the Dryad Data Repository: http://dx.doi.org/10.5061/dryad.p342d [35].